Abstract
Objective:
The aim of this study is to investigate whether the primary tumour response to neoadjuvant chemotherapy (NAC), based on the increase in the ADC-values (apparent diffusion coefficient) within the breast lesion, could help to predict axillary complete response.
Methods:
We retrospectively included 74 patients who were treated with NAC followed by surgery at Lucus Augusti Hospital between January 2015 and September 2020. Simple logistic regression was used to evaluate the factors associated with axillary pathological complete response, including the changes in breast tumour ADC-values due to the treatment.
Results:
Axillary complete response was correlated with negative oestrogen receptor status, Her2 positivity and response of primary tumour. It was achieved in 31% of the patients. In addition, the increase in the tumour ADC-values with NAC was higher for responders. Among the tumours that demonstrated an increase in ADC-value >0.92 ×10−3 mm2/s, 42.8% (15/35) showed axillary complete response. Eight (20.5%) breast cancers with an increase in ADC below the cut-off value were found to have no metastatic nodes after treatment (p = 0.038).
Conclusion:
Our results suggest that the performance of models predicting axillary response to NAC can be improved by adding the tumour response determined also using diffusion-weighted imaging.
Advances in knowledge:
For the fist time, we investigate the relation between tumour response to NAC, assessed using diffusion-weighted imaging, and axillary pathologic complete response.
Introduction
In the past two decades, the axillary management of patients with breast cancer has significantly changed and its surgical approach has evolved toward less invasive strategies.
Neoadjuvant chemotherapy (NAC) is been increasingly used to downstage disease in the axilla in node-positive (cN+) patients. It has been published that axillary pathologic complete response (pCR) can be achieved in 30–40% of cases.1 According to the results of four prospective trials, sentinel lymph node biopsy (SLNB) could be reliable for axillary staging in cases that become node negative following NAC (ypN0), if at least three sentinel lymph nodes (LNs) are retrieved and dual-tracer mapping is used.2–5 Therefore, axillary lymph node dissection (ALND) could be omitted in these patients, preventing morbidity and complications associated to this surgical technique.
MRI is the imaging modality best suited to assess the response of locally advanced breast cancer to NAC.6 Diffusion-weighted (DW) sequences have demonstrated to increase the accuracy of the MRI in predicting pCR to treatment.7 Diffusion-weighted imaging (DWI) is a functional imaging technique that measures the mobility of the water molecules within the tissues, providing information about cellularity and cell membranes integrity. The value of water diffusion can be quantitatively measured using the apparent diffusion coefficient (ADC). Neoplasms are characterized by relatively high cellular density, which implies restricted water movement and low ADC-value.8 Chemotherapy produces changes in cell density and apoptosis and, therefore, an increase in the ADC inside the tumour.
However, the evaluation of axillary response to NAC using breast MRI is less accurate.9 Many studies have tried to predict, non-invasively, pCR of the axillary LN helping to select those patients candidates to SLNB. It has been suggested that axillary response correlates with clinical N stage, Her2 positivity, and response of primary tumour.10 Nevertheless, to the best of our knowledge, the relation between tumour response to NAC, assessed using DWI, and axillary pCR has not been yet evaluated.
The aim of this study is to investigate whether the primary tumour response to NAC, based on the increase in the ADC-values within the breast lesion, could help to efficiently select those patients who would benefit from SLNB after NAC and predict axillary pCR.
Methods and materials
Our institutional ethics committee approved this retrospective study. Informed consent was waived, as this was a noninterventional study using and authorized healthcare database with routinely collected and anonymous data.
We included all females diagnosed with advanced breast cancer (BC) and pathologically proven axillary node metastasis scheduled to neoadjuvant treatment, followed by breast surgery with SLNB and/or ALND at Lucus Augusti hospital between January 2015 and September 2020. All patients underwent baseline and post-chemotherapy breast MRI examinations and pre-NAC axillary ultrasound. Exclusion criteria were as follows: patients who did not go to surgery, those who had not surgery and final pathologic examination in our institution (Universitary hospital Lucus Augusti) and absence of pre- and/or post-treatment MRI.
