Abstract
Objective:
The purpose of this study is to evaluate the value of fluorine-18-fludeoxyglucose positron emission tomography (18F-FDG PET)/CT in the diagnosis and treatment evaluation of ocular adnexal mucosa-associated lymphoid tissue (MALT) lymphoma.
Methods:
70 patients with OAML who received radiotherapy were recruited in our study. All the patients had the 18F-FDG PET/CT examination before the treatment. We retrospectively reviewed the medical records, pathological reports, laboratory results, and imaging features of all patients. The associations between 18F-FDG PET/CT parameters and Epstein-Barr virus antibodies, treatment response, MRI data, and Ki-67 expression were investigated.
Results:
The PET/CT scan indicated that 80% (56/70) of the patients showed orbital FDG avidity. The median level of maximum standardized uptake value (SUVmax) of the lesions was 4.65 ± 3.00 (range:1.2–13.5). 92.0% (46/50) of the mass-forming lesions showed 18F-FDG avidity, while only 50.0% (10/20) of the non-massive lesions had 18F-FDG avidity (χ2 = 13.23, p=0.01). The SUVmax in orbit, conjunctiva, and lacrimal gland lymphoma were 5.6, 2.9, and 3.7, respectively. A significant difference was identified of SUVmax among the three locations’ lymphoma using one-way ANOVA analysis (F = 5.039, p = 0.01). After completion of radiotherapy, the complete remission rate was achieved in 30.8% (4/13) of the patients without 18F-FDG avidity, and 70.4% (38/54) in cases with 18F-FDG avidity (χ2 = 5.43, p = 0.02). The correlation between high Ki-67 score and 18F-FDG avidity was confirmed (χ2 = 3.916, p = 0.048); however, no significant correlation was found between the SUVmax and Ki-67 score of the lesions (p = 0.971). Three patients (3/70, 4.3%) were upregulated the stage via PET/CT.
Conclusion:
18F-FDG PET/CT had some potential values in the diagnosis and assessment of treatment response in patients with OAML.
Advances in knowledge:
The value of 18F-FDG PET/CT for patients with OAML.
Introduction
Ocular adnexal lymphoma (OAL) is the most common ocular tumor and constitutes approximately 2% of non-Hodgkin lymphomas and 5–15% of extranodal lymphomas.1 The most common subtype of OAL is the mucosa-associated lymphoid tissue (MALT) lymphoma, and it accounts for 50–98%.2 The etiology of ocular adnexal MALT lymphoma (OAML) is unknown, while some cases can be associated with autoimmune disease, and in some geographic areas, it is associated with infection. OAML presents indolent and shows favorable overall and disease-free survival rates after appropriate treatment.3,4
Fluorine-18-fludeoxyglucose positron emission tomography/CT (18F-FDG PET/CT) acts as a critical imaging tool applied for diagnosis, staging, assessment of treatment response, and follow-up in a wide range of malignant diseases, including Hodgkin and non-Hodgkin lymphoma.5 18F-FDG PET/CT combines glucose metabolism with anatomical images provided by CT, capable of providing the anatomical and functional characteristics of lymphomas. Alejandra et al6 reported that 4/6 (66.6%) of the ocular adnexal lymphoma patients were upstaged under PET scan in addition to CT and/or MRI in a retrospective study. A maximum standardized uptake value (SUVmax) in 18F-FDG PET/CT of 2.5 or higher in lesions is generally considered to be indicative of malignant tissue. High tumor SUVmax acts as an indicator of more aggressive lesions or lymph node metastases in malignant tumors.7,8
The lesions showing 18F-FDG avidity in PET/CT scan relies on multiple parameters. MALT lymphoma has a low to moderate 18F-FDG avidity, and only around 50–80% of MALT lymphoma indicated a PET-positivity.9 In patients with non-conjunctival origin OAML, 83.3% of cases were found 18F-FDG avidity tumors.10 However, no data of proportion of 18F-FDG avidity in superficial conjunctival lesions were reported. Accordingly, PET/CT in the assessment of MALT lymphoma remains limited and controversial. A growing number of studies demonstrated that a higher SUVmax before the treatment might be a prognosis of worse overall survival (OS),9,11 while a study of 110 patients with MALT lymphoma showed that pretreatment PET/CT was not predictive power of PFS or OS, and the only positive PET/CT at the end of the treatment was associated with reduced PFS, other than OS.12 Likewise, a study reported that PET/CT metabolic parameters in MALT lymphoma were not related to survival.13 So far, few studies were reported to evaluate the value of PET/CT in the diagnosis, treatment response, and prognosis in patients with OAML. The present study aims to investigate the correlation between the characteristics of 18F-FDG PET/CT in OAML and epidemiological, pathological, imaging parameters, and treatment response. Then we assess the role of 18F-FDG PET/CT in the diagnosis and the prognosis after the treatment.
Methods and materials
Patient selection
We retrospectively evaluated 70 patients in one institution (Eye, Ear, Nose & Throat Hospital of Fudan University) from April 2015 to September 2020. 18F-FDG PET/CT scan were performed for all the collected cases. All the patients had a histopathological diagnosis with OAML in accordance with the World Health Organization criteria. We reviewed the medical records, pathological reports, laboratory results, and imaging characteristics of all 70 patients. Tumor staging was classified according to the Ann-Abor staging. Furthermore, the demographic data, lactate dehydrogenase, Epstein-Barr virus (EBV) antibodies, tumor treatment response, MRI data, and Ki-67 score were also collected. Ki-67 expression level was defined as the high group (≥15%) and the low group (<15%) for the Ki-67 score by employing the immunohistochemistry method. All patients were performed with the contrasted MRI or CT scan of orbit, and the MRI is preferred as possible. We analyzed the correlation between the clinicopathological characteristics and 18F-FDG PET/CT characteristics, including SUVmax and the FDG avidity.
