Abstract
Despite limited evidence on clinical efficacy and increasing resistance problems, topical antibiotics are still used in everyday clinical practice. However, topical antiseptic agents such, as octenidine and polyhexanide, often have a broader efficacy spectrum. They also have a broader target tropism because of their non-specific cellular mechanisms of action. Repeated use of topical antibiotics also carries the risk of contact sensitization, which could limit potential subsequent use as systemic antibiotics. Contact allergy is a clinically relevant problem, particularly in patients with barrier-damaged skin, pre-existing dermatosis, or occupational exposure. It can be concluded that with the use of modern antiseptics, topical antibiotic therapy is rarely indicated and should be avoided, not only because of the risk of contact sensitization but also because of the unfavorable and potentially consequential resistance problem.
Keywords: topical antibiotics, antiseptics, contact allergy, epicutaneous patch testing, neomycin, fusidic acid, octenidine, polyhexanide, allergy
Introduction
Topical antibiotics (AB) are generally used for the treatment of superficial infections of the skin, eye, and ear [1]. Their use in dermatology is also well established in conditions such as acne vulgaris and rosacea [2]. Although local AB are popular in clinical practice, limited evidence on clinical efficacy means that today, prescribing can only be recommended for a few indications [3]. Theoretical advantages of local antibiotic therapy for skin infections are the deposition of a high antimicrobial drug concentration at the site of infection as well as the reduction of the risk of systemic toxicity [3, 4]. In addition, agents that are not available for systemic use can be applied locally [2]. However, the use of topical AB is only useful for superficial (staphylogenic) skin infections, as there is usually insufficient penetration of the active ingredients into deeper skin layers [3]. The use in other diseases, such as rosacea or acne, must be considered in a differentiated manner, since an immunomodulatory rather than an anti-infective effect is intended. In many cases, combined preparations of topical AB and glucocorticosteroid are used unnecessarily for the treatment of inflammatory skin conditions – for example in eczema diseases – without a skin infection even being present [3]. Apart from the limited evidence on clinical efficacy, the usefulness of local antibiotic therapy must be critically questioned because of increasing bacterial resistance [3]. Consistent local antiseptic treatment with active substances, such as octenidine or polyhexanide, often covers a wider spectrum of efficacy. Modern antiseptics also have a broader target tropism because of their non-specific cellular mechanisms of action, and are therefore much less likely to lead to the development of resistance compared with locally applied AB. Long-term or repeated use also increases the risk of contact sensitization (CS) and thus limits the subsequent systemic use of the antibiotic [1].
Contact allergies to topical antibiotics
The prevalence of contact allergy (CA) is generally quite low but may be underdiagnosed. CA is a clinically relevant problem, especially in high-risk patients [1, 2]. CS resulting from repeated topical antibiotic therapies most commonly develop from uncritical self-treatment, occupational exposure, or are iatrogenic [1]. An overview of antibiotics as a cause of contact allergies can also be found in Table 1. Particular risk factors are the use of topical AB on barrier-damaged skin or in the presence of pre-existing dermatoses. Repeated and occlusive therapy in intertriginous skin areas also increase the risk of contact allergy (CA) [1, 2, 5]. Studies have shown that patients with chronic venous insufficiency, chronic otitis externa, post-operative or post-traumatic wounds, and chronic eczema lesions treated with topical AB are particularly predisposed to the development of allergic contact dermatitis (CD) [5, 6]. AB in topical ophthalmic treatments can lead to periocular eczema as a consequence of CS [7, 8]. CA should be considered if there is no improvement or even a paradoxical worsening of the local findings during therapy with topical AB. In the case of acute allergic CD, edematous, erythematous, and pruritic papules and vesicles appear at the site of contact allergen application [1].
Table 1. Antibiotics as triggers of contact allergies and their special features.