We extracted from the database clinical, radiological and pathologic data, including patient’s age, tumour histologic type and nuclear grade, BC molecular subtype, baseline and preoperative MRI findings (mass or non-mass enhancement and ADC-value), number of suspicious axillary LN on ultrasound at diagnosis, percentage change in tumour size, type of breast and axillary surgery and number of positive and total number of nodes removed.
Patients were managed according to our Breast Unit’s protocol. Criteria for NAC comprise: tumours greater than 3 cm; locally advanced disease in the breast (cT4) and/or in the nodes (cN2/N3); and patients with cT1-T2 (<3 cm) cN0-1 BC and small breasts, to increase the possibility of breast-conserving surgery.
Axillary ultrasound was done using a 12-MHz high-frequency linear-array transducer (Affinity 70G, Philips Healthcare). LN that showed round shape, absence of the fatty hilum and increased concentric or focal cortical thickness greater than 3 mm were considered suspicious for malignancy. To confirm metastatic involvement we performed sonographically guided 14-Gauge core needle biopsy of one of the nodes. Before 2016, in all patients who had presented as cN +pre-NAC ALND was performed. In 2016, we updated our protocol and in cases with only one or two metastatic LN a gel-based marker (Hydromark®) was placed in the positive nodes under ultrasound guidance. After NAC, we judged these patients to be node-negative (cN0), and therefore eligible for SLNB, when palpation and ultrasound examination showed no suspicious nodes. Patients who initially presented with three or more suspicious LN underwent ALND regardless of the axillary response to NAC.
Breast MRI examinations were performed with the patients in the prone position using a 1.5 T unit (Signa Excite, GE, Healthcare, Milwaukee, WI) with a bilateral 8-channel breast coil. Our imaging protocol included an axial non-fat-saturated T1 weighted fast spin-echo sequence and a short-tau inversion recovery (STIR) axial sequence. DWI was performed using a DW STIR ecoplanar imaging sequence with b-values of 0 and 800 s/mm2 on the axial plane.11 For dynamic contrast-enhanced MRI, T1 weighted fat-suppressed three-dimensional (3D) fast spoiled gradient-echo axial sequences were acquired before and after the intravenous administration of 20 ml gadopentetate dimeglumine (Magnevist; Berlex Laboratories, Wayne, NJ).
All MR images were transferred to a workstation (Advanced Windows version 4.4, General Electric). We obtained ADC maps from the DW sequence using the commercial software FuncTool, GE Healthcare. The ADC-value of each lesion was obtained on the ADC map, guided by the hyperintensity at the high-b value DWI, the T2W sequences, the T1W pre-contrast images and by the first post-contrast MRI sequence (as anatomical references), selecting a slice without artefacts and that included the biggest solid portion of the tumour using a small round 2D-ROI randomly drawn at different locations of the lesion and choosing the lowest ADC-value obtained. We measured the ADC-values at pre- and post-treatment MRI. The median differences in these values before and after neoadjuvant chemotherapy (ΔADC) were calculated.
One breast-dedicated radiologist, with 7 years of breast MRI experience, evaluated the images. Lesions were described according to the current BI-RADS lexicon12 and the RECIST, v. 1.1, was used to evaluate the response to NAC on post-contrast sequences. We considered radiological complete response when no enhancement and no restricted diffusion were seen in previous tumour location.
Histological type, histological grade and molecular subtype of the BC were determined from the core biopsy samples before NAC, by an experienced pathologist, who was blinded to the study and who also examined the surgical specimens to assessed the treatment response after NAC.
From 2016, wire localization of clipped nodes was combined with SLNB after chemotherapy and prior to surgery. The SLNB technique was performed using dual tracing modality (a combination of 99mTc sulfur colloid and isosulfan blue dye). In the setting of a positive SLNB (with micro- or macrometastasis) we proceed with a completion ALND.
