Abstract
Adolescence is a critical period of structural and functional neural maturation among regions serving the cognitive control of emotion. Evidence suggests that this process is guided by developmental changes in amygdala and striatum structure and shifts in functional connectivity between subcortical (SC) and cognitive control (CC) networks. Herein, we investigate the extent to which such developmental shifts in structure and function reciprocally predict one another over time. 179 youth (9–15 years-old) completed annual MRI scans for three years. Amygdala and striatum volumes and connectivity within and between SC and CC resting state networks were measured for each year. We tested for reciprocal predictability of within-person and between-person changes in structure and function using random-intercept cross-lagged panel models. Within-person shifts in amygdala volumes in a given year significantly and specifically predicted deviations in SC-CC connectivity in the following year, such that an increase in volume was associated with decreased SC-CC connectivity the following year. Deviations in connectivity did not predict changes in amygdala volumes over time. Conversely, broader group-level shifts in SC-CC connectivity were predictive of subsequent deviations in striatal volumes. We did not see any cross-predictability among amygdala or striatum volumes and within-network connectivity measures. Within-person shifts in amygdala structure year-to-year robustly predicted weaker SC-CC connectivity in subsequent years, whereas broader increases in SC-CC connectivity predicted smaller striatal volumes over time. These specific structure function relationships may contribute to the development of emotional control across adolescence.
Keywords: Striatum, Emotional control, Development, Structure-function relationships, Structural Equation Modeling (SEM), longitudinal
1. Introduction
Many recent studies have focused on understanding functional resting-state brain networks, and their patterns of intra- and interconnectivity. Briefly, resting-state networks are groups of brain regions whose activity patterns correlate during unstructured time (i.e., rest), and are commonly assessed using functional MRI (fMRI; Lv et al., 2018; Shen, 2015). Despite being assessed at rest, many of the identified networks are implicated in cognitive, emotional, and sensorimotor functioning during tasks (Dwyer et al., 2014; Marek et al., 2015; Rosazza and Minati, 2011). For example, a commonly identified “cognitive control” network is comprised of structures spanning mainly frontal and parietal regions, which are commonly active during tasks requiring attentional control, emotional control, and high-level reasoning (Agcaoglu et al., 2019; Heller et al., 2016; Jung and Haier, 2007; Petersen and Posner, 2012; Spooner et al., 2019; Taylor et al., 2021, 2020). Likewise, the “subcortical” network, although somewhat more ambiguously named with respect to function, is commonly implicated in a wide array of abilities like emotional control and reward processing (Agcaoglu et al., 2019; Cerliani et al., 2015; Gabard-Durnam et al., 2014; Heller et al., 2016). Functional connectivity between these two networks is known to be associated with cognitive control in the face of emotional cues (Heller et al., 2016), and is developmentally sensitive (Casey et al., 2019; van Duijvenvoorde et al., 2016).
Throughout childhood and adolescence, the neural circuitry underlying emotional control is continually being refined, with some evidence suggesting a shift from subcortically-driven (e.g., amygdala, striatum) to more cortically-driven mechanisms (e.g., prefrontal cortex) underlying decision-making processes in the face of emotional stimuli (Casey et al., 2016; Casey, 2015; Heller et al., 2016; van Duijvenvoorde et al., 2016). The net result of this maturation is a modification of behavior away from impulsive reactions to more methodical, rational responses to emotional cues. Research suggests that some degree of reduction in subcortical-cortical connectivity, with a shift in balance toward cortically-guided mechanisms as a function of age could be considered normative, ultimately supporting more mature cognitive-emotional functioning in the case of healthy development (i.e., free of psychopathologies; Cerliani et al., 2015; Pfeifer and Allen, 2012; Rubia, 2013). However, the literature in this area is decidedly mixed (Lucian, 2013). For instance, several studies have shown that decreased subcortico-cortical connectivity is associated with depression in adolescents and adults (Connolly et al., 2017; Heller et al., 2009). Alternatively, others have shown that increases in connectivity are associated with greater cognitive control in the face of emotional cues (Heller et al., 2016). Although there is limited consensus on the nature of “normative” change in connectivity patterns, there is generalized agreement that the nature of connectivity between cortical and subcortical regions of the brain is changing over the course of adolescent development; much recent work highlights a shift toward cortically-driven mechanisms of cognitive control of emotions (Casey et al., 2019).
This shift from “bottom-up” to “top-down” connectivity patterns serving emotional control across development is believed to result from maturational changes in brain structure, though the exact mechanisms are yet unclear (Casey, 2015; van Duijvenvoorde et al., 2016). One candidate structure that may contribute to changes in emotional control-related functional connectivity is the amygdala (Bickart et al., 2014; Giedd et al., 1996; Graham et al., 2016; Sato et al., 2020). The amygdala has been repeatedly identified as a key hub in emotional reactivity, motivation, and decision-making (Bickart et al., 2014; He et al., 2017; Jung et al., 2018; Luciana and Collins, 2012; Ochsner et al., 2012; Ochsner and Gross, 2005; Sato et al., 2020; van Duijvenvoorde et al., 2016; Warnell et al., 2018). Research has shown only minor deviations in amygdala structure (e.g., volume) across development, though such modulations can have systemic, varied impacts on overall cognitive-emotional functioning and well-being (Gabard-Durnam et al., 2014; Graham et al., 2016; He et al., 2017; Rogers et al., 2017).
Studies reporting associations between amygdala structure and functional outcomes have been mixed. For instance, anxiety symptoms and anxious behaviors have been associated with both larger and smaller amygdala volumes in youths (Qin et al., 2014; Warnell et al., 2018). Volumetric variability has also been differentially associated with risk tolerance (Jung et al., 2018) and aberrant threat processing (Saxbe et al., 2018). The disparity in associations between amygdala structure and psychological health is in part due to the fact that these individual variations are dependent on experiences like exposure to stress and trauma at different stages of development, as well as epigenetic factors like familial predisposition to psychological disorders (Evans et al., 2016; Merz et al., 2018; Monk, 2008; Pechtel et al., 2014). As such, it is notoriously difficult to disentangle the nature of these structure-function associations. Still, some research suggests that minute individual-level variability in amygdala structure over time may actually be driven by changes in functional connectivity within key brain networks across development (Saygin et al., 2015).
Thus, although limited, the available evidence hints at a reciprocal pattern of neural development underlying the maturation of cognitive-emotional control. In other words, changes in functional connectivity over time may shape individual variability in amygdala structure, and such volumetric shifts in the amygdala may impact the refinement of functional network connectivity across development (Casey, 2015; Saygin et al., 2015; van Duijvenvoorde et al., 2016). However, studies to date have yet to directly test this; any reciprocal relationship between amygdala volume and functional connectivity pertinent to the cognitive control of emotion in developing youth remains speculative.
The purpose of the present investigation was to determine whether amygdala volumes relate to the strength of intra- and inter-network connectivity (i.e. functional connectivity or its network analog functional network connectivity or FNC) within subcortical (SC) and cognitive control (CC) networks, both of which are heavily implicated in the cognitive control of emotion and have been robustly constructed and vetted in prior work (Agcaoglu et al., 2019). We leveraged data from a large multi-site study of typically developing children and adolescents who were assessed annually for three years. Based on the extant literature, we hypothesized reciprocal predictability of changes in amygdala volumes and longitudinal patterns of FNC over time. Given the exploratory nature of this work, we additionally assessed relationships between FNC and other relevant neural substrates, including regions within the SC and CC functional networks and other critical limbic structures, as well as relationships between the amygdala and other FNC measured during resting state. This allowed us to assess the specificity of the relationships detected between the targeted SC and CC networks and amygdala volumes.
2. Materials and methods
2.1. Participants
In total, 215 typically developing youth between the ages of 9 and 15 years-old (M = 11.72 years, SD = 1.78; 106 male) were recruited from two data collection sites to participate in the Developmental Chronnecto-Genomics Study (Dev-CoG; Stephen et al., 2021). Each participant was invited to complete MRI scans annually for three consecutive years. Exclusionary criteria for the study, determined through parent report, included diagnosis of a neurological, developmental, or substance use disorder, use of pharmaceuticals that impact central nervous system functioning, and presence of metal that could not be removed from the body, including braces, permanent retainers, and implanted medical devices. Before beginning study procedures, all parents of youth participants signed informed consent forms, and youth signed assent forms. All study protocols were approved by the appropriate institutional review board for each study site.