This study was approved by the institutional review board of Eye, Ear, Nose & Throat Hospital of Fudan University in accordance with the Declaration of Helsinki, and informed consent for research was obtained from patients.
18F-FDG PET/CT scan
All patients underwent the whole-body-18F-FDG PET/CT scans 2–4 weeks before radiotherapy, and the scans were performed in different institutions using the scanners. All the patients fasted for at least 4 h before receiving an intravenous injection of 2.22–3.7 MBq/kg 18F-FDG in Siemens Biograph mCT-s64 and 1.85–3.7 MBq/kg 18F-FDG in General Electric Discovery VCT, and scanning began 60 min later. Low-dose CT acquisition was performed first, and a PET scan was carried out immediately. The acquisition time was 2–4 min per bed position. Typically, the body was imaged from head to toe in 8–10 min.
By drawing a region of interest (ROI) over the area of maximum activity in the detectable lesions, the standardized uptake value (SUV) was determined according to the ratio of FDG concentration in the tumor to FDG normalized to body weight. SUVmax was defined as the single maximum pixel count in the defined ROI. For semi-quantitative analysis, the SUVmax was determined as follows: SUV = ROI activity (MBq/ml)×body wt (g) / injected dose (MBq).
FDG avid lymphoma was defined as a tumor showing segmental or diffuse increased FDG uptake with abnormal thickening on CT of PET-CT images. FDG avidity is according to the judgment of comparison between the lesion and normal background control.
MRI protocol
The contrasted MRI scan of orbit before the treatment and three months after the completion of radiotherapy was recommended. Orbital MRI was performed on a 3-Tesla system (Verio; Siemens, Germany). MRI sequence and parameters were presented below. Enhanced T1 weighted imaging (T1WI:) repetition time (TR) = 3.73 ms, echo time (TE) = 1.42 ms, field of view (FOV) = 24×23, slice thickness = 3 mm. Diffusion-weighted imaging: B = 01000 s / mm2, TR = 3600 ms, TE = 65 ms, FOV = 24×24, layer thickness = 3 mm. Apparent diffusion coefficient (ADC) maps were generated, and a T1W turbo spin-echo or, in case of breathing difficulties, a fast gradient-echo sequence was achieved for anatomical and morphological correlations.
Radiation therapy
A head-and-neck mask was made for the immobilization at the time of the CT simulation (Philips Brilliance, Holland). The CT scan was performed from 2 to 3 cm above the frontal sinus to the chin with a slice thickness of 2.5 mm. Treatment planning was performed on a three-dimensional (3D) CT image-based planning system (Varian Eclipse, USA; Eye, Ear, Nose & Throat Hospital of Fudan University, Shanghai, China). The gross tumor volume (GTV) was defined as the gross extent of the tumor by imaging and physical examination; the clinical target volume (CTV) covered the partial or the whole orbit based on the tumor burden.
Patients were irradiated using intensity-modulated radiation therapy (IMRT) or conventional three-dimensional-conformed radiation therapy (3D-CRT) or electron beam. A 6 MV linear accelerator or 6 MV electron beam irradiation to the primary tumor was delivered. GTV was implemented based on a schedule of 1.8–2.0 Gy per fraction, CTV as 1.7–1.8 Gy per fraction, in five daily fractions per week.
After approximately 20–27 Gy doses of radiation, all the patients had another CT scans. To evaluate the treatment response, the tumor volumes were determined and compared between the beginning of radiotherapy and the middle of the whole radiotherapy.
Statistical analysis
A χ2 test was used to evaluate the correlation between ki-67, imaging data, tumor remission, and PET/CT parameters. Numeric variables were described as mean ± standard deviation (M ± SD), and categorical variables were expressed as a percentage (%). T-tests were compared between two samples with normal distribution and homogeneity of variance. Moreover, by employing the χ2 test, the categorical variables between groups were compared. Two-tailored p < 0.05 was considered with statistical significance. Statistical analyses were performed using SPSS software v. 29.0 (IBM Corp., Armonk, NY)
Results
Patients’ characteristics
A total of 70 patients with OAML were identified for the final analysis. There were 31 females and 39 males, and they aged from 27 to 85 years old (median: 54.4 years). The baseline characteristics of these patients were summarized in Table 1. The most common site of the lesion was the retrobulbar area (orbit) in 36 patients (51.4%) and followed by conjunctiva in 25 patients (35.7%) and lacrimal gland in 9 cases (12.9%). The majority of patients (55/70, 77.1%) were staged into IE according to Ann-Arbor; one patient with right parotid gland lesion was classified into Stage ⅡE (1/70, 1.4%); one patient with inguinal lymph nodes involvement was staged into ⅢE (1/70, 1.4%); and the other 14 (14/70, 20.0 %) were staged into ⅣE. 13 (13/70, 18.6%) patients were found with bilateral orbit lesions. Serum lactate dehydrogenase levels were elevated in two cases (2/70, 2.9%).
Table 1.
