Abstract
Background:
The current standard method to measure intracardiac oxygen (O2) saturation is by invasive catheterization. Accurate noninvasive blood O2 saturation by MRI could potentially reduce the duration and risk of invasive diagnostic procedures.
Purpose:
To noninvasively determine blood oxygen saturation in the heart with MRI and compare the accuracy with catheter measurements.
Study Type:
Prospective.
Subjects:
Thirty-two patients referred for right heart catheterization (RHC) and five healthy subjects.
Field Strength/Sequence:
T2-prepared single-shot balanced steady-state free-precession at 1.5T.
Assessment:
MR signals in venous and arterial blood, hematocrit, and arterial O2 saturation from a pulse oximeter were jointly processed to fit the Luz–Meiboom model and estimate blood O2 saturation in the right heart. Interstudy reproducibility was evaluated in volunteers and patients. Interobserver reproducibility among three readers was assessed using data from volunteers and 10 patients. Accuracy of MR oximetry was compared to RHC in all patients.
Statistical Tests:
Coefficient of variation, intraclass correlation coefficient, Bland–Altman analysis, Pearson’s correlation.
Results:
The coefficient of variation for interstudy reproducibility of O2 saturation was 2.6% on average in volunteers and 3.2% in patients. Interobserver reproducibility among three observers yielded intraclass correlation coefficients of 0.81 and 0.87 respectively for RV and MPA O2 saturation. O2 saturation (y = 0.85x + 0.13, R = 0.78) and (a-v)O2 difference (y = 0.71x + 0.90, R = 0.69) by MR and RHC were significantly correlated (N = 32, P < 0.05 in both cases) in patients. MR slightly overestimated O2 saturation compared to RHC with 2% ± 5% bias and limits of agreement between −7% and 12%.
Data Conclusion:
MR oximetry is repeatable and reproducible. Good agreement was shown between MR and catheter venous O2 saturation and (a-v)O2 difference in a cohort whose venous O2 ranged from abnormally low to high levels, with most values in the normal physiological range.
Level of Evidence:
2.
Technical Efficacy Stage:
2.
BLOOD OXYGEN (O2) saturation measured in cardiac chambers and vessels is clinically used to quantify shunt fraction, and therefore to guide pre- and post-interventional management in congenital heart defects.1,2 (a-v)O2, the difference in blood O2 content measured between arterial and mixed venous blood, is also used to evaluate cardiac output (CO) via the Fick method in heart failure and pulmonary hypertension (PHTN) and to characterize exertional dyspnea and exercise-induced PHTN in conjunction with exercise.3 The current clinical approach to measure intracardiac blood O2 saturation is by invasive catheterization. While catheterization in most adult patients requires only mild to moderate sedation and is performed as a relatively safe outpatient procedure,4 children often require general anesthesia and hospitalization. The complication rates are as high as 25% in patients under 2 years of age.5–7
Cardiac magnetic resonance (MR) is increasingly being used in conjunction with diagnostic catheterization for comprehensive cardiovascular assessment.8–10 Deoxygenation causes a concomitant decrease in blood T1, T2, and T2*11–15 and methods for MR-based measurement of intracardiac O2 saturation have been previously proposed based on the paramagnetic nature of deoxyhemoglobin.16–18 Noninvasive MR oximetry could augment MR diagnostic capabilities to characterize myocardial energetics in heart failure and to distinguish cardiac vs. pulmonary etiology of dyspnea and PHTN. The overall frequency, duration, risks, and costs involved with diagnostic catheterization could potentially be reduced, especially when serial or repeated evaluation is desired such as with pediatric and adult congenital heart disease patients and heart transplant recipients.
Wright et al16 proposed a method to measure blood O2 saturation in vivo based on blood T2 relaxation. This technique relied on a simplification of the Luz–Meiboom (L-M) model19 to incorporate a global calibration factor, K, derived from T2 measurements of in vitro blood samples oxygenated to different levels. The method has not come into widespread use due to the elaborate calibration process required.20–22 An alternative method has been proposed23 that requires no in vitro or population-based calibration, and instead estimates the multiple unknown parameters of the L-M model. This method has been previously demonstrated and validated in an animal hypoxemia model against catheter-derived values. In the current study we present a modified, patient-adaptive version of this technique, including modifications designed to make data acquisition and postprocessing more efficient. We term this method adaptive MR oximetry, as the fitting of the L-M model is driven by patient-specific MR image data and measured physiological inputs, and provides the flexibility to estimate patient-specific outputs, ie, the O2 saturation and other nuisance parameters of the L-M model.
Materials and Methods
It has been previously shown in an animal study23 that blood O2 saturation can be measured using distinct T2 maps, each map generated from source images acquired with a specific inter-echo spacing (τ180). In this manner, all unknown parameters of the L-M model could be estimated, providing a patient-specific measurement of O2 saturation. There are two key differences in the technique employed in this work. First, no intermediate T2 maps were generated within the data-processing chain; multiple T2-prepared images, each acquired at a specific τ180, were acquired and directly processed to fit the L-M model. The MR blood signal in each image thus reflects transverse decay caused by τ180, as well as the T2 preparation time. Second, Gaussian priors rather than hard constraints were placed on the parameter estimates to discourage convergence to parameter bounds or other local minima and to relax the solution space; the wider range of possible parameter values should provide accuracy under varied cardiovascular conditions. The acquisition scheme in this work also provides images across a wider range of τ180 (from 5–25 msec) within a total acquisition time of 30 seconds, resulting in an imaging protocol that can be easily incorporated into a routine MR exam. Furthermore, a semiautomatic pixel exclusion criterion was applied to help avoid pixels with obvious dephasing due to complex flow or other sources.
MR Protocol
Following screening and informed consent, each subject was prepared for imaging by placement of ECG electrodes for cardiac triggering and patient monitoring. All volunteer and patient MR examinations were performed at 1.5T (Magnetom Avanto, Siemens Healthineers, Erlangen, Germany). An MR-compatible finger pulse oximeter probe (Medrad MR Vital Signs Patient Monitor, Bayer Healthcare, Whippany, NJ) was used to monitor arterial O2 saturation (SpO2); the average SpO2 during MR oximetry image acquisition was recorded.