We defined breast cancer pCR as no invasive disease in both breast and axillary LN after NAC (ypT0/is, ypN0). We considered as axillary responders those patients who achieved pathological complete response in the LN in the axilla (ypN0).
Statistical analysis
Statistical analysis was performed using commercially available software (SPSS for Windows, V.22.0, IBM-SPSS, Chicago, Illinois, USA). The results were presented in contingency tables. Absolute and relative frequencies of the variables were calculated for descriptive analysis. All continuous variables were expressed as median and interquartile range (IQR). We used the Kolmogorov–Smirnov test for the study of data distribution and continuous variables were compared with the Student’s t test or Mann–Whitney U non-parametric test. For categorical variables, we used the χ2 test or Fisher’s exact test.
Receiver operating characteristic (ROC) analyses was performed to determine the optimum ΔADC threshold value to discriminate axillary pCR and non-pCR.
Finally, simple logistic regression was used to evaluate the factors associated with axillary pathological complete response after NAC.
Results
Between January 2015 and September 2020, 74 node-positive breast tumours were treated with NAC in our institution. Of these, 23 patients achieved axillary pCR after treatment (31%) and 51 had residual axillary metastasis (ypN+).
Table 1 compares the clinicopathological characteristics of both groups. Median age was 51.5 years (IQR 44–66.5 years) and it was not significantly different between ypN0 patients and those with ypN +tumours (p = 0.24). Tumours with negative ER status and those Her2 enriched were significantly more common in females who achieved axillary pCR. There was a trend for a higher percentage of high Ki-67 expression among responders, however, without statistical significance in our sample (p = 0.053). Conversely, histologic grade or type, PR status, clinical tumour stage, number of LN seen on US, MRI enhancement or baseline ADC-value did not significantly differ in the two groups.
Table 1.
Characteristics of patients and tumours treated with neoadjuvant chemotherapy
| Characteristic | All patients N = 74 |
Axillary non-pCR N = 51 (69%) |
Axillary pCR N = 23 (31%) |
p-value |
|---|---|---|---|---|
| Age (yr) | 51.5 (44–66.5) | 53 (44–68) | 48 (44–62) | 0.24 |
| Breast surgery | 0.209 | |||
| Breast-conserving surgery | 37 (50) | 23 (45.1) | 14 (61) | |
| Mastectomy | 37 (50) | 28 (16.9) | 9 (39) | |
| Axillary surgery | 0.006 | |||
| SLNB | 10 (13.5) | 3 (5.9) | 7 (30.4) | |
| ALND | 58 (78.4) | 42 (25.3) | 16 (69.6) | |
| SLNB + ALND | 6 (8.1) | 6 (11.8) | 0 | |
| Clinical tumour stage | 0.726 | |||
| cT2 | 47 (63.5) | 33 (64.7) | 14 (61) | |
| cT3 | 27 (36.5) | 18 (35.3) | 9 (39) | |
| Primary tumour size (cm)* | 4.2 (2.98–6.05) | 3.9 (2.9–6.2) | 4.8 (3.3–5.8) | 0.248 |
| Histologic type | 0.16 | |||
| IDC | 65 (87.8) | 43 (84.3) | 22 (95.7) | |
| ILC | 8 (10.8) | 7 (13.7) | 1 (4.3) | |