Characteristics of patients with OAML and the treatment factors
| Variables | No. of patients (%), n = 70 |
|---|---|
| Age (y) | |
| <60 | 42 (60.0) |
| ≥60 | 28 (40.0) |
| Gender | |
| Male | 39 (55.7) |
| Female | 31 (44.3) |
| IPI score | |
| 0–1 | 54 (77.1) |
| >1 | 16 (22.9) |
| Performance status | |
| 0–1 | 70(100) |
| >1 | 0 (0) |
| Sites | |
| Orbit | 36 (51.4) |
| Conjunctiva | 25 (35.7) |
| Lacrimal gland | 9 (12.9) |
| Symptoms | |
| Exophthalmos | 30 (42.3) |
| Epiphora | 22 (21.0) |
| Palpable mass | 15 (22.5) |
| Diplopia | 4 (5.6) |
| Ptosis | 3 (4.2) |
| Pain | 3 (4.2) |
| Stage | |
| IE | 54 (77.1) |
| ⅡE | 1 (1.4) |
| ⅢE | 1 (1.4) |
| ⅣE | 14 (20.0) |
| IR technique | |
| Electron beam | 12 (17.1) |
| 3D-CRT | 3 (4.3) |
| IMRT | 41 (58.6) |
| VMAT | 14 (20.0) |
3D-CRT: three-dimensional conformal radiotherapy, IMRT: intensity-modulated radiation therapy, IPI: international prognostic index, IR: irradiation therapy,OMAL: ocular adnexal mucosa-associated lymphoid tissue lymphoma, S: surgery, VMAT: volumetric-modulated arc radiation therapy.
The international prognostic index (IPI) score was evaluated, and 77.1% (54/70) scored as IPI 0–1. All the patients had good performance status (PS) at diagnosis rated as 0–1. 29 patients were performed with EBV antibodies test, including EBV/EA-IgA, EBV/Zta-IgA, EBV/NA-1 IgA, and EBV/VCA-IgA, and the results were slightly elevated in only 2 cases (6.9%). Epstein-Barr virus early RNA (EBER) was checked by the immunohistochemistry method in 27 patients. One patient (1/27, 3.7%) presented positive EBER.
All the patients were treated with definitive radiation therapy, and none of them accepted chemotherapy or Rituximab. 34 patients (34/70, 48.6%) had partial excision of the tumor. A total dose of radiation therapy is 27–41.4 Gy, in 1.8–2.0 Gy per fraction, which were delivered with multiple fields of conformal radiotherapy. The median RT dose for OAML was 32.4 Gy (range 27–41.4 Gy, average 32.6).
PET/CT
All the patients had 18F-FDG PET/CT assessment at initial diagnosis, while 59 and 11 patients had enhanced contrasted orbital MRI and CT scans, respectively. Of the 70 patients, 56 patients (80%) had abnormal PET/CT findings in the orbit. The lesions were identified as OAML according to the contrasted MRI, CT scan, and pathological results. Therefore, the FDG avidity in patients with OAML was 80.0%. The median level of SUVmax of lesions was 4.65 ± 3.00 (range: 1.2–13.5). 92.0% (46/50) of the mass-forming lesions showed 18F-FDG avidity, while 50.0% (10/20) of the non-massive lesions had 18F-FDG avidity (χ2 = 13.23, p < 0.01). The average SUVmax in non-massive lesions is slightly lower than that in the mass-forming group (3.72 vs 4.85, p = 0.28). Tumor invasions were reported in other locations beyond the ocular adnexa in three patients (4.3%) using PET/CT, and the clinical stage was up-regulated. Among all the 34 OAML cases executed functional MRI, 32 lesions (94.1%, 32/34) indicated FDG avid in PET/CT. The mean ADC value in 34 OAML lesions was 0.67 ± 0.37×10−3 mm2/s (range, 0.17–1.88 × 10−3), and there was no significant correlation between SUVmax and ADC (r = −0.028, p = 0.879) (Figure 1).
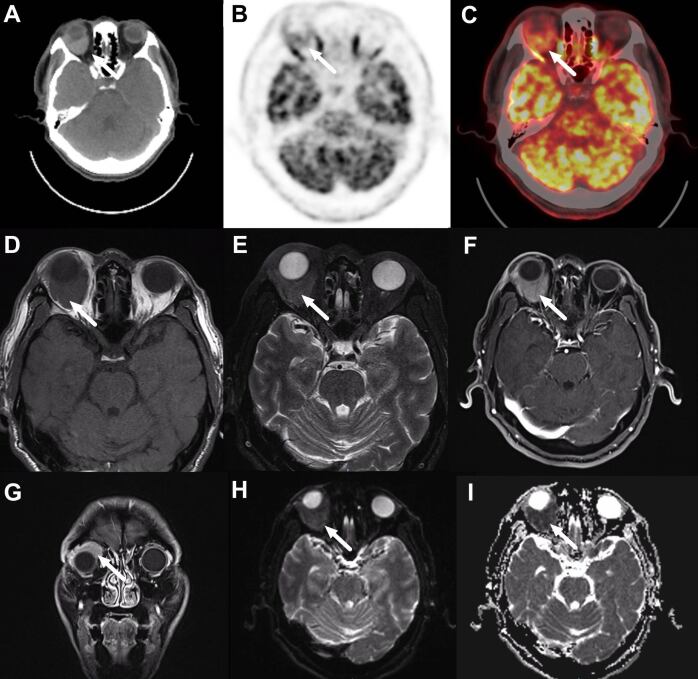
Figure 1.
