Abstract
Objectives:
To evaluate the effects of combat sports on cerebellar function in adolescents based on resting-state functional MRI (rs-fMRI).
Methods:
Rs-fMRI data were acquired from the combat sports (CS) group (n = 32, aged 14.2 ± 1.1 years) and non-athlete healthy control (HC) group (n = 29, aged 14.8 ± 0.9 years). The amplitude of low-frequency fluctuation (ALFF), regional homogeneity (ReHo), and functional connectivity (FC) within the cerebellum was calculated and then compared between the two groups.
Results:
None of these participants displayed intracranial lesions on conventional MRI and microhemorrhages on SWI. Compared with the HC group, the CS group showed decreased ALFF and ReHo in the bilateral cerebellum, mainly located in the inferior regions of the cerebellum (Cerebellum_8, Cerebellum_9, Cerebellum_7b, and Cerebellum_Crus2). While increased FC was found within the cerebellar network, mainly located in the superior regions near the midline (bilateral Cerebellum_6, Cerebellum_Crus1_R, and Vermis_6). There is no internetwork FC change between the CEN and other networks.
Conclusion:
This study confirmed extensive effects of combat sports on cerebellar rs-fMRI in adolescents, which could enhance the understanding of cerebellar regulatory mechanism under combat conditions, and provide additional information about cerebellar protective inhibition and compensatory adaptation.
Advances in knowledge:
Adolescent combat participants are an ideal model to study training-induced brain plasticity and vulnerability. Relative to task-related fMRI, rs-fMRI can bring more information about cerebellar regulation and explain the Central Governor Model more comprehensively.
Introduction
The sports types and exercise intensities modulate the brain differently, resulting in various physiological responses and behavioral outputs. Low-intensity exercise is related to a stable physiological state, while high-intensity exercise can bring about some significant metabolic disorders and additional neuromodulations.1 The Central Governor Model is a mature exercise physiological model, which emphasizes that the brain maintains homeostasis and prevents physiological failure by regulating afferent feedback and anticipatory feedforward.2 The cerebellum is an important motor regulatory center in the Central Governor Model, which controls postural balance, movement coordination, and motor learning by integrating motor-related information.3 At present, most sports-related fMRI studies focus on supratentorial regions, but cerebellar regulations have not been studied systematically. For sports-related concussions, resting-state fMRI (rs-fMRI) showed local deactivation or disrupted functional connectivity (FC) within the cerebellar network (CEN).4,5 Besides, the resting-state connections between the dentate nucleus and cerebellar cortex could be also altered by physical exercise.6 While task-related fMRI studies have shown that cerebellar activation was associated with the exercise workload and performance.7,8 As the exercise intensity increased, cerebellar activation would be terminated.9 Besides, the complex motor plan could activate the lobules IV-VII.10 However, most of these studies were limited to a single task or simple movement. It is difficult to reflect the actual situation of combat sports. As a heavy competitive project, combat sports have the higher demands on the confrontational intensity and exercise load to complete some complex skills.11 It is still unclear how the cerebellum is involved in motor control under combat conditions. Therefore, we hypothesize that combat sports may affect the cerebellar resting-state function in adolescence. Furthermore, we investigate whether combat sports also have negative effects on cerebellar rs-fMRI, just like the task-related fMRI at high exercise intensities.7–9 Rs-fMRI analysis can be divided into functional segregation and functional integration, which provide functional information from different aspects.12 The functional segmentation mainly includes an amplitude of low-frequency fluctuation (ALFF) and regional homogeneity (ReHo), focusing on specific functional locations and local neural activation. Functional integration mainly refers to functional connectivity (FC), focusing on the functional coordination and correlation between different brain regions. The complexity of brain function can not be explained by a single theory. Combining functional segmentation and functional integration can provide more complementary information to evaluate the effects of combat sports on brain function. Therefore, we test our hypotheses by analyzing ALFF, ReHo, and independent component analysis (ICA)-based FC in this study.
Methods and materials
Participants
This study was approved by the ethics committee of our hospital, and written informed consent of each participant’s legal guardian was obtained. Thirty-two healthy participants from a sports school were enrolled in the combat sports (CS) group (30 males, 2 females; age range, 13–16 years old; height range, 155–170 cm; weight range, 49–59 kg; years of education, 6–8 years), including Judo (18 cases) and wrestling (14 cases). In this sports school, they have been trained for more than 24 months (time range, 26–39 months; meantime, 34.1 ± 2.5 months), with a training frequency of four hours per day, five days per week (20 h per week). Twenty-nine non-athlete healthy participants from an ordinary middle school matched for age, sex, height, weight, and years of education, were enrolled in the healthy control (HC) group. The Montreal Cognitive Assessment (MoCa) and Rivermead Post-Concussion Symptoms Questionnaire (RPSQ) were completed by two professional neurologists for all participants to exclude possible cognitive impairment or concussion. The inclusion criteria were as followed: healthy adolescents without a concussion or cognitive impairments (13–16 years old, MoCa scores > 26, RPSQ scores = 0); more than 24 months of training (for the CS group); without any contact sports experience (for the HC group), right-handed. The exclusion criteria included a history of brain injury, neuropsychological or neurological disorders, MRI contraindications, excessive head motion (translation >3 mm, or rotation >3°), intracranial lesions on conventional MRI, and microhemorrhage on susceptibility-weighted imaging (SWI).