| Antibiotics as triggers of contact allergies | Special features | |
|---|---|---|
| Aminoglycoside antibiotics | Gentamicin Neomycin |
In some countries, in combination preparations with steroids, antifungals, and/or bacitracin |
| Polypeptide antibiotics | Bacitracin | Occasional co-sensitization with neomycin by combined use |
| “Late” test reactions in epicutaneous patch testing | ||
| Immediate-type allergic reactions have also been described | ||
| Polymyxin B | In some countries in combination preparations with bacitracin and neomycin. | |
| “Late” test reactions in epicutaneous patch testing | ||
| Lincosamide antibiotics | Clindamycin | Rare as a cause of allergic contact dermatitis |
| Occasional atypical pictures of contact allergy (EEM-like, rosacea-like rash). | ||
| Macrolide antibiotics | Erythromycin | Very rare as a cause of allergic contact dermatitis |
| β-lactam antibiotics | Penicillins Cefalosporins |
Contact allergy due to occupational exposure, e.g. during the preparation of infusions to be administered systemically |
| Immediate-type allergic reactions possible | ||
| Various | Fusidic acid | Increased risk of contact allergy in patients with chronic leg ulcers, stasis dermatitis, otitis externa |
| Mupirocin | Rarely a cause of allergic contact dermatitis | |
| Safe alternative in the case of contact sensitization to neomycin and bacitracin. | ||
| No immunological cross reactions | ||
| Metronidazole | Occasional atypical clinical pictures of type IV allergy (fixed AME, SDRIFE). | |
| Chloramphenicol | In the past, allergic contact dermatitis often via application in eye drops | |
| Topical use in Europe rare nowadays | ||
| Nitrofurazone | In the past, occupational exposure via animal feed additive | |
| Still marketed in Germany (furacin-sol 0.2% ointment) | ||
| Oxytetracycline | No data on current prevalence of contact sensitization | |
| Immunological cross-reactions to other tetracyclines possible | ||
| Clioquinol | Contact sensitization is rare | |
| Immunological cross-reactions to other halogenated hydroxyquinolines have been described | ||
| Ozenoxacin | Approved 2019 as a topical antibiotic in Europe | |
| So far, no increased risk of contact sensitization | ||
| Retapamulin | Contact allergies described so far only in isolated cases | |
CA, as a result of occupational exposure, is often triggered by antibiotics that are usually administered systemically, for example, via improper handling, such as repeated accidental skin contact when preparing antibiotic infusions to be administered systemically (β-lactam antibiotics, especially cephalosporins) without protective gloves [9]. A transfer of the allergen via the hands to another part of the body may result in “dislocated” eczema on forearms or face [5, 9]. Occupationally induced aerogenic CD and generalized exanthema have also been described in the literature [9, 10]. In principle, occupationally induced allergic reactions of the immediate-type with urticaria and anaphylaxis due to antibiotics are also possible and have been described. The risk of sensitization by occupational exposure is not only relevant for healthcare workers, but also for workers in the pharmaceutical industry and in agriculture. Natural and semi-synthetic penicillins are among the more common allergens responsible for allergic CD [10]. In principle, it can be assumed that generalized drug reactions (drug exanthema) in acquired CS are also possible if the corresponding AB or a related substance is taken systemically. De Castro Martinez et al. [12] reported a case of systemic CD to fusidic acid with previous sensitization via the skin. By analogy, it is conceivable that sensitization via systemic administration of an AB also leads to allergic CD on subsequent skin contact [9]. Whether an atopic disposition is increasingly associated with allergic CD remains controversial [13, 14, 15]. The same applies to the risk of CS to topical AB [1].
Among topical ABs, aminoglycosides, such as neomycin, gentamycin, and framycetin, appear to have the highest risk of CS [2]; other triggers include bacitracin, chloramphenicol, clindamycin, and erythromycin [1, 16]. Co-sensitization to multiple substances that are structurally unrelated but contained in the same preparation is also possible. This has been repeatedly described for neomycin and bacitracin [16].
Aminoglycoside antibiotics
Aminoglycoside antibiotics as topical preparations are not only used in dermatology but also in ophthalmology and otolaryngology [6, 17]. A high prevalence of CA to gentamicin is found in patients with chronic venous insufficiency and otitis externa. Another frequently used topical aminoglycoside antibiotic is neomycin, not least because of low therapeutic costs. As a broad-spectrum antibiotic with bactericidal activity against Gram-negative (not effective against Pseudomonas aeruginosa and anaerobic bacteria) and Gram-positive bacteria, particularly Staphylococcus aureus, neomycin is widely prescribed for the treatment of superficial skin infections [2]. In ophthalmology, it is used in the form of eye drops for bacterial conjunctivitis, and in otolaryngology for the treatment of otitis externa [6]. Unlike in Germany, neomycin (as well as gentamicin) is available in the USA as an over-the-counter preparation in combined steroid formulations (dexamethasone), with antifungal agents (nystatin) as well as together with bacitracin [16, 18, 19]. It is therefore one of the most common contact allergens in North America, with sensitization rates exceeding 8% [6]. An analysis of epicutaneous patch test data in 10 European countries in 2005/2006 showed sensitization rates for neomycin to be between 1.1 and 3.8% [20]. It is also significant as a contact allergen in children (in the USA and in some European countries) [21]. Due to the combined use with bacitracin, there are also many cases of co-sensitization to neomycin and bacitracin. The combination of neomycin and glucocorticosteroid may mask the clinical picture of allergic CD [5]. Because of their structural relationship to each other, aminoglycoside antibiotics are characterized by a high rate of immunologic cross-reactions [16]. Thus, in the case of CS to neomycin, cross-reactions to other aminoglycoside antibiotics, such as amikacin, framycetin, gentamicin, tobramycin, kanamycin, and butirosin, are common [1, 7]. Notably, in epicutaneous patch testing with aminoglycoside antibiotics, the maximum of a positive test reaction is often reached after 7 days, so late readings are very important [6]. In 2019, CA after topical application of paromomycin was reported for the first time by an Italian research group [22]. Streptomycin is used systemically to treat tuberculosis, other mycobacterial infections, and infections caused by enterococci and streptococci. CA to streptomycin is observed in healthcare professionals and pharmaceutical workers [1]. Spectinomycin has a different chemical structure among aminoglycosides and therefore shows minimal cross-reactivity [16].