A 47-year-old male patient with ocular adnexal mucosa-associated lymphoid tissue lymphoma in the right orbit. CT scan (A),18F-FDG positron emission tomography scanning (B), and fused images (C) show focal FDG uptake in right orbit (arrow, SUVmax 7.1) on 18F-FDG PET/CT scan. Axial T2- (D) and T2 weighted (E) MRI scans show an iso-intense mass in the right retrobulbar space (arrow). Axial (F) and coronal (G) post-contrast T1 weighted MRI scan demonstrates the corresponding mass with heterogeneous enhancement and vague outlines (arrow). The tumor is around the right eyeball and pushes in the upper lateral direction. (H, I) The mass shows restricted diffusion on diffusion-weighted images, and the intensity depends on the apparent diffusion coefficients (arrow).18F-FDG, fluorine-18-fludeoxyglucose; PET, positron emission tomography; SUVmax, maximum standardized uptake value.
Correlation between 18F-FDG PET/CT and Ki-67 expression
42.9% of patients (24/56) with high Ki-67 score (≥15%) had positive PET/CT avidity, while only 7.7% of (2/14) patients with low Ki-67 score (<15%) had positive PET/CT avidity. The correlation between high Ki-67 score and 18F-FDG avidity were also confirmed by χ2 test (χ2 = 3.916, p = 0.048). The average Ki-67 score in 18F-FDG PET/CT avidity group was 13.5, which was slightly higher than that of 10.3 in cases without 18F-FDG PET/CT avidity group (p = 0.254). In cases with 18F-FDG avid PET/CT, the average SUVmax in cases with a high Ki-67 score was 4.9, and was 4.5 in cases with a low Ki-67 score. No significant difference was reported between them (p = 0.581). Furthermore, linear regression showed that the SUVmax was not associated with the Ki-67 score in patients with OAML (r = 0.04, p = 0.754).
Correlation between 18F-FDG PET/CT and tumor size and location
68 patients presented with measurable lesions, and the tumor volumes were measured based on the simulation-CT and MRI. The average volume of the primary tumor was 9.97 mm3. The lesions with FDG avidity had significantly larger tumor volume than those without FDG avidity (11.59 vs 3.09, p = 0.00). However, in cases with positive 18F-FDG PET/CT avidity, SUVmax did not correlate significantly with primary tumor size (r = 0.256, p = 0.06).
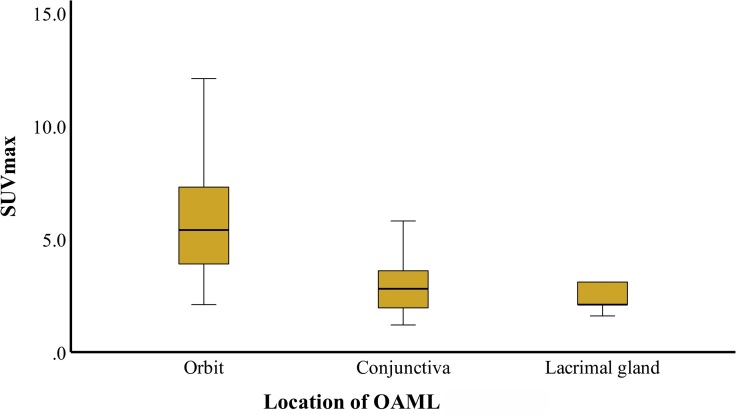
According to tumor locations, 18F-FDG PET/CT avidity was shown in 52% of patients (13/25) with conjunctiva lesions, 94.4% (34/36) of orbital lesions, and 100% (9/9) of lacrimal gland lesions. The SUVmax in the three groups was 2.9, 5.6, and 3.7, respectively, and a significant difference was identified among them using one-way ANOVA analysis (F = 5.039, p = 0.01) (Figure 2).
Figure 2.
SUVmax according to tumor location. The SUVmax of orbit mass is significantly elevated than that of the conjunctiva and lacrimal gland in patients with ocular adnexal mucosa-associated lymphoid tissue lymphoma. SUVmax, maximum standardized uptake value
Correlation between 18F-FDG PET/CT and treatment response
Overall, treatment responses were assessed using CT scans or MRI. 51 patients had the assessment of treatment response after 20–27 Gy during radiotherapy. In comparison with the primary tumor volume, and which 66.4% the tumor volume in the 18F-FDG avidity lesions had a decline rate of 66.4%, and was 62.5% in lesions without 18F-FDG avidity (p = 0.52). The post-treatment response was assessed in 1–3 months after the completion of radiotherapy. 69 patients underwent the treatment response evaluation, 42 (62.7%) patients achieved a complete remission (CR), and 25 (37.3%) patients achieved partial remission (PR). 30.8% of patients (4/13) without 18F-FDG avidity presented CR, while 70.4% of those (38/54) with18F-FDG avidity presented CR (χ2 = 5.43, p = 0.02).