MRI acquisition
The MRI data were obtained on a 3.0T scanner (GE Discovery MR750, Milwaukee, USA) equipped with an eight-channel-phased array head coil. Rs-fMRI data were acquired using gradient-echo-planar imaging sequences: repetition time (TR) = 2000 ms, echo time (TE) = 30 ms, flip angle (FA) = 90°, matrix = 64×64, field of view (FOV) = 240×240 mm2, slice thickness = 3 mm, slice gap = 0 mm, slice number = 40. A total of 240 volumes were collected from each participant. The functional scans took 8 min. Conventional cranial MRI scans were performed on all participants to exclude intracranial lesions. SWI sequences (TR/TE = 27 ms/20 ms, FOV = 230 × 230 mm2, matrix = 256 × 256, slice thickness = 2.5 mm, slice gap = 0 mm, slice number = 48, FA = 15°) were implemented to detect microhemorrhage. The participants should stay awake, eyes closed, without actively moving and thinking. The earplugs and sponge cushions were used to avoid noise and head movement.
Data preprocessing and analysis
Resting-State fMRI Data Analysis Toolbox plus V. 1.23 (RESTplus V1.23, http://www.restfmri.net/forum/RESTplus) was applied for data preprocessing. The first 10 volumes were removed from each time series to ensure the participants’ adaptation and magnetized equilibrium. The remaining images were slice-timing corrected and head motion calibrated. Considering that teenagers were very active and their head movements were difficult to control, the data with head movement >3.0 mm translation (x, y, z) or >3.0° rotation (pitch, roll, yaw) were discarded.13 Four subjects had been excluded before the analysis. Then, the functional images were spatially normalized to a standard EPI template from the Montreal Neurological Institute (MNI) stereotaxic space (resampled voxel size = 3×3×3 mm3). Spatial smoothing was conducted for ALFF and FC (but not for ReHo), with a Gaussian kernel of 6 mm full-width at half-maximum (FWHM). The linear trend and nuisance covariates (six head motion parameters, white matter signal, and cerebrospinal fluid signal) were regressed out. Band-pass (0.01–0.08 Hz) filtering was used to ensure those meaningful spontaneous neural activities. For this study, the subsequent analysis was confined to the cerebellum.
ALFF calculation
ALFF and ReHo calculations were performed by the RESTplus (V1.23). After data preprocessing, a fast Fourier transform (FFT) was used to transform the filtered time series of each voxel into a frequency domain, and then, a power spectrum was obtained. For the original time series, the power of a given frequency is proportional to the square of its amplitude. Therefore, the square root was calculated at each frequency of the power spectrum and averaged across 0.01–0.08 Hz at each voxel. This averaged square root was taken as the ALFF.14 Finally, the ALFF of each voxel was divided by the global average ALFF value to achieve standardization.
ReHo calculation
Kendall Coefficient of Concordance (KCC) is a correlation metric to calculate the correlative degree of multiple grade variables. It can measure the similarity of time series to purify a given functional cluster and reveal the functional complexity to some extent. ReHo objectively reflects the functional consistency of local spontaneous activation through KCC. KCC values were generated by computing the consistency of the time series between a given voxel and its nearest 26 neighbors.15 The individual ReHo maps were obtained by calculating individual KCC values within the whole brain voxels. Then, these individual KCC values were divided by their own global average KCC for normalization to reduce the influence of individual variation within the whole-brain mask. Finally, a spatial smooth with a Gaussian kernel of 6 mm FWHM was conducted on these standardized ReHo maps. Spatial smoothing after ReHo calculation can avoid artificially increasing local similarity of original time series, and appropriately improve the spatial signal to noise ratio and interindividual matching degree.