Volunteer Cohort
Interstudy reproducibility of MR O2 saturation was evaluated in five healthy volunteers. Hematocrit was determined from a blood sample drawn ~15 minutes before the MR exam. The volunteers did not undergo right heart catheterization (RHC) and therefore had no reference venous O2 saturation.
Patient Enrollment
The study was approved by the local Institutional Review Board and was HIPAA-compliant. Patients clinically indicated for RHC and without MR contraindications were eligible for participation. Patients were recruited if: 1) they consented to a research cardiac MR exam prior to RHC on the same day, or 2) were clinically scheduled for both RHC and cardiac MR within a 7-day period and consented to the addition of oximetry scans to their clinical MR exam. Fifty adult patients were enrolled. Nine patients could not complete both exams and an additional nine patients were excluded based on chart review. These included patients in whom supplemental O2 levels or hematocrit were different between MR and RHC, intraprocedural sedation caused significant variations in heart rate and blood pressure during the exam, or medications that could alter hemodynamic status, such as inotropes, vasopressors, and vasodilators were initiated or discontinued in the interval between the two exams. Of the 32 patients included for final comparison, 12 had MR oximetry added to their clinical scan, while a research cardiac MR was performed in the other 20. Two of the 12 clinical patients underwent continuous hemodynamic monitoring with a Swan Ganz catheter during heart failure therapy adjustment; in these patients the mixed venous co-oximetry sample closest in time to the MR was used as reference. For all patients, hematocrit measured closest in time to MR was used. MR and RHC exams were conducted on the same day in 28 patients, and within 1 day for the others. The two exams were performed in room air for 27 patients, and with matching supplemental O2 for the others (range across patients, 2–6 L/min).
Patient-Adaptive MR Oximetry
Six T2-prepared single-shot balanced steady-state free-precession (bSSFP) images were acquired in each imaging plane, at τ180 of 0, 5, 10, 15, 20, and 25 msec. The nonselective T2 preparation module consisted of an excitation pulse (90x), a fixed number (n = 8) of composite refocusing pulses (90x-180y-90x), and a composite tip-up pulse (270x-360−x). The refocusing pulses were weighted in a Malcolm–Levitt phase cycling scheme24 known to minimize B0 and B1 errors, and reduce sensitivity to flow.25,26 The resulting T2 preparation times (T2p = n * τ180) acquired were 0, 40, 80, 120, 160, and 200 msec. Other image acquisition parameters included a field of view 266–369 × 288–425 mm, acquisition matrix 70–104 × 128, average pixel size 3.0 mm × 3.8 mm2, slice thickness 10 mm, 1 NEX, bSSFP readout parameters repetition time / echo time (TR/TE) 2.08/0.89 msec, flip angle 70°, bandwidth 1500 Hz/pixel, 5 linear ramp-up pulses with no α/2 pulse at the end of readout, rate 2 generalized autocalibrating partial parallel acquisition (GRAPPA) with 36 autocalibration signal (ACS) reference lines, linear k-space ordering, 6/8 partial Fourier, and imaging duration ~110 msec. The wait time between T2-prepared images was at least 5 seconds (>3 × T1blood of 1500 msec) to ensure adequate T1 recovery. The total acquisition time for a given imaging plane was ~30 seconds. All images were ECG-triggered, acquired during free-breathing in late diastole, and prior to administration of any contrast agent. Images were acquired in a short-axis view of the right and left ventricles (RV and LV), and cross-sectional views of the aorta and main pulmonary artery (MPA) and superior vena cava (SVC). Short-axis images were acquired in all 32 patients, while cross-sectional images to measure MPA O2 saturation were only acquired in 22 patients (all research patients and two clinical patients), as MPA imaging planes were not routinely acquired for clinical assessment. For these 22 patients, RV and MPA MR O2 saturation were averaged to compare with the single catheter measurement. The overall image and data acquisition strategy used to estimate O2 saturation is shown in Fig. 1.
FIGURE 1:
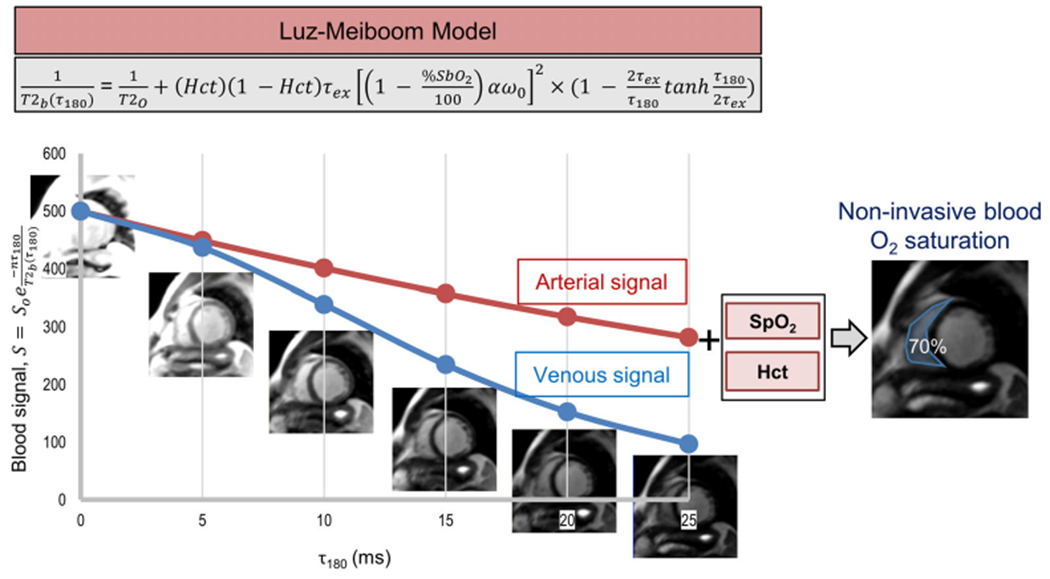
Overall image acquisition strategy for patient-adaptive MR oximetry. Multiple T2-prepared images are acquired at different inter-echo spacing (τ180), in order to create a system of equations to fit the Luz–Meiboom model. Additional measurable parameters in the model, hematocrit, and arterial O2 saturation from a finger pulse oximeter are provided as patient-specific inputs to improve the accuracy of O2 saturation estimate in the chambers and vessels of the right heart.