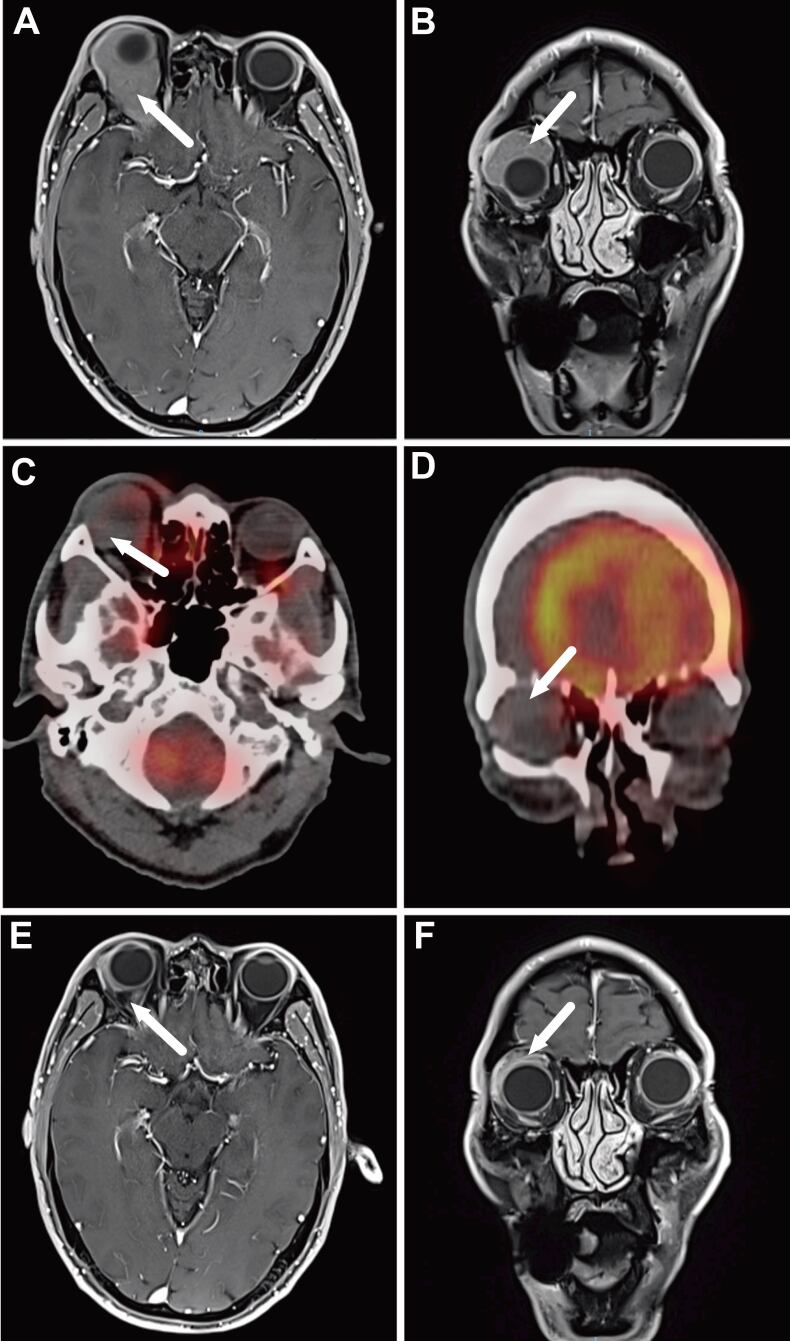
For patients who had measurable mass, the tumor volumes were measured before radiation therapy and interim radiation therapy (around the dose of 20–27 Gy). The correlation between SUVmax and tumor remission rate was evaluated with Pearson correlation analysis (r = 0.121, p = 0.445) (Figure 3).
Figure 3.
A 57-year-old male patient with ocular adnexal mucosa-associated lymphoid tissue lymphoma in the right orbit. Axial (A) and coronal (B) post-contrast T1 weighted MRI scan demonstrate the corresponding mass with heterogeneous enhancement and vague outlines around the right eyeball; the tumor pushes in the upper lateral direction and makes the right eyeball protruded (arrow). Axial (D) and coronal (E) show no FDG avidity of lesion in PET/CT scan. Axial (E) and coronal (F) post-contrast T1 weighted MRI scan shows the tumor achieved a complete response after the completion of radiation therapy, and the protruding eyeball retracted to the normal position. FDG, fludeoxyglucose; PET, positron emission tomography.
Discussion
This study assessed the value of 18FDG-PET/CT in the diagnosis and treatment evaluation of OAML. It is known that OAML is a low-grade tumor, and the value of 18FDG-PET/CT is controversial.14 Accordingly, 70 cases with OAML in one institution were retrospectively studied, and the correlations of semi-parameters and the characteristics of 18F-FDG PET/CT were investigated between the common clinical parameters from MRI, pathological results, laboratory examinations, and tumor response to treatments. 18F-FDG avidity varied significantly in different studies, which might be related to the location of lesions, morphologic characteristics, histologic characteristics, and clinical-stage classification.14 Lesions presenting as protrusions, polyps, or mass-forming were more 18F-FDG avid than the superficial, chronic gastritis-like, as well as low thick lesions.14
Positive FDG abnormal uptake in the present study was observed in 80% of the patients with OAML, consistent with the results of other reported studies.15 Park et al10 reported that 83.3% (50/60) of the OAML were FDG-avid tumors. However, all the conjunctival MALT lymphoma were ruled out for the analysis in that research. In the present study, 18F-FDG PET/CT avidity was reported in 52% of patients (13/25) with conjunctiva lesions, 95.6% of patients with mass, which were subcategorized to be 94.4% (34/36) of orbital lesions, and 100% (9/9) of lacrimal gland lesions. There was a significant difference in the varied locations which might result from the non-massive tumors’ less active metabolic cells enrichment. The assessment of FDG avidity complied with clinical experience, and we recommend merging the MRI and CT scan to determine ROI. The primary reference criterion of FDG avidity is that the brightness of FDG uptake in lesions exceeded the rest of the normal parenchyma (without lesions identified on hybrid imaging) and compared to the contralateral region. To avoid the potential effect exerted by inflammation on the FDG uptake, the PET/CT scan were performed at least 2 weeks after biopsy. Furthermore, the combination of MR images and PET/CT scans is critical to discriminating the lymphoma from the inflammation in several lesions.
EBV antibodies and EBER, representing the EBV infected status, were both approximately normal in all the tested cases. Unlike diffused large B-cell lymphoma and nature kill T cell lymphoma in the head and neck, EBV affection is not the pathogenic factor in patients with OAML. Thus, the correlation between EBV infection and 18F-FGD PET/CT parameters is unlikely to be analyzed.