ICA-based FC
After slice-timing correction, head motion calibration, spatial normalization, and smoothing, these preprocessed images were used for ICA-based FC. ICA was performed by the Group ICA of fMRI Toolbox (GIFT, V3.0b, http://mialab.mrn.org/software/gift/index.html), which contained data reduction, application of the ICA algorithm, and back-reconstruction for each subject.16 The data dimensionality reduction was performed by the principal component analysis (PCA). The minimum description length (MDL) was used to determine the number of independent components (ICs). IC separation was completed by the infomax algorithm in ICASSO. Finally, back-reconstruction was performed to obtain these spatial maps and corresponding time series. Thus, IC time courses and spatial maps were acquired for each participant. The participant-specific maps were converted to Z-scores on an average spatial component of each group. In this study, 19 meaningful ICs were classified into eight functional networks. Through visual inspection of aggregate spatial maps and average power spectra, the CEN was selected by reaching a consensus among three experienced neuroradiologists who reviewed these IC maps separately.17,18
Statistical analysis
Demographic and neuropsychological findings were compared between the two groups by IBM SPSS v. 22.0 (SPSS, Chicago, IL, USA). The independent t-test or chi-square test was used for these continuous or dichotomous variables, respectively. p < 0.05 was defined as statistically significant. For ALFF, ReHo, and intranetwork FC analyses, comparisons between the two groups were performed using the two-sample t-tests by SPM 12 (statistical parametric mapping, http://www.fil.ion.ucl.ac.uk/spm/). The between-group effects were thresholded at p < 0.05, using false discovery rate (FDR, voxel-wise) correction. Age, sex, height, weight, and years of education were used as covariates. For FC within CEN, a single-sample t-test was firstly used to obtain the z-maps for both groups by SPM 12 to determine a union mask. Then, a between-group comparison of the z-maps of CEN was conducted by a two-sample t-test within the union mask. The significant threshold for single- and two-sample t-tests were set at p < 0.05, corrected by FDR correction. To evaluate intragroup differences of ALFF, ReHo, and intranetwork FC within the CS group, two-sample t-tests were also performed between Judo and wrestling participants by SPM 12. In the CS group, Pearson correlation was analyzed between these functional indicators and training time by SPSS v. 22.0. The brain regions with a significant between-group difference were selected as the regions of interest to extract ALFF value, ReHo value, and mean z-scores. Then, correlation analyses were performed between them and training time.
Results
Participants and basic data
There were no significant demographic and neuropsychological differences between the two groups, achieving an intergroup matching. The detailed demographic and neuropsychological information of the two groups was shown in Table 1. None of these participants displayed intracranial lesions on conventional MRI and microhemorrhages on SWI. There was no significant difference in ALFF, ReHo, and intranetwork FC between Judo and wrestling participants in the CS group. The changed ALFF, ReHo, and intranetwork FC in the CS group did not have any significant correlation with training time.
Table 1.
Demographic and neuropsychological findings of both groups
| Demographic and clinical findings | CS group | HC group | P |
|---|---|---|---|
| Age (years) | 14.2 ± 1.1 | 14.8 ± 0.9 | 0.523 |
| Sex (Male/Female) | 30/2 | 28/1 | 0.802 |
| Height (cm) | 164.65 ± 4.35 | 164.31 ± 3.94 | 0.691 |
| Weight (kg) | 55.63 ± 1.6 | 54.82 ± 2.2 | 0.665 |
| Years of education | 7.5 ± 0.4 | 7.6 ± 0.3 | 0.899 |
| MoCa scores | 28.85 ± 0.81 | 28.92 ± 0.85 | 0.725 |
| RPSQ scores | 0 | 0 | >0.99 |
CS, combat sports; HC, healthy control; MoCa, Montreal Cognitive Assessment; RPSQ, Rivermead Post-concussion Symptoms Questionnaire.
Values are given as n or mean ± SD.
ALFF
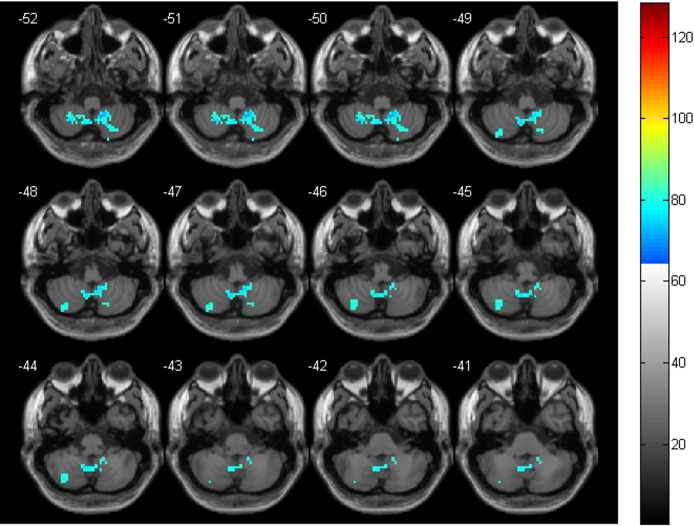
Compared with the HC group, the CS group only demonstrated decreased ALFF in the bilateral cerebellum. All of them were located in the inferior cerebellum (bilateral Cerebellum_8 and Cerebellum_9). See Table 2 and Figure 1 for details.