2.2. Structural MRI acquisition and analysis
Participants underwent a structural T1-weighted MRI scan during each visit. Children recruited at one site were scanned using a Siemens 3T Skyra scanner, and those at the second site were scanned using a Siemens 3T TIM Trio. Structural T1-weighted MR images at both sites were acquired with a 32-channel head coil and a MP-RAGE sequence with the following parameters: TR = 2400 ms; TE = 1.94 ms; flip angle = 8°; FOV = 256 mm; slice thickness = 1 mm (no gap); base resolution = 256; 192 slices; voxel size = 1 × 1 × 1 mm. The T1-weighted structural brain images of all participants were processed using Freesurfer software version 5.3 (http://surfer.nmr.mgh.harvard.edu). Gray matter volume estimates were computed for the 70 Desikan-Killiany atlas regions (34 regions per hemisphere, plus left and right hemisphere). We followed the ENIGMA protocol for quality assurance, including performing visual checks on all cortical segmentations (http://enigma.usc.edu/protocols/imaging-protocols) and checking for motion among other artifacts. Participants with large motion artifacts were excluded. In addition, histograms of all regional values were computed for visual inspection; no cortical segmentations were flagged during these quality control inspections. For each year of the study, we extracted the left and right amygdala volumes. Bilateral volumes were averaged and normalized by dividing by the total brain volume per participant to avoid bias due to differences in head size. In follow-up exploratory analyses we examined hippocampus, striatum, and medial orbitofrontal cortex volumes, which were also averaged across hemispheres and corrected for total brain volume. Regions were selected based on their known integration with cognitive-emotional control mechanisms (Fox et al., 2015), as well as their direct involvement in the identified SC and CC networks (Agcaoglu et al., 2019).
2.3. Functional MRI acquisition and processing
Functional and structural MRI acquisition occurred during the same scan session in each year of the study. To assess functional network connectivity, a total of 650 volumes of echo planar imaging BOLD data were collected during eyes open rest with the following parameters: TR = 0.46 s, TE = 29 ms, FA = 44°, and a slice thickness of 3 mm with no gap. Rs-fMRI scans were acquired using a standard gradient-echo echo planar imaging paradigm; site 1: FOV of 268 × 268 mm (82 × 82 matrix), 48 sequential axial slices; site 2: FOV of 246 × 246 mm (82 × 82 matrix), 56 sequential axial slices.
Complete details of the preprocessing and analysis are detailed in Supplementary Materials, and in a previous publications (Agcaoglu et al., 2020, 2019). Briefly, scans were corrected for head motion and differences in slice timing, followed by despiking to reduce outliers. Data were warped into Montreal Neurological Institute (MNI) space (http://www.mni.mcgill.ca), and subsequently rewarped to a study-specific template due to the age range of the participants (Agcaoglu et al., 2020, 2019). Group independent component analysis (ICA; Calhoun et al., 2001; Calhoun and Adali, 2012) of the preprocessed functional data yielded 150 spatially-independent components, 51 of which were identified as components comprising seven different resting state networks. FNC was measured as the average Pearson correlation between different resting state network time courses (Supplementary Table S7). The present study focused on the connectivity within and between the SC and CC networks. The SC network was comprised of connectivity among putamen and thalamic areas, and the CC network was comprised of connectivity among largely frontal and parietal regions, as well as some key temporal areas (for complete details, see Supplementary Table S7). For exploratory analyses, we also examined FNC within the visual, auditory, and sensorimotor networks.
2.4. Statistical analysis
The present study aimed to explore the extent to which amygdala volumes and both within and between-network FNC among the subcortical and cognitive control networks reciprocally impact one another over time in typically developing youth. Thus, we utilized a random intercept cross-lagged panel approach (RI-CLPM; Hamaker et al., 2015; Kline, 2005; Mund and Nestler, 2019) to explore the interrelationships between FNC and amygdala volumes both at the stable trait level, and at the within-person state level (see Fig. 1).
Fig. 1.
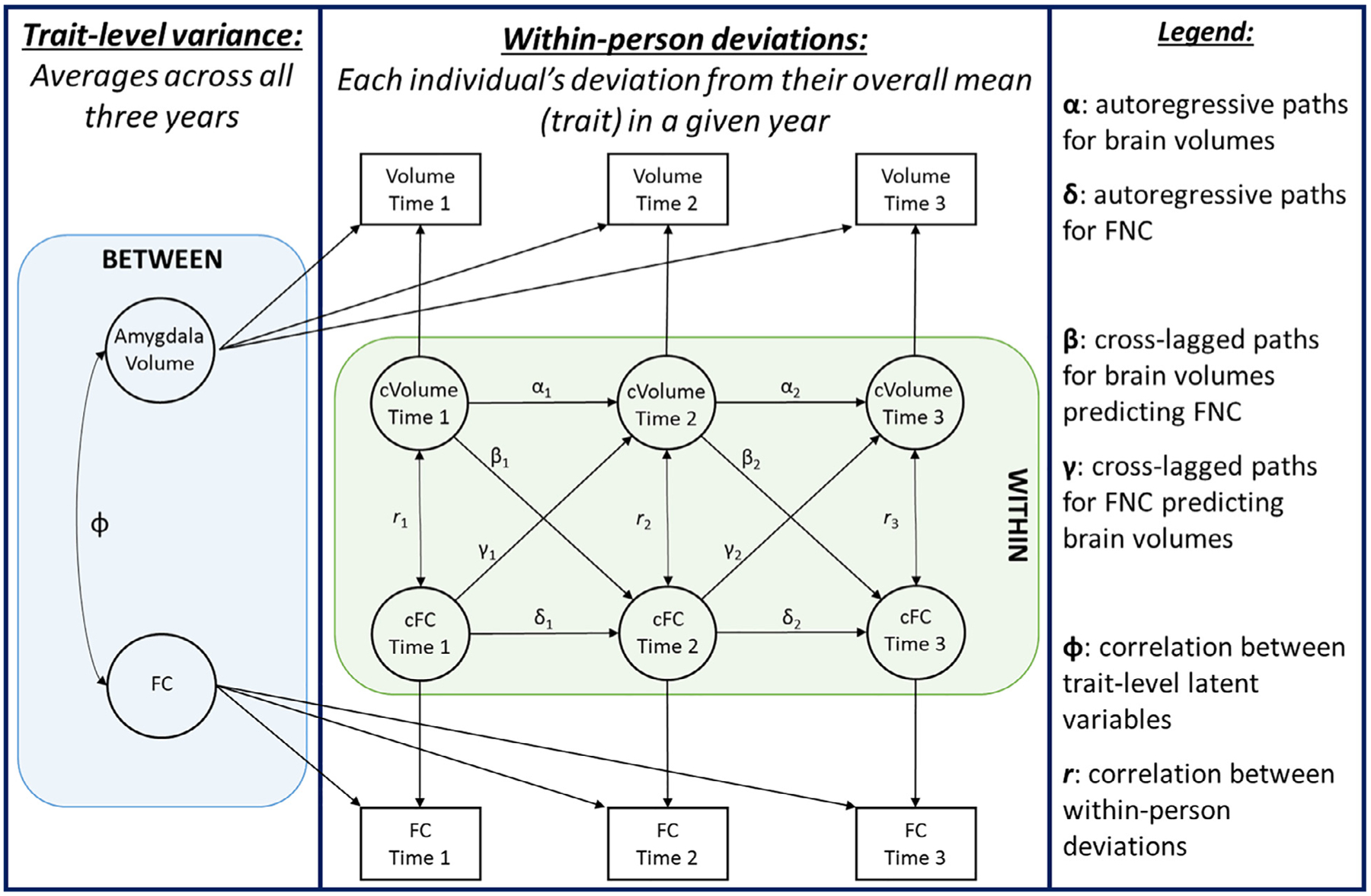
Conceptual design of the random-intercept cross-lagged panel model (RI-CLPM). Between-person latent trait variables are defined using data from observed variables across all three time points, and essentially represent an individual’s mean over time. Between-person latent variables are correlated. Time-specific within-person deviations are modeled at the within-person level. Within-person deviations are modeled with autoregressive paths, and cross-lagged paths, and within-time correlations. Double-headed arrows show correlations, and single-headed arrows show predictive paths. “FC” = functional connectivity; “cFC” = centered within-person deviation in functional connectivity; “cVolume” = centered within-person deviation in brain volume; squares represent manifest (observed) variables; circles represent latent variables.
Briefly, the model structure defines a random intercept for each measure of interest, which subsumes between-level (trait-based) variance in each measure over time. The remaining within-person variance in each measure is state-based and represents minute individual differences (i.e., temporary increases or decreases from one’s typical score) that occur within each time point. The model structure accounts for stability of within-person variance in a given measure over time (autoregressive paths) as well as the dynamic influences of measures on each other (cross-lagged paths).
We followed best practices for defining our model in the present study (Dietvorst et al., 2018; Fetvadjiev and He, 2019; Hamaker et al., 2015; Nelemans et al., 2019). We started with a freely estimated model in which all autoregressive paths, cross-lagged paths, and within-person correlations were allowed to freely vary. We then constrained the model in a stepwise manner to assess the non-stationarity of the underlying within-person processes. After each constraint, we assessed change in model fit using a chi-square difference test. After determining the most parsimonious model with the best fit, we compared the RI-CLPM to a standard cross-lagged panel model (i.e., a model without the between-person latent variables), and compared model fit to determine whether accounting for trait-level effects improved model fit. In other words, the more traditional CLPM does not disentangle within- from between-person level variance, and instead operates at the coarser grain of “group-level” statistics.
