Key Points
Question
In patients with acute ischemic stroke, does mobile stroke unit (MSU) use lead to better functional outcomes than usual care?
Findings
In this systematic review and meta-analysis including 14 articles, MSU use was significantly associated with an approximately 65% increase in the odds of excellent outcome at 90 days after adjustment for potential confounders, a higher proportion of intravenous thrombolysis, and a reduction of half an hour in onset-to-thrombolysis time, without safety concerns.
Meanings
These results provide evidence that MSU use improves functional outcome compared with usual care and may help guideline writing committees and decision makers to organize prehospital care.
This systematic review and meta-analysis investigates whether mobile stroke unit use is associated with better functional outcomes in patients with acute ischemic stroke.
Abstract
Importance
So far, uncertainty remains as to whether there is sufficient cumulative evidence that mobile stroke unit (MSU; specialized ambulance equipped with computed tomography scanner, point-of-care laboratory, and neurological expertise) use leads to better functional outcomes compared with usual care.
Objective
To determine with a systematic review and meta-analysis of the literature whether MSU use is associated with better functional outcomes in patients with acute ischemic stroke (AIS).
Data Sources
MEDLINE, Cochrane Library, and Embase from 1960 to 2021.
Study Selection
Studies comparing MSU deployment and usual care for patients with suspected stroke were eligible for analysis, excluding case series and case-control studies.
Data Extraction and Synthesis
Independent data extraction by 2 observers, following the PRISMA and MOOSE reporting guidelines. The risk of bias in each study was determined using the ROBINS-I and RoB2 tools. In the case of articles with partially overlapping study populations, unpublished disentangled results were obtained. Data were pooled in random-effects meta-analyses.
Main Outcomes and Measures
The primary outcome was excellent outcome as measured with the modified Rankin Scale (mRS; score of 0 to 1 at 90 days).
Results
Compared with usual care, MSU use was associated with excellent outcome (adjusted odds ratio [OR], 1.64; 95% CI, 1.27-2.13; P < .001; 5 studies; n = 3228), reduced disability over the full range of the mRS (adjusted common OR, 1.39; 95% CI, 1.14-1.70; P = .001; 3 studies; n = 1563), good outcome (mRS score of 0 to 2: crude OR, 1.25; 95% CI, 1.09-1.44; P = .001; 6 studies; n = 3266), shorter onset-to-intravenous thrombolysis (IVT) times (median reduction, 31 minutes [95% CI, 23-39]; P < .001; 13 studies; n = 3322), delivery of IVT (crude OR, 1.83; 95% CI, 1.58-2.12; P < .001; 7 studies; n = 4790), and IVT within 60 minutes of symptom onset (crude OR, 7.71; 95% CI, 4.17-14.25; P < .001; 8 studies; n = 3351). MSU use was not associated with an increased risk of all-cause mortality at 7 days or at 90 days or with higher proportions of symptomatic intracranial hemorrhage after IVT.
Conclusions and Relevance
Compared with usual care, MSU use was associated with an approximately 65% increase in the odds of excellent outcome and a 30-minute reduction in onset-to-IVT times, without safety concerns. These results should help guideline writing committees and policy makers.
Introduction
In patients with acute ischemic stroke (AIS), the effectiveness of intravenous thrombolysis (IVT)1 and mechanical thrombectomy (MT)2 is highly time dependent (“save a minute, save a day”),3,4,5 and shortening delays to start of such reperfusion therapies has become a major target in acute stroke management around the globe.6,7 While improved organization of in-hospital procedures has led to massive reductions in door-to-needle8 and door-to-puncture9 times, telemedicine networking is increasingly used to avoid delays through interhospital transfers from hospitals with no full-time neurovascular expertise available onsite.10 Yet none of these approaches address the prehospital phase of stroke management. This prompted the proof of a concept of shifting specialized stroke workup and treatment into the prehospital setting by introducing specialized ambulances equipped with computed tomography (CT) scanners, point-of-care laboratory, telemedicine connection, and neurological expertise on mobile stroke units (MSUs).11,12 Safety and effects on shortening time to thrombolysis have been investigated across MSU implementations in different settings and countries.12,13,14,15,16,17,18 Although time savings were substantial, it remained unclear whether and to what extent earlier treatment would translate to better clinical outcomes.19 Recently published trials comparing MSU care with conventional care20,21 are now providing answers to these urgent questions. To inform clinicians and decision makers as to the magnitude of benefit from shifting acute stroke management into the prehospital phase of stroke using MSUs and whether MSU implementation should be included in guidelines, we performed a systematic review and meta-analysis of all randomized clinical trials (RCTs) and controlled studies providing evidence for comparison of MSU vs usual care in patients with acute ischemic stroke.
Methods
This study was registered in PROSPERO (CRD42021276038) and prepared in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA)22 and the Meta-analysis of Observational Studies in Epidemiology (MOOSE)23 reporting guidelines. All data necessary to other researchers for purposes of reproducing our results are provided in the present work. Unless specified otherwise, the population of interest for efficacy analyses was patients with confirmed AIS. Functional outcomes were assessed with the mRS score at 90 days.24 Our primary end point was excellent outcome (mRS score of 0 to 1).25,26,27,28,29 Secondary efficacy outcomes were good outcome (mRS score of 0 to 2), reduced disability (improvement of 1 or more points over the whole range of the mRS), the proportion of patients treated with IVT, and the proportion of golden-hour thrombolysis (IVT started within 60 minutes of symptom onset) among IVT-treated patients. The following time metrics were assessed in patients treated with reperfusion therapies: duration from symptom onset (or time last known well) to IVT bolus administration (onset-to-IVT time) or arterial puncture (onset-to-MT time), and duration from emergency medical service (EMS) first ambulance dispatch activation to IVT bolus (alarm-to-IVT time) or arterial puncture time (alarm-to-MT time). The population of interest for safety analyses were all patients with suspected stroke. Safety outcomes were all-cause mortality at 7 and 90 days, symptomatic intracranial hemorrhage (according to the definition of each study) among IVT-treated patients, and the proportion of IVT-treated patients ultimately diagnosed as stroke mimics.
Literature Search, Study Selection, Data Extraction, and Quality Assessment
We searched MEDLINE, Cochrane Library, and Embase for articles published up to September 2021 using the following combination of keywords: “mobile,” “stroke,” “unit,” “treatment,” “ambulance,” and “prehospital” (eMethods in the Supplement). Controlled studies comparing MSU deployment and usual care for prehospital management of adult patients with suspected acute ischemic stroke (less than 6-hour duration) were eligible for inclusion. Prospective and retrospective studies with a contemporaneous or historical control group could be included, but the analyses were stratified by study design. Exclusion criteria were lack of a control group, indirect comparison based on a different population, studies focusing on intracranial hemorrhage, case series, case-control studies, and conference abstracts. In the case of articles with partially overlapping sets of participants, we obtained disentangled results from the investigators so that no patient was counted twice.
Two investigators (G. Turc and M.H.) independently screened the abstracts of all records, excluded irrelevant articles, and obtained the full texts of the remaining articles. Using standardized forms, relevant data were independently extracted by the same investigators in duplicate. Whenever needed, we contacted the authors of included studies to obtain unpublished results regarding outcomes of interest that had been prospectively collected but not reported in the original publication. The risk of bias of individual studies was assessed by 2 authors (G. Turc and M.H.) who did not take part in any included study, using the Cochrane’s Risk of Bias 2 (RoB2) and the Risk of Bias in Non-Randomized Studies of Interventions (ROBINS-I) tools for the RCTs and the nonrandomized studies, respectively.