The contrasted MRI exhibits the advantage in distinguishing the tumors from their surrounding soft tissues in OAML. In this study, all the lesions were clearly shown in the MRI, even if the superficial tumors in the conjunctivas. However, the sensitivity of detection was 80% for the whole cases in 18F-FGD PET/CT, decreasing to 52% for patients with conjunctival lesions. In comparison with 18F-FGD PET/CT, MRI could provide more valuable information for OAML. However, for patients with conjunctival OAML, the combination of ocular examination with MRI is critical to more effectively identifying the tumors.
DWI, a functional MRI technique dependent of the restriction of water movement in hypercellular tumors as impacted by extracellular space narrowing, can indirectly assess cell density. As indicated from the evidence, MRI, particularly with the addition of DWI, may be a feasible alternative to 18F-FDG-PET/CT for follow-up and treatment response assessment in patients with FDG-avid lymphoma.16 For FDG-avid MALT lymphoma, 18F-FDG PET/CT shows moderately superior to DWI-MRI in the outcome prediction. However, ADC value may act as an alternative parameter for assessing interim treatment response in MALT lymphoma.17 The superficial and tiny lesions could hardly be presented in DWI, and 100% (34/34) of the visible variations in DWI were mass-forming cases in this study. Several pieces of literature reported that a significant inverse correlation between the SUVmax and mean ADC values in malignant tumors.18–20 However, insignificant correlation between SUVmax and ADC values (r = −0.028, p = 0.879) was observed in OAML.
The Ki-67 protein was encoded by gene MKI67 and first identified as an antigen in Hodgkin’s lymphoma cell nuclei by Gerdes et al.21 Ki-67 affects cell cycle progression, including interphase and mitosis, and it has extensively acted as a proliferation marker for numerous malignant human tumors for decades.22–26 Given that FDG uptakes in lymphoma lesions generally reflect lymphoid cell activity and aggressiveness of the tumor, we studied the correlation between FDG avidity and Ki-67. It is investigated the lesions with a high Ki-67 score had positive 18F-FDG avidity in MALT tumors.27 Albano et al28 reported that 75% of extragastric MALT lymphoma were FDG avid, and the FDG avidity was associated with the Ki-67 score index. The present study indicated a significant correlation between 18F-FDG avidity and Ki-67 score in patients with a high proliferative score. Furthermore, SUVmax was proved to be moderate to strongly correlated with the expression of Ki-67 in various kinds of tumors.29,30 However, SUVmax was not correlated with the Ki-67 score in OAML, according to data from the present study.
Several therapeutic options are presented for OAML, radiotherapy is the best choice for early-stage diseases, and chemotherapy should be delivered for advanced-stage illnesses. Mass resection is an alternative if feasible, but it is rarely performed for retrobulbar lesions.31 Matsuo et al reported four patients presenting with MLAT in conjunctiva developed the clinical relapse after initial resection during long-term follow-up, and all the patients had not been delivered with additional therapy.32 In the present study, all the patients received IMRT, 3D conformal radiation therapy, or electron beams. After the treatment was completed, 62.7% of patients achieved CR, and 37.3% of patients achieved PR regardless of tumor stages. Radiotherapy is the optimal treatment for OAML at any stage. Among those patients, cases had 18F-FDG avidity was prone to be CR (70.4% vs 30.8%), and SUVmax was not correlated with tumor remission rate. 18F-FDG avidity lesions are probably more sensitive to radiotherapy. Accordingly, whether the lesion with strong FDG avidity could be treated with a lower radiation dose? Song et al33 demonstrated that the baseline SUV value had also been significantly elevated in the treatment failure group compared with that in the complete response group in gastric MALT. In the present study, whether the uptake of FDG could be used for the prognosis for long-term survival rate needs follow-up data.
All the data in this study were from two different scanners, the majority were from Siemens Biograph mCT-s64, and several were from General Electric Discovery VCT-s64. We have calculated all the data from patients with or without GE scanners and found that all the results were very similar. Accordingly, the results are not significantly affected by the scanners in our research.
The present study had several limitations. First, it is a retrospective study with a bias of case selection. Second, some cases accepted biopsy other than dissection, and the treatment mode was classified as without surgery. Third, the long-term follow-up data were not collected.
Conclusions
In conclusion, 80% of the ocular adnexal MALT presented with FDG avidity, and the median SUVmax in FDG avidity lesions was 4.65 ± 3.00. Given the value of 18F-FDG PET/CT in OAML, we recommend more cases to have the examination of 18FDG-PET/CT before the treatment for diagnosis and the prediction of treatment response. However, for the conjunctival lesions, the 18F-FDG-PET/CT does not appear to be useful for the diagnosis of OAML. Lesions with 18F-FDG avidity was more prone to be CR after the treatment. Furthermore, PET/CT upregulated the tumor stage by screening other lesions beyond the ocular adnexa.
Footnotes
Funding: This work was supported by the National Natural Science Foundation of China (Grant No. 81960551).
The authors Weifang Wang and Xiaochen Ni contributed equally to the work.
Contributor Information
Weifang Wang, Email: 13916229507@163.com.
Xiaochen Ni, Email: kevin_nxc@163.com.
Tianci Tang, Email: francois_tang@163.com.
Jie Wang, Email: wangjie3955366@163.com.
Yi Li, Email: liyi3443@hotmail.com.
Xinmao Song, Email: muqinger@sina.com.