Polypeptide antibiotics
These include the polymyxins and also bacitracin. Because of high toxicity, systemic use is rarely justifiable. Bacitracin is produced by Bacillus subtilis, inhibits bacterial cell wall synthesis, is effective against Gram-positive bacteria and is used for the prevention and therapy of superficial skin infections. Because of potential nephrotoxicity, the substance is restricted to topical use [4]. Bacitracin was declared “Contact Allergen of the Year 2003” by the American Contact Dermatitis Society in 2003. Since “late” test reactions are common in epicutaneous patch testing, it can be assumed that CS to bacitracin has been overlooked more frequently in the past [23]. Of note in this context is that immediate-type allergic reactions are also possible. Thus, anaphylaxis has been described in the context of intra-operative bacitracin irrigation [24] or by use in topical preparations [25]. Polymyxin B binds to the cell membranes of bacteria and disrupts their osmotic properties. Its antibiotic activity encompasses Gram-negative bacteria including Pseudomonas. For decades, polymyxin B has been used in topical preparations to treat skin, eye, and ear infections, often in combination with other antimicrobial agents. Polymyxin B, bacitracin, and neomycin are sold in combination (“one cream treats all”) as over-the-counter preparations in some countries [26]. Polymyxin B had previously been thought to be a rather weak sensitizer. However, in a retrospective cohort study of 795 patients in Canada (where polymyxin is available only on prescription), in whom epicutaneous patch testing was performed, a prevalence of CS of as much as 2.3% was seen [26]. Moreover, in analogy to bacitracin and neomycin, it could be observed that positive epicutaneous patch test reactions are often not noticeable until day 4, so it is recommended to always perform late readings with topical AB [23, 26]. Although bacitracin and polymyxin B are cyclic polypeptides, they differ in chemical structure in a way that immunologic cross-reactions are not very likely [26]. Virginiamycin is another cyclic polypeptide that is occasionally used in Europe for topical treatment of infections with Gram-positive bacteria. It is still used as a growth promoter in cattle, pigs, and poultry in some countries and thus may cause occupational contact dermatitis in livestock workers [1]. Pristinamycin is a related streptogramin AB. Factor M of virginiamycin is identical to fraction IIA of pristinamycin. Therefore, cross-reactions of the two ABs are common [1]. However, virginiamycin and pristinamycin are hardly used in humans today.
Lincosamide antibiotics
Clindamycin is a semi-synthetic derivative of lincomycin, inhibits bacterial protein synthesis, and is effective against aerobic Gram-positive cocci and some anaerobic and microaerophilic Gram-negative and Gram-positive micro-organisms [1]. Topical applications of clindamycin include the treatment of acne vulgaris and bacterial vaginosis. However, despite frequent long-term use, it is rarely a trigger of allergic contact dermatitis [27]. CA to a 1% alcoholic clindamycin solution for the treatment of facial acne was first described in 1978 [28]. Since acne therapy often involves the use of a variety of other topical preparations, a diagnosis is often delayed. Manifestations of atypical clinical pictures of CA, such as rosacea-like rash [29] or erythema multiforme-like skin lesions [30], have been described. Therefore, in the case of a paradoxical worsening as well as a change of the clinical appearance of acne despite therapy, CA should always be considered [28]. Immunological cross-reactions between clindamycin and lincomycin, which is not approved for human medicine in Germany, are possible.