Complete details of model fitting for each of the three models tested (amygdala volume related to 1) intrinsic SC, 2) intrinsic CC, and 3) between-network SC-CC connectivity) are provided in the Supplementary Materials. Only the results of the best fitting models are described further in the main text. Model fit was assessed using common standards (Hu and Bentler, 1999), including a non-statistically significant chi-square, root mean square error of approximation (RMSEA) < .06, comparative fit index (CFI) > .95, and standardized root mean residual (SRMR) < .08. Analyses were completed in Mplus version 8.1 using full-information maximum likelihood (FIML) estimation for missing data.
Of note, we incorporated control variables of age at time 1, sex (0 = male, 1 = female), and data collection site (0 = site 1, 1 = site 2). We expected study site would be best modeled as a control variable on between-level variables (if the best-fitting model was a RI-CLPM), and that age and sex would be best modeled as control variables on within-person variables given the relatively dynamic nature of age- and sex-related neurological maturation over time. However, we did test whether control variables were better suited to the between-person variables in RI-CLPMs given the exploratory nature of the data. Testing of control variables is detailed in Supplementary Materials.
3. Results
3.1. Demographics and descriptive statistics
In total, 36 participants were excluded from final analyses (n = 10 had no data available for any MRI scans; n = 26 had either no structural MRI or no resting-state FNC available for any year of the study). Thus, the final sample was comprised of 179 youth (M = 11.90 years, SD = 1.70; 92 male). For a detailed overview of recruitment, scans completed, and retention over years of the study, see Supplemental Figure S1. Of note, youth who had data did not differ from those who did not have quality data or who discontinued participation across years of the study with respect to sex or study site (χ2 = .006 to 2.09, ps = .15 to .94). Youths who continued versus those who discontinued in Year 2 did not differ by age (t = .58, p = .56), though youths who continued on to year 3 of the study were slightly younger at the time of their first visit (t = 2.27, p = .024; Mcont. = 11.51 years, SD = 1.66; Mdiscont. = 12.11 years, SD = 1.69). The average time between visit 1 and visit 2 was 1.13 years (SD = .20), and between visit 2 and visit 3 was 1.08 years (SD = .21). Descriptive data and correlations between amygdala volumes and SC-CC connectivity among the final sample are provided in Table 1 and Supplementary Table S1, respectively.
Table 1.
Descriptive statistics for the measures input into the random-intercept cross-lagged panel model, including amygdala volumes (mm3 × 10−3), and functional connectivity between the subcortical (SC) and cognitive control (CC) networks taken from eyes-open resting state fMRI.
| Measure | N | M | SD | Range |
|---|---|---|---|---|
| Amygdala volume T1 | 171 | 1.05 | .13 | .11 to 1.40 |
| Amygdala volume T2 | 119 | 1.02 | .23 | .19 to 1.40 |
| Amygdala volume T3 | 71 | .96 | .28 | .21 to 1.32 |
| SC connectivity T1 | 172 | .51 | .16 | .15 to .98 |
| SC connectivity T2 | 118 | .53 | .16 | .15 to .89 |
| SC connectivity T3 | 64 | .48 | .17 | .12 to .81 |
| CC connectivity T1 | 172 | .095 | .076 | −.048 to .35 |
| CC connectivity T2 | 118 | .11 | .099 | −.021 to .43 |
| CC connectivity T3 | 64 | .11 | .098 | −.026 to .42 |
| SC-CC connectivity T1 | 172 | .094 | .086 | −.13 to .37 |
| SC-CC connectivity T2 | 118 | .092 | .12 | −.25 to .42 |
| SC-CC connectivity T3 | 64 | .048 | .13 | −.43 to .36 |
“N” = the number of participants who had adequate data for a given variable and a given study time point. “SC” = subcortical network intrinsic connectivity; “CC” = cognitive control network intrinsic connectivity; “SC-CC” = subcortical-to-cognitive control between-network connectivity; “T1” = time 1; “T2” = time 2; “T3” = time 3
3.2. Intrinsic connectivity models
A fully constrained traditional cross-lagged panel model (CLPM) was the best-fitting structure for the models of both SC and CC intrinsic connectivity related to amygdala volumes. In other words, neither model incorporated the latent between-person trait effect, and the autoregressive paths, cross-lagged predictions, and within-person correlations were all constrained over time (see Table 2, Supplementary Table S2 and Fig. S2). Thus, the results can be interpreted as “group-level” findings. Of note, the inclusion of age, sex, and site as control variables significantly harmed model fit, thus the final model did not incorporate these variables.
Table 2.
Cross-lagged panel model results linking amygdala volumes and intrinsic SC and CC network functional connectivity.
| Subcortical Network | Cognitive Control Network | |||||
|---|---|---|---|---|---|---|
| Parameter Estimated | b(SE) | r/β | p | b(SE) | r/β | p |
| amygdala volume → connectivity | .006 (.10) | .004 and .008 | .96 | .075 (.065) | .087 and .16 | .25 |
| connectivity → amygdala volume | .035 (.082) | .025 and .019 | .67 | −.077 (.17) | −.027 and −.026 | .64 |
| autoregressive paths: amygdala volume | 1.11 (.10) | .58 and .83 | < .001 | 1.11 (.10) | .58 and .83 | < .001 |
| autoregressive paths: connectivity | .42 (.070) | .43 and .41 | < .001 | .50 (.090) | .39 and .48 | < .001 |
| within-person correlations time 1 | .002 (.001) | .10 | .20 | −.001 (.001) | −.089 | .26 |
| time 2 and time 3 | −.003 (.005) | −.13 and −.13 | .20 | .002 (.003) | .13 and .15 | .51 |
Amygdala volumes were linearly transformed by multiplying mean volumes (adjusted for total brain volume per participant) by 1000; unstandardized coefficients reflect the relationships between intrinsic network connectivity and linearly transformed amygdala volumes; “b” = unstandardized parameter estimate; “SE” = standard error about the unstandardized parameter estimate; “r/β” = standardized parameter estimate (correlation/prediction, respectively), listed for T1 → T2 then T2 → T3 when applicable
Results for the SC network connectivity model indicated that amygdala volumes were stable over time (αs = .58 and .83, ps < .001), as was SC network connectivity (δs = .43 and .41, ps < .001). Cross-lagged predictions revealed no significant reciprocal effects between amygdala volumes and connectivity (βs and γs = .004 to .019, ps = .67 to .96). Correlations at each time point were small, and none reached statistical significance (rs = −.13 to .10, ps = .20 to .52). The findings were similar for the CC network connectivity model. CC network connectivity was stable over time (δs = .39 and .48, ps < .001); stability of amygdala volumes was the same as in the SC network model (αs = .58 and .83, ps < .001). Again, there were no reciprocal influences among amygdala volumes and CC network connectivity over time (βs and γs = −.027 to .16, ps = .25 to .64). And again, correlations at each time point were small and non-statistically significant (rs = −.089 to .15, ps = .26 to .51).
3.3. SC-CC internetwork connectivity model
The best-fitting model of between-network SC-CC connectivity related to amygdala volumes was a full RI-CLPM that included constrained autoregressive and cross-lagged predictions and constrained within-person correlations over time. The inclusion of between-person (trait) effects, which were regressed onto control variables of age at time 1, sex, and study site, were key to the model’s excellent overall fit (χ2 (14) = 20.98, p = .10; RMSEA = .05, 90% CI [.00, .10]; CFI = .95; SRMR = .09). Results of the final model are detailed in Fig. 2 and Table 3.
Fig. 2.