REFERENCES
- 1. Raderer M, Kiesewetter B, Ferreri AJM. Clinicopathologic characteristics and treatment of marginal zone lymphoma of mucosa-associated lymphoid tissue (malt lymphoma). CA Cancer J Clin 2016; 66: 153–71. doi: 10.3322/caac.21330 [DOI] [PubMed] [Google Scholar]
- 2. Stefanovic A, Lossos IS. Extranodal marginal zone lymphoma of the ocular adnexa. Blood 2009; 114: 501–10. doi: 10.1182/blood-2008-12-195453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Desai A, Joag MG, Lekakis L, Chapman JR, Vega F, Tibshirani R, et al. Long-term course of patients with primary ocular adnexal malt lymphoma: a large single-institution cohort study. Blood 2017; 129: 324–32. doi: 10.1182/blood-2016-05-714584 [DOI] [PubMed] [Google Scholar]
- 4. Nam SW, Woo KI, Kim YD. Characteristics of primary extranodal marginal zone b-cell lymphoma in korea: conjunctiva versus other ocular adnexa. Br J Ophthalmol 2018; 102: 502–8. doi: 10.1136/bjophthalmol-2017-310741 [DOI] [PubMed] [Google Scholar]
- 5. Cheson BD, Fisher RI, Barrington SF, Cavalli F, Schwartz LH, Zucca E, et al. Recommendations for initial evaluation, staging, and response assessment of hodgkin and non-hodgkin lymphoma: the lugano classification. J Clin Oncol 2014; 32: 3059–68. doi: 10.1200/JCO.2013.54.8800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Valenzuela AA, Allen C, Grimes D, Wong D, Sullivan TJ. Positron emission tomography in the detection and staging of ocular adnexal lymphoproliferative disease. Ophthalmology 2006; 113: 2331–37. doi: 10.1016/j.ophtha.2006.05.059 [DOI] [PubMed] [Google Scholar]
- 7. Ngeow JYY, Quek RHH, Ng DCE, Hee SW, Tao M, Lim LC, et al. High suv uptake on fdg-pet/ct predicts for an aggressive b-cell lymphoma in a prospective study of primary fdg-pet/ct staging in lymphoma. Ann Oncol 2009; 20: 1543–47. doi: 10.1093/annonc/mdp030 [DOI] [PubMed] [Google Scholar]
- 8. Kitajima K, Suenaga Y, Minamikawa T, Komori T, Otsuki N, Nibu K-. I, et al. Clinical significance of suvmax in (18)f-fdg pet/ct scan for detecting nodal metastases in patients with oral squamous cell carcinoma. Springerplus 2015; 4: 718. doi: 10.1186/s40064-015-1521-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Qi S, Huang MY, Yang Y, Schöder H, Teckie S, Noy A, et al. Uptake of [18f]fluorodeoxyglucose in initial positron-emission tomography predicts survival in malt lymphoma. Blood Adv 2018; 2: 649–55. doi: 10.1182/bloodadvances.2017013698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Park HL, O JH, Park SY, Jung S-. E, Park G, Choi B-. O, et al. Role of f-18 fdg pet/ct in non-conjunctival origin ocular adnexal mucosa-associated lymphoid tissue (malt) lymphomas. EJNMMI Res 2019; 9(1): 99. doi: 10.1186/s13550-019-0562-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hwang JP, Lim I, Byun BH, Kim BI, Choi CW, Lim SM. Prognostic value of suvmax measured by pretreatment 18f-fdg pet/ct in patients with primary gastric lymphoma. Nucl Med Commun 2016; 37: 1267–72. doi: 10.1097/MNM.0000000000000579 [DOI] [PubMed] [Google Scholar]
- 12. Vaxman I, Bernstine H, Kleinstern G, Hendin N, Shimony S, Domachevsky L, et al. FDG pet/ct as a diagnostic and prognostic tool for the evaluation of marginal zone lymphoma. Hematol Oncol 2019; 37: 168–75. doi: 10.1002/hon.2578 [DOI] [PubMed] [Google Scholar]
- 13. Albano D, Bosio G, Camoni L, Farina M, Re A, Tucci A, et al. Prognostic role of baseline 18 f-fdg pet/ct parameters in malt lymphoma. Hematol Oncol 2019; 37: 39–46. doi: 10.1002/hon.2563 [DOI] [PubMed] [Google Scholar]
- 14. Albano D, Durmo R, Treglia G, Giubbini R, Bertagna F. 18F-fdg pet/ct or pet role in malt lymphoma: an open issue not yet solved-a critical review. Clin Lymphoma Myeloma Leuk 2020; 20: 137–46. doi: 10.1016/j.clml.2019.10.006 [DOI] [PubMed] [Google Scholar]
- 15. Zanni M, Moulin-Romsee G, Servois V, Validire P, Bénamor M, Plancher C, et al. Value of 18fdg pet scan in staging of ocular adnexal lymphomas: a large single-center experience. Hematology 2012; 17: 76–84. doi: 10.1179/102453312X13221316477813 [DOI] [PubMed] [Google Scholar]
- 16. Mayerhoefer ME, Karanikas G, Kletter K, Prosch H, Kiesewetter B, Skrabs C, et al. Evaluation of diffusion-weighted magnetic resonance imaging for follow-up and treatment response assessment of lymphoma: results of an 18f-fdg-pet/ct-controlled prospective study in 64 patients. Clin Cancer Res 2015; 21: 2506–13. doi: 10.1158/1078-0432.CCR-14-2454 [DOI] [PubMed] [Google Scholar]