Macrolides
The macrolide antibiotic erythromycin inhibits bacterial protein synthesis. It is actively effective against most aerobic and anaerobic Gram-positive and some Gram-negative bacteria. Topically, erythromycin is used in the treatment of acne vulgaris, rosacea, and perioral dermatitis as well as superficial skin and eye infections. The occurrence of allergic CD is very rare [2, 31].
Beta-lactam antibiotics
β-lactam antibiotics inhibit mucopeptide synthesis in the bacterial cell wall. They are rarely used in topical pharmaceuticals. Previously reported cases of CA often relate to healthcare workers and the pharmaceutical industry or pharmaceutical manufacturing. Among semi-synthetic penicillins, ampicillin is an occasional cause of occupational CA [1]. CS to cephalosporins is also more commonly found in the context of occupational exposure. Immediate-type allergic reactions have also been described [11]. Immunologic cross-reactions within a cephalosporin group of the same generation are common. Knowing the sensitizing potential of penicillin, topical use is now widely avoided. In Malaysia, where topical penicillin was formerly available as an over-the-counter agent, it was the most common cause of AB-induced allergic contact dermatitis in 1976 [32]. Cross-reactions between penicillins, semi-synthetic penicillins, and cephalosporins are theoretically possible due to the common β-lactam ring, but are rarely observed in practice because, in the vast majority of cases, the immunological cross-reactions can be explained by side-chain sensitization [33].
Various antibiotics
Fusidic acid, a topical AB used to treat skin infections caused by Gram-positive bacteria, mainly Staphylococcus aureus, appears to rarely trigger CS [17, 34] and is therefore widely considered an alternative to topical aminoglycoside antibiotics. There is an increased risk of sensitization when fusidic acid is used to treat chronic leg ulcers, stasis dermatitis, and otitis externa [34].
Mupirocin is produced by Pseudomonas fluorescens, inhibits bacterial protein synthesis, and is effective against aerobic Gram-positive bacteria. Topical applications include treatment of skin infections and also elimination of staphylococci in the nasal vestibule. The occurrence of allergic CD is apparently very rare [35]. To date, only a few cases have been published, the first being in 1995 for a patient who had applied mupirocin topically for the treatment of chronic leg ulcers [35, 36]. In cases of CS to neomycin and/or bacitracin, mupirocin can be used as a safe alternative because it is the only representative of this pharmacologic class of agents and has a unique structure among topical ABs. Immunological cross-reactions have not been observed [16].
Metronidazole is a synthetic nitroimidazole derivative for the treatment of infections with anaerobic bacteria and protozoa. In addition, the substance has direct anti-inflammatory and immunosuppressive properties, which is why it is also used in the topical treatment of inflammatory dermatoses such as rosacea and perioral dermatitis. Topically, metronidazole is also used in the treatment of bacterial vaginosis, trichomoniasis, and occasionally in wound healing disorders of the skin. After intra-vaginal application as an ovule, drug reactions have been observed under the clinical picture of fixed-drug reaction [37] and SDRIFE (symmetrical drug-related intertriginous and flexural exanthema) [38]. Allergic CD on the face has been described in association with the use of metronidazole-containing topical preparations for the treatment of facial dermatoses such as rosacea and acneiform skin symptoms [39]. Cross-reactions to imidazole antifungals have been discussed. However, reliable data on this do not appear to exist to date [1, 40].
Chloramphenicol inhibits bacterial protein synthesis. Overall, topical use in Europe has decreased significantly in recent decades, and the sensitization potential is low. Allergic CD used to be induced via repeated application of chloramphenicol-containing eye drops [41].
Nitrofurazone (Nitrofural) is a broad-spectrum antibiotic from the nitrofuran group. Topical application used to be for the treatment of skin infections, ulcers, and burns. It is still marketed in Germany under the name “Furacin-Sol 0,2% Salbe”, approved for the treatment of superficial skin and wound infections. Due to a high incidence of allergic reactions, its use has been increasingly abandoned in Western countries [42]. Nitrofurazone was formerly used in veterinary medicine as an animal feed additive. Therefore, occupational exposure was generally present [42]. In the meantime, nitrofurans may no longer be used in food-producing animals in the EU, so that the substance is no longer important as an animal feed additive [43].
Oxytetracycline inhibits bacterial protein synthesis and is effective against many aerobic and anaerobic Gram-negative and Gram-positive bacteria, including Rickettsia, Chlamydia, Mycoplasma, and Spirochetes. Local application is also used for the treatment of acute and chronic bacterial infections of the anterior segment of the eye and superficial skin infections. CS with immunologic cross-reactions to other tetracyclines are generally possible [1]. Recent data on the current prevalence of CS to oxytetracycline are not available.