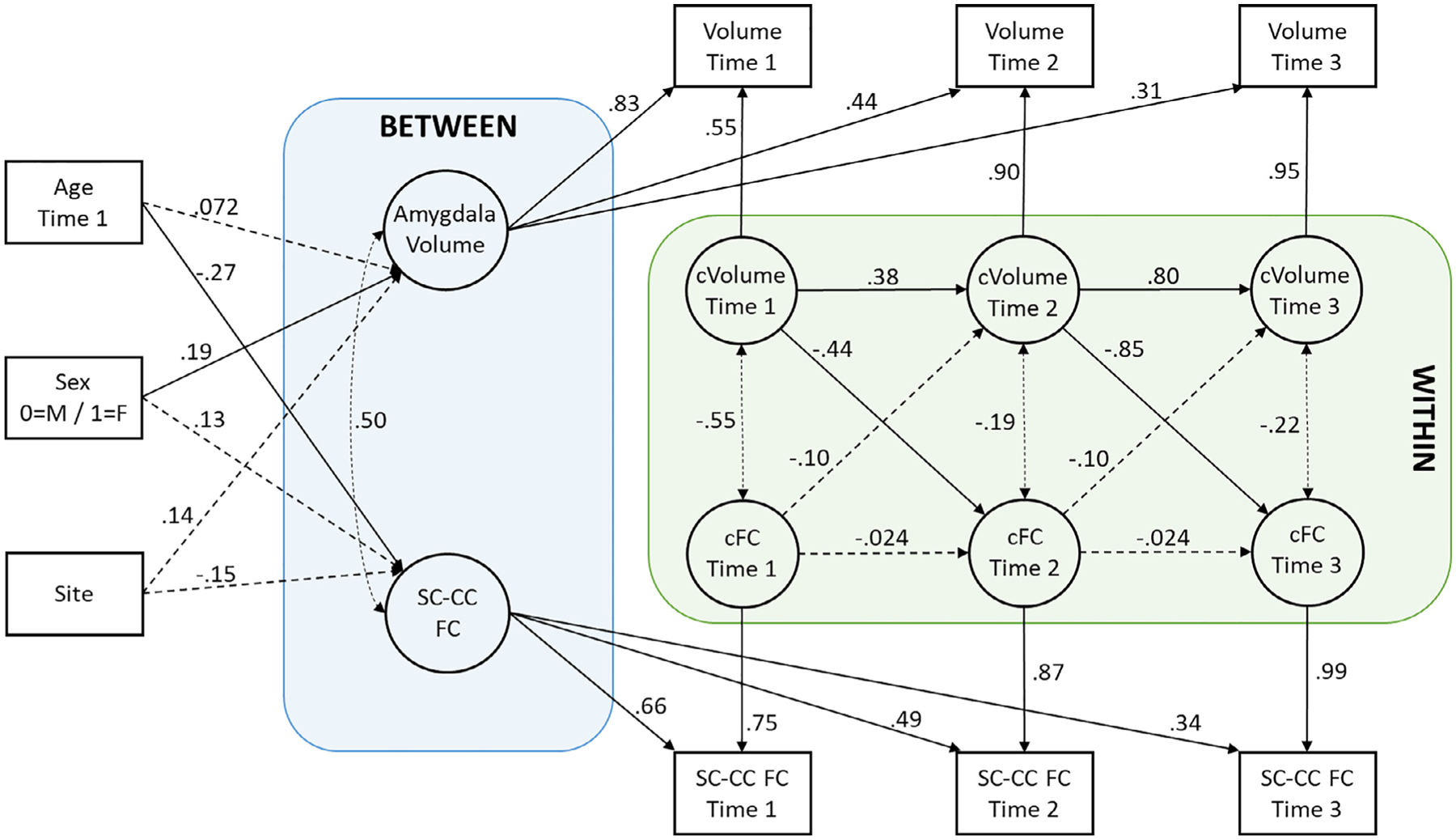
Results of the random-intercept cross-lagged panel model illustrating associations between amygdala volumes and between-network SC-CC connectivity controlling for age, sex (0 = “male”, 1 = “female”), and data collection site. All reported parameters are standardized coefficients. Solid lines indicate statistically significant relationships at the p < .05 level, whereas dashed lines indicate non-statistically significant relationships. Double-headed arrows show correlations, and single-headed arrows show predictive paths. “FC” = functional connectivity; “cFC” = centered within-person deviation in functional connectivity between the SC and CC networks; “cVolume” = centered within-person deviation in amygdala volume; squares represent manifest (observed) variables; circles represent latent variables.
Table 3.
Random-intercept cross-lagged panel model results linking amygdala volumes and subcortical-to-cognitive control network functional network connectivity, including control variables of age at time 1, study site, and sex, all on between-level latent variables.
| Parameter Estimated | b(SE) | r/β | p |
|---|---|---|---|
| amygdala volume → SC-CC connectivity | 1.71 (.17) | −.44 and −.85 | < .001 |
| SC-CC connectivity → amygdala volume | −.29 (.27) | −.10 and −.10 | .29 |
| autoregressive paths: amygdala volume | 1.16 (.16) | .38 and .80 | < .001 |
| autoregressive paths: SC-CC connectivity | −.038 (.17) | −.024 and −.024 | .83 |
| between-level correlation | .002 (.003) | .50 | .45 |
| within-person correlations | |||
| time 1 | −.002 (.003) | −.55 | .49 |
| time 2 and time 3 | −.003 (.003) | −.19 and −.22 | .32 |
| Control variables on FNC | |||
| age at time 1 | −.009 (.003) | −.27 | .010 |
| sex | .014 (.011) | −.15 | .22 |
| study site | −.016 (.012) | .13 | .16 |
| Control variables on amygdala volume | |||
| age at time 1 | .004 (.005) | .072 | .41 |
| sex | .035 (.016) | .19 | .029 |
| study site | .025 (.017) | .14 | .13 |
Amygdala volumes were linearly transformed by multiplying mean volumes (adjusted for total brain volume per participant) by 1000; unstandardized coefficients reflect the relationships between SC-CC connectivity and linearly transformed amygdala volumes; “b” = unstandardized parameter estimate; “SE” = standard error about the unstandardized parameter estimate; “r/β” = standardized parameter estimate (correlation/prediction, respectively) listed for time 1 → time 2 then time 2 → time 3 when applicable; “SC-CC” = functional connectivity between the subcortical and cognitive control networks.
The between-person effects represent the stable trait-level variance across all three years of the study; the correlation between them indicates the degree to which trait-level variance in amygdala volumes is related to trait-level variance in SC-CC connectivity. There was a strong positive correlation between the two traits suggesting that youth who generally had larger amygdala volumes also tended to have stronger SC-CC connectivity, though the effect was not statistically significant (φ = .50, p = .45; Figs. 2 and 3).
Fig. 3.
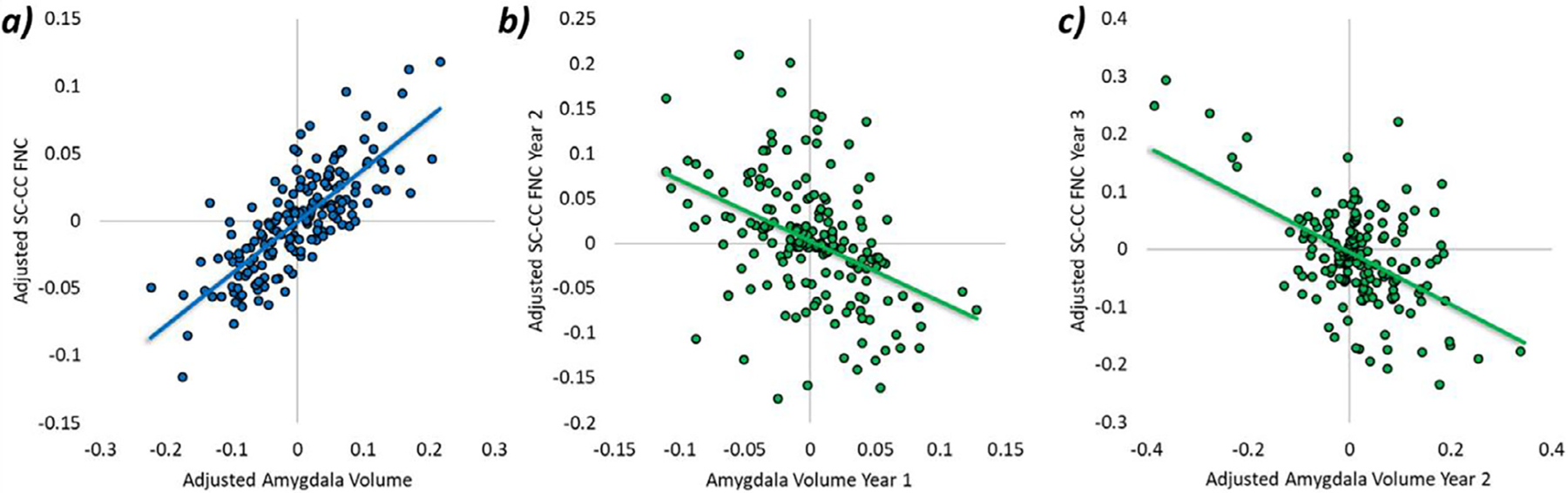
Scatterplots demonstrating (a) the between-person association between amygdala volumes and SC-CC FNC, as well as (b) the significant cross-predictive paths wherein within-person variability in amygdala volumes was associated with changes in SC-CC FNC in subsequent years. Between-person variables were adjusted for age, sex, and site. Within-person variables were adjusted for preceding predictive variables (e.g., autoregressive paths) as illustrated in model Fig. 2.
Of note, there was a significant effect of age on FNC, such that older youth tended to have lower (or more negative) average SC-CC connectivity. Additionally, there was an effect of sex on amygdala volumes, which implied that females tended to have larger amygdala volumes (corrected for total brain volume) on average relative to males. There were no significant effects of data collection site (Table 3).
Examination of within-person stability (i.e., autoregressive paths) indicated that individual-level deviations in amygdala volumes tracked over time; youth who generally had larger amygdala volumes at one point continued to have larger amygdala volumes at subsequent time points (see Table 3, Figs. 2 and 3). The same stability was not found for SC-CC connectivity metrics; individual-level deviations in connectivity over time were not significantly predicted by deviations in prior years. Interestingly, cross-lagged predictions revealed significant effects of amygdala volumes on SC-CC connectivity. Youth who had increases in amygdala volumes in a given year relative to their overall average volumes exhibited decreased SC-CC connectivity in the subsequent year (see Table 3 and Fig. 2). Importantly, within-person deviations in SC-CC connectivity were not significantly associated with subsequent individual-level deviations in amygdala volumes. With respect to within-person correlations, there was a small-to-moderate negative association between individual-level deviations in amygdala volumes and SC-CC connectivity. However, none of the correlations were statistically significant (rs = −.55 to −.19, ps = .32 to .49).