Statistical Analysis
The principal summary measure for binary outcomes was the odds ratio (OR) and the corresponding 95% CI. Associations between treatment and reduced disability (improvement of 1 or more points over the whole range of the mRS at 90 days) were described and summarized as common ORs. To limit the risk of confounding, we favored pooling adjusted ORs whenever possible. In case the OR for a binary or ordinal outcome was not mentioned in the original publication, we calculated it using crude logistic regression based on raw published numbers. The Firth correction was used to compute the OR if zero event was observed in one of the treatment groups. Regarding time metrics, we hypothesized that most results in published studies would be reported as median (IQR) rather than mean (SD) because of a nonnormal underlying distribution. Therefore, we decided to use the difference of medians as principal summary measure for all time metrics. We used the quantile estimation method to estimate the underlying distribution and the variance of the difference of medians in each study, as described by McGrath et al.30 We decided a priori to use random-effects models to calculate pooled estimates because we assumed that the true effect size may differ across studies. The DerSimonian and Laird method was used for all analyses. Heterogeneity across studies was assessed using Cochran Q test (reported as a P value) and the I2 statistics. Heterogeneity was classified as moderate (I2 of 30% or more), substantial (I2 of 50% or more), or considerable (I2 of 75% or more).31 Potential sources of heterogeneity were investigated by stratifying studies according to their design (3 predefined subgroups): RCTs, large (more than 500 participants) prospective controlled nonrandomized studies with blinded assessment of functional outcome, and other observational studies; stratifying studies according to their population (inclusion/exclusion of stroke mimics; inclusion/exclusion of patients not treated with IVT). Sensitivity analyses including all patients with available 90-day mRS scores (irrespective of the final diagnosis) and excluding studies with historical controls were conducted. Potential publication bias was visually investigated using funnel plots. Whenever appropriate, the rank correlation test32 and the Egger regression test,33 using the standard error of the observed outcomes as predictor, were used to check for funnel plot asymmetry. All statistical tests were 2-tailed, and significance was set at P < .05. Statistical analysis was performed using R version 4.1.1 (R Foundation for Statistical Computing) and Stata version 16.0 (StataCorp).
Results
The PRISMA flowchart of literature search and study selection is presented in eFigure 1 in the Supplement. We identified 14 articles meeting the inclusion criteria. Of these, 4 articles reported results of 3 RCTs (2 articles reported results of different outcomes of the Pre-Hospital Acute Neurological Therapy and Optimization of Medical Care in Stroke Patients - Study [PHANTOM-S] trial),12,34,35,36 2 corresponded to large prospective nonrandomized intervention studies with blinded assessment of functional outcome (Berlin Prehospital Or Usual Delivery of Acute Stroke Care [B_PROUD]20 and Benefits of Stroke Treatment Delivered Using a Mobile Stroke Unit [BEST-MSU]21), and 8 to other observational studies.13,16,37,38,39,40,41,42 No study featured randomization at the patient level, as the intervention to be applied to patients enrolled in each of the 3 RCTs was randomly assigned week-wise (cluster-randomized trials).12,34,35 All but 3 studies13,38,40 used a contemporaneous control group. There was no overlap in participants across studies, except regarding published results from the Berlin group.13,34,36,37 We therefore disentangled the different study cohorts from Berlin based on individual participant data and only considered results from nonoverlapping sets of participants for meta-analyses. Key features of included studies are summarized in eTable 1 in the Supplement.
Functional Outcome
Functional outcome at 90 days (mRS score), assessed in a blinded fashion, was the primary outcome of 2 large prospective nonrandomized controlled studies, B_PROUD and BEST-MSU.20,21 According to the ROBINS-I tool, we concluded that these 2 studies were at low risk of bias (eFigure 2 in the Supplement). Excellent outcome was also available in 3 other studies.16,35,37 The meta-analysis of these 5 studies (3228 patients) suggested that MSU was superior to usual care regarding excellent outcome in adjusted (pooled OR, 1.64; 95% CI, 1.27-2.13; P < .001; I2 = 48%; Table; Figure 1; eTable 2 in the Supplement) and crude analyses (pooled OR, 1.37; 95% CI, 1.19-1.58; I2 = 0%; P < .001; eFigure 3 in the Supplement). Of note, there were some differences in study populations. In B_PROUD, the main analysis was based on patients with a final diagnosis of AIS or transient ischemic attack (TIA) with neurological symptoms at EMS arrival irrespective of treatment with IVT, whereas BEST-MSU focused on patients deemed to be eligible for IVT, some of whom had a final diagnosis of stroke mimic. In contrast, patients with AIS, TIA, intracranial hemorrhage, or stroke mimic were eligible for the RCT by Helwig et al,35 provided that vascular imaging was available. Finally, only patients treated with IVT for AIS were included in the study by Kunz et al,37 whereas Larsen et al16 only collected 90-day mRS scores for patients treated with IVT, irrespective of the final diagnosis. Pooled ORs were similar between studies including or excluding stroke mimics and between studies including or excluding patients not treated with IVT (data not shown). In a sensitivity analysis including all patients irrespective of the final diagnosis and for whom 90-day mRS scores were available, MSU use remained significantly associated with excellent outcome (pooled crude OR, 1.37; 95% CI, 1.21-1.56; P < .001; I2 = 0; 5 studies16,20,21,35,37; 4028 patients).
Table. Summary of Findings (Mobile Stroke Unit vs Usual Care).
| Outcome | Pooled effect size (95% CI) | P value | I2, % | Studies (patients), No. |
|---|---|---|---|---|
| Excellent outcome, mRS score of 0-1 at 90 d | Adjusted OR, 1.64 (1.27-2.13) | <.001 | 48 | 5 (3228)16,20,21,35,37 |
| Crude OR, 1.37 (1.19-1.58) | <.001 | 0 | 5 (3228)16,20,21,35,37 | |
| Reduced disability, lower mRS score at 90 d | Adjusted common OR, 1.39 (1.14-1.70) | .001 | 0 | 3 (1563)16,20,35 |
| Crude common OR, 1.30 (1.12-1.50) | .001 | 21 | 5 (3228)16,20,21,35,37 | |
| Good outcome, mRS score of 0-2 at 90 d | Crude OR, 1.25 (1.09-1.44) | .001 | 0 | 6 (3266)16,20,21,35,37,41 |
| Proportion of IVT among patients with AIS | Crude OR, 1.83 (1.58-2.12) | <.001 | 13 | 7 (4790)12,16,20,21,34,35,39 |
| IVT within the golden hour | Crude OR, 7.71 (4.17-14.25) | <.001 | 75 | 8 (3351)16,20,21,36,37,38,40,42 |
| Onset-to-IVT time, min | Difference of medians, 31 (23-39) | <.001 | 47 | 13 (3322)12,13,16,20,21,34,35,37,38,39,40,41,42 |
| Alarm-to-IVT time, min | Difference of medians, 29 (25-33) | <.001 | 78 | 13 (3319)12,13,16,20,21,34,35,37,38,39,40,41,42 |
| Onset-to-MT time, min | Difference of medians, 27 (–17 to 71) | .23 | 86 | 5 (666)16,20,21,35,40 |
| Alarm-to-MT time, min | Difference of medians, 14 (–15 to 43) | .35 | 89 | 5 (671)16,20,21,35,40 |
| Death | ||||
| 7 d | Crude OR, 0.74 (0.51-1.09) | .13 | 33 | 9 (8599)12,13,16,20,21,34,35,37,42 |
| 90 d | Crude OR, 0.82 (0.58-1.17) | .28 | 56 | 7 (3924)16,20,21,34,37,41,42 |
| Symptomatic intracranial hemorrhage after IVT | Crude OR, 0.80 (0.52-1.24) | .32 | 0 | 5 (1977)16,20,37,41,42 |
| Stroke mimic among IVT-treated patients | Crude OR, 1.22 (0.70-2.14) | .48 | 0 | 3 (666)16,34,39 |
Abbreviations: IVT, intravenous thrombolysis; mRS, modified Rankin Scale; MT, mechanical thrombectomy; OR, odds ratio.
Figure 1. Pooled Adjusted Odds Ratio (OR) for Excellent Outcome at 90 Days (Modified Rankin Scale Score of 0 to 1) in Patients With Mobile Stroke Unit (MSU) Deployment vs Usual Care (Random-Effects Meta-analysis).