Clioquinol is a halogenated hydroxyquinoline AB. It inhibits the growth of Gram-positive cocci (staphylococci, enterococci), various fungal pathogens (microsporon), Trichophyton, Candida albicans) and is also amebicidal. It was formerly used topically in the treatment of eczema and fungal infections. Sensitization has been observed in patients with chronic leg ulcers [1]. Clioquinol is a rare sensitizer and seems to be used much less frequently in recent years, not least because of the substance’s yellow intrinsic coloration 44]. Thus, based on data from the European Surveillance System on Contact Allergies (ESSCA), it was proposed in 2018 to remove clioquinol from the European Baseline Series [44]. Immunologic cross-reactions to other topical and systemic halogenated hydroxyquinolines, such as iodochlorhydroxyquin, iodoquinol, broxyquinoline, chlorquinaldol, and chlorhydroxyquinolines have been described [45]. In clioquinol-sensitized patients, positive epicutaneous patch test reactions to various antimalarials, such as quinine, chloroquine, and amodiaquine, have been observed [46].
Ozenoxacin is a bactericidal AB from the quinolone group, approved in 2017 in the USA and in 2019 as a 1% cream in Europe for the short-term treatment of non-bullous impetigo from 6 months of age. An increased risk of relevant CS has apparently not been observed so far [47].
Retapamulin is a semi-synthetic derivative of pleuromutilin with activity against staphylococci and streptococci. It is approved in Europe as a 1% topical preparation for the treatment of impetigo and minor infected wounds [3]. CA to retapamulin is apparently very rare and has been described only in isolated cases [48].
Diagnosis and management of allergy to topical antibiotics
After CA has healed, epicutaneous patch testing is the most important tool for further allergological clarification (Figure 1). Current test concentrations of commercially available antibiotics according to recommendations of the German Contact Dermatitis Research Group (DKG) can be found in Table 2. In many cases, it is recommended to test not only the active ingredients but also the individual components such as preservatives, additives, and vehicles of the substances used [5]. Since a large proportion of the ABs in question are not available as commercial test preparations, it may be advisable to carry out testing with the patient’s own substances after informing the patient accordingly. The regulatory requirements of the German Medicinal Products Act (The Drug Law) (Arzneimittelgesetz, AMG) must be observed [49]. Since delayed patch test reactions often occur, readings after 1 week and/or later are indispensable in order to not overlook late reactions.
Figure 1. Epicutaneous patch test chloramphenicol 5% Vas.: +++ test reaction after 72 hours.
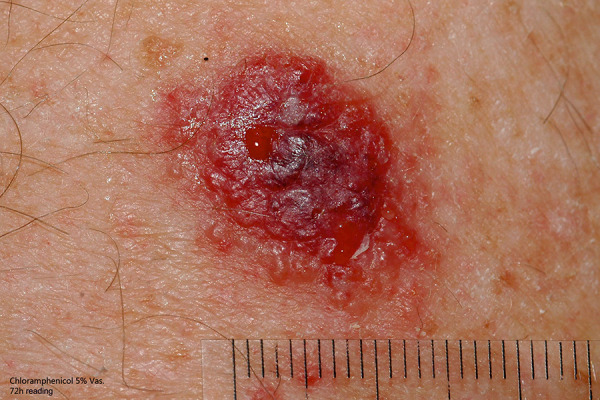
Table 2. Test concentrations of various commercially available antibiotics according to recommendations of the German Contact Allergy Group (DKG) – as of January 2022 (https://dkg.ivdk.org/testreihen.html#a005).
| Active ingredient | Test concentration | Vehicle (Vas.: Vaseline) |
|---|---|---|
| Bacitracin | 20% | Vas. |
| Gentamicin sulfate | 20% | Vas. |
| Oxytetracycline | 3% | Vas. |
| Framycetin sulfate | 10% | Vas. |
| Fusidic acid (Na. salt) | 2% | Vas. |
| Neomycin sulfate | 20% | Vas. |
| Polymyxin B sulfate | 3% | Vas. |
| Chloramphenicol | 5% | Vas. |
| Kanamycin sulfate | 10% | Vas. |
| Clioquinol (Iodochlorhydroxyquin) | 5% | Vas. |
After diagnosis, the patient must be informed about the substances to be avoided. This also includes information about possible immunological cross-reactions.