The T2-prepared source images were motion-corrected inline using a nonrigid registration algorithm,27 and further processed in Matlab (R2017a, The MathWorks Inc., Natick, MA) for MR oximetry. To minimize user bias in defining regions of interest (ROIs), a two-step threshold was implemented to avoid pixels with artifacts or low signal (Fig. 2, see detailed illustration in Fig. S1). A single reader (Observer 1, J.V., with 7 years of MR experience) completed analysis on all volunteer and patient images. The reader initially drew contours to outline the RV, LV, aorta, and MPA. The first threshold, the lesser of the median or an empirical threshold based on the pixel intensity distribution within a blood pool, was applied to the τ180 = 5 msec image to eliminate papillary muscle and pixels potentially containing a mix of papillary muscle and blood. The second threshold sought to identify and exclude pixels with rapid signal decay outside of the expected L-M model behavior. Based on simulation of the T2-prepared blood signal behavior, a conservative pixelwise threshold was set at 20% of the MR signal intensity from the image acquired at τ180 = 0 msec. Mean MR signal intensity in each T2-prepared image was determined from the retained pixels and used as the L-M model input.
FIGURE 2:
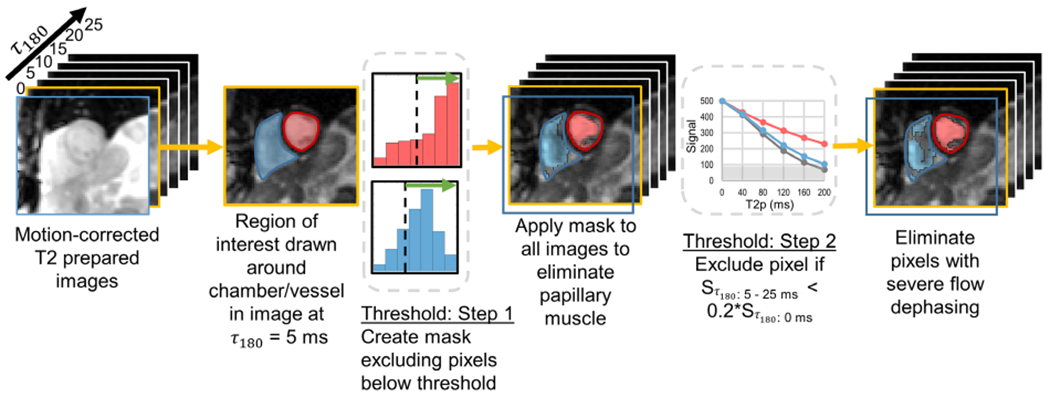
An illustration showing the choice of pixels to be included for estimation of O2 saturation from the T2-prepared images with a process equivalent to a three-parameter fit-based exclusion. A region of interest (ROI) outlining a chamber or vessel (short axis view with the right and left ventricles—RV and LV—shown in this illustration) was defined on the image at τ180 = 5 msec. In Step 1, a threshold was defined to exclude pixels with signal intensities below a threshold specified based on the histogram of pixels within the ROI. This mask was then applied across all six images. Pixels retained in the RV and LV are shown in blue and red, respectively. In the second step, a pixel was only retained if the signal intensity across all the five T2 preparation times (corresponding to τ180 5–25 msec) was greater than 20% of the pixel intensity in first image (τ180 = 0 msec). As shown in the graph, the gray pixel would be eliminated in all T2-prepared images since the MR signal was below the threshold (gray box). In this example, additional pixels were eliminated in the RV, while the LV was not affected with the second threshold.
Estimation of MR O2 Saturation
To estimate the unknown blood O2 saturation of the RV, MPA, and SVC, the mean MR signals from the six T2-prepared images and one arterial reference region (LV for RV and SVC, and aorta for MPA) were used jointly to fit the L-M model. Arterial O2 saturation obtained by pulse oximetry during MR and measured hematocrit were used as patient-specific parameters in model fitting. The L-M model implemented in this study is given by:
| (1) |
| (2) |
where T2b(τ180), the τ180 dependent T2 relaxation time of arterial or venous blood; T2O, T2 relaxation time of fully oxygenated blood; Pa, the fraction of protons in erythrocytes or plasma, the two sites undergoing chemical exchange; τex, the water proton exchange time between erythrocytes and plasma; α, a dimensionless parameter dependent on the susceptibility difference of deoxy- and oxyhemoglobin and the geometry and shape of the erythrocytes; ω0 the proton resonance frequency (determined by the MRI system field strength, 4 × 108 rad/s at 1.5T); %SbO2, the arterial or venous blood O2 saturation; τ180, the interecho spacing of the n number of 180° refocusing pulses in the T2 preparation module; S, the measured MR signal intensity of blood in a single T2-prepared bSSFP image with a specific τ180 and a corresponding T2 preparation duration of n* τ180; and S0, the steady-state signal. The term represents the frequency difference between the protons in erythrocytes and plasma. We equate Pa to hematocrit (Hct), the volume fraction of erythrocytes in whole blood, in this implementation.
Thus, 12 equations were used together to estimate the unknown O2 saturation—six corresponding to the MR signal from the six τ180 images for the venous blood of interest, and six from the reference arterial blood. There are six unknown parameters, namely: venous O2 saturation, S0 for the venous blood, S0 for the reference arterial blood, α, T2O, and τex. The six unknowns were estimated individually for each dataset using a nonlinear least squares fit of Eq. 2. Arterial blood signal and the corresponding SpO2 were used to provide a good T2O estimate, which, in turn, improves the accuracy of the estimated venous O2 saturation. The bounds on O2 saturation were set between 0 and 1, as values beyond this range are physiologically impossible. To reduce reliance on parameter bounds and yet discourage convergence to local minima, Gaussian priors, expressed in terms of mean (μ), and standard deviation (σ), were assumed for estimation of the remaining five nuisance parameters. For venous and arterial S0, an ROI-specific μ was set based on the mean signal measured in the bSSFP image at τ180 = 0 msec, while σ was set as (1.5*S0). For parameters α, T2O, and τex, (μ, σ) were empirically set as (0.4, 0.1), (300, 120) msec, and (8, 1) msec, respectively.
Reproducibility and Accuracy
To test for interstudy reproducibility, oximetry images were acquired five times in each imaging plane (short-axis and cross-sectional views of the aorta and MPA) in five healthy volunteers. Repetitions were acquired ~1 minute apart. Two repeat acquisitions of the RV and LV were also acquired in six of the patients studied. Interobserver reproducibility was evaluated in the five volunteers and in a subset of 10 randomly selected patients. Three readers (J.V. with seven, J.C. with five, and Y.P. with 1 year of MR experience) analyzed these images and were blinded to each other’s results.