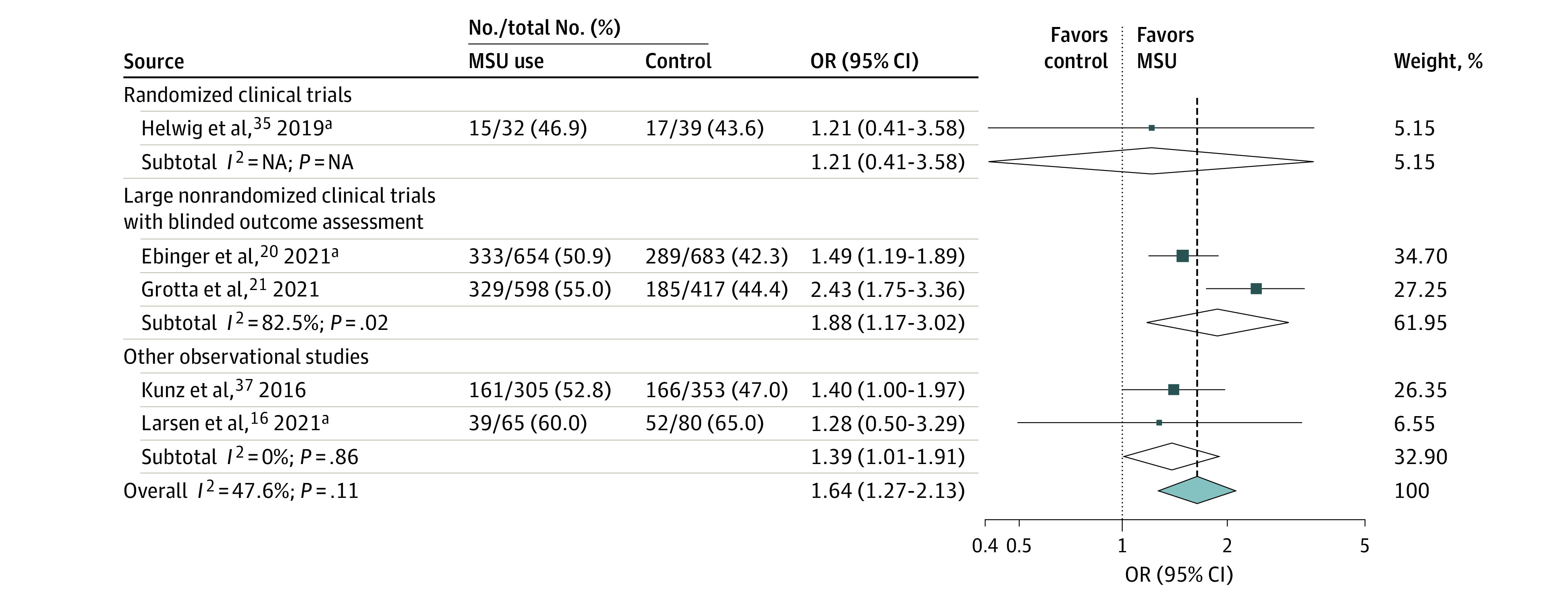
Results are expressed as number of patients with a modified Rankin Scale score of 0 to 1 divided by the total number of patients in each treatment group. Adjustment variables differed across studies (eTable 2 in the Supplement). The test for heterogeneity between subgroups was P = .39. NA indicates not applicable.
aPreviously unpublished data, excluding patients with stroke mimic in the study by Larsen et al.16 When including patients with stroke mimic, the adjusted OR for excellent outcome in the study by Larsen et al16 was 1.45 (95% CI, 0.60-3.51). Results from Helwig et al35 correspond to a post hoc analysis based on individual participant data, with adjustment on age and baseline National Institutes of Health Stroke Scale score.
The pooled common OR for reduced disability was 1.39 (95% CI, 1.14-1.70; P = .001; I2 = 0%) in adjusted analysis (3 studies16,20; 1563 patients) and 1.30 (95% CI, 1.12-1.50; P = .001; I2 = 21%) in unadjusted analysis (5 studies16,20,21,37; 3228 patients; eFigure 4 in the Supplement). Data on good outcome were available in 6 studies.16,20,21,35,37,41 The corresponding pooled crude OR was 1.25 (95% CI, 1.09-1.44; P = .001; I2 = 0%; 3266 patients; eFigure 5 in the Supplement).
Other Efficacy End Points
Data on the proportion of IVT among patients with AIS were provided in 7 studies comprising 4790 patients.12,16,20,21,34,35 The pooled crude OR for MSU vs usual care regarding this end point was 1.83 (95% CI, 1.58-2.12; P < .001; I2 = 13%; Figure 2). The proportion of golden-hour thrombolysis among IVT-treated patients was reported in 8 studies (3351 patients),16,20,21,36,37,38,40 with a pooled crude OR of 7.71 (95% CI, 4.17-14.25; P < .001; I2 = 75%; Figure 3).
Figure 2. Pooled Crude Odds Ratio (OR) Associated With the Proportions of Intravenous Thrombolysis Among Patients With Acute Ischemic Stroke According to Treatment Group (Mobile Stroke Unit [MSU] Deployment vs Usual Care, Random-Effects Meta-analysis).
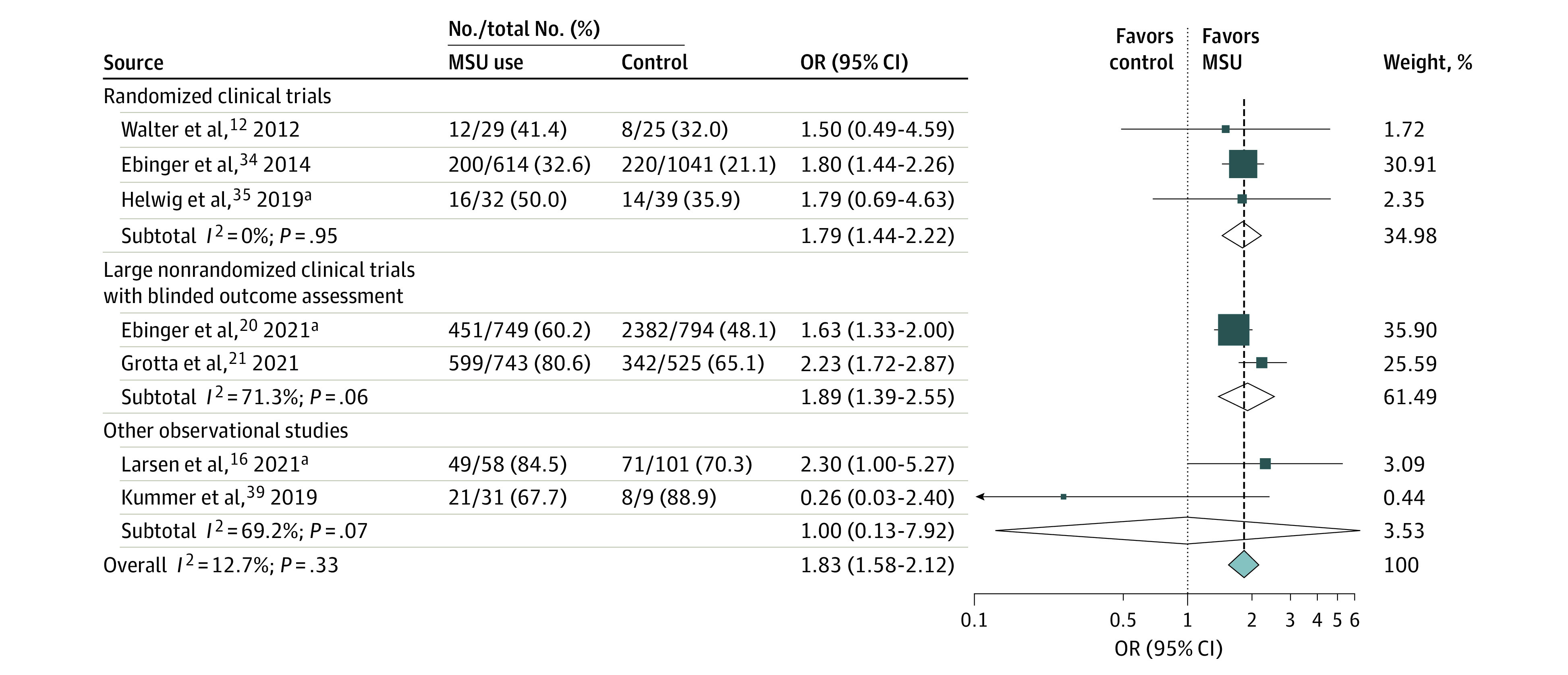
Results are expressed as number of patients treated with intravenous thrombolysis divided by the number of patients with acute ischemic stroke in each treatment group. The test for heterogeneity between subgroups was P = .98.
aPreviously unpublished data, excluding patients with stroke mimic in the study by Larsen et al.16
Figure 3. Pooled Crude Odds Ratio (OR) Associated With the Proportions of Golden-Hour Intravenous Thrombolysis According to Treatment Group (Mobile Stroke Unit [MSU] Deployment vs Usual Care, Random-Effects Meta-analysis).