Conclusion
Repeated or long-term use of topical antibiotics and an existing skin barrier defect are risk factors for the development of CA. In many cases, topical antibiotic treatment is not necessary with the use of modern antiseptics. Therefore, the indication must always be critically questioned not only because of the sensitization potential but also because of the unfavorable and potentially consequential resistance problem.
Funding
None.
Conflict of interest
J. Wohlrab has received grants for scientific projects, clinical studies, lectures and/or consulting from the following relevant companies in the last 5 years: Allergika, Almirall, Aristo, BayPharma, Dermapharm, Galderma, GSK, Helm, Hexal, Infectopharm, Jenapharm, Klinge, Leo, Medac, Medice, Mibe, MSD, Mylan, Novartis, Pierre Fabre, Pfizer, Skinomics, Wolff.
B. Kreft has received grants for scientific projects, clinical studies, lectures or consulting in the last 5 years outside the submitted work from Jenapharm, Novartis, Biogen.
References
- 1. Gehrig KA Warshaw EM Allergic contact dermatitis to topical antibiotics: Epidemiology, responsible allergens, and management. J Am Acad Dermatol. 2008; 58: 1–21. [DOI] [PubMed] [Google Scholar]
- 2. Bonamonte D De Marco A Giuffrida R Conforti C Barlusconi C Foti C Romita P Topical antibiotics in the dermatological clinical practice: Indications, efficacy, and adverse effects. Dermatol Ther (Heidelb). 2020; 33:e13824. [DOI] [PubMed] [Google Scholar]
- 3. Williamson DA Carter GP Howden BP Current and emerging topical antibacterials and antiseptics: agents, action, and resistance patterns. Clin Microbiol Rev. 2017; 30: 827–860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kaye ET Topical antibacterial agents. Infect Dis Clin North Am. 2000; 14: 321–339. [DOI] [PubMed] [Google Scholar]
- 5. Davis MD Unusual patterns in contact dermatitis: medicaments. Dermatol Clin. 2009; 27: 289–297 vi.. [DOI] [PubMed] [Google Scholar]
- 6. Coppola-Fasick G Nazario S Recurrent otitis externa owing to allergic contact dermatitis to topical medications. Ann Allergy Asthma Immunol. 2020; 125: 616–617. [DOI] [PubMed] [Google Scholar]
- 7. Gilissen L De Decker L Hulshagen T Goossens A Allergic contact dermatitis caused by topical ophthalmic medications: Keep an eye on it! Contact Dermat. 2019; 80: 291–297. [DOI] [PubMed] [Google Scholar]
- 8. Kreft B Wohlrab J Ophthalmika. Allergo J. 2018; 27: 16–18. [Google Scholar]
- 9. Pinheiro V Pestana C Pinho A Antunes I Gonçalo M Occupational allergic contact dermatitis caused by antibiotics in healthcare workers - relationship with non-immediate drug eruptions. Contact Dermat. 2018; 78: 281–286. [DOI] [PubMed] [Google Scholar]
- 10. Minciullo PL Imbesi S Tigano V Gangemi S Airborne contact dermatitis to drugs. Allergol Immunopathol (Madr). 2013; 41: 121–126. [DOI] [PubMed] [Google Scholar]
- 11. Merget R Sander I Fartasch M van Kampen V Röseler S Merk H Wurpts G Raulf M Brüning T Occupational generalized urticaria and anaphylaxis after inhalation of cefuroxime in a nurse. Am J Ind Med. 2018; 61: 261–266. [DOI] [PubMed] [Google Scholar]
- 12. De Castro Martinez FJ Ruiz FJ Tornero P De Barrio M Prieto A Systemic contact dermatitis due to fusidic acid. Contact Dermat. 2006; 54: 169. [DOI] [PubMed] [Google Scholar]
- 13. Forkel S Cevik N Schill T Worm M Mahler V Weisshaar E Vieluf D Pfützner W Löffler H Schön MP Geier J Buhl T Atopische Hautdiathese ist stärker mit spezifischen Kontaktallergien assoziiert als atopische Dermatitis. J Dtsch Dermatol Ges. 2021; 19: 231–240. [DOI] [PubMed] [Google Scholar]