Table 2.
Between-group differences of ALFF, ReHo, and FC in the cerebellum
| AAL brain regions | Peak MNI coordinate (x,y,z) | t value | Number of voxels | |
|---|---|---|---|---|
| ALFF | Cerebellum_9_R, Cerebellum_9_L, Cerebellum_8_R, Cerebellum_8_L |
12 −51–41 | −6.4411 | 158 |
| ReHo | Cerebellum_8_R, Cerebellum_8_L, Cerebellum_9_R, Cerebellum_9_L, Cerebellum_7b_R, Cerebellum_7b_L, Cerebellum_Crus2_R, Cerebellum_Crus2_L, Cerebellum_Crus1_R |
−18–33 −60 | −9.9404 | 679 |
| FC | Cerebellum_Crus2_L | −24–84 −36 | 4.757 | 46 |
| Cerebellum_6_R, Cerebellum_6_L, Vermis_6, Cerebellum_Crus1_R |
−6–48 −30 | 5.7766 | 420 |
AAL, Anatomical Automatic Labeling; ALFF, the amplitude of low-frequency fluctuation; FC, functional connectivity; MNI, Montreal Neurological Institute;ReHo, regional homogeneity.
CS - HC, p < 0.05, FDR correction.
Figure 1.
The significant ALFF differences in cerebellum between the two groups. The cool colors indicate the brain areas with reduced ALFF in the CS group, compared with the HC group (p < 0.05, FDR correction)
ReHo
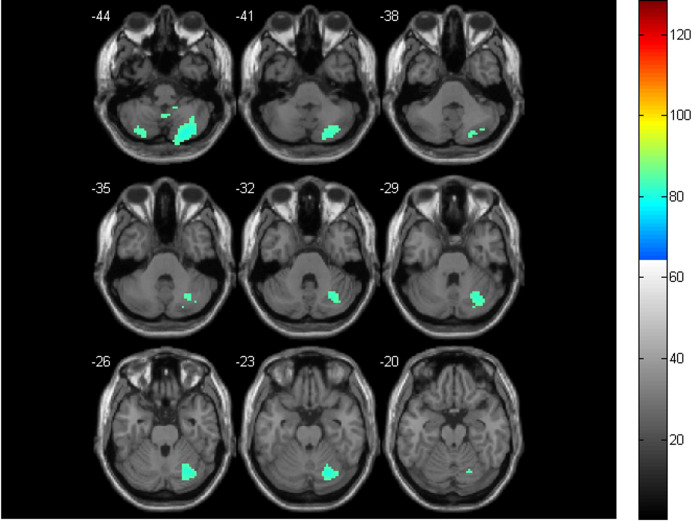
Relative to the HC group, the CS group only showed decreased ReHo in the bilateral cerebellum. Most of them were located in the inferior cerebellum (bilateral Cerebellum_8, Cerebellum_9, Cerebellum_Crus2, and Cerebellum_7b). The others were located in the superior cerebellum (Cerebellum_Crus1_R). See Table 2 and Figure 2 for details.
Figure 2.
The significant ReHo differences in cerebellum between the two groups. The cool colors indicate the brain areas with reduced ReHo in the CS group, relative to the HC group (p < 0.05, FDR correction)
FC within CEN
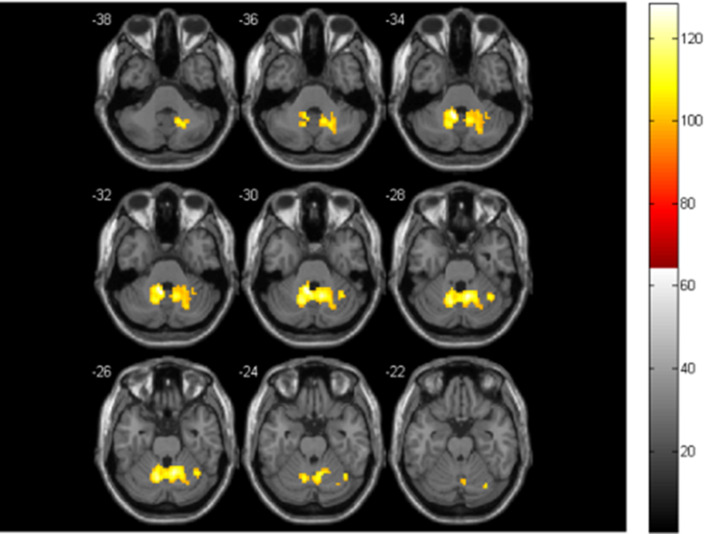
Compared with the HC group, the CS group only exhibited increased FC in the CEN. Most of them were located in the superior cerebellum (bilateral Cerebellum_6, Cerebellum_Crus1_R, and Vermis_6). The rest of them were located in the inferior cerebellum (Cerebellum_Crus2_L). Most of them were found near the midline. See Table 2 and Figure 3 for details. Besides, there was no significant change in FC between CEN and other functional networks in the CS group.