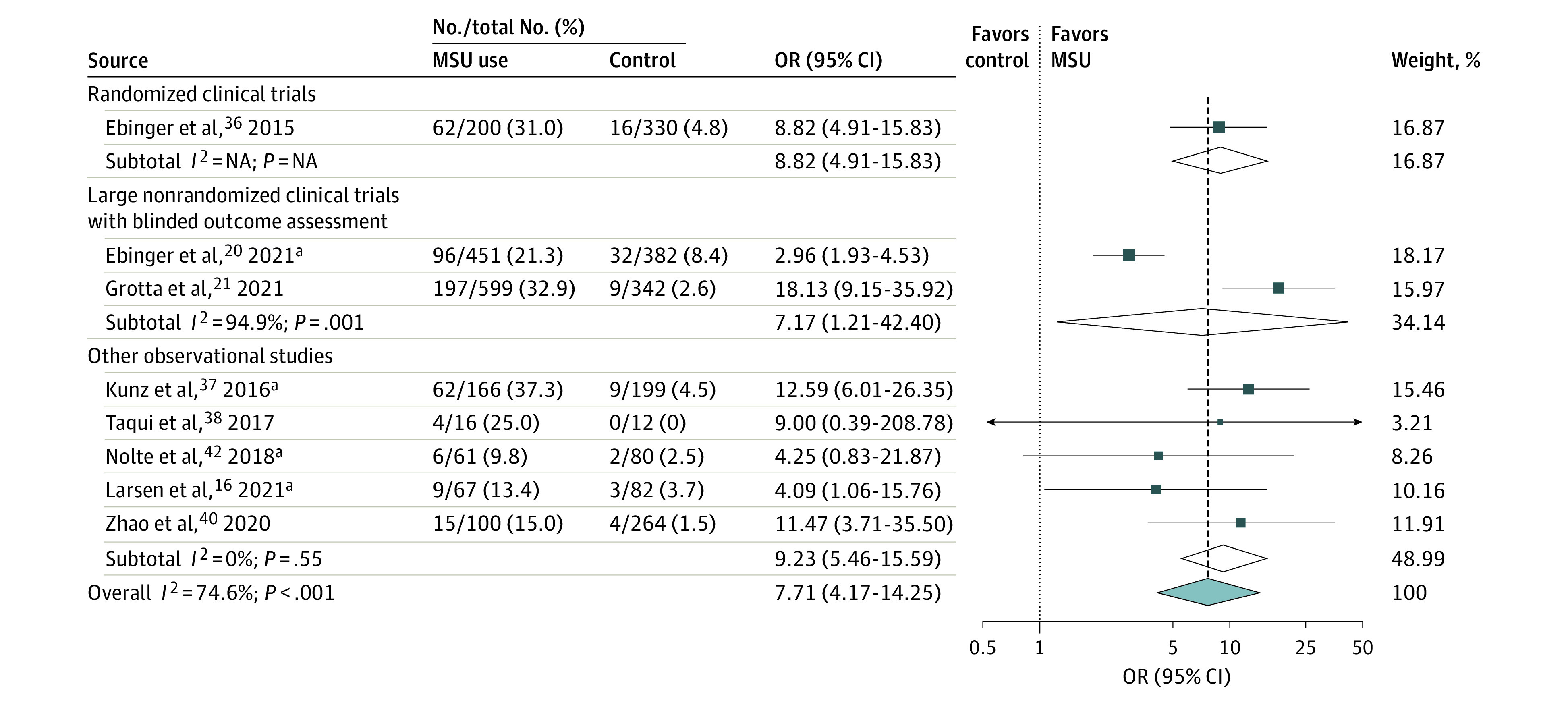
Results are expressed as number of patients treated with intravenous thrombolysis within 60 minutes of symptom onset divided by number of patients treated with intravenous thrombolysis in each treatment group. The test for heterogeneity between subgroups was P = .08. NA indicates not applicable.
aPreviously unpublished (disentangled) data, excluding patients with stroke mimic in the study by Larsen et al.16 When including patients with stroke mimic, the crude OR for golden-hour thrombolysis in the study by Larsen et al16 was 4.62 (95% CI, 1.46-14.59). Furthermore, there was partial overlap in participants between the studies by Ebinger et al,36 Kunz et al,37 and Nolte et al.42
Time Metrics
The meta-analyses of the association of MSU deployment vs usual care with time metrics related to IVT comprised 3322 patients from 13 studies (Table; eFigure 6 in the Supplement).12,13,20,21,34,35,37,38,39,40,41,42 The pooled median reduction in onset-to-IVT time was 31 minutes (95% CI, 23-39; P < .001; I2 = 47%; Figure 4), without evidence of publication bias (eFigure 7 in the Supplement). Excluding studies with historical control groups led to similar results. When considering only the 8 studies focusing on patients with a final diagnosis of AIS (n = 2024), the pooled median reduction in onset-to-IVT time was 26 minutes (95% CI, 16-36; P < .001; I2 = 41%). The pooled median reduction in alarm-to-IVT was 29 minutes (95% CI, 25-33; P < .001; I2 = 78%; eFigure 8 in the Supplement), without evidence of publication bias (eFigure 9 in the Supplement).
Figure 4. Pooled Difference of Medians of Time From Symptom Onset or Last Known Well to Intravenous Thrombolysis in Patients With Mobile Stroke Unit (MSU) Deployment vs Usual Care (Random-Effects Meta-analysis).
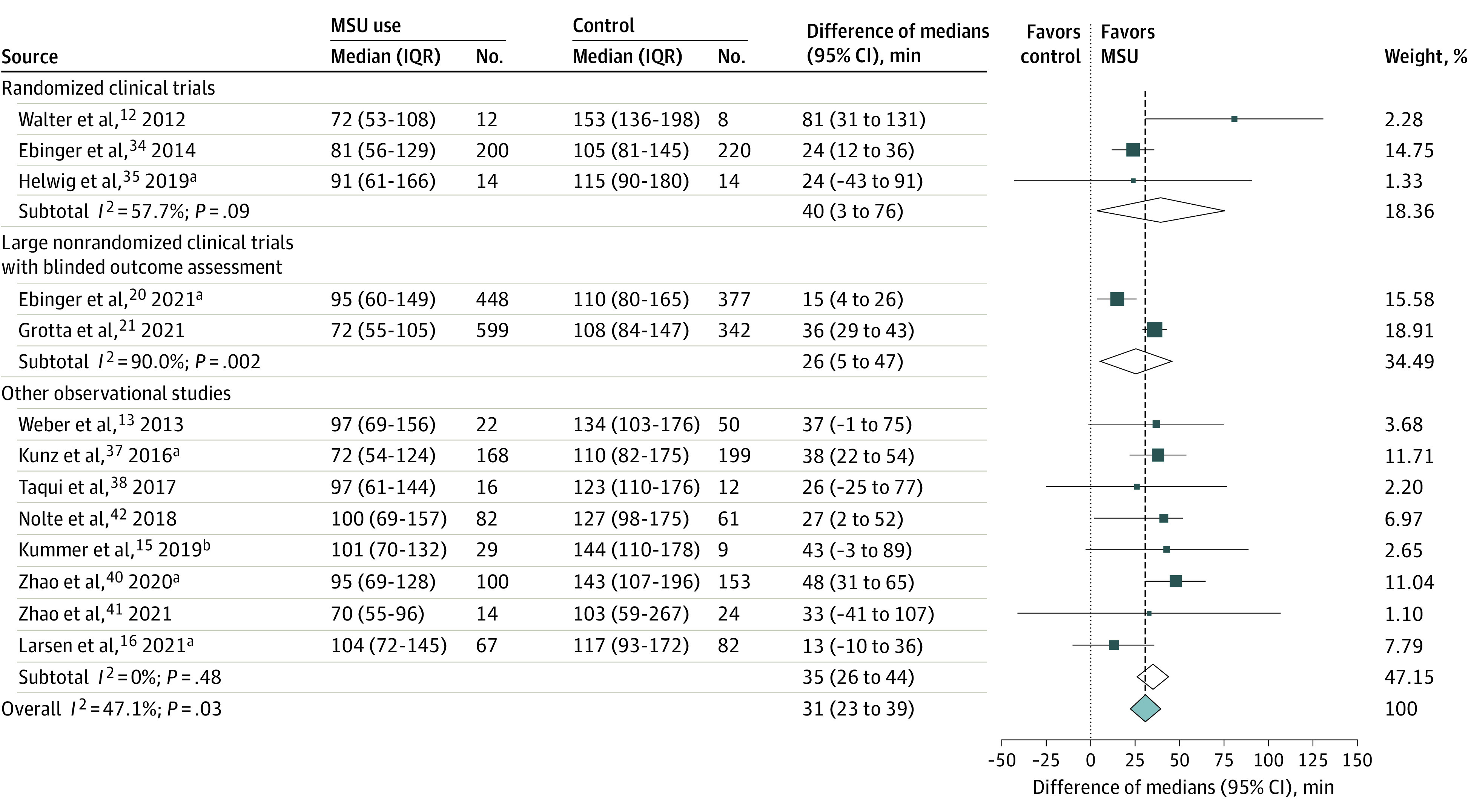
The test for heterogeneity between subgroups was P = .48.