In volunteers, MR O2 saturation was determined in the RV and MPA for each repeated acquisition. In patients, MR O2 saturation was estimated in the RV, MPA, and SVC. RV and MPA O2 saturations were averaged to provide a single mixed venous value in patients. This was compared to the mixed venous O2 saturation by RHC to test for accuracy. MR O2 saturation of SVC was compared to the corresponding RHC measurement. The (a-v)O2 difference, expressed in mL/100 mL of blood, was calculated for each patient (excluding SVC measurements) using the formula:
| (3) |
For MR (a-v)O2, Hb is the hemoglobin measured closest in time to MRI. The arterial O2 saturation, SaO2, was measured with a pulse oximeter and the venous O2 saturation, SvO2, was estimated as described above. This was compared to the corresponding catheter-based (a-v)O2 difference, where hemoglobin, SaO2, and SvO2 (MPA) were measured during RHC.
Cardiac output (CO), in L/min, was also determined from adaptive MR oximetry using the Fick equation:
| (4) |
where is the whole body oxygen consumption (mL/min). The value reported for each patient during RHC (using the Dehmer formula, = 125* body surface area) was used to calculate CO from MR oximetry. CO determined using the Fick method from MR oximetry and RHC were compared to CO determined from the reference method of breath-held segmented 2D phase contrast (PC) MRI images of the aorta in patients. CO was quantitatively determined from PC MR images using SuiteHeart software (Neosoft, Pewaukee, WI).
Statistical Analysis
Statistical analysis was performed with MedCalc Statistical Software v. 18.5 (MedCalc Software, Ostend, Belgium; https://www.medcalc.org; 2018). All continuous data are presented as mean ± SD. For interstudy reproducibility, the coefficient of variation (CV) in O2 saturation was determined for each volunteer as the ratio of SD to the average O2 saturation for the five repeat acquisitions. For patients, interstudy CV was calculated as the ratio of SD of the difference between the two measurements to the population mean. Intraclass correlation coefficient (ICC) and 95% confidence interval (CI) of O2 saturation determined by the three observers was calculated for interobserver reproducibility. The mean difference in MR and RHC O2 saturation for the subset of patients tested for interobserver reproducibility was also determined for each observer. To determine accuracy, MR O2 saturation and the (a-v)O2 difference in all patients were tested against RHC using linear regression and Bland–Altman analysis (P < 0.05 significant). The Pearson correlation coefficient, R, was used to report the linear association. Absolute differences between MR and RHC O2 saturation were also determined. MR and RHC measurements of SVC O2 saturation were compared separately. CO from RHC and MR oximetry were compared to 2D PC MRI using Bonferroni-corrected repeated measures analysis of variance.
Results
Interstudy Reproducibility
The mean volunteer (N = 5, two females) age was 31 ± 13 (20–53) years. Average heart rate and SpO2 at time of MR was 77 ± 14 (60–95) bpm and 97% ± 1% (96–98%), respectively. The measured hematocrit was 42.8 ± 3.6 (39.8–48.9). The mean, SD, and range of O2 saturation across volunteers and CV determined for each subject from the five repeat acquisitions by three observers are reported in Table 1 as a measure of interstudy reproducibility. Interstudy CV determined from the patient data by a single observer was 3.2%.
TABLE 1.
Interstudy Reproducibility Characteristics
| RV (N = 5x5) |
MPA (N = 5x5) |
|||
|---|---|---|---|---|
| O2 Saturation, % | CV, % | O2 Saturation, % | CV, % | |
|
| ||||
| Observer 1 | 78 ± 6 | 2.97 ± 1.67 | 77 ± 2 | 2.24 ± 0.70 |
|
| ||||
| (70–83) | (1.25–5.30) | (73–79) | (1.51–3.36) | |
|
| ||||
| Observer 2 | 78 ± 7 | 2.61 ± 1.01 | 75 ± 2 | 1.99 ± 0.55 |
|
| ||||
| (69–86) | (1.53–3.89) | (73–78) | (1.12–2.57) | |
|
| ||||
| Observer 3 | 81 ± 7 | 3.41 ± 2.31 | 75 ± 2 | 2.49 ± 0.61 |
|
| ||||
| (72–87) | (1.17–7.30) | (72–78) | (1.55–3.21) | |
CV = coefficient of variation; MPA = main pulmonary artery; RV = right ventricle.
Interobserver Reproducibility
The single-measures ICC for all volunteer and patient data included in interobserver analyses were 0.81 (95% CI, 0.64–0.90) for RV O2 saturation (N = 35) and 0.87 (95% CI, 0.73–0.94) for MPA O2 saturation (N = 32). O2 saturation determined by RHC ranged from 49–77% in the subset of 10 patients included for reproducibility analysis. The mean difference in O2 saturation from reference RHC values for patients were 3% ± 6% for Observer 1, 4% ± 6% for Observer 2, and 4% ± 6% for Observer 3.
Accuracy Compared to RHC (Patient Results)
Patient demographics and baseline characteristics are shown in Table 2. All MRI exams were performed without any sedation; 17 patients underwent RHC with moderate sedation, and the remainder without sedation. One-third of the patients had sternal wires from transplant surgery, one patient had a coronary stent, and another had a PFO occluder. SpO2 was greater than 90% for all patients and did not differ significantly between MR and RHC; the largest difference measured between RHC and MR was 5% (absolute difference, 1% ± 1%). A same-day laboratory measurement of hematocrit was available, within 10 minutes to 16 hours of MRI for 24 patients and within 1 to 13 days from MRI for the other eight patients.
TABLE 2.