- 14. Hamann CR Hamann D Egeberg A Johansen JD Silverberg J Thyssen JP Association between atopic dermatitis and contact sensitization: A systematic review and meta-analysis. J Am Acad Dermatol. 2017; 77: 70–78. [DOI] [PubMed] [Google Scholar]
- 15. Rastogi S Patel KR Singam V Silverberg JI Allergic contact dermatitis to personal care products and topical medications in adults with atopic dermatitis. J Am Acad Dermatol. 2018; 79: 1028–1033.e6. [DOI] [PubMed] [Google Scholar]
- 16. Nguyen HL Yiannias JA Contact dermatitis to medications and skin products. Clin Rev Allergy Immunol. 2019; 56: 41–59. [DOI] [PubMed] [Google Scholar]
- 17. Uter W Spiewak R Cooper SM Wilkinson M Sánchez Pérez J Schnuch A Schuttelaar ML Contact allergy to ingredients of topical medications: results of the European Surveillance System on Contact Allergies (ESSCA), 2009 – 2012. Pharmacoepidemiol Drug Saf. 2016; 25: 1305–1312. [DOI] [PubMed] [Google Scholar]
- 18. Dumycz K Osinka K Feleszko W Contact allergens in topical corticosteroid vehicles: analysis of product composition. Contact Dermat. 2017; 76: 254–255. [DOI] [PubMed] [Google Scholar]
- 19. Zug KA Pham AK Belsito DV DeKoven JG DeLeo VA Fowler JF Fransway AF Maibach HI Marks JG Mathias CG Pratt MD Sasseville D Storrs FJ Taylor JS Warshaw EM Zirwas MJ Patch testing in children from 2005 to 2012: results from the North American contact dermatitis group. Dermatitis. 2014; 25: 345–355. [DOI] [PubMed] [Google Scholar]
- 20. Uter W Rämsch C Aberer W Ayala F Balato A Beliauskiene A Fortina AB Bircher A Brasch J Chowdhury MM Coenraads PJ Schuttelaar ML Cooper S Corradin MT Elsner P English JS Fartasch M Mahler V Frosch PJ Fuchs T The European baseline series in 10 European countries, 2005/2006-results of the European surveillance system on contact allergies (ESSCA). Contact Dermat. 2009; 61: 31–38. [DOI] [PubMed] [Google Scholar]
- 21. Seidenari S Giusti F Pepe P Mantovani L Contact sensitization in 1094 children undergoing patch testing over a 7-year period. Pediatr Dermatol. 2005; 22: 1–5. [DOI] [PubMed] [Google Scholar]
- 22. Veraldi S Benzecry V Faraci AG Nazzaro G Allergic contact dermatitis caused by paromomycin. Contact Dermat. 2019; 81: 393–394. [DOI] [PubMed] [Google Scholar]
- 23. Katz BE Fisher AA Bacitracin: a unique topical antibiotic sensitizer. J Am Acad Dermatol. 1987; 17: 1016–1024. [DOI] [PubMed] [Google Scholar]
- 24. Damm S Intraoperative anaphylaxis associated with bacitracin irrigation. Am J Health Syst Pharm. 2011; 68: 323–327. [DOI] [PubMed] [Google Scholar]
- 25. Schechter JF Wilkinson RD Del Carpio J Anaphylaxis following the use of bacitracin ointment. Report of a case and review of the literature. Arch Dermatol. 1984; 120: 909–911. [PubMed] [Google Scholar]
- 26. Alfalah M Zargham H Moreau L Stanciu M Sasseville D Contact Allergy to Polymyxin B Among Patients Referred for Patch Testing. Dermatitis. 2016; 27: 119–122. [DOI] [PubMed] [Google Scholar]
- 27. Voller LM Kullberg SA Warshaw EM Axillary allergic contact dermatitis to topical clindamycin. Contact Dermat. 2020; 82: 313–314. [DOI] [PubMed] [Google Scholar]
- 28. Coskey RJ Contact dermatitis due to clindamycin. Arch Dermatol. 1978; 114: 446. [PubMed] [Google Scholar]
- 29. de Kort WJ de Groot AC Clindamycin allergy presenting as rosacea. Contact Dermat. 1989; 20: 72–73. [DOI] [PubMed] [Google Scholar]
- 30. Munoz D Del Pozo MD Audicana M Fernandez E Fernandez De Corres LF Erythema-multiforme-like eruption from antibiotics of 3 different groups. Contact Dermat. 1996; 34: 227–228. [DOI] [PubMed] [Google Scholar]
- 31. Valsecchi R Pansera B Reseghetti A Contact allergy to erythromycin. Contact Dermat. 1996; 34: 428. [DOI] [PubMed] [Google Scholar]
- 32. Nagreh DS Contact dermatitis from proprietary preparations in Malaysia. Int J Dermatol. 1976; 15: 34–35. [DOI] [PubMed] [Google Scholar]