aPreviously unpublished (disentangled) data. There was partial overlap in participants between the PHANTOM-S study34 and the study by Kunz et al.37
bEstimated from published mean (SD) values (101 [46] minutes vs 144 [50] minutes),39 assuming normal distribution.
The meta-analyses of the association of MSU deployment vs usual care with time metrics related to MT comprised 671 patients from 5 studies (Table).16,20,21,35,40 There was no overall evidence of a significant reduction in onset-to-MT time (pooled median difference, 27 minutes; 95% CI, –17 to 71; P = .23; I2 = 86%; eFigure 10 in the Supplement). The pooled median reduction in alarm-to-MT time was 14 minutes (95% CI, –15 to 43; P = .35). However, there was a considerable heterogeneity across studies, with the small RCT by Helwig et al35 and the observational study by Zhao et al40 suggesting a potential benefit of MSU in reducing alarm-to-MT time, whereas the BEST-MSU21 and B_PROUD20 studies pointed toward the opposite direction (I2 = 89%; P for heterogeneity < .001; eFigure 11 in the Supplement).
Safety Outcomes
All-cause mortality at 7 days was obtained from 9 studies comprising 8599 patients12,13,16,20,21,34,35,37 and did not differ between treatment groups (pooled crude OR, 0.74; 95% CI, 0.51-1.09; P = .13; I2 = 33%; Table). However, significant heterogeneity between population study subgroups was observed: MSU was associated with lower all-cause mortality at 7 days in studies focusing on IVT-treated patients (pooled crude OR, 0.45; 95% CI, 0.27-0.76; I2 = 0%) but not in studies also including patients not treated with IVT (pooled crude OR, 0.97; 95% CI, 0.64-1.45; I2 = 22%) (P for interaction = .01; eFigure 12 in the Supplement).
All-cause mortality at 90 days, reported in 7 studies comprising 3924 patients, did not differ between treatment groups (pooled crude OR, 0.82; 95% CI, 0.58-1.17; P = .28; I2 = 56%; Table). No heterogeneity was observed between studies including or excluding stroke mimics (eFigure 13 in the Supplement). Symptomatic intracranial hemorrhage among IVT-treated patients did not significantly differ between treatment groups (MSU vs usual care: pooled crude OR, 0.80; 95% CI, 0.52-1.24; P = .32; I2 = 0%; 1977 patients from 5 studies;16,20,37,41,42; eFigure 14 in the Supplement). Finally, the proportion of stroke mimics among patients treated with IVT was not different after MSU deployment or usual care (pooled crude OR, 1.22; 95% CI, 0.70-2.14; P = .48; I2 = 0%; 666 patients from 3 studies;16,34,39; Table).
Discussion
Compared with usual care, MSU use was consistently associated with higher probabilities of excellent outcome and reduced disability at 90 days, in adjusted and crude analyses. MSU deployment was associated with a reduction of approximately half an hour in both onset-to-IVT and alarm-to-IVT times and also led to a higher proportion of IVT among patients with AIS, of whom up to one-third were treated within 60 minutes of symptom onset. Finally, this meta-analysis did not suggest significant safety concerns associated with MSU use.
The reported symptom onset-to-IVT times in the intervention group of MSU studies, ranging from 70 to 104 minutes, were much shorter than any onset-to-IVT times reported from hospital-based thrombolysis registries.8,43,44,45 The median reduction of approximately 30 minutes in onset-to-IVT time translated into clearly better functional outcomes of patients candidates for IVT in the large prospective controlled outcome studies.20,21 The extent of these outcome improvements with a pooled OR for excellent outcome of 1.64 (95% CI, 1.24-2.13) for MSU compared with usual care may appear surprisingly high, given that the pooled OR for excellent outcome in the recent meta-analysis of RCTs of IVT vs control was only 1.28 (95% CI, 1.15-1.42).46 However, treating patients in an MSU currently seems to be the only realistic way to treat a substantial proportion of patients within 60 minutes after stroke onset.36,37 Previously, little was known about the effects of thrombolysis in this hyperacute time window, and the standard time-is-brain relationship curve was shown starting at 60 minutes after symptom onset.29 All available subgroup analyses in MSU studies20,21,36 have shown that the beneficial effects of IVT treatment in this golden hour are much higher than in the time windows beyond the first hour that were eventually evaluated in the RCTs of IVT vs control.47,48 Of note, MSU use was significantly associated with a lower 7-day mortality rate in studies focusing on IVT-treated patients. This association did not reach significance when considering mortality at 90 days, but our analysis lacked statistical power for this outcome. There may be other effects than faster IVT treatment contributing to better outcome through patient management in MSU. For example, the prehospital diagnosis of stroke subtype allows for earlier blood pressure management and better routing to the most appropriate hospital.49,50
We did not find evidence of a reduction in MT-related metrics with MSU use and even observed longer alarm-to-MT times in the subgroup of large prospective controlled studies (B_PROUD and BEST-MSU). This association was no longer significant when considering mean times instead of median times in a post hoc analysis (eFigure 15 in the Supplement). Specific criteria for performing CT angiography in the MSU were unfortunately not detailed, may not have been standardized, and are likely to slightly differ across studies (eMethods in the Supplement). A considerable heterogeneity across included studies was observed for MT-related metrics, which reflects different management strategies at hospital arrival—ie, whether the patients are delivered directly to the angiography suite or to the emergency department with repeated imaging, which was done in most hospitals in the B_PROUD and BEST-MSU studies. One reason for repeated imaging is that most MSUs cannot image the extracranial circulation. This issue may be resolved in the future by using MSUs equipped with head/neck CT angiography that can substantially increase the yield of mechanical thrombectomy by bypassing the emergency department.51
Strengths and Limitations
The main strength of this review is the use of all published data with consistent findings across studies. An added strength is the collection of unpublished results from peer-reviewed publications, which allowed us to obtain a more comprehensive and homogeneous analysis of clinically relevant outcomes, disentangle the results of several studies with partially overlapping sets of participants,52 and conduct adjusted analyses of functional outcome, therefore reducing the risk of confounding.