Patient Characteristics
| Patient characteristics (N = 32) | Mean ± SD or N (%) | Range |
|---|---|---|
| Age, years | 55 ± 13 | 24–76 |
| Gender | ||
| Males | 18 (56%) | |
| Females | 14 (44%) | |
| Race | ||
| Caucasian | 30 (94%) | |
| African-American | 1 (3%) | |
| Asian | 1 (3%) | |
| Ethnicity | ||
| Not Hispanic | 32 (100%) | |
| Weight, kg | 84.0 ± 18.3 | 42.2–121.1 |
| Height, cm | 172.0 ± 9.9 | 149.9–188.0 |
| BMI, kg/m2 | 28.5 ± 6.2 | 16.0–46.8 |
| Time between MRI and RHC, days | 0.1 ± 0.3 | 0–1 |
| Indication for RHC | ||
| Heart failure | 3 (9%) | |
| PHTN | 6 (19%) | |
| HF + PHTN | 2 (6%) | |
| Post heart transplant monitoring | 10 (31%) | |
| Congenital | 5 (16%) | |
| ASD | 3 (9%) | |
| PFO | 1 (3%) | |
| Dextrocardia | 1 (3%) | |
| Respiratory | 3 (9%) | |
| Other | 3 (9%) | |
| Hct (day of MRI)a | 38.9 ± 4.9 | 23.5–46.6 |
| SpO2 at MRI | 97 ± 2 | 93–100 |
| HR at MRI | 81 ± 15 | 43–114 |
| FiO2, | ||
| Room air, | 27 (84%) | |
| Supplemental O2 | 5 (16%) | |
Eight patients had a laboratory measurement of hematocrit on a different day.
ASD = atrial septal defect; BMI = body mass index; FiO2 = fraction of inspired oxygen; Hct = hematocrit; HF = heart failure; HR = heart rate; MRI = magnetic resonance imaging; PFO = patent foramen ovale; PHTN = pulmonary hypertension; RHC = right heart catheterization; SD = standard deviation; SpO2 = peripheral arterial oxygen saturation.
MPA O2 saturation measured during RHC ranged from 49–85%; values fell within the physiologically normal range of mixed venous O2 saturation for 28 of the 32 patients. O2 saturation was below normal (<60%) by RHC in two patients presenting with systolic heart failure and right heart failure due to severe PHTN, respectively. While physiologically low, their corresponding MR estimates were higher (MR vs. RHC, 65% vs. 58%, and 58% vs. 49%). A step-up in mixed venous O2 saturation was demonstrated in two of the three atrial septal defect (ASD) patients with RHC (although MR oximetry correlated with MR Qp:Qs in the third patient who underwent ASD closure following diagnostic assessment). PV and SVC O2 saturation required to determine MR oximetry-based Qp:Qs were not acquired in these patients. MR and RHC O2 saturation, and Qp:Qs shunt ratio determined from RHC and 2D PC MRI, are reported in Table 3.
TABLE 3.
O2 Saturation and Qp:Qs Ratio of Patients With ASD
| Mixed venous O2 saturation (%) |
Qp:Qsa |
|||
|---|---|---|---|---|
| Patient | MRI | RHC | 2D PC MRI | RHC |
|
| ||||
| 1 | 88 | 85 | 2:1 | 2.3:1 |
|
| ||||
| 2 | 92 | 82 | 1.8:1 | 1.8:1 |
|
| ||||
| 3 | 80 | 71 | 1.2:1 | NA |
NA, Qp:Qs not determined.
ASD = atrial septal defect; MRI = magnetic resonance imaging; PC = phase contrast; Qp = pulmonic flow; Qs = systemic flow; RHC = right heart catheterization.
O2 saturation by MR and RHC were significantly correlated (y = 0.85x + 0.13, R = 0.78, P < 0.05) (Fig. 3a). The bias was 2% ± 5% (95% CI, 0.6–4%, P < 0.05) and limits of agreement ranged from −7 to 12% (Fig. 3b). The absolute difference between O2 saturation by MR and RHC was 4% ± 3% (range, 0–15%). The (a-v)O2 difference also demonstrated a significant positive correlation (y = 0.71x + 0.90, R = 0.69, P < 0.05, Fig. 3c). The bias in (a-v)O2 difference was −0.53 ± 0.97 mL/100 mL (95% CI, −0.88 to −0.18, P < 0.05) and the limits of agreement were between −2.43 to 1.37 mL O2/100 mL (Fig. 3d). The difference between MR and RHC varied from −3.11 to 1.05 mL O2/100 mL.
FIGURE 3:
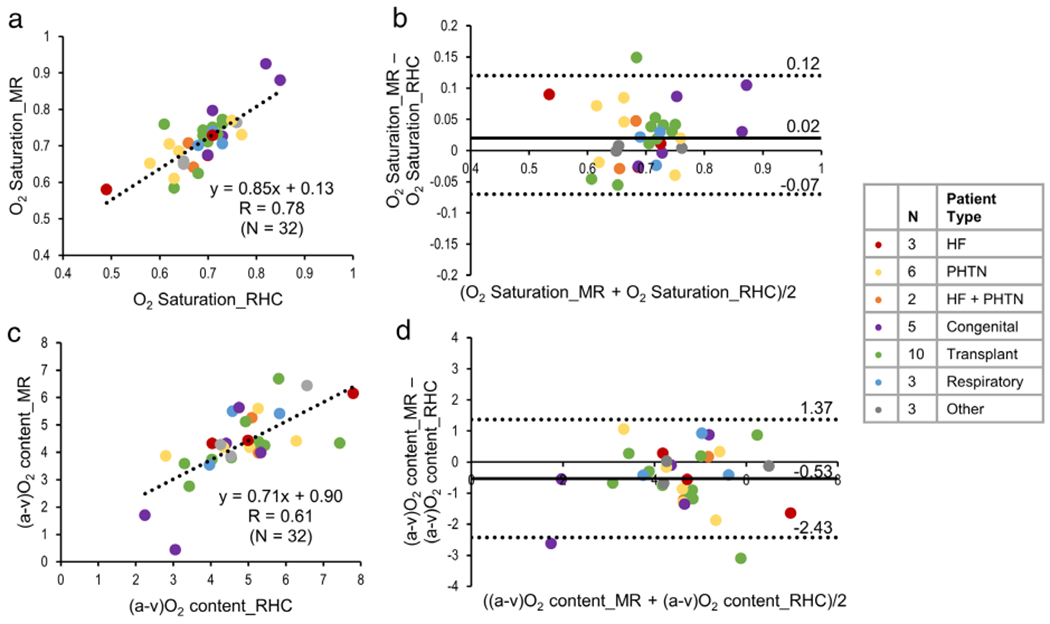
Linear regression (a) and Bland–Altman (b) plots showing the comparison of O2 saturation estimated from MR oximetry against invasive RHC results. (c,d) The linear regression and Bland–Altman plots for (a-v)O2 difference (mL O2/100 mL of blood) estimated by MR against RHC. The Pearson correlation coefficient, R, is reported in (a,c). Specific patient pathologies are highlighted by the different colors.