Figure 3.
The significant FC differences within the CEN between the two groups. The warm colors represent the brain areas with increased FC in the CS group, compared with the HC group (p < 0.05, FDR correction)
Discussion
To evaluate intragroup differences of ALFF, ReHo, and intranetwork FC in the CS group, we performed two-sample t-tests on judo and wrestling participants, which showed no significant difference. In the CS group, wrestling and judo participants have similar exercising properties. Their similar combating nature, training time, and exercise intensity result in no significant difference in these functional indicators. In the current study, the differences of cerebellar rs-fMRI between the two groups were distributed bilaterally and extensively. It was different from those local changes caused by injury or disease, which were mostly confined to some susceptible brain regions. All participants in the CS group were healthy adolescents without intracranial lesions and microhemorrhages, whose motor-related functions were more balanced and comprehensive after professional training.19 However, these fMRI changes did not correlate with training time. Most of the combat participants were from the same grade, with similar training time and exercise load. Previous task-related fMRI studies revealed that cerebellar activation could be terminated during high-intensity exercises, but without decreased activation.9 While in this rs-fMRI study, compared with the HC group, the CS group showed not only decreased ALFF and ReHo but also increased FC within CEN. Maybe these two fMRI methods are essentially different. Task-related fMRI is a complex block design and event-related design, reflecting the quick and immediate influences on the brain, with a functional directivity. While rs-fMRI is a simple, continuous, and task-free design, focusing more on the accumulative and remodeling effects, with a spontaneous regularity. Furthermore, task-related fMRI is greatly affected by task design and is difficult for baseline control, while rs-fMRI is an indirect reflection of functional state and susceptible to various physiological and physical noises.12,20
ALFF and ReHo are voxel-based analytic procedures for local activation, which belong to functional segmentation. ALFF measures spontaneous activity in the resting state from an energy perspective, which is the basis of functional coordination.14,21 ReHo obtains the synchronization of time series between a given voxel and its nearest 26 voxels through Kendall efficient of concordance (KCC), which is used to evaluate the regional consistency and local centrality of brain function.15,22 These two simple and repeatable methods do not need a priori hypothesis and have become the important indicators of rs-fMRI. The decreased ALFF and ReHo indicated the reduced spontaneous activation and regional synchronization, respectively.21,22 Besides, this study also found that decreased ALFF and ReHo were overlapped in bilateral inferior regions (Cerebellum_8–9, correlated to the complex action and motor perception). It suggested that combat sports deactivated a larger group of neighboring neurons at the same time-frequency to complete a functional regulation in these regions.12,22 The combination of ReHo and ALFF could provide complementary information about neuronal metabolism.12 The purpose of professional training is to make players get suprathreshold stimuli and continuously provoke athletic potential. But this improvement is not endless.23 The high-intensity confrontation and accompanying physiological instability could promote the cerebellum to compare executive intention with the actual physical state.22,24 As an important motor regulatory center, the cerebellum can break the cerebral motor commands through local protective inhibition (decreased ALFF and ReHo) to prevent excessive exercise and physiological failure.25 This is in line with the Central Governor Model. In the CS group, decreased cerebellar ALFF and ReHo did not mean the weakened function in the conventional sense, but a self-adjustment of cerebellar function under high-intensity combat conditions. Therefore, these symmetrically reduced activations should not be confused with the pathological manifestations of combat-related brain injury to avoid excessive medical intervention.