| Other | 1 (1.4) | 1 (2) | 0 | |
| Histologic grade | 0.206 | |||
| Low-intermediate | 61 (82.4) | 44 (86.3) | 17 (73.9) | |
| High | 13 (17.6) | 7 (13.7) | 6 (26.1) | |
| ER | 0.025 | |||
| Positive | 62 (83.8) | 46 (90.2%) | 16 (69.6) | |
| Negative | 12 (16.2) | 5 (9.8%) | 7 (30.4) | |
| PR | 0.236 | |||
| Positive | 49 (66.2) | 36 (70.6%) | 13 (56.5) | |
| Negative | 25 (33.8) | 15 (29.4%) | 10 (43.5) | |
| Her2 | <0.001 | |||
| Positive | 29 (39.2) | 10 (19.6%) | 19 (82.6) | |
| Negative | 45 (60.8) | 41 (80.4%) | 4 (17.4) | |
| Ki-67 | 0.053 | |||
| Low | 16 (21.6) | 13 (25.5%) | 3 (13) | |
| High | 51 (68.9) | 31 (60.8%) | 20 (87) | |
| Intermediate | 7 (9.5) | 7 (13.7%) | 0 | |
| Molecular subtype | <0.001 | |||
| Luminal A | 12 (16.2) | 11 (21.6%) | 1 (4.3) | |
| Luminal B | 28 (37.8) | 26 (51%) | 2 (8.7) | |
| Her2 | 30 (40.5) | 11 (21.6%) | 19 (82.6) | |
| TNBC | 4 (5.4) | 3 (5.9%) | 1 (4.3) | |
| MRI findings | 0.302 | |||
| ME | 44 (59.5) | 30 (18.1%) | 14 (60.9) | |
| NME | 29 (39.2) | 21 (12.7%) | 8 (34.8) | |
| ME +NME | 1 (1.4) | 0 | 1 (4.3) | |
| Baseline ADC* | 0.87 (0.71–0.99) | 0.85 (0.7–0.98) | 0.89 (0.79–1.00) | 0.403 |
| Number of LN on ultrasound | 0.318 | |||
| 1–2 | 45 (60.8) | 33 (64.7%) | 12 (52.2) | |
| >2 | 29 (39.2) | 18 (35.3%) | 11 (47.8) | |
| Tumour size reduction (%)* | 58.8 (21.1–98.6) | 45.9 (17.0–82.6) | 100.0 (32.9–100.0) | 0.020 |
| ADC increase* | 0.9 (0.32–1.31) | 0.7 (0.23–1.24) | 1.1 (0.16–2.9) | 0.058 |
| Tumor and axilla pCR** | 21 (28.4) | 7 (13.7) | 14 (60.9) | <0.001 |
ADC = apparent diffusion coefficient; ALND = axillary lymph node dissection; ER = oestrogen receptor; HER2 = human epidermal growth factor receptor 2; IDC = invasive ductal carcinoma; ILC = invasive lobular carcinoma;LN, lymph node; ME = mass enhancement; NME = non mass enhancement; PR = progesterone receptor;SLNB = sentinel lymph node biopsy; TNBC = triple-negative breast cancer; pCR = pathologic complete response.
p-value of significant difference by chi-square, Fisher exact, Student t-test or Mann–Whitney U test.
Pathologic complete response in the breast and absence of axillary lymph node metastases (ypT0ypN0) Unless otherwise indicated, data are the number of patients, with percentages in parentheses.
Median and interquartile range.
The average percentage of tumour size reduction was significantly higher in the patients with axillary pCR 100% (32.9–100.0) vs 45.9% (17.0–82.6); p < 0.020. Breast lesions with pCR were more likely to achieve also axillary pCR. The response rate was 60.9% (14/23) in responders and 13.7% (7/51) in non-responders (p < 0.001).
Analysing the tumour ADC-values, the median increase in the ADC-value in non-responders was 0.70 (IQR: 0.26–1.22), and in those with no residual axillary metastasis was 1.10 (IQR: 0.45–1.51). The increase in the tumour ADC-values with NAC was higher for responders (Figure 1). Nevertheless, the difference was not statistically significant (p = 0.058), probably because of the small sample size.
Figure 1.