Further analysis including only patients (N = 28) with same-day MR and RHC revealed a higher correlation for O2 saturation (y = 0.94x + 0.06, R = 0.85, P < 0.05) and (a-v) O2 difference (y = 0.87x + 0.17, R = 0.77, P < 0.05).
Matching MR and RHC measurements of SVC O2 saturation were available in four patients; the difference ranged from 1–9% (MR vs. RHC values were 0.74 vs. 0.75, 0.76 vs. 0.67, 0.66 vs. 0.68 and 0.70 vs. 0.75 respectively).
The estimated values for the nuisance parameters from all samples (N = 32 in the RV, N = 22 in the MPA, and N = 4 in the SVC) were 0.39 ± 0.02 (0.24 to 0.41) for α, 224 ± 21 (173 to 267) msec for T2O, and 7.5 ± 0.84 (2.4 to 8.3) msec for τex respectively.
Twenty-nine patients had CO determined from RHC (2.1 to 10.8 L/min), MR oximetry (3.2 to 9.6 L/min), and 2D PC MRI (3.3 to 9.1 L/min). CO could not be calculated by MR oximetry in one patient whose MR venous O2 saturation measured 92%. In the other 28 patients, CO determined by MR oximetry matched slightly closer to 2D PC MRI (mean difference, 0.2 ± 1.0 L/min, range, −1.8 to 2.3 L/min, P = 1.0) than RHC (mean difference, −0.3 ± 1.4 L/min, range, −2.7 to 3.6 L/min, P = 0.71).
Discussion
Reproducibility and Accuracy
The present study evaluated the feasibility and accuracy of blood O2 saturation estimated by noninvasive, patient-adaptive MR oximetry against clinical standard catheter-based sampling and blood gas analysis. Excellent interstudy and interobserver reproducibility was shown in volunteers and patients. A high positive correlation with a small but significant bias between MR oximetry and RHC O2 saturation was shown in patients; catheter measurements of venous O2 saturation spanned abnormally low to high levels, with most values within the normal physiological range for this cohort.
The catheter O2 saturation measurement was not used to estimate any model parameters. Instead, the noninvasive arterial O2 saturation measured by pulse oximetry during MR served as a patient-specific input to improve estimation of the unknown parameters in the L-M model. The τ180-dependent MR signal behavior is relatively flat within the normal arterial range of 95–100%, and therefore fluctuations in the pulse oximeter measurement do not appreciably affect MR oximetry results. Below arterial O2 saturation of 95%, a downward bias greater than 5% in the pulse oximeter reading could have a larger impact on the accuracy of the MR O2 saturation estimate.
Hematocrit is also used as a patient-specific input and has a quadratic influence on O2 saturation in the L-M model. As a result, hematocrit values within the range of 40–60% minimally alter the estimated O2 saturation; however, every 5% increase or decrease in hematocrit beyond this range results in an ~2% drop in the O2 saturation estimate. The use of patient-specific inputs rather than application of a fixed, population-based calibration factors leads to an adaptive approach where the data drives the fit to the model.
Confidence of MR Oximetry From the Present Study
The implied assumption in this study that RV and MPA O2 saturations were equivalent was reasonable, as none of the patients had ventricular septal defects (VSD). Thus, MR O2 saturation in both RV and MPA were averaged to provide a single mixed venous O2 saturation to compare to the single catheter measurement in the MPA. Although the clinical reference standard, catheterization represents a noisy ground truth for blood O2 saturation in this comparison study, as the point blood sampling within a chamber can introduce measurement variability. Interstudy reproducibility of MR oximetry in both volunteers and patients were comparable to the reported catheter variability of 2% in the MPA.1,28 However, application in pediatric patients and patients with congenital cardiovascular abnormalities would pose additional challenges; complex flow patterns, stents, occluders, and other implanted metallic devices locally affect the MRI signal and may reduce accuracy compared to healthy volunteers and patients with structurally normal hearts. Despite the wide confidence limits in patients and the limited sample size for specific pathologies, MR oximetry accurately identified shunt patients, determined normal venous O2 saturation in transplant patients, and characterized the tendency towards hypoxemia in heart failure and PHTN patients. Studies with simultaneous cardiac MR-invasive catheter measurements are warranted in these patients with potentially unstable hemodynamics to further demonstrate oximetry accuracy.
Clinical Translatability
Noninvasive MR blood oximetry has a number of potentially important clinical applications. Generally, its role is anticipated to be to noninvasively determine O2 saturation as complimentary information to standard MR evaluation of cardiac morphology, function, flow, and viability.
This technique could play an important role in pediatric congenital heart disease, where despite the imaging challenges associated with smaller body size, higher cardiac and respiratory rates, and subject motion, MRI is increasingly used for pre- and post-operative management as a surrogate to invasive catheterization. A noninvasive MR oximetry run could substantially reduce radiation exposure during diagnostic cardiac catheterization for serial evaluation. MR measurements can be repeated at no additional patient risk to improve accuracy. Shunt quantification by PC-MRI can be internally validated with MR oximetry, and when combined with flow, could potentially be used to assess lung perfusion. MR oximetry could determine O2 saturation in regions where RHC results are uncertain or a catheter measurement may be difficult or risky to obtain. MR oximetry in fetal surveillance could also assist in postnatal intervention planning.29
MR oximetry may also provide value in patients with heart failure and PHTN, which are both associated with hypoxemia. A reduced mixed venous O2 saturation and increased (a-v)O2 difference reflects poor O2 delivery and/or greater systemic O2 extraction. O2 measurement in specific cardiac chambers could help to establish the etiology of hypoxemia (cardiac vs. pulmonary), and potentially be used to tailor therapy and monitor treatment in ways not currently feasible. MR oximetry, as part of an MR-compatible exercise test, may identify and characterize exertional dyspnea, exercise-induced PHTN, and myocardial energetics in heart failure. A comprehensive cardiac MR exam augmented with myocardial O2 consumption and tissue characterization could also provide a noninvasive alternative to frequent catheterization in heart transplant patients.