Several limitations need to be considered. First, the current meta-analysis explores study results in a still young field of research with limited available literature. Nevertheless, the numbers reported are substantial and the associations with time metrics and functional outcome are consistent. Second, the assessed functional outcomes largely rely on 2 nonrandomized large prospective controlled studies that used alternating weeks and availability of MSU for allocation of patients. Randomization at the patient level appeared indeed unfeasible in the prehospital setting of acute stroke. Based on the ROBINS-I tool, we concluded that the overall risk of bias for these 2 studies was low. The chosen method of allocation cannot completely exclude bias in sample composition, eg, by including more patients with TIA in one arm. However, in both studies, precautions were implemented to avoid such bias by not excluding patients with ischemic stroke or TIA who had reversible symptoms at first ambulance arrival in B_PROUD and by adjudicating patients qualifying for IVT at time of ambulance arrival in BEST-MSU. Third, our study has the limitations of aggregate data meta-analyses. In particular, the definitions of some end points, such as symptomatic intracranial hemorrhage, varied across studies. However, the assessment of functional outcome was based on the mRS in all studies, including those patients with secondary intracranial hemorrhage. Fourth, the current meta-analysis is mainly focused on the outcome of patients with ischemic stroke, although the approach of MSU care will always also affect patients with nonischemic stroke or stroke mimics. While the available data do not allow conclusions of beneficial effects for patients without AIS, they do not suggest an increased risk of adverse outcome in patients with hemorrhagic stroke or those without stroke.12,34,53 Furthermore, MSU use remained significantly associated with better functional outcomes when analyzing all enrolled patients, including stroke mimics and intracranial hemorrhages, in the BEST-MSU study.21 Also, PHANTOM-S and B-PROUD included patients with MSU cancellation (20% and 26%, respectively) in their analyses and still found favorable MSU outcomes using this intention-to-treat approach. Fifth, most of the data used in this meta-analysis are derived from studies conducted in metropolitan areas with well-established EMS. Hence, the results may not be generalizable to nonurban sites or to countries without established EMS infrastructure. Sixth, our meta-analysis did not take into account health economics’ aspects, given that MSUs are associated with sizeable investment and sustainment costs.54,55,56 As health economics analyses are part of the study protocols of the recent large prospective controlled studies,21,57 the respective evaluations are expected in the near future.
Conclusions
In conclusion, compared with usual care, MSU use was associated with an approximately 65% increase in the odds of excellent outcome, a higher proportion of treatment with IVT, and a 30-minute reduction in onset-to-IVT times, without safety concerns. These results should help guideline writing committees and decision makers to shape the future of prehospital stroke care. However, MSU implementation is associated with costs and requires optimal integration into regional emergency response services. Further studies will be needed to determine in which local environments the deployment of MSUs would be the most useful.
eMethods.
eTable 1. Key features of included studies.
eTable 2. Adjustment variables in each study reporting adjusted results for excellent outcome or reduced disability.
eFigure 1. PRISMA flow chart.
eFigure 2. Risk of bias of each included study for excellent functional outcome (mRS score of 0-1 at 90 days), according to the RoB2 tool for cluster-randomized trials (panel A) and the ROBINS-I tool for nonrandomized studies (panel B).
eFigure 3. Pooled odds ratio for excellent outcome (mRS score of 0-1 at 90 days) in patients with MSU deployment vs usual care (random-effects meta-analysis, crude ORs).
eFigure 4. Pooled odds ratio for reduced disability (shift analysis over the whole range of the mRS scores at 90 days) in patients with MSU deployment vs usual care (random-effects meta-analysis, crude ORs).
eFigure 5. Pooled odds ratio for good outcome (mRS score of 0-2 at 90 days) in patients with MSU deployment vs usual care (random-effects meta-analysis, crude ORs).
eFigure 6. Risk of bias of each included study for alarm-to-IVT time, according to the RoB2 tool for cluster-randomized trials (panel A) and the ROBINS-I tool for nonrandomized studies (panel B).
eFigure 7. Funnel plot of studies included in the meta-analysis of the median reduction of symptom onset or last known well to IVT time in patients with MSU deployment vs usual care.
eFigure 8. Pooled difference of medians of alarm (ambulance dispatch)-to-IVT time in patients with MSU deployment vs usual care (random-effects meta-analysis).
eFigure 9. Funnel plot of studies included in the meta-analysis of the median reduction of alarm (ambulance dispatch)-to-IVT time in patients with MSU deployment vs usual care.
eFigure 10. Pooled difference of medians of symptom onset/last known well-to-MT time in patients with MSU deployment vs usual care (random-effects meta-analysis).
eFigure 11. Pooled difference of medians of alarm (ambulance dispatch)-to-MT time in patients with MSU deployment vs usual care (random-effects meta-analysis).
eFigure 12. Pooled odds ratio for all-cause mortality 7 days after MSU deployment vs usual care (random-effects meta-analysis, crude ORs).
eFigure 13. Pooled odds ratio for all-cause mortality 90 days after MSU deployment vs usual care (random-effects meta-analysis, crude ORs).
eFigure 14. Pooled odds ratio for symptomatic intracerebral hemorrhage in patients with MSU deployment vs usual care (random-effects meta-analysis, crude ORs).
eFigure 15. Pooled difference of means of alarm (ambulance dispatch)-to-MT time in patients with MSU deployment vs usual care (post-hoc analysis; random-effects model).
eReferences.
References
- 1.Berge E, Whiteley W, Audebert H, et al. European Stroke Organisation (ESO) guidelines on intravenous thrombolysis for acute ischaemic stroke. Eur Stroke J. 2021;6(1):I-LXII. doi: 10.1177/2396987321989865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Turc G, Bhogal P, Fischer U, et al. European Stroke Organisation (ESO)—European Society for Minimally Invasive Neurological Therapy (ESMINT) guidelines on mechanical thrombectomy in acute ischaemic stroke: endorsed by Stroke Alliance for Europe (SAFE). Eur Stroke J. 2019;4(1):6-12. doi: 10.1177/2396987319832140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Meretoja A, Keshtkaran M, Saver JL, et al. Stroke thrombolysis: save a minute, save a day. Stroke. 2014;45(4):1053-1058. doi: 10.1161/STROKEAHA.113.002910 [DOI] [PubMed] [Google Scholar]
- 4.Lees KR, Bluhmki E, von Kummer R, et al. ; ECASS, ATLANTIS, NINDS and EPITHET rt-PA Study Group . Time to treatment with intravenous alteplase and outcome in stroke: an updated pooled analysis of ECASS, ATLANTIS, NINDS, and EPITHET trials. Lancet. 2010;375(9727):1695-1703. doi: 10.1016/S0140-6736(10)60491-6 [DOI] [PubMed] [Google Scholar]
- 5.Saver JL, Goyal M, van der Lugt A, et al. ; HERMES Collaborators . Time to treatment with endovascular thrombectomy and outcomes from ischemic stroke: a meta-analysis. JAMA. 2016;316(12):1279-1288. doi: 10.1001/jama.2016.13647 [DOI] [PubMed] [Google Scholar]