- 17. Mayerhoefer ME, Karanikas G, Kletter K, Kiesewetter B, Weber M, Rausch I, et al. Can interim 18f-fdg pet or diffusion-weighted mri predict end-of-treatment outcome in fdg-avid malt lymphoma after rituximab-based therapy?: a preliminary study in 15 patients. Clin Nucl Med 2016; 41: 837–43. doi: 10.1097/RLU.0000000000001395 [DOI] [PubMed] [Google Scholar]
- 18. Kitajima K, Yamano T, Fukushima K, Miyoshi Y, Hirota S, Kawanaka Y, et al. Correlation of the suvmax of fdg-pet and adc values of diffusion-weighted mr imaging with pathologic prognostic factors in breast carcinoma. Eur J Radiol 2016; 85: 943–49. doi: 10.1016/j.ejrad.2016.02.015 [DOI] [PubMed] [Google Scholar]
- 19. Lee S-. Y, Jee W-. H, Yoo IR, Jung J-. Y, Im S-. A, Chung Y-. G, et al. Comparison of 3t diffusion-weighted mri and 18f-fdg pet/ct in musculoskeletal tumours: quantitative analysis of apparent diffusion coefficients and standardized uptake values. Br J Radiol 2019; 92: 1102. doi: 10.1259/bjr.20181051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Usuda K, Funasaki A, Sekimura A, Motono N, Matoba M, Doai M, et al. FDG-pet/ct and diffusion-weighted imaging for resected lung cancer: correlation of maximum standardized uptake value and apparent diffusion coefficient value with prognostic factors. Med Oncol 2018; 35: 66. doi: 10.1007/s12032-018-1128-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gerdes J, Schwab U, Lemke H, Stein H. Production of a mouse monoclonal antibody reactive with a human nuclear antigen associated with cell proliferation. Int J Cancer 1983; 31: 13–20. doi: 10.1002/ijc.2910310104 [DOI] [PubMed] [Google Scholar]
- 22. Sun X, Kaufman PD. Ki-67: more than a proliferation marker. Chromosoma 2018; 127: 175–86. doi: 10.1007/s00412-018-0659-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sobecki M, Mrouj K, Colinge J, Gerbe F, Jay P, Krasinska L, et al. Cell-cycle regulation accounts for variability in ki-67 expression levels. Cancer Res 2017; 77: 2722–34. doi: 10.1158/0008-5472.CAN-16-0707 [DOI] [PubMed] [Google Scholar]
- 24. Berlin A, Castro-Mesta JF, Rodriguez-Romo L, Hernandez-Barajas D, González-Guerrero JF, Rodríguez-Fernández IA, et al. Prognostic role of ki-67 score in localized prostate cancer: a systematic review and meta-analysis. Urol Oncol 2017; 35: 499–506. doi: 10.1016/j.urolonc.2017.05.004 [DOI] [PubMed] [Google Scholar]
- 25. Li L, Han D, Wang X, Wang Q, Tian J, Yao J, et al. Prognostic values of ki-67 in neoadjuvant setting for breast cancer: a systematic review and meta-analysis. Future Oncol 2017; 13: 1021–34. doi: 10.2217/fon-2016-0428 [DOI] [PubMed] [Google Scholar]
- 26. Jakobsen JN, Sørensen JB. Clinical impact of ki-67 labeling index in non-small cell lung cancer. Lung Cancer 2013; 79: 1–7. doi: 10.1016/j.lungcan.2012.10.008 [DOI] [PubMed] [Google Scholar]
- 27. Albano D, Giubbini R, Bertagna F. 18F-fdg pet/ct in splenic marginal zone lymphoma. Abdom Radiol 2018; 43: 2721–27. doi: 10.1007/s00261-018-1542-z [DOI] [PubMed] [Google Scholar]
- 28. Albano D, Bosio G, Giubbini R, Bertagna F. 18F-fdg pet/ct and extragastric malt lymphoma: role of ki-67 score and plasmacytic differentiation. Leuk Lymphoma 2017; 58: 2328–34. doi: 10.1080/10428194.2017.1298754 [DOI] [PubMed] [Google Scholar]
- 29. Surov A, Meyer HJ, Wienke A. Associations between pet parameters and expression of ki-67 in breast cancer. Transl Oncol 2019; 12: 375–80. doi: 10.1016/j.tranon.2018.11.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Shen G, Ma H, Pang F, Ren P, Kuang A. Correlations of 18f-fdg and 18f-flt uptake on pet with ki-67 expression in patients with lung cancer: a meta-analysis. Acta Radiol 2018; 59: 188–95. doi: 10.1177/0284185117706609 [DOI] [PubMed] [Google Scholar]
- 31. Tsai HK, Li S, Ng AK, Silver B, Stevenson MA, Mauch PM. Role of radiation therapy in the treatment of stage i/ii mucosa-associated lymphoid tissue lymphoma. Ann Oncol 2007; 18: 672–78. doi: 10.1093/annonc/mdl468 [DOI] [PubMed] [Google Scholar]
- 32. Matsuo T, Ichimura K, Tanaka T, Kaji M. Conjunctival lymphoma can be detected by fdg pet. Clin Nucl Med 2012; 37: 516–19. doi: 10.1097/RLU.0b013e31823ea96d [DOI] [PubMed] [Google Scholar]
- 33. Song KH, Yun M, Kim J-H, Yang WI, Kang DR, Chung JB, et al. Role of f-fdg pet scans in patients with helicobacter pylori-infected gastric low-grade malt lymphoma. Gut Liver 2011; 5: 308–14. doi: 10.5009/gnl.2011.5.3.308 [DOI] [PMC free article] [PubMed] [Google Scholar]