3.4. Additional exploratory comparison models
We tested a series of exploratory models to probe the specificity of effects detected in our main analyses, which were focused on potential reciprocity in amygdala volumes and SC and CC FNC. In one set of models, we examined the associations between amygdala volumes and intrinsic FC in three other networks (auditory, sensorimotor, and visual networks). Results are reported in Supplementary Table S4. In brief, there were no significant cross-predictions between volumes and networks in any of the models.
In a second set of exploratory models, we separately tested associations between hippocampal, striatum, and medial orbitofrontal cortex volumes and our three primary connectivity metrics of interest (SC, CC, and SC-CC). Full results are reported in Table 4 and in Supplementary Tables S5–6. There were no significant associations between hippocampal volumes or medial orbitofrontal cortex volumes and any FNC measures of interest, as indicated by cross-predictive paths in the best-fitting models (Tables S5 and S6). Only striatum volumes showed significant associations with between-network SC-CC connectivity (Table 4).
Table 4.
Results of the exploratory cross-lagged panel models investigating associations between striatum volumes and functional network connectivity within and between the subcortical and cognitive control networks.
| Cognitive Control Network | Subcortical Network | Subcortical-Cognitive Control Between- Network | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Parameter Estimated | b | r/β | p | b | r/β | p | b | r/β | p |
| striatum → FC | — | — | — | .003 | .039 and .085 | .57 | −.001 | −.031 and −.047 | .67 |
| FC → striatum | — | — | — | 1.62 | .051 and .040 | .47 | −9.56 | −.16 and −.16 | .004 |
| autoregressive paths: striatum | — | — | — | .84 | .39 and .65 | < .001 | .84 | .39 and .63 | < .001 |
| autoregressive paths: FC | — | — | — | .41 | .40 and .41 | < .001 | .54 | .42 and .38 | < .001 |
| within-person correlations | |||||||||
| time 1 | — | — | — | .02 | .057 | .46 | −.001 | −.004 | .96 |
| time 2 and time 3 | — | — | — | −.24 | −.37 and −.34 | .08 | .028 | .065 | .75 |
| time 3a | — | — | — | — | — | — | −.54 | −.79 | .001 |
| Model Fit | [no convergence] | χ2 (9) = 9.52, p = .39 RMSEA = .02, 90% CI[.00, .09] CFI = .99 SRMR = .11 | χ2 (8) = 9.73, p = .28 RMSEA = .04, 90% CI[.00, .10] CFI = .97 SRMR = .12 | ||||||
Only the model examining associations between striatum volume and subcortical-cognitive control between network connectivity indicated that Time 2 and Time 3 correlations should be estimated uniquely, rather than constraining them to be equal.Striatum volumes were linearly transformed by multiplying mean volumes (adjusted for total brain volume per participant) by 1000; unstandardized coefficients reflect the relationships between CC connectivity and linearly transformed striatum volumes; “b” = unstandardized parameter estimate; “r/β” = standardized parameter estimate (correlation/prediction, respectively); “FC” = functional connectivity within the specified network.
The final model, which had good fit, was a traditional CLPM (i.e., group-level) in which correlations freely varied across time points, but autoregressive paths and cross-predictive paths were constrained to be equal over time. Most interestingly, FNC significantly predicted striatal volumes over time, such that greater SC-CC between-network connectivity at one time tended to precede smaller striatal volumes in the subsequent year of the study. Additionally, striatum volumes and FC measures were both stable over time. Generally, larger striatal volumes at one time point were associated with larger volumes at the subsequent point, and stronger SC-CC connectivity at one point was associated with stronger connectivity at the following time (Table 4). Correlations between FNC and striatum volumes were only significant at time 3 and suggested that greater SC-CC connectivity was associated with smaller striatum volumes.
4. Discussion
The present study investigated whether amygdala volumes and patterns of intra- and inter-network FNC in the SC and CC networks were reciprocally related over time in a large cohort of typically developing youth. Our key finding was that individual-level changes in amygdala structure predicted shifts in FNC over time, but the opposite relationship (i.e., FNC predicting volumetric changes) was virtually non-existent. Specifically, we found that within-person increases in amygdala volumes in a given year robustly predicted decreases in functional connectivity between the SC and CC networks in subsequent years. However, within-person variability in connectivity metrics did not predict subsequent deviations in amygdala volumes in kind. Interestingly, our exploratory follow-up analyses linking striatum volumes to FNC indicated robust predictive associations whereby group-level increases in SC-CC connectivity in one year were associated with significantly smaller striatum volumes in subsequent years. We discuss our findings in detail below.
Our data indicated a robust effect wherein individual-level deviations (from average) in amygdala volume in one year predicted fluctuations in SC-CC connectivity the following year, which largely supported previous literature suggesting that structural morphology drives the refinement of network connectivity in the brain (Casey et al., 2016; Casey, 2015; van Duijvenvoorde et al., 2016). Some prior reports have suggested that connectivity between SC and CC networks decreases over time during normative development, signaling the shift from “bottom-up” to “top-down” control of emotion (Cerliani et al., 2015; Rubia, 2013; van Duijvenvoorde et al., 2019). Thus, our data might suggest that within-person increases in amygdala volumes support the expected refinement in FNC across development. Perhaps a temporary proliferation in gray matter at one point provides a foundation for developing and pruning connections throughout the brain, thereby shaping the nature of internetwork connectivity (Baker et al., 2015; Huttenlocher and Dabholkar, 1997; Kharitonova et al., 2013). This process is seen in other areas of neurocognitive development and functioning, such as memory formation and recall abilities (Liljenström, 2010), and the development of reading skills (Linkersdörfer et al., 2014).
On the other hand, there is also the possibility that our data are suggestive of risk for future psychopathology, like the onset of mood disorders. Some prior studies have shown that decreased subcortico-cortical connectivity is associated with depression in adolescents (e.g., Connolly et al., 2017; Heller et al., 2009). In particular, the detected individual-level variability in SC-CC connectivity in the present study may be capturing nuanced with-person deviations indicative of psychopathological risk. Future works could include self-reported psychological symptom measurements coincident with neuroimaging to better assess the nature of these neurological shifts. Further work is needed to disentangle the functional relevance of these data, but herein we provide a lens with which to understand distinct aspects of structure-function relationships in developing youth.
It is critical to keep in mind that these effects were found at the individual-level. That is, within-person changes in amygdala volumes were associated with subsequent within-person shifts n SC-CC connectivity. This effect was best captured at the within-person level rather than the group-level, which might suggest more nuanced individual-level drivers of the neurological changes. Future works should dive deeper into the mechanisms through which individual-level variability arises and manifests in longitudinal changes in FNC. For instance, personal environmental factors (White et al., 2019), exposures to major stressors/events (Blair et al., 2019), and shifts in pubertal status (Fung et al., 2020) are all known to influence neural structure and function, and may all play critical roles in the individual-level changes detected in the present study. Future investigations detailing these types of personal factors will help shed light on the degree to which these neurological shifts are normative, protective, or otherwise.
Interestingly, age and sex effects were best modeled as control variables on the group-level effects of FNC and amygdala volumes, rather than the individual-level effects as we had hypothesized. The data suggest that these factors may be more broadly applicable at the group level, and that changes at the individual-level are more specific to personal factors that vary from one person to another. Previous work has identified a number of individual factors that can impact both neural structure and function, including exposure to stress and trauma (Cohodes et al., 2021; McLaughlin et al., 2019), genetics (Berardi et al., 2015), and socioeconomic disadvantage (Barch et al., 2016; Rakesh et al., 2021; Whittle et al., 2017). Further, other indices of development and maturation might provide more unique insights into within-person variability in structure-function relationships. For instance, previous studies have shown unique effects of pubertal staging and sex hormones on aspects of brain structure and function (Fung et al., 2020; Koolschijn et al., 2014; McHenry et al., 2014; Petro et al., 2021; van Duijvenvoorde et al., 2019). Thus, it is reasonable to hypothesize that these metrics, which were not fully captured in the present study, may also influence the nature of structure-function relationships. Future work should continue to explore how such personal factors specifically shape the trajectory of within-person changes in structure-function relationships.