- 6.Norrving B, Barrick J, Davalos A, et al. Action plan for stroke in Europe 2018-2030. Eur Stroke J. 2018;3(4):309-336. doi: 10.1177/2396987318808719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Powers WJ, Rabinstein AA, Ackerson T, et al. Guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 guidelines for the early management of acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2019;50(12):e344-e418. doi: 10.1161/STR.0000000000000211 [DOI] [PubMed] [Google Scholar]
- 8.Meretoja A, Strbian D, Mustanoja S, Tatlisumak T, Lindsberg PJ, Kaste M. Reducing in-hospital delay to 20 minutes in stroke thrombolysis. Neurology. 2012;79(4):306-313. doi: 10.1212/WNL.0b013e31825d6011 [DOI] [PubMed] [Google Scholar]
- 9.Psychogios MN, Maier IL, Tsogkas I, et al. One-stop management of 230 consecutive acute stroke patients: report of procedural times and clinical outcome. J Clin Med. 2019;8(12):8. doi: 10.3390/jcm8122185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Müller-Barna P, Schwamm LH, Haberl RL. Telestroke increases use of acute stroke therapy. Curr Opin Neurol. 2012;25(1):5-10. doi: 10.1097/WCO.0b013e32834d5fe4 [DOI] [PubMed] [Google Scholar]
- 11.Fassbender K, Walter S, Liu Y, et al. “Mobile stroke unit” for hyperacute stroke treatment. Stroke. 2003;34(6):e44. doi: 10.1161/01.STR.0000075573.22885.3B [DOI] [PubMed] [Google Scholar]
- 12.Walter S, Kostopoulos P, Haass A, et al. Diagnosis and treatment of patients with stroke in a mobile stroke unit versus in hospital: a randomised controlled trial. Lancet Neurol. 2012;11(5):397-404. doi: 10.1016/S1474-4422(12)70057-1 [DOI] [PubMed] [Google Scholar]
- 13.Weber JE, Ebinger M, Rozanski M, et al. ; STEMO-Consortium . Prehospital thrombolysis in acute stroke: results of the PHANTOM-S pilot study. Neurology. 2013;80(2):163-168. doi: 10.1212/WNL.0b013e31827b90e5 [DOI] [PubMed] [Google Scholar]
- 14.Parker SA, Bowry R, Wu TC, et al. Establishing the first mobile stroke unit in the United States. Stroke. 2015;46(5):1384-1391. doi: 10.1161/STROKEAHA.114.007993 [DOI] [PubMed] [Google Scholar]
- 15.Gomes JA, Ahrens CL, Hussain MS, Winners S, Rasmussen PA, Uchino K; Cleveland Pre-Hospital Acute Stroke Treatment Study Group . Prehospital reversal of warfarin-related coagulopathy in intracerebral hemorrhage in a mobile stroke treatment unit. Stroke. 2015;46(5):e118-e120. doi: 10.1161/STROKEAHA.115.008483 [DOI] [PubMed] [Google Scholar]
- 16.Larsen K, Jaeger HS, Tveit LH, et al. Ultraearly thrombolysis by an anesthesiologist in a mobile stroke unit: a prospective, controlled intervention study. Eur J Neurol. 2021;28(8):2488-2496. doi: 10.1111/ene.14877 [DOI] [PubMed] [Google Scholar]
- 17.Fassbender K, Grotta JC, Walter S, Grunwald IQ, Ragoschke-Schumm A, Saver JL. Mobile stroke units for prehospital thrombolysis, triage, and beyond: benefits and challenges. Lancet Neurol. 2017;16(3):227-237. doi: 10.1016/S1474-4422(17)30008-X [DOI] [PubMed] [Google Scholar]
- 18.Kate MP, Jeerakathil T, Buck BH, et al. Pre-hospital triage of suspected acute stroke patients in a mobile stroke unit in the rural Alberta. Sci Rep. 2021;11(1):4988. doi: 10.1038/s41598-021-84441-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kunz A, Nolte CH, Erdur H, et al. Effects of ultraearly intravenous thrombolysis on outcomes in ischemic stroke: the STEMO (Stroke Emergency Mobile) group. Circulation. 2017;135(18):1765-1767. doi: 10.1161/CIRCULATIONAHA.117.027693 [DOI] [PubMed] [Google Scholar]
- 20.Ebinger M, Siegerink B, Kunz A, et al. ; Berlin_PRehospital Or Usual Delivery in stroke care (B_PROUD) Study Group . Association between dispatch of mobile stroke units and functional outcomes among patients with acute ischemic stroke in Berlin. JAMA. 2021;325(5):454-466. doi: 10.1001/jama.2020.26345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grotta JC, Yamal JM, Parker SA, et al. Prospective, multicenter, controlled trial of mobile stroke units. N Engl J Med. 2021;385(11):971-981. doi: 10.1056/NEJMoa2103879 [DOI] [PubMed] [Google Scholar]
- 22.Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372(n71):n71. doi: 10.1136/bmj.n71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stroup DF, Berlin JA, Morton SC, et al. ; Meta-analysis Of Observational Studies in Epidemiology (MOOSE) Group. Meta-analysis of observational studies in epidemiology: a proposal for reporting. JAMA. 2000;283(15):2008-2012. doi: 10.1001/jama.283.15.2008 [DOI] [PubMed] [Google Scholar]
- 24.Saver JL, Chaisinanunkul N, Campbell BCV, et al. ; XIth Stroke Treatment Academic Industry Roundtable . Standardized nomenclature for modified Rankin Scale global disability outcomes: consensus recommendations from Stroke Therapy Academic Industry Roundtable XI. Stroke. 2021;52(9):3054-3062. doi: 10.1161/STROKEAHA.121.034480 [DOI] [PubMed] [Google Scholar]
- 25.The National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group. Tissue plasminogen activator for acute ischemic stroke. N Engl J Med. 1995;333:1581-1587. doi: 10.1056/NEJM199512143332401 [DOI] [PubMed] [Google Scholar]
- 26.Hacke W, Kaste M, Bluhmki E, et al. ; ECASS Investigators . Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. N Engl J Med. 2008;359(13):1317-1329. doi: 10.1056/NEJMoa0804656 [DOI] [PubMed] [Google Scholar]
- 27.Thomalla G, Simonsen CZ, Boutitie F, et al. ; WAKE-UP Investigators . MRI-guided thrombolysis for stroke with unknown time of onset. N Engl J Med. 2018;379(7):611-622. doi: 10.1056/NEJMoa1804355 [DOI] [PubMed] [Google Scholar]
- 28.Ma H, Campbell BCV, Parsons MW, et al. ; EXTEND Investigators . Thrombolysis guided by perfusion imaging up to 9 hours after onset of stroke. N Engl J Med. 2019;380(19):1795-1803. doi: 10.1056/NEJMoa1813046 [DOI] [PubMed] [Google Scholar]
- 29.Emberson J, Lees KR, Lyden P, et al. ; Stroke Thrombolysis Trialists’ Collaborative Group . Effect of treatment delay, age, and stroke severity on the effects of intravenous thrombolysis with alteplase for acute ischaemic stroke: a meta-analysis of individual patient data from randomised trials. Lancet. 2014;384(9958):1929-1935. doi: 10.1016/S0140-6736(14)60584-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McGrath S, Sohn H, Steele R, Benedetti A. Meta-analysis of the difference of medians. Biom J. 2020;62:69-98. [DOI] [PubMed] [Google Scholar]
- 31.Higgins JPT, Thomas J, Chandler J, et al. , eds. Cochrane Handbook for Systematic Reviews of Interventions. 2nd ed. John Wiley & Sons; 2019. [Google Scholar]
- 32.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50:1088-1101. [PubMed] [Google Scholar]
- 33.Sterne JAC, Egger M. Regression methods to detect publication and other bias in meta-analysis. In: Rothstein HR, Sutton AJ, Borenstein M, eds. Publication Bias in Meta-Analysis: Prevention, Assessment and Adjustment. Wiley; 2005:99-110. [Google Scholar]
- 34.Ebinger M, Winter B, Wendt M, et al. ; STEMO Consortium . Effect of the use of ambulance-based thrombolysis on time to thrombolysis in acute ischemic stroke: a randomized clinical trial. JAMA. 2014;311(16):1622-1631. doi: 10.1001/jama.2014.2850 [DOI] [PubMed] [Google Scholar]
- 35.Helwig SA, Ragoschke-Schumm A, Schwindling L, et al. Prehospital stroke management optimized by use of clinical scoring vs mobile stroke unit for triage of patients with stroke: a randomized clinical trial. JAMA Neurol. 2019;76(12):1484-1492. doi: 10.1001/jamaneurol.2019.2829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ebinger M, Kunz A, Wendt M, et al. Effects of golden hour thrombolysis: a Prehospital Acute Neurological Treatment and Optimization of Medical Care in Stroke (PHANTOM-S) substudy. JAMA Neurol. 2015;72(1):25-30. doi: 10.1001/jamaneurol.2014.3188 [DOI] [PubMed] [Google Scholar]
- 37.Kunz A, Ebinger M, Geisler F, et al. Functional outcomes of pre-hospital thrombolysis in a mobile stroke treatment unit compared with conventional care: an observational registry study. Lancet Neurol. 2016;15(10):1035-1043. doi: 10.1016/S1474-4422(16)30129-6 [DOI] [PubMed] [Google Scholar]