- 33. Wurpts G Aberer W Dickel H Brehler R Jakob T Kreft B Mahler V Merk HF Mülleneisen N Ott H Pfützner W Röseler S Ruëff F Sitter H Sunderkötter C Trautmann A Treudler R Wedi B Worm M Brockow K Guideline on diagnostic procedures for suspected hypersensitivity to beta-lactam antibiotics: Guideline of the German Society for Allergology and Clinical Immunology (DGAKI) in collaboration with the German Society of Allergology (AeDA), German Society for Pediatric Allergology and Environmental Medicine (GPA), the German Contact Dermatitis Research Group (DKG), the Austrian Society for Allergology and Immunology (ÖGAI), and the Paul-Ehrlich Society for Chemotherapy (PEG). Allergol Select. 2020; 4: 11–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. de Groot AC Contact allergy to sodium fusidate. Contact Dermat. 1982; 8: 429. [DOI] [PubMed] [Google Scholar]
- 35. Assier H Hirsch G Wolkenstein P Chosidow O Severe contact allergy to mupirocin in a polysensitized patient. Contact Dermat. 2019; 80: 397–398. [DOI] [PubMed] [Google Scholar]
- 36. Eedy DJ Mupirocin allergy in the setting of venous ulceration. Contact Dermat. 1995; 32: 240–241. [DOI] [PubMed] [Google Scholar]
- 37. Hermida MD Consalvo L Lapadula MM Della Giovanna P Cabrera HN Bullous fixed drug eruption induced by intravaginal metronidazole ovules, with positive topical provocation test findings. Arch Dermatol. 2011; 147: 250–251. [DOI] [PubMed] [Google Scholar]
- 38. Kumagai J Nakamura A Ogawa S Washio K Intravaginal metronidazole ovule-related allergic contact dermatitis. Contact Dermat. 2021; 85: 85–86. [DOI] [PubMed] [Google Scholar]
- 39. Madsen JT Thormann J Kerre S Andersen KE Goossens A Allergic contact dermatitis to topical metronidazole – 3 cases. Contact Dermat. 2007; 56: 364–366. [DOI] [PubMed] [Google Scholar]
- 40. Izu R Aguirre A González M Díaz-Pérez JL Contact dermatitis from tioconazole with cross-sensitivity to other imidazoles. Contact Dermat. 1992; 26: 130–131. [DOI] [PubMed] [Google Scholar]
- 41. Urrutia I Audícana M Echechipía S Gastaminza G Bernaola G Fernández de Corrès L Sensitization to chloramphenicol. Contact Dermat. 1992; 26: 66–67. [DOI] [PubMed] [Google Scholar]
- 42. Ballmer-Weber BK Elsner P Contact allergy to nitrofurazone. Contact Dermat. 1994; 31: 274–275. [DOI] [PubMed] [Google Scholar]
- 43. BVL-Report 13.6 – Bericht zur Lebensmittelsicherheit 2017. Bundesamt für Verbraucherschutz und Lebensmittelsicherheit. https://www.bvl.bund.de.
- 44. Wilkinson M Gallo R Goossens A Johansen JD Rustemeyer T Sánchez-Pérez J Schuttelaar ML Uter W A proposal to create an extension to the European baseline series. Contact Dermat. 2018; 78: 101–108. [DOI] [PubMed] [Google Scholar]
- 45. Rodríguez A Cabrerizo S Barranco R de Frutos C de Barrio M Contact cross-sensitization among quinolines. Allergy. 2001; 56: 795. [DOI] [PubMed] [Google Scholar]
- 46. Soesman-van Waadenoijen Kernekamp A van Ketel WG Persistence of patch test reactions to clioquinol (Vioform) and cross-sensitization. Contact Dermat. 1980; 6: 455–460. [DOI] [PubMed] [Google Scholar]
- 47. Gropper S Cepero AL Dosik JS LaStella P Siemetzki H Wigger-Alberti W Cumulative irritation, sensitizing potential, phototoxicity and photoallergy of ozenoxacin in healthy adult volunteers. Future Microbiol. 2014; 9: S23–S31. [DOI] [PubMed] [Google Scholar]
- 48. Schalock PC Allergic contact cermatitis to retapamulin ointment. Contact Dermat. 2009; 61: 126. [DOI] [PubMed] [Google Scholar]
- 49. Zweites Gesetz zur Änderung arzneimittelrechtlicher und anderer Vorschriften vom 19.10.2012 (sog. „16. AMG-Novelle”) im Bundesgesetzblatt verkündet (BGBl I S. 2192) am 25.10.2012.