Contrary to the extant literature (e.g., Saygin et al., 2015), we did not find that FNC was a driver of changes in amygdala volumes across development. However, we did note a robust group-level cross-predictive association wherein generalized increases in SC-CC FNC were associated with subsequent decreases in striatal volumes. Importantly, these findings were distinct from the amygdala findings previously described, which were based on individual-level shifts. The fact that striatum-related findings were best described at the group level suggests that these neurological shifts are more consistent across the full study sample, and may be described in terms of more “common” or “typical” changes in brain structure and function across adolescence. The striatum in a known driver in such processes as habit formation and reinforcement learning, with much recent literature focused on reward processing (Cardinal et al., 2002; Casey et al., 2019; Costa et al., 2016; Ousdal et al., 2012). Importantly, some research suggests that decreases in striatal volumes during adolescence are normative, and that aberrations in this pattern of decreasing volumes with time are associated with the onset of depressive disorders (Ostby et al., 2009; Whittle et al., 2014). Thus, one might conclude from the present study that broad, group-level increases in SC-CC connectivity over time are potentially beneficial to healthy development and refinement of neural substrates critical in mental health. Of course, this requires further investigation and targeted approaches to assessing neurocognitive and psychological linkages to shifts in SC-CC connectivity over time.
Given models of development suggesting that adolescence is characterized by a shift from subcortico-subcortical, to subcortico-cortical, and finally to cortico-cortical drivers of emotional control (Casey et al., 2019; Rosenberg et al., 2018), is it plausible that broad increases in SC-CC connectivity in this period of early adolescence could be integral in shaping and refining neural structure within key substrates including the striatum. Of note, all of our findings in the present study were specific to SC-CC FNC, with limited structure-function associations between any of the explored neural structures and intrinsic connectivity in the tested networks. It is possible that different definitions of the functional networks, or exploration of different regional volumes may yield more fruitful discussion of structure-function relationships with respect to intrinsic network connectivity metrics. Additionally, further work should explore how these relationships between connectivity and structure shift in later periods of adolescence, when we would expect youth would be reaching maturity and shifting to cortico-cortical connectivity as the primary driver of emotional processing. It is possible that in later periods of development, we might see a shift in which intrinsic connectivity in the CC network becomes a more major player, and more consistently associated with shifts in neural structure.
Before closing we must note several limitations of the present investigation. First, we utilized resting state rather than task-based FNC metrics, which limits the generalizability of our findings to cognitive constructs of interest. Prior studies have shown that resting FNC data is well-associated with task-based measures (Dwyer et al., 2014). However, future work should consider examining the nature of shifts in FNC elicited during tasks targeting specific neurocognitive systems to further decipher the nature of structure-function relationships over time. Further, studies should consider employing neuropsychological testing or other behavioral measures in this type of work, with interpretability in mind. Utilizing robust neuropsychological assessments may enable the identification of the cognitive and emotional systems affected by specific trajectories of change in structure-function maturation. Another potential limitation is the definition of functional networks in the current study. We utilized robustly defined networks that were previously identified by Agcaoglu et al. (2020, 2019). However, this represents only one possible configuration of what are broad networks, which are disparately defined across the literature. Further investigation of other network configurations would be fruitful for determining the generalizability of these findings. Further, different structural delineations could shed additional light on the noted structure-function relationships. For instance, explorations of structural connectivity via diffusion tensor imaging would afford a new lens for understanding how neural structure and function influence one another over time. Finally, additional work is needed to understand what mechanisms may be driving the noted neurodevelopmental shifts over time. For instance, examination of clinical symptom sets (e.g., anxiety, depression), environment (e.g., stressors/traumatic experiences, enrichment opportunities), and other individual differences (e.g., genetics, hormonal fluctuations and pubertal staging) may drive changes at the within-person level. It would also be interesting to determine whether such associations between structural morphology and FNC over time exist in both younger and older age groups.
In sum, the present study sought to determine whether aspects of structural and functional neural maturation reciprocally influenced one another over time in a large cohort of typically developing youth. We found that individual-level changes in amygdala volumes predicted fluctuations in SC-CC connectivity in subsequent years, with no evidence for the reciprocal relationship. However, group-level variations in SC-CC connectivity were associated with subsequent morphological variability in striatal volumes. These findings contribute a novel, robust view into longitudinal trajectories of neural maturation in structure-function relationships underlying emotional control in youth.
Supplementary Material
Acknowledgements
This work was supported by the National Science Foundation (#1539067) and the National Institutes of Health (P20-GM-144641, R01-MH-121101, R01-MH-116782, R01-MH-118013, P20-GM-103472, R01-EB-020407). The funders had no role in the study design, collection, analysis, or interpretation of data, nor did they influence writing the report or the decision to submit this work for publication. The authors report no conflicts of interest. The data presented in this manuscript have not been published or presented elsewhere.
Footnotes
Declaration of Competing Interest
The authors report no financial interests or potential conflicts of interest.
Credit authorship contribution statement
Brittany K. Taylor: Conceptualization, Formal analysis, Investigation, Methodology, Software, Validation, Visualization, Writing – original draft, Writing – review & editing. Michaela R. Frenzel: Data curation, Methodology, Project administration, Writing – review & editing. Jacob A. Eastman: Data curation, Methodology, Writing – review & editing. Christine M. Embury: Project administration, Resources, Writing – review & editing. Oktay Agcaoglu: Formal analysis, Software, Writing – review & editing. Yu-Ping Wang: Funding acquisition, Writing – review & editing. Julia M. Stephen: Data curation, Funding acquisition, Investigation, Methodology, Project administration, Writing – review & editing. Vince D. Calhoun: Data curation, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Writing – review & editing. Tony W. Wilson: Data curation, Funding acquisition, Investigation, Methodology, Project administration, Resources, Supervision, Validation, Writing – review & editing.
Supplementary materials
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.neuroimage.2021.118852.
Data availability
All data are available upon request to the corresponding author (TWW). Data will be made publicly available upon study completion.
References
- Agcaoglu O, Wilson TW, Wang Y, Stephen J, Calhoun VD, 2019. Resting state connectivity differences in eyes open versus eyes closed conditions. Hum Brain Mapp. 40, 2488–2498. doi: 10.1002/hbm.24539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agcaoglu O, Wilson TW, Wang Y-P, Stephen JM, Calhoun VD, 2020. Dynamic resting-state connectivity differences in eyes open versus eyes closed conditions. Brain Connect 10, 504–519. doi: 10.1089/brain.2020.0768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker STE, Lubman DI, Yücel M, Allen NB, Whittle S, Fulcher BD, Zalesky A, Fornito A, 2015. Developmental changes in brain network hub connectivity in late adolescence. J. Neurosci 35, 9078–9087. doi: 10.1523/JNEUROSCI.5043-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barch D, Pagliaccio D, Belden A, Harms MP, Gaffrey M, Sylvester CM, Till-man R, Luby J, 2016. Effect of hippocampal and amygdala connectivity on the relationship between preschool poverty and school-age depression. AJP 173, 625–634. doi: 10.1176/appi.ajp.2015.15081014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berardi N, Sale A, Maffei L, 2015. Brain structural and functional development: genetics and experience. Devel. Med. Child Neurol 57, 4–9. doi: 10.1111/dmcn.12691. [DOI] [PubMed] [Google Scholar]