- 38.Taqui A, Cerejo R, Itrat A, et al. ; Cleveland Pre-Hospital Acute Stroke Treatment (PHAST) Group . Reduction in time to treatment in prehospital telemedicine evaluation and thrombolysis. Neurology. 2017;88(14):1305-1312. doi: 10.1212/WNL.0000000000003786 [DOI] [PubMed] [Google Scholar]
- 39.Kummer BR, Lerario MP, Hunter MD, et al. Geographic analysis of mobile stroke unit treatment in a dense urban area: the New York City METRONOME registry. J Am Heart Assoc. 2019;8(24):e013529. doi: 10.1161/JAHA.119.013529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhao H, Coote S, Easton D, et al. Melbourne mobile stroke unit and reperfusion therapy: greater clinical impact of thrombectomy than thrombolysis. Stroke. 2020;51(3):922-930. doi: 10.1161/STROKEAHA.119.027843 [DOI] [PubMed] [Google Scholar]
- 41.Zhou T, Zhu L, Wang M, et al. Application of mobile stroke unit in prehospital thrombolysis of acute stroke: experience from China. Cerebrovasc Dis. 2021;50(5):520-525. doi: 10.1159/000514370 [DOI] [PubMed] [Google Scholar]
- 42.Nolte CH, Ebinger M, Scheitz JF, et al. Effects of prehospital thrombolysis in stroke patients with prestroke dependency. Stroke. 2018;49(3):646-651. doi: 10.1161/STROKEAHA.117.019060 [DOI] [PubMed] [Google Scholar]
- 43.Zonneveld TP, Richard E, Vergouwen MD, et al. Blood pressure-lowering treatment for preventing recurrent stroke, major vascular events, and dementia in patients with a history of stroke or transient ischaemic attack. Cochrane Database Syst Rev. 2018;(7):CD007858. doi: 10.1002/14651858.CD007858.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hubert GJ, Meretoja A, Audebert HJ, Tatlisumak T, Zeman F, Boy S, et al. Comparison of stroke thrombolysis rates and delays in a centralized (Helsinki, Finland) and decentralized (TEMPIS telestroke unit network, Germany) setting. Int J Stroke. 2014;9:81. [Google Scholar]
- 45.Meretoja A, Weir L, Ugalde M, et al. Helsinki model cut stroke thrombolysis delays to 25 minutes in Melbourne in only 4 months. Neurology. 2013;81(12):1071-1076. doi: 10.1212/WNL.0b013e3182a4a4d2 [DOI] [PubMed] [Google Scholar]
- 46.Lees KR, Emberson J, Blackwell L, et al. ; Stroke Thrombolysis Trialists’ Collaborators Group . Effects of alteplase for acute stroke on the distribution of functional outcomes: a pooled analysis of 9 trials. Stroke. 2016;47(9):2373-2379. doi: 10.1161/STROKEAHA.116.013644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Grotta JC. tPA for stroke: important progress in achieving faster treatment. JAMA. 2014;311(16):1615-1617. doi: 10.1001/jama.2014.3322 [DOI] [PubMed] [Google Scholar]
- 48.Tsivgoulis G, Geisler F, Katsanos AH, et al. Ultraearly intravenous thrombolysis for acute ischemic stroke in mobile stroke unit and hospital settings. Stroke. 2018;49(8):1996-1999. doi: 10.1161/STROKEAHA.118.021536 [DOI] [PubMed] [Google Scholar]
- 49.Fassbender K, Balucani C, Walter S, Levine SR, Haass A, Grotta J. Streamlining of prehospital stroke management: the golden hour. Lancet Neurol. 2013;12(6):585-596. doi: 10.1016/S1474-4422(13)70100-5 [DOI] [PubMed] [Google Scholar]
- 50.Audebert HJ, Saver JL, Starkman S, Lees KR, Endres M. Prehospital stroke care: new prospects for treatment and clinical research. Neurology. 2013;81(5):501-508. doi: 10.1212/WNL.0b013e31829e0fdd [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Alexandrov AW, Arthur AS, Bryndziar T, et al. High-resolution CT with arch/neck/head CT angiography on a mobile stroke unit. J Neurointerv Surg. 2021;neurintsurg-2021-017697. doi: 10.1136/neurintsurg-2021-017697 [DOI] [PubMed] [Google Scholar]
- 52.Senn SJ. Overstating the evidence: double counting in meta-analysis and related problems. BMC Med Res Methodol. 2009;9:10. doi: 10.1186/1471-2288-9-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wendt M, Ebinger M, Kunz A, et al. ; STEMO Consortium . Improved prehospital triage of patients with stroke in a specialized stroke ambulance: results of the pre-hospital acute neurological therapy and optimization of medical care in stroke study. Stroke. 2015;46(3):740-745. doi: 10.1161/STROKEAHA.114.008159 [DOI] [PubMed] [Google Scholar]
- 54.Dietrich M, Walter S, Ragoschke-Schumm A, et al. Is prehospital treatment of acute stroke too expensive? an economic evaluation based on the first trial. Cerebrovasc Dis. 2014;38(6):457-463. doi: 10.1159/000371427 [DOI] [PubMed] [Google Scholar]
- 55.Gyrd-Hansen D, Olsen KR, Bollweg K, Kronborg C, Ebinger M, Audebert HJ. Cost-effectiveness estimate of prehospital thrombolysis: results of the PHANTOM-S study. Neurology. 2015;84(11):1090-1097. doi: 10.1212/WNL.0000000000001366 [DOI] [PubMed] [Google Scholar]
- 56.Kim J, Easton D, Zhao H, et al. Economic evaluation of the Melbourne Mobile Stroke Unit. Int J Stroke. 2021;16(4):466-475. doi: 10.1177/1747493020929944 [DOI] [PubMed] [Google Scholar]
- 57.Ebinger M, Harmel P, Nolte CH, Grittner U, Siegerink B, Audebert HJ. Berlin prehospital or usual delivery of acute stroke care - study protocol. Int J Stroke. 2017;12(6):653-658. doi: 10.1177/1747493017700152 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods.
eTable 1. Key features of included studies.
eTable 2. Adjustment variables in each study reporting adjusted results for excellent outcome or reduced disability.
eFigure 1. PRISMA flow chart.
eFigure 2. Risk of bias of each included study for excellent functional outcome (mRS score of 0-1 at 90 days), according to the RoB2 tool for cluster-randomized trials (panel A) and the ROBINS-I tool for nonrandomized studies (panel B).
eFigure 3. Pooled odds ratio for excellent outcome (mRS score of 0-1 at 90 days) in patients with MSU deployment vs usual care (random-effects meta-analysis, crude ORs).
eFigure 4. Pooled odds ratio for reduced disability (shift analysis over the whole range of the mRS scores at 90 days) in patients with MSU deployment vs usual care (random-effects meta-analysis, crude ORs).
eFigure 5. Pooled odds ratio for good outcome (mRS score of 0-2 at 90 days) in patients with MSU deployment vs usual care (random-effects meta-analysis, crude ORs).
eFigure 6. Risk of bias of each included study for alarm-to-IVT time, according to the RoB2 tool for cluster-randomized trials (panel A) and the ROBINS-I tool for nonrandomized studies (panel B).
eFigure 7. Funnel plot of studies included in the meta-analysis of the median reduction of symptom onset or last known well to IVT time in patients with MSU deployment vs usual care.
eFigure 8. Pooled difference of medians of alarm (ambulance dispatch)-to-IVT time in patients with MSU deployment vs usual care (random-effects meta-analysis).
eFigure 9. Funnel plot of studies included in the meta-analysis of the median reduction of alarm (ambulance dispatch)-to-IVT time in patients with MSU deployment vs usual care.
eFigure 10. Pooled difference of medians of symptom onset/last known well-to-MT time in patients with MSU deployment vs usual care (random-effects meta-analysis).
eFigure 11. Pooled difference of medians of alarm (ambulance dispatch)-to-MT time in patients with MSU deployment vs usual care (random-effects meta-analysis).
eFigure 12. Pooled odds ratio for all-cause mortality 7 days after MSU deployment vs usual care (random-effects meta-analysis, crude ORs).
eFigure 13. Pooled odds ratio for all-cause mortality 90 days after MSU deployment vs usual care (random-effects meta-analysis, crude ORs).
eFigure 14. Pooled odds ratio for symptomatic intracerebral hemorrhage in patients with MSU deployment vs usual care (random-effects meta-analysis, crude ORs).
eFigure 15. Pooled difference of means of alarm (ambulance dispatch)-to-MT time in patients with MSU deployment vs usual care (post-hoc analysis; random-effects model).
eReferences.