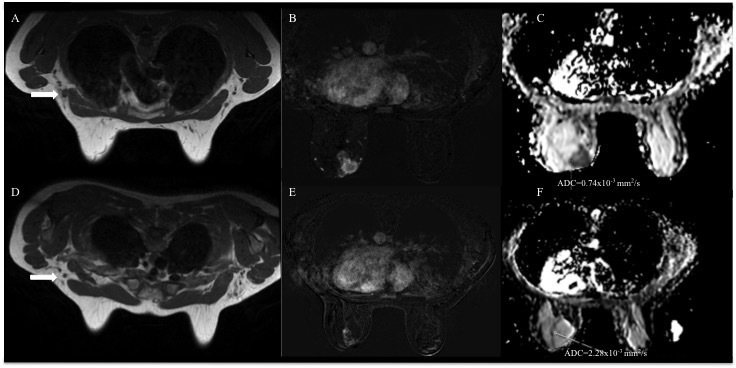
Representative images from baseline MRI (A-C) and post-neoadjuvant q chemotherapy (NAC) MRI (D-F) of a 47-year-old female with clinical T2N + stage Grade 2 invasive ductal carcinoma in the left breast, which was hormone receptor and human epidermal growth factor receptor two positive. Axial T1 weighted images from breast MRI (A, D); axial fat-saturated post-contrast subtracted T1 weighted images (B,E); and axial ADC maps (C, F). Before NAC, a 5 cm mass enhancement in the left breast with enlarged left axillary lymph nodes (arrow in A) was demonstrated. Breast tumour baseline ADC-value was 0.74 × 10−3 mm2/s (C). After treatment with docetaxel, carboplatin, trastuzumab and pertuzumab, the mass size was slightly reduced (E) and the lymph nodes decreased in size (arrow in D). Tumour ADC increased to 2.28 × 10−3 mm2/s (F). After breast conserving surgery axillary lymph node dissection, pathologic stage was ypT2ypN0. ADC, apparent diffusion coefficient; NAC, neoadjuvant chemotherapy.
The optimal threshold of ΔADC for differentiating ypN0 and ypN +was determined by maximizing the Youden index, and the obtained value was 0.92 × 10−3 mm2/s. Among the tumours that demonstrated a ΔADC above the cut-off value, 42.8% (15/35) showed axillary pCR. Eight (20.5%) breast cancers with ΔADC < 0.92 × 10−3 mm2/s were found to have no metastatic nodes after NAC (Table 2). The difference was statistically significant (p = 0.038). The ΔADC demonstrated an AUC 0.64 (95% confidence interval, CI: 0.50–0.77) (Figure 2).
Table 2.
Optimal threshold of the increase in breast tumour ADC-values after neoadjuvant chemotherapy for differentiating ypN0 and ypN+, determined by maximizing the Youden index
| Total | Axillary pCR | P-value | |
|---|---|---|---|
| ΔADC < 0.92 | 39 | 8 (20.5) | 0.038 |
| ΔADC > 0.92 | 35 | 15 (42.8) |
ADC, apparent diffusion coefficient.
Data are the number of patients, with percentages in parentheses. p-value of significant difference by χ2 test.
ADC = increase in the breast tumour ADC-values after neoadjuvant chemotherapy; pCR = pathological complete response; ypN0 = axillary complete response after neoadjuvant chemotherapy; ypN += residual axillary metastasis after neoadjuvant chemotherapy.
Figure 2.
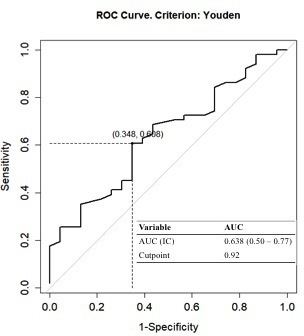
ROC curves depicting sensitivity and specificity of the increase in breast tumour ADC-values after neoadjuvant chemotherapy for evaluating axillary pathological complete response. ADC, apparent diffusion coefficient; ROC, receiver operating characteristic.
Discussion
Currently, many patients with axillary LN metastasis before NAC undergo ALND, a surgical technique associated with complications that may last a long time or become permanent, impairing the quality of life of breast cancer survivors (lymphedema, arm pain, reduced arm movement...).13
To identify patients with axillary pCR after NAC, who can avoid ALND, surgeons use the SLNB, that has demonstrated to have an acceptable false negative rate if two or more SLNs are detected, a dual-tracer is used for mapping, and if the axillary LN initially identified as being a nodal metastasis is removed.2–5,14 However, the accuracy of the SLNB and targeted node excision can be improved if we add non-invasive predicting factors based on imaging.