- Bickart KC, Dickerson BC, Feldman Barrett L, 2014. The amygdala as a hub in brain networks that support social life. Neuropsychologia 63, 235–248. doi: 10.1016/j.neuropsychologia.2014.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair KS, Aloi J, Crum K, Meffert H, White SF, Taylor BK, Leiker EK, Thornton LC, Tyler PM, Shah N, Johnson K, Abdel-Rahim H, Lukoff J, Dobbertin M, Pope K, Pollak S, Blair RJ, 2019. Association of different types of childhood maltreatment with emotional responding and response control among youths. JAMA Netw. Open 2, e194604. doi: 10.1001/jamanetworkopen.2019.4604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calhoun VD, Adali T, 2012. Multisubject independent component analysis of FMRI: a decade of intrinsic networks, default mode, and neurodiagnostic discovery. IEEE Rev. Biomed. Eng 5, 60–73. doi: 10.1109/RBME.2012.2211076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calhoun VD, Adali T, Pearlson GD, Pekar JJ, 2001. A method for making group inferences from functional MRI data using independent component analysis. Human Brain Mapp. 14, 140–151. doi: 10.1002/hbm.1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardinal RN, Parkinson JA, Hall J, Everitt BJ, 2002. Emotion and motivation: the role of the amygdala, ventral striatum, and prefrontal cortex. Neurosci. Biobehav. Rev 26, 321–352. doi: 10.1016/S0149-7634(02)00007-6. [DOI] [PubMed] [Google Scholar]
- Casey B, Galván A, Somerville LH, 2016. Beyond simple models of adolescence to an integrated circuit-based account: a commentary. Devel. Cognit. Neurosci 17, 128–130. doi: 10.1016/j.dcn.2015.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey BJ, 2015. Beyond simple models of self-control to circuit-based accounts of adolescent behavior. Ann. Rev. Psychol 66, 295–319. doi: 10.1146/annurev-psych-010814-015156. [DOI] [PubMed] [Google Scholar]
- Casey BJ, Heller AS, Gee DG, Cohen AO, 2019. Development of the emotional brain. Neurosci. Lett 693, 29–34. doi: 10.1016/j.neulet.2017.11.055, Functional Neuroimaging of the Emotional Brain. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerliani L, Mennes M, Thomas RM, Martino AD, Thioux M, Keysers C, 2015. Increased functional connectivity between subcortical and cortical resting-state networks in autism spectrum disorder. JAMA Psych. 72, 767–777. doi: 10.1001/jamapsy-chiatry.2015.0101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohodes EM, Kitt ER, Baskin-Sommers A, Gee DG, 2021. Influences of early-life stress on frontolimbic circuitry: harnessing a dimensional approach to eluci-date the effects of heterogeneity in stress exposure. Devel. Psychobiol 63, 153–172. doi: 10.1002/dev.21969. [DOI] [PubMed] [Google Scholar]
- Connolly CG, Ho TC, Blom EH, LeWinn KZ, Sacchet MD, Tymofiyeva O, Simmons AN, Yang TT, 2017. Resting-state functional connectivity of the amygdala and longitudinal changes in depression severity in adolescent depression. J.Affect. Disord 207, 86–94. doi: 10.1016/j.jad.2016.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa VD, Dal Monte O, Lucas DR, Murray EA, Averbeck BB, 2016. Amygdala and ventral striatum make distinct contributions to reinforcement learning. Neuron 92, 505–517. doi: 10.1016/j.neuron.2016.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietvorst E, Hiemstra M, Hillegers MHJ, Keijsers L, 2018. Adolescent perceptions of parental privacy invasion and adolescent secrecy: an illustration of Simpson’s paradox. Child Development 89, 2081–2090. doi: 10.1111/cdev.13002. [DOI] [PubMed] [Google Scholar]
- Dwyer DB, Harrison BJ, Yücel M, Whittle S, Zalesky A, Pantelis C, Allen NB, Fornito A, 2014. Large-scale brain network dynamics supporting adolescent cognitive control. J. Neurosci 34, 14096–14107. doi: 10.1523/JNEUROSCI.1634-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans GW, Swain JE, King AP, Wang X, Javanbakht A, Ho SS, Angstadt M, Phan KL, Xie H, Liberzon I, 2016. Childhood cumulative risk exposure and adult amygdala volume and function. J. Neurosci. Res 94, 535–543. doi: 10.1002/jnr.23681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fetvadjiev VH, He J, 2019. The longitudinal links of personality traits, values, and well-being and self-esteem: a five-wave study of a nationally representative sample. J. Personal. Soc. Psychol 117, 448. [DOI] [PubMed] [Google Scholar]
- Fox AS, Oler JA, Tromp DPM, Fudge JL, Kalin NH, 2015. Extending the amygdala in theories of threat processing. Trends Neurosci. 38, 319–329. doi: 10.1016/j.tins.2015.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fung MH, Taylor BK, Frenzel MR, Eastman JA, Wang Y-P, Calhoun VD, Stephen JM, Wilson TW, 2020. Pubertal testosterone tracks the developmental trajectory of neural oscillatory activity serving visuospatial processing. Cerebral Cortex Bhaa 169. doi: 10.1093/cercor/bhaa169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabard-Durnam LJ, Flannery J, Goff B, Gee DG, Humphreys KL, Telzer E, Hare T, Tottenham N, 2014. The development of human amygdala functional connectivity at rest from 4 to 23years: a cross-sectional study. NeuroImage 95, 193–207. doi: 10.1016/j.neuroimage.2014.03.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giedd JN, Vaituzis AC, Hamburger SD, Lange N, Rajapakse JC, Kaysen D, Vauss YC, Rapoport JL, 1996. Quantitative MRI of the temporal lobe, amygdala, and hippocampus in normal human development: Ages 4–18 years. J. Comparat. Neurol 366, 223–230 . [DOI] [PubMed] [Google Scholar]
- Graham AM, Buss C, Rasmussen JM, Rudolph MD, Demeter DV, Gilmore JH, Styner M, Entringer S, Wadhwa PD, Fair DA, 2016. Implications of newborn amygdala connectivity for fear and cognitive development at 6-months-of-age. Devel. Cognit. Neurosci., Flux Congress 2014 (18), 12–25. doi: 10.1016/j.dcn.2015.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamaker EL, Kuiper RM, Grasman RPPP, 2015. A critique of the cross-lagged panel model. Psychol. Methods 20, 102–116. doi: 10.1037/a0038889. [DOI] [PubMed] [Google Scholar]
- He H, Sui J, Du Y, Yu Q, Lin D, Drevets WC, Savitz JB, Yang J, Victor TA, Calhoun VD, 2017. Co-altered functional networks and brain structure in unmedicated patients with bipolar and major depressive disorders. Brain Struct. Funct 222, 4051–4064. doi: 10.1007/s00429-017-1451-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heller AS, Cohen AO, Dreyfuss MFW, Casey BJ, 2016. Changes in cortico-subcortical and subcortico-subcortical connectivity impact cognitive control to emotional cues across development. Soc. Cogn. Affect Neurosci 11, 1910–1918. doi: 10.1093/scan/nsw097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heller AS, Johnstone T, Shackman AJ, Light SN, Peterson MJ, Kolden GG, Kalin NH, Davidson RJ, 2009. Reduced capacity to sustain positive emotion in major depression reflects diminished maintenance of fronto-striatal brain activation. Proc. Natl. Acad. Sci 106, 6. doi: 10.1073/pnas.0910651106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu L, Bentler PM, 1999. Cutoff criteria for fit indexes in covariance structure analysis: conventional criteria versus new alternatives. Struct. Eq. Model. A Multidiscipl. J 6, 1–55. doi: 10.1080/10705519909540118. [DOI] [Google Scholar]
- Huttenlocher PR, Dabholkar AS, 1997. Regional differences in synapto-genesis in human cerebral cortex. J. Comparat. Neurol 387, 167–178 . [DOI] [PubMed] [Google Scholar]
- Jung RE, Haier RJ, 2007. The parieto-frontal integration theory (P-FIT) of intelligence: converging neuroimaging evidence. Behavioral, in: Retrieved from Http://Www.Gse.Uci.Edu/Docs/Leak_Duncan_Li_Timing_Paper_APPAM_102810.Pdf Lessov-Schlaggar. Psychological, pp. 1174–1177. [DOI] [PubMed] [Google Scholar]
- Jung WH, Lee S, Lerman C, Kable JW, 2018. Amygdala functional and structural connectivity predicts individual risk tolerance. Neuron 98, 394–404. doi: 10.1016/j.neuron.2018.03.019, e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kharitonova M, Martin RE, Gabrieli JDE, Sheridan MA, 2013. Cortical gray-matter thinning is associated with age-related improvements on executive function tasks. Devel. Cognit. Neurosci 6, 61–71. doi: 10.1016/j.dcn.2013.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kline RB, 2005. Principles and practice of structural equation modeling. Principles and Practice of Structural Equation Modeling, 2nd ed. Guilford Press, New York, NY, US. [Google Scholar]
- Koolschijn PCMP, Peper JS, Crone EA, 2014. The influence of sex steroids on structural brain maturation in adolescence. PLoS ONE 9, e83929. doi: 10.1371/journal.pone.0083929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liljenström H, 2010. Network effects of synaptic modifications. Pharmacopsychiatry 43, S67–S81. doi: 10.1055/s-0030-1252058. [DOI] [PubMed] [Google Scholar]
- Linkersdörfer J, Jurcoane A, Lindberg S, Kaiser J, Hasselhorn M, Fiebach CJ, Lonnemann J, 2014. The association between gray matter volume and reading proficiency: a longitudinal study of beginning readers. J. Cogn. Neurosci 27, 308–318. doi: 10.1162/jocn_a_00710. [DOI] [PubMed] [Google Scholar]