Notably, in the CS group, decreased ALFF and ReHo were accompanied by increased FC within CEN. Increased FC reflects the increased temporal dependency between spatially distant neuronal activities, meaning enhanced informational transmission and integration.26 Moreover, it was distributed with some regularities. Decreased ALFF and ReHo were mostly in the inferior cerebellum, while increased FC was near the midline of the superior cerebellum. The cerebellar subregions are responsible for different motor functions, and the key regions are located medially.27 Decreased ALFF and ReHo in the Cerebellum_8–9 subregions might weaken complex limb movements and motor perception. While increased FC near the midline of the cerebellum could strengthen the body balance and motor coordination.28 This is consistent with the functional compensatory theory to maintain a basic state.29 Furthermore, the Cerebellum_6, Cerebellum_Crus1, and Crus2 are very important in motor learning.30 Compared with the HC group, complex combat training can enhance adaptive learning, increasing the FC within these regions. This also suggests the higher neuroplasticity in adolescents.31,32 Previous studies have found that long-term physical training can cause increased FC among cerebellar, motor, sensory, and advanced cognitive networks.33 However, our results did not show such a tendency. For young combat athletes, the cerebellar function is more inclined to the traditional motor control, through the cerebellar regulation to intervene with the cerebral motor management.20 Furthermore, the adolescents in the CS group are in the initial stage of motor learning and have not yet formed large-scale functional interactions between the cerebellar network and others.30
The head can be impacted repeatedly in combat sports, which brings the potential risk of subconcussion, especially for the immature brain in adolescence.34,35 However, the subconcussion is a vague subclinical state with a history of repeated head hits, but without obvious clinical symptoms or signs.36 Therefore, using this concept to interpret these functional alterations between the two groups should be cautious. Besides, the instability of brain functional development in adolescents may be another reason.35,37 A longitudinal study is necessary. Limited by experimental conditions, we could not quantify the confrontational intensity of combat training, only detecting its general effects on rs-fMRI. Participants in this study were all healthy adolescents without clinical abnormal tendencies. MoCa and RPSQ were only for the preliminary behavioral-psychological evaluation. There were fewer female participants in this study. It is in line with the actual participation of combat sports, although the influence of sex differences on brain function does exist.38
Conclusion
In conclusion, combat sports may affect cerebellar function extensively. Relative to task-related fMRI (terminated activation at high exercise intensities), rs-fMRI can bring more information about cerebellar regulation and explain the Central Governor Model more comprehensively. Combat sports have similar effects on cerebellar rs-fMRI (decreased ALFF and ReHo) and may cause compensatory functional changes (increased FC within the CEN). According to the Central Governor Model and functional compensatory theory, these functional changes should be cerebellar adaptation and compensation to combat training. Although there are no functional interactions between the CEN and other networks, large-scale structural covariance networks may be affected by confrontational sports. Future research should focus on long-term longitudinal observation, increase in sample size, and refinement of exercise types, and apply more sophisticated data analysis to dynamically observe the effects of combat sports on adolescent brain function.
Contributor Information
Wei Li, Email: liweiqd830127@163.com.
Xin Kong, Email: 874807413@qq.com.
Jun Ma, Email: 15106566170@163.com.
REFERENCES
- 1. Liu JZ, Shan ZY, Zhang LD, Sahgal V, Brown RW, Yue GH, et al. Human brain activation during sustained and intermittent submaximal fatigue muscle contractions: an fmri study. J Neurophysiol 2003; 90: 300–312. doi: 10.1152/jn.00821.2002 [DOI] [PubMed] [Google Scholar]