Several published articles describe predictive models to identify patients suitable for SLNB after NAC. Our results are consistent with theirs, and axillary response to NAC was correlated with negative oestrogen receptor status, Her2 positivity and pCR of primary tumour.10 Although our results did not reach statistical significance, there was a trend for a higher percentage of high Ki-67 expression, which indicates a higher level of cell proliferation, among responders.
Some previous studies demonstrate that the tumour response rate is one of the most significant independent predictors of axillary pCR in response to NAC.10 To assess the tumour response, they use only the lesion diameter measured on MRI, which has been shown to be the proper imaging modality to monitor response to treatment.6 However, cytotoxic effects of chemotherapy (apoptosis, cell lysis), in addition to reduce the breast lesion size, also increase the diffusion of water inside the tumour, which is reflected in an increase of ADC-values.
Our results suggest that the performance of models predicting axillary response to NAC can be improved by adding the tumour response determined also using DWI. In our study, an increase in tumour ADC-value of >0.92 ×10−3 mm2/s yielded the highest accuracy in predicting axillary pCR. The diagnostic accuracy of ΔADC-values in discriminating patient’s axillary pCR from residual axillary nodes metastasis revealed an AUC of 0.64 (95% CI: 0.50–0.77). The small sample size might be a contributing factor to this poor value.
The value DWI in predicting axillary response after NAC was previously investigated by Belli et al.15 They took a different approach, as they measured the ADC-values of the nodes before and after treatment, concluding that DWI has a potential role in identifying residual axillary macrometastases after chemotherapy.
The statistical power of the current study is limited by the small sample size. Due to these weakness, a multivariate regression analysis was unable to be performed. We could not compare the ADC-values between those females with pCR of the breast tumour or axillary lymph nodes only, pCR of both breast and axilla and non-pCR. Besides, we were not able to stratify the patients according to molecular subtype. Another major limitation is the retrospective design.
Conclusion
In conclusion, although our results did not reach statistical significance, this study suggests that the accurate prediction of axillary nodal status after NAC may be improved by using the ADC-values, along with tumour size reduction, to assess the BC response to NAC. We recommend this parameter to be included in the predictive models of future prospective studies.
Contributor Information
Lucia Graña-López, Email: Lucia.Grana.Lopez@sergas.es.
Tania Pérez-Ramos, Email: tania.perez.ramos@sergas.es.
Fiz Andrés Maciñeira, Email: Fiz.Andres.Macineira.Bertran.Lis@sergas.es.
Ángeles Villares, Email: angeles.villares.armas@sergas.es.
Manuel Vázquez-Caruncho, Email: manuel.vazquez.caruncho@sergas.es.
REFERENCES
- 1.Montagna G, Mamtani A, Knezevic A, Brogi E, Barrio AV, Morrow M. Selecting node-positive patients for axillary downstaging with neoadjuvant chemotherapy. Ann Surg Oncol 2020; 27: 4515–22. doi: 10.1245/s10434-020-08650-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boughey JC, McCall LM, Ballman KV, Mittendorf EA, Ahrendt GM, Wilke LG, et al. Tumor biology correlates with rates of breast-conserving surgery and pathologic complete response after neoadjuvant chemotherapy for breast cancer: findings from the ACOSOG Z1071 (Alliance) prospective multicenter clinical trial. Ann Surg 2014; 260: 608–14. doi: 10.1097/SLA.0000000000000924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Classe J-M, Loaec C, Gimbergues P, Alran S, de Lara CT, Dupre PF, et al. Sentinel lymph node biopsy without axillary lymphadenectomy after neoadjuvant chemotherapy is accurate and safe for selected patients: the GANEA 2 study. Breast Cancer Res Treat 2019; 173: 343–52. doi: 10.1007/s10549-018-5004-7 [DOI] [PubMed] [Google Scholar]