- Lucian M, 2013. Adolescent brain development in normality and psychopathology. Dev Psychopathol. 25, 1325–1345. doi: 10.1017/S0954579413000643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luciana M, Collins PF, 2012. Incentive motivation, cognitive control, and the adolescent brain: is it time for a paradigm shift? Child Devel. Perspect 6, 392–399. doi: 10.1111/j.1750-8606.2012.00252.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lv H, Wang Z, Tong E, Williams LM, Zaharchuk G, Zeineh M, Goldstein-Piekarski AN, Ball TM, Liao C, Wintermark M, 2018. Resting-state functional MRI: everything that nonexperts have always wanted to know. Am. J. Neuroradiol doi: 10.3174/ajnr.A5527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marek S, Hwang K, Foran W, Hallquist MN, Luna B, 2015. The contribution of network organization and integration to the development of cognitive control. PLoS Biol. 13. doi: 10.1371/journal.pbio.1002328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McHenry J, Carrier N, Hull E, Kabbaj M, 2014. Sex differences in anxiety and depression: role of testosterone. Front Neuroendocrinol. 35, 42–57. doi: 10.1016/j.yfrne.2013.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin KA, Weissman D, Bitrán D, 2019. Childhood adversity and neural development: a systematic review. Ann. Rev. Devel. Psychol 1, 277–312. doi: 10.1146/annurev-devpsych-121318-084950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merz EC, Tottenham N, Noble KG, 2018. Socioeconomic status, amygdala volume, and internalizing symptoms in children and adolescents. J. Clinical Child Adolescent Psychol 47, 312–323. doi: 10.1080/15374416.2017.1326122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monk C, 2008. The development of emotion-related neural circuitry in health and psychopathology. Devel. psychopathol 20, 1231–1250. doi: 10.1017/S095457940800059X. [DOI] [PubMed] [Google Scholar]
- Mund M, Nestler S, 2019. Beyond the Cross-Lagged Panel Model: Next-generation statistical tools for analyzing interdependencies across the life course. Adv. Life Course Res. Theoret. Methodol. Front. Life Course Res 41, 100249. doi: 10.1016/j.alcr.2018.10.002. [DOI] [PubMed] [Google Scholar]
- Nelemans SA, Keijsers L, Colpin H, Leeuwen K, Bijttebier P, Verschueren K, Goossens L, 2019. Transactional links between social anxiety symptoms and parenting across adolescence: between- and within-person associations. Child Dev CDEV doi: 10.1111/cdev.13236, 13236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochsner KN, Gross JJ, 2005. The cognitive control of emotion. Trends Cognit. Sci 9, 242–249. doi: 10.1016/j.tics.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Silvers JA, Buhle JT, 2012. Functional imaging studies of emotion regulation: a synthetic review and evolving model of the cognitive control of emotion. Ann. NY Acad. Sci 1251, E1–24. doi: 10.1111/j.1749-6632.2012.06751.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostby Y, Tamnes CK, Fjell AM, Westlye LT, Due-Tonnessen P, Walhovd KB, 2009. Heterogeneity in subcortical brain development: a structural magnetic resonance imaging study of brain maturation from 8 to 30 Years. J. Neurosci 29, 11772–11782. doi: 10.1523/JNEUROSCI.1242-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ousdal OT, Reckless GE, Server A, Andreassen OA, Jensen J, 2012. Effect of relevance on amygdala activation and association with the ventral striatum. NeuroImage 62, 95–101. doi: 10.1016/j.neuroimage.2012.04.035. [DOI] [PubMed] [Google Scholar]
- Pechtel P, Lyons-Ruth K, Anderson CM, Teicher MH, 2014. Sensitive periods of amygdala development: the role of maltreatment in preadolescence. Neuroimage 97, 236–244. doi: 10.1016/j.neuroimage.2014.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen SE, Posner MI, 2012. The attention system of the human brain: 20 years after. Ann. Rev. Neurosci 35, 73–89. doi: 10.1146/annurev-neuro-062111-150525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petro NM, Tottenham N, Neta M, 2021. Exploring valence bias as a metric for fron-toamygdalar connectivity and depressive symptoms in childhood. Devel. Psychobiol 1–16. doi: 10.1002/dev.22084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeifer JH, Allen NB, 2012. Arrested development? Reconsidering dual-systems models of brain function in adolescence and disorders. Trends. Cogn. Sci 16, 322–329. doi: 10.1016/j.tics.2012.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin S, Young CB, Duan X, Chen T, Supekar K, Menon V, 2014. Amgydala subregional structure and intrinsic functional connectivity predicts individual differences in anxiety during early childhood. Biol. Psychiatry 75, 892–900. doi: 10.1016/j.biopsych.2013.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakesh D, Seguin C, Zalesky A, Cropley V, Whittle S, 2021. Associations between neighborhood disadvantage, resting-state functional connectivity, and behavior in the adolescent brain cognitive development study: the moderating role of positive family and school environments. Biological Psychiatry: Cognitive Neuroscience and Neuroimaging S2451902221000859. 10.1016/j.bpsc.2021.03.008 [DOI] [PubMed] [Google Scholar]
- Rogers CE, Sylvester CM, Mintz C, Kenley JK, Shimony JS, Barch DM, Smyser CD, 2017. Neonatal amygdala functional connectivity at rest in healthy and preterm infants and early internalizing symptoms. J. Am. Acad. Child Adolescent Psych 56, 157–166. doi: 10.1016/j.jaac.2016.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosazza C, Minati L, 2011. Resting-state brain networks: literature review and clinical applications. Neurol. Sci 32, 773–785. doi: 10.1007/s10072-011-0636-y. [DOI] [PubMed] [Google Scholar]
- Rosenberg MD, Casey BJ, Holmes AJ, 2018. Prediction complements explanation in understanding the developing brain. Nature Commun. 9, 589. doi: 10.1038/s41467-018-02887-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubia K, 2013. Functional brain imaging across development. Eur. Child Adolesc. Psych 22, 719–731. doi: 10.1007/s00787-012-0291-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato W, Kochiyama T, Uono S, Sawada R, Yoshikawa S, 2020. Amygdala activity related to perceived social support. Sci. Rep 10, 1–9. doi: 10.1038/s41598-020-59758-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxbe D, Khoddam H, Piero LD, Stoycos SA, Gimbel SI, Margolin G, Kaplan JT, 2018. Community violence exposure in early adolescence: longitudinal associations with hippocampal and amygdala volume and resting state connectivity. Devel. Sci 21, e12686. doi: 10.1111/desc.12686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saygin ZM, Osher DE, Koldewyn K, Martin RE, Finn A, Saxe R, Gabrieli JDE, Sheridan M, 2015. Structural connectivity of the developing human amygdala. PLoS One 10. doi: 10.1371/journal.pone.0125170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen HH, 2015. Core concept: resting-state connectivity. Proc. Natl. Acad. Sci. USA 112, 14115–14116. doi: 10.1073/pnas.1518785112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spooner RK, Eastman JA, Rezich MT, Wilson TW, 2019. HD-tDCS dissociates fronto-visual theta lateralization during visual selective attention. J. Physiol, JP278788 doi: 10.1113/JP278788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephen JM, Solis I, Janowich J, Stern M, Frenzel MR, Eastman JA, Mills MS, Embury CM, Coolidge NM, Heinrichs-Graham E, Mayer A, Liu J, Wang YP, Wilson TW, Calhoun VD, 2021. The developmental chronnecto-genomics (Dev-CoG) study: a multimodal study on the developing brain. NeuroImage 225, 117438. doi: 10.1016/j.neuroimage.2020.117438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor BK, Eastman JA, Frenzel MR, Embury CM, Wang Y-P, Calhoun VD, Stephen JM, Wilson TW, 2021. Neural oscillations underlying selective attention follow sexually divergent developmental trajectories during adolescence. Devel. Cognit. Neurosci 49, 100961. doi: 10.1016/j.dcn.2021.100961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor BK, Embury CM, Heinrichs-Graham E, Frenzel MR, Eastman JA, Wiesman AI, Wang Y-P, Calhoun VD, Stephen JM, Wilson TW, 2020. Neural oscillatory dynamics serving abstract reasoning reveal robust sex differences in typically-developing children and adolescents. Devel. Cogn. Neurosci 100770. doi: 10.1016/j.dcn.2020.100770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Duijvenvoorde ACK, Peters S, Braams BR, Crone EA, 2016. What motivates adolescents? Neural responses to rewards and their influence on adolescents’ risk taking, learning, and cognitive control. Neurosci. Biobehav. Rev 70, 135–147. doi: 10.1016/j.neubiorev.2016.06.037, The Adolescent Brain. [DOI] [PubMed] [Google Scholar]
- van Duijvenvoorde ACK, Westhoff B, Vos F.de, Wierenga LM, Crone EA, 2019. A three-wave longitudinal study of subcortical–cortical resting-state connectivity in adolescence: testing age- and puberty-related changes. Human Brain Mapp. 40, 3769–3783. doi: 10.1002/hbm.24630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warnell KR, Pecukonis M, Redcay E, 2018. Developmental relations between amygdala volume and anxiety traits: Effects of informant, sex, and age. Devel. Psychopathol 30, 1503–1515. doi: 10.1017/S0954579417001626. [DOI] [PubMed] [Google Scholar]
- White SF, Voss JL, Chiang JJ, Wang L, McLaughlin KA, Miller GE, 2019. Exposure to violence and low family income are associated with heightened amygdala responsiveness to threat among adolescents. Dev. Cogn. Neurosci 40, 100709. doi: 10.1016/j.dcn.2019.100709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittle S, Lichter R, Dennison M, Vijayakumar N, Schwartz O, Byrne ML, Simmons JG, Yücel M, Pantelis C, McGorry P, Allen NB, 2014. Structural brain development and depression onset during adolescence: a prospective longitudinal study. AJP 171, 564–571. doi: 10.1176/appi.ajp.2013.13070920. [DOI] [PubMed] [Google Scholar]
- Whittle S, Vijayakumar N, Simmons JG, Dennison M, Schwartz O, Pantelis C, Sheeber L, Byrne ML, Allen NB, 2017. Role of positive parenting in the association between neighborhood social disadvantage and brain development across adolescence. JAMA Psych. 74, 824. doi: 10.1001/jamapsychiatry.2017.1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data are available upon request to the corresponding author (TWW). Data will be made publicly available upon study completion.