- 2. Lambert EV, St Clair Gibson A, Noakes TD. Complex systems model of fatigue: integrative homoeostatic control of peripheral physiological systems during exercise in humans. Br J Sports Med 2005; 39: 52–62. doi: 10.1136/bjsm.2003.011247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Buckner RL, Krienen FM, Castellanos A, Diaz JC, Yeo BTT, et al. The organization of the human cerebellum estimated by intrinsic functional connectivity. J Neurophysiol 2011; 106: 2322–45. doi: 10.1152/jn.00339.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Palacios EM, Sala-Llonch R, Junque C, Roig T, Tormos JM, Bargallo N, et al. Resting-state functional magnetic resonance imaging activity and connectivity and cognitive outcome in traumatic brain injury. JAMA Neurol 2013; 70: 845–51. doi: 10.1001/jamaneurol.2013.38 [DOI] [PubMed] [Google Scholar]
- 5. Li F, Lu L, Shang S, Hu L, Chen H, Wang P, et al. Disrupted functional network connectivity predicts cognitive impairment after acute mild traumatic brain injury. CNS Neurosci Ther 2020; 26: 1083–91. doi: 10.1111/cns.13430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Xu-Wilson M, Chen-Harris H, Zee DS, Shadmehr R, et al. Cerebellar contributions to adaptive control of saccades in humans. J Neurosci 2009; 29: 12930–39. doi: 10.1523/JNEUROSCI.3115-09.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Alahyane N, Fonteille V, Urquizar C, Salemme R, Nighoghossian N, Pelisson D, et al. Separate neural substrates in the human cerebellum for sensory-motor adaptation of reactive and of scanning voluntary saccades. Cerebellum 2008; 7: 595–601. doi: 10.1007/s12311-008-0065-5 [DOI] [PubMed] [Google Scholar]
- 8. Manto M, Bower JM, Conforto AB, Delgado-García JM, da Guarda SNF, Gerwig M, et al. Consensus paper: roles of the cerebellum in motor control--the diversity of ideas on cerebellar involvement in movement. Cerebellum 2012; 11: 457–87. doi: 10.1007/s12311-011-0331-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Fontes EB, Bortolotti H, Grandjean da Costa K, Machado de Campos B, Castanho GK, Hohl R, et al. Modulation of cortical and subcortical brain areas at low and high exercise intensities. Br J Sports Med 2020; 54: 110–15. doi: 10.1136/bjsports-2018-100295 [DOI] [PubMed] [Google Scholar]
- 10. Chan RCK, Huang J, Di X. Dexterous movement complexity and cerebellar activation: a meta-analysis. Brain Res Rev 2009; 59: 316–23. doi: 10.1016/j.brainresrev.2008.09.003 [DOI] [PubMed] [Google Scholar]
- 11. Verhagen E, van Stralen MM, van Mechelen W. Behaviour, the key factor for sports injury prevention. Sports Med 2010; 40: 899–906. doi: 10.2165/11536890-000000000-00000 [DOI] [PubMed] [Google Scholar]
- 12. Lv H, Wang Z, Tong E, Williams LM, Zaharchuk G, Zeineh M, et al. Resting-state functional mri: everything that nonexperts have always wanted to know. AJNR Am J Neuroradiol 2018; 39: 1390–99. doi: 10.3174/ajnr.A5527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dinomais M, Lignon G, Chinier E, Richard I, Ter Minassian A, Tich SNT, et al. Effect of observation of simple hand movement on brain activations in patients with unilateral cerebral palsy: an fmri study. Res Dev Disabil 2013; 34: 1928–37. doi: 10.1016/j.ridd.2013.03.020 [DOI] [PubMed] [Google Scholar]
- 14. Zang Y-F, He Y, Zhu C-Z, Cao Q-J, Sui M-Q, Liang M, et al. Altered baseline brain activity in children with adhd revealed by resting-state functional mri. Brain Dev 2007; 29: 83–91. doi: 10.1016/j.braindev.2006.07.002 [DOI] [PubMed] [Google Scholar]
- 15. Zang Y, Jiang T, Lu Y, He Y, Tian L, et al. Regional homogeneity approach to fmri data analysis. Neuroimage 2004; 22: 394–400. doi: 10.1016/j.neuroimage.2003.12.030 [DOI] [PubMed] [Google Scholar]
- 16. Jafri MJ, Pearlson GD, Stevens M, Calhoun VD, et al. A method for functional network connectivity among spatially independent resting-state components in schizophrenia. Neuroimage 2008; 39: 1666–81. doi: 10.1016/j.neuroimage.2007.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Allen EA, Erhardt EB, Damaraju E, Gruner W, Segall JM, Silva RF, et al. A baseline for the multivariate comparison of resting-state networks. Front Syst Neurosci 2011; 5: 1–23. doi: 10.3389/fnsys.2011.00002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Luo C, Li Q, Lai Y, Xia Y, Qin Y, Liao W, et al. Altered functional connectivity in default mode network in absence epilepsy: a resting-state fmri study. Hum Brain Mapp 2011; 32: 438–49. doi: 10.1002/hbm.21034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Dietrich A. Transient hypofrontality as a mechanism for the psychological effects of exercise. Psychiatry Res 2006; 145: 79–83. doi: 10.1016/j.psychres.2005.07.033 [DOI] [PubMed] [Google Scholar]
- 20. Costigan SA, Eather N, Plotnikoff RC, Taaffe DR, Lubans DR, et al. High-intensity interval training for improving health-related fitness in adolescents: a systematic review and meta-analysis. Br J Sports Med 2015; 49: 1253–61. doi: 10.1136/bjsports-2014-094490 [DOI] [PubMed] [Google Scholar]