MR oximetry combined with the hemodynamic assessments made possible by 4D flow30–32 could substantially augment, and in some cases replace, diagnostic catheterization by providing quantitative evaluation of cardiac function, hemodynamics and tissue characterization, as well as O2 saturation, delivery, and consumption across the patient lifespan.
Limitations
Despite the use of nonselective T2 preparation pulse schemes known to minimize sensitivity to field inhomogeneity and flow,25,26 we observed signal loss primarily in the left atrium and pulmonary veins,33 rendering these regions unreliable for MR oximetry. The present study was also limited to single 2D slices acquired perpendicular to a chamber or vessel. Extending the technique to 3D volumetric acquisition would facilitate comprehensive evaluation across all cardiac chambers and vessels.
Blood signal heterogeneity may lead to a greater dependency on the user to select regions for analysis, leading to increased interobserver variability. The semiautomated analysis approach implemented in this study to eliminate pixels with potentially abnormal signal loss does not guarantee a selection of blood pixels devoid of flow-induced dephasing; however, it makes the analysis more data-driven and less user-biased. Metallic implants may also cause irreversible dephasing beyond what can be refocused by T2 preparation; however, correlation of MR oximetry with catheterization was reasonable despite the presence of wires and implants in this patient cohort. Additional postprocessing strategies combining flow information could optimize pixel selection and therefore further improve the accuracy of MR O2 saturation.
As the L-M model implies, the sensitivity of T2-weighted blood signal decreases with increasing blood O2 saturation. This could reduce the accuracy at higher O2 saturation levels, and may have contributed to the greater than average error observed in the ASD patients. Reliance on the L-M model, which may not fully capture the biophysical properties of blood and the associated magnetic behavior, could also impact overall measurement accuracy.
Novel methods such as augmenting the model fit with a neural network approach34 may increase the robustness of adaptive MR oximetry. In-line processing of MR oximetry images and user-friendly analysis tools would further help to facilitate clinical use.
The study applied rigorous exclusion criteria to minimize physiological variability between exams; however, differences between nonsimultaneous MR and catheter measurements may have contributed to the variability in our results. Future evaluation in patients undergoing simultaneous MR and catheterization is warranted. The present study included only one patient with sickle cell anemia, and the impact of other hemoglobin variants such as HbA1c, known to cause overestimation of O2 saturation by pulse oximetry, was not evaluated. The majority of patients in the current study were Caucasians; thus, hematologic differences that may be present across different populations, eg, sickle cell disease in African Americans, were not evaluated. The accuracy of MR oximetry in patients with hemoglobin variants that affect oxygen affinity needs to be further assessed. As most patients had O2 saturation within the normal physiological range, this small study cohort was not adequate to evaluate the sensitivity and specificity of MR oximetry to abnormally high and low O2 saturation. Continued evaluation in a larger, racially diverse population, in patients with stents and other metallic implants, and in cohorts of patients with specific cardiac conditions and congenital anomalies is warranted to establish the sensitivity, specificity, and accuracy of this technique.
Conclusion
A patient-adaptive MR oximetry method was applied to noninvasively determine intracardiac and intravascular O2 saturation. Patient-specific measurable inputs—MR data, hematocrit, and SpO2—drive model fitting to generate patient-specific outputs—the unknown O2 saturation and nuisance parameters. The technique was shown to be repeatable and reproducible. MR O2 saturation and (a-v)O2 showed good agreement with invasive catheter measurements in a small cohort of adult patients, with values ranging from abnormally low to high O2 saturation, but mostly within the normal physiological range. Adaptive MR oximetry would be a useful component in the comprehensive, noninvasive MR evaluation of patients with a variety of cardiovascular conditions that can impact blood oxygenation.
Supplementary Material
Acknowledgments
The authors thank Debbie Scandling for help with the patient studies and Ning Jin, PhD, of Siemens Healthcare for providing technical support.
Contract grant sponsor: National Heart Lung and Blood Institute; Contract grant number: 5T32HL098039–07 (to J.V.); receive research support from Contract grant sponsor: Siemens Healthineers (to O.P.S., R.A., and S.V.R.); Contract grant sponsor: Robert F. Wolfe and Edgar T. Wolfe Foundation (to O.P.S.); Contract grant sponsor: National Institute of Biomedical Imaging and Bioengineering; Contract grant number: R21EB021638 (to O.P.S. and R.A.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Additional supporting information may be found in the online version of this article
References
- 1.Antman EM, Marsh JD, Green LH, Grossman W. Blood oxygen measurements in the assessment of intracardiac left to right shunts: A critical appraisal of methodology. Am J Cardiol 1980;46:265–271. [DOI] [PubMed] [Google Scholar]
- 2.Hillis LD, Firth BG, Winniford MD. Variability of right-sided cardiac oxygen saturations in adults with and without left-to-right intracardiac shunting. Am J Cardiol 1986;58:129–132. [DOI] [PubMed] [Google Scholar]
- 3.Kandel G, Aberman A. Mixed venous oxygen saturation. Its role in the assessment of the critically ill patient. Arch Intern Med 1983;143:1400–1402. [DOI] [PubMed] [Google Scholar]
- 4.Hoeper MM, Lee SH, Voswinckel R, et al. Complications of right heart catheterization procedures in patients with pulmonary hypertension in experienced centers. J Am Coll Cardiol 2006;48:2546–2552. [DOI] [PubMed] [Google Scholar]