- 4.Kuehn T, Bauerfeind I, Fehm T, Fleige B, Hausschild M, Helms G, et al. Sentinel-lymph-node biopsy in patients with breast cancer before and after neoadjuvant chemotherapy (SENTINA): a prospective, multicentre cohort study. Lancet Oncol 2013; 14: 609–18. doi: 10.1016/S1470-2045(13)70166-9 [DOI] [PubMed] [Google Scholar]
- 5.Boileau J-F, Poirier B, Basik M, Holloway CMB, Gaboury L, Sideris L, et al. Sentinel node biopsy after neoadjuvant chemotherapy in biopsy-proven node-positive breast cancer: the Sn FNAC study. J Clin Oncol 2015; 33: 258–64. doi: 10.1200/JCO.2014.55.7827 [DOI] [PubMed] [Google Scholar]
- 6. De Los Santos JF, Cantor A, Amos KD, Forero A, Golshan M, Horton JK, et al. Magnetic resonance imaging as a predictor of pathologic response in patients treated with neoadjuvant systemic treatment for operable breast cancer. Translational Breast Cancer Research Consortium trial 017. Cancer 2013; 119: 1776–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Abdel Razek AAK, Gaballa G, Denewer A, Tawakol I. Diffusion weighted MR imaging of the breast. Acad Radiol 2010; 17: 382–6. doi: 10.1016/j.acra.2009.10.014 [DOI] [PubMed] [Google Scholar]
- 8.Padhani AR, Liu G, Koh DM, Chenevert TL, Thoeny HC, Takahara T, et al. Diffusion-Weighted magnetic resonance imaging as a cancer biomarker: consensus and recommendations. Neoplasia 2009; 11: 102–25. doi: 10.1593/neo.81328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schipper RJ, Moossdorff M, Beets-Tan RGH, Smidt ML, Lobbes MBI. Noninvasive nodal restaging in clinically node positive breast cancer patients after neoadjuvant systemic therapy: a systematic review. Eur J Radiol 2015; 84: 41–7. doi: 10.1016/j.ejrad.2014.09.020 [DOI] [PubMed] [Google Scholar]
- 10.Kim R, Chang JM, Lee H-B, Lee SH, Kim S-Y, Kim ES, et al. Predicting axillary response to neoadjuvant chemotherapy: breast MRI and US in patients with node-positive breast cancer. Radiology 2019; 293: 49–57. doi: 10.1148/radiol.2019190014 [DOI] [PubMed] [Google Scholar]
- 11.- Baltzer P, Mann RM, Iima M, et al. EUSOBI International breast diffusion-weighted imaging Working Group. diffusion-weighted imaging of the breast-a consensus and mission statement from the EUSOBI International breast diffusion-weighted imaging Working group. Eur Radiol 2020; 30: 1436–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.- D`Orsi CJ, Sickles EA, Mendelson EB, Morris EA. et al.ACR BI-RADS Atlas. Breast Imaging Reporting and Data System. Reston, Va: American College of Radiology; 2013. [Google Scholar]
- 13.Lucci A, McCall LM, Beitsch PD, Whitworth PW, Reintgen DS, Blumencranz PW, et al. Surgical complications associated with sentinel lymph node dissection (SLND) plus axillary lymph node dissection compared with SLND alone in the American College of surgeons Oncology Group trial Z0011. J Clin Oncol 2007; 25: 3657–63. doi: 10.1200/JCO.2006.07.4062 [DOI] [PubMed] [Google Scholar]
- 14.Caudle AS, Yang WT, Krishnamurthy S, Mittendorf EA, Black DM, Gilcrease MZ, et al. Improved axillary evaluation following neoadjuvant therapy for patients with node-positive breast cancer using selective evaluation of clipped nodes: implementation of targeted axillary dissection. J Clin Oncol 2016; 34: 1072–8. doi: 10.1200/JCO.2015.64.0094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Belli P, Bufi E, Buccheri C, Rinaldi P, Giuliani M, Romani M, et al. Role of DWI assessing nodal involvement and response to neoadjuvant chemotherapy in advanced breast cancer. Eur Rev Med Pharmacol Sci 2017; 21: 695–705. [PubMed] [Google Scholar]