- 21. Zou Q-. H, Zhu C-. Z, Yang Y, Zuo X-. N, Long X-. Y, Cao Q-. J, et al. An improved approach to detection of amplitude of low-frequency fluctuation (alff) for resting-state fmri: fractional alff. J Neurosci Methods 2008; 172: 137–41. doi: 10.1016/j.jneumeth.2008.04.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Friston KJ. Modalities, modes, and models in functional neuroimaging. Science 2009; 326: 399–403. doi: 10.1126/science.1174521 [DOI] [PubMed] [Google Scholar]
- 23. Hilty L, Jäncke L, Luechinger R, Boutellier U, Lutz K, et al. Limitation of physical performance in a muscle fatiguing handgrip exercise is mediated by thalamo-insular activity. Hum Brain Mapp 2011; 32: 2151–60. doi: 10.1002/hbm.21177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Debaere F, Wenderoth N, Sunaert S, Van Hecke P, Swinnen SP, et al. Cerebellar and premotor function in bimanual coordination: parametric neural responses to spatiotemporal complexity and cycling frequency. Neuroimage 2004; 21: 1416–27. doi: 10.1016/j.neuroimage.2003.12.011 [DOI] [PubMed] [Google Scholar]
- 25. Fontes EB, Okano AH, De Guio F, Schabort EJ, Min LL, Basset FA, et al. Brain activity and perceived exertion during cycling exercise: an fmri study. Br J Sports Med 2015; 49: 556–60. doi: 10.1136/bjsports-2012-091924 [DOI] [PubMed] [Google Scholar]
- 26. Lee MH, Smyser CD, Shimony JS. Resting-state fmri: a review of methods and clinical applications. AJNR Am J Neuroradiol 2013; 34: 1866–72. doi: 10.3174/ajnr.A3263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Apps R, Hawkes R, Aoki S, Bengtsson F, Brown AM, Chen G, et al. Cerebellar modules and their role as operational cerebellar processing units: a consensus paper [corrected]. Cerebellum 2018; 17: 654–82. doi: 10.1007/s12311-018-0952-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Yang Z, Ye C, Bogovic JA, Carass A, Jedynak BM, Ying SH, et al. Automated cerebellar lobule segmentation with application to cerebellar structural analysis in cerebellar disease. Neuroimage 2016; 127: 435–44. doi: 10.1016/j.neuroimage.2015.09.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Noakes TD. Time to move beyond a brainless exercise physiology: the evidence for complex regulation of human exercise performance. Appl Physiol Nutr Metab 2011; 36: 23–35. doi: 10.1139/H10-082 [DOI] [PubMed] [Google Scholar]
- 30. Ito M. Mechanisms of motor learning in the cerebellum. Brain Res 2000; 886: 237–45. doi: 10.1016/s0006-8993(00)03142-5 [DOI] [PubMed] [Google Scholar]
- 31. Chan RCK, Rao H, Chen EEH, Ye B, Zhang C, et al. The neural basis of motor sequencing: an fmri study of healthy subjects. Neurosci Lett 2006; 398: 189–94. doi: 10.1016/j.neulet.2006.01.014 [DOI] [PubMed] [Google Scholar]
- 32. Riecker A, Gröschel K, Ackermann H, Steinbrink C, Witte O, Kastrup A, et al. Functional significance of age-related differences in motor activation patterns. Neuroimage 2006; 32: 1345–54. doi: 10.1016/j.neuroimage.2006.05.021 [DOI] [PubMed] [Google Scholar]
- 33. Habas C, Kamdar N, Nguyen D, Prater K, Beckmann CF, Menon V, et al. Distinct cerebellar contributions to intrinsic connectivity networks. J Neurosci 2009; 29: 8586–94. doi: 10.1523/JNEUROSCI.1868-09.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Martini D, Eckner J, Kutcher J, Broglio SP, et al. Subconcussive head impact biomechanics: comparing differing offensive schemes. Med Sci Sports Exerc 2013; 45: 755–61. doi: 10.1249/MSS.0b013e3182798758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Li W, Kong X, Zhanng Y, Luo Y, Ma J, et al. Effects of combat sports on functional network connectivity in adolescents. Neuroradiology 2021; 63: 1863–71. doi: 10.1007/s00234-021-02713-y [DOI] [PubMed] [Google Scholar]
- 36. Poole VN, Breedlove EL, Shenk TE, Abbas K, Robinson ME, Leverenz LJ, et al. Sub-concussive hit characteristics predict deviant brain metabolism in football athletes. Dev Neuropsychol 2015; 40: 12–17. doi: 10.1080/87565641.2014.984810 [DOI] [PubMed] [Google Scholar]
- 37. Giedd JN, Blumenthal J, Jeffries NO, Castellanos FX, Liu H, Zijdenbos A, et al. Brain development during childhood and adolescence: a longitudinal mri study. Nat Neurosci 1999; 2: 861–63. doi: 10.1038/13158 [DOI] [PubMed] [Google Scholar]
- 38. McCuen E, Svaldi D, Breedlove K, Kraz N, Cummiskey B, Breedlove EL, et al. Collegiate women’s soccer players suffer greater cumulative head impacts than their high school counterparts. J Biomech 2015; 48: 3720–23. doi: 10.1016/j.jbiomech.2015.08.003 [DOI] [PubMed] [Google Scholar]