- 5.Zuckerman WA, Turner ME, Kerstein J, et al. Safety of cardiac catheterization at a center specializing in the care of patients with pulmonary arterial hypertension. Pulm Circ 2013;3:831–839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Connors AF Jr, Speroff T, Dawson NV, et al. The effectiveness of right heart catheterization in the initial care of critically ill patients. SUPPORT investigators. JAMA 1996;276:889–897. [DOI] [PubMed] [Google Scholar]
- 7.Chittock DR, Dhingra VK, Ronco JJ, et al. Severity of illness and risk of death associated with pulmonary artery catheter use. Crit Care Med 2004;32:911–915. [DOI] [PubMed] [Google Scholar]
- 8.Fogel MA, Pawlowski TW, Whitehead KK, et al. Cardiac magnetic resonance and the need for routine cardiac catheterization in single ventricle patients prior to Fontan: A comparison of 3 groups: Pre-Fontan CMR versus cath evaluation. J Am Coll Cardiol 2012;60:1094–1102. [DOI] [PubMed] [Google Scholar]
- 9.Brown DW, Gauvreau K, Powell AJ, et al. Cardiac magnetic resonance versus routine cardiac catheterization before bidirectional glenn anastomosis in infants with functional single ventricle: A prospective randomized trial. Circulation 2007;116:2718–2725. [DOI] [PubMed] [Google Scholar]
- 10.Ntsinjana HN, Hughes ML, Taylor AM. The role of cardiovascular magnetic resonance in pediatric congenital heart disease. J Cardiovasc Magn Reson 2011;13:51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brooks RA, Brunetti A, Alger JR, Di Chiro G. On the origin of paramagnetic inhomogeneity effects in blood. Magn Reson Med 1989;12:241–248. [DOI] [PubMed] [Google Scholar]
- 12.Thulborn KR, Waterton JC, Matthews PM, Radda GK. Oxygenation dependence of the transverse relaxation time of water protons in whole blood at high field. Biochim Biophys Acta 1982;714:265–270. [DOI] [PubMed] [Google Scholar]
- 13.Li D, Waight DJ, Wang Y. In vivo correlation between blood T2* and oxygen saturation. J Magn Reson Imaging 1998;8:1236–1239. [DOI] [PubMed] [Google Scholar]
- 14.Silvennoinen MJ, Clingman CS, Golay X, Kauppinen RA, van Zijl PC. Comparison of the dependence of blood R2 and R2* on oxygen saturation at 1.5 and 4.7 Tesla. Magn Reson Med 2003;49:47–60. [DOI] [PubMed] [Google Scholar]
- 15.Silvennoinen MJ, Kettunen MI, Kauppinen RA. Effects of hematocrit and oxygen saturation level on blood spin-lattice relaxation. Magn Reson Med 2003;49:568–571. [DOI] [PubMed] [Google Scholar]
- 16.Wright GA, Hu BS, Macovski A. 1991 I.I. Rabi award. Estimating oxygen saturation of blood in vivo with MR imaging at 1.5 T. J Magn Reson Imaging 1991;1:275–283. [DOI] [PubMed] [Google Scholar]
- 17.Li D, Wang Y, Waight DJ. Blood oxygen saturation assessment in vivo using T2* estimation. Magn Reson Med 1998;39:685–690. [DOI] [PubMed] [Google Scholar]
- 18.Wedegartner U, Kooijman H, Yamamura J, et al. In vivo MRI measurement of fetal blood oxygen saturation in cardiac ventricles of fetal sheep: A feasibility study. Magn Reson Med 2010;64:32–41. [DOI] [PubMed] [Google Scholar]
- 19.Luz Z, Meiboom S. Nuclear magnetic resonance study of the protolysis of trimethylammonium ion in aqueous solution—Order of the reaction with respect to solvent. J Chem Phys 1963;39:366–370. [Google Scholar]
- 20.Nield LE, Qi X, Yoo SJ, Valsangiacomo ER, Hornberger LK, Wright GA. MRI-based blood oxygen saturation measurements in infants and children with congenital heart disease. Pediatr Radiol 2002;32:518–522. [DOI] [PubMed] [Google Scholar]
- 21.Nield LE, Qi XL, Valsangiacomo ER, et al. In vivo MRI measurement of blood oxygen saturation in children with congenital heart disease. Pediatr Radiol 2005;35:179–185. [DOI] [PubMed] [Google Scholar]
- 22.Qi X, Wright GA. Using population data to calibrate MRI-based blood oxygen saturation measurements in CHD patients and volunteers. In: Proc 8th Annual Meeting ISMRM, Denver; 2000. p 1570. [Google Scholar]
- 23.Varghese J, Potter LC, LaFountain R, et al. CMR-based blood oximetry via multi-parametric estimation using multiple T2 measurements. J Cardiovasc Magn Reson 2017;19:88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Levitt MH, Freeman R. Compensation for pulse imperfections in NMR spin-echo experiments. J Magn Reson 1969;43:65–80. [Google Scholar]
- 25.Brittain JH, Hu BS, Wright GA, Meyer CH, Macovski A, Nishimura DG. Coronary angiography with magnetization-prepared T2 contrast. Magn Reson Med 1995;33:689–696. [DOI] [PubMed] [Google Scholar]
- 26.Wright GA, Nishimura DG, Macovski A. Flow-independent magnetic resonance projection angiography. Magn Reson Med 1991;17:126–140. [DOI] [PubMed] [Google Scholar]
- 27.Chefd’hotel C, Hermosillo G, Faugeras O. Flows of diffeomorphisms for multimodal image registration. Washington, DC: In: Proc IEEE International Symposium on Biomedical Imaging; 2002. p 753–756. [Google Scholar]
- 28.Barratt-Boyes BG, Wood EH. The oxygen saturation of blood in the venae cavae, right-heart chambers, and pulmonary vessels of healthy subjects. J Lab Clin Med 1957;50:93–106. [PubMed] [Google Scholar]
- 29.Sun L, Marini D, Saini B, Schrauben E, Macgowan CK, Seed M. Understanding fetal hemodynamics using cardiovascular magnetic resonance imaging. Fetal Diagn Ther 2020;47:354–362. [DOI] [PubMed] [Google Scholar]
- 30.Rengier F, Delles M, Eichhorn J, et al. Noninvasive pressure difference mapping derived from 4D flow MRI in patients with unrepaired and repaired aortic coarctation. Cardiovasc Diagn Ther 2014;4:97–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kheyfets VO, Schafer M, Podgorski CA, et al. 4D magnetic resonance flow imaging for estimating pulmonary vascular resistance in pulmonary hypertension. J Magn Reson Imaging 2016;44:914–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schafer M, Barker AJ, Kheyfets V, et al. Helicity and vorticity of pulmonary arterial flow in patients with pulmonary hypertension: Quantitative analysis of flow formations. J Am Heart Assoc 2017;6:e007010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hu P, Stoeck CT, Smink J, et al. Noncontrast SSFP pulmonary vein magnetic resonance angiography: Impact of off-resonance and flow. J Magn Reson Imaging 2010;32:1255–1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Varghese J, Ahmad R, Raman SV, Potter LC, Simonetti OP. Non-invasive quantitative estimation of blood oxygen saturation with MRI: Feasibility of machine learning. Paris: Joint Annual Meeting ISMRM-ESMRMB 2018; 2018. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


