Abstract
Objective:
Xerostomia is the most common treatment-related toxicity after radiotherapy (RT) for head and neck carcinoma, reducing the quality of life of patients due to a decrease in salivary gland function.
Methods:
Salivary gland scintigraphy was performed to quantitatively evaluate the salivary gland functions in patients undergoing RT. It was done chronologically for 62 salivary glands of 31 patients before RT and retested 12 months later.
Results:
The salivary gland functions of most patients deteriorated post-RT and recovered when the radiation dose to the salivary gland was not high. The mean dose to the salivary gland was found to be the most reliable factor in deteriorating salivary gland function, and the tolerance dose was determined to be 46 Gy. The recovery rate of salivary gland function after 1 year of RT was 72% in the RT alone group (n = 10), 56% in the conformal radiotherapy group (n = 15), and 44% in the bioradiotherapy group (n = 6).
Conclusion:
Scintigraphy revealed that the salivary glands recovered from post-RT hypofunction when decreased doses were administered. The determined tolerance dose of 46 Gy may guide the approach to minimizing associated xerostomia in RT.
Advances in knowledge:
In this study, the average tolerated dose to the salivary glands was 46 Gy.
Introduction
Radiotherapy (RT) plays an important role in the treatment of head and neck cancers. A considerable radiation dose is necessary to eradicate cancer spreading in the neck lymphatic route in advanced cases. Whole neck radiation therapy (WNRT), which covers the entire neck lymphatic system, has mainly been used for this purpose. It provides almost the same dose of radiation to the salivary gland when three-dimensional conformal radiotherapy (3D-CRT) is used. Subsequently, the function of the salivary gland is destroyed, resulting in permanent xerostomia. Intensity-modulated radiotherapy (IMRT) enables the irradiation of high doses to the neck lymphatic routes while reducing doses to the salivary gland and is becoming more widely available due to technological improvements.1 This technique can set pre-determined doses to target organs, as well as organs at risk, such as the salivary gland in this case. However, the adequate dose–volume relationship is not well understood. In this study, we used salivary scintigraphy to mathematically investigate the tolerance dose of RT in the salivary gland.
Methods and materials
Patient eligibility
The ethical committee of Tokyo Medical University Hospital approved this study (IRB number SH3686), and all patients provided written informed consent to participate in the study.
The eligibility criteria included the following: (1) age of 20 years or older, (2) World Health Organization Performance Status of 0 or 1, and (3) diagnosis of carcinoma by cytology or histology. The exclusion criteria included the following: (1) patients who suffered from autoimmune diseases such as Sjogren’s syndrome, (2) patients who previously received RT to the head and neck region, and (3) patients whose prognosis was survival less than 6 months.
Radiotherapy methods, target definition, and dose prescription
All patients were immobilized in the supine position using a thermoplastic mask (Immobilization Systems, Orfit Industries NV, Wijnegem, Belgium) covering the head, neck, and shoulders to reduce positioning errors during treatment. Planning CT was performed with 5 mm thickness and 5 mm intervals from the level of the parietal lobe through the tracheal bifurcation. CT images were then transferred to a target contouring software (MIM Maestro v. 6.1, MIM Software Inc., Cleveland, OH) and overlaid with useful images from magnetic resonance imaging, contrast-enhanced CT, and positron emission tomography (PET)-CT. These overlaid images, which contained information about targets and critical organs, were sent to the IMRT treatment planning system (Xio v. 4.6 System, Elekta AB, Stockholm, Sweden). For IMRT planning, five beams at the angles of 36°, 108°, 180°, 252°, and 324° were selected. RT was administered 5 days a week with a daily dose of 2.0 Gy.
Three different clinical target volumes (CTVs) were defined. CTV1 was defined as the gross tumor volume in the primary site along with the gross lymph node volume, with a margin of approximately 5 mm each. In post-operative patients, CTV1 is the area of the post-operative tumor bed and extranodal extension. CTV2 was defined as the neck node volume in the primary site according to the Radiation Therapy Oncology Group consensus guidelines. CTV3 was defined as the prophylactic lymph node volume at the contra primary site. Similarly, different planning target volumes (PTVs) were defined. PTV1, PTV2, and PTV3 were determined to be CTV1, CTV2, and CTV3 plus a 5 mm margin in all directions, respectively. The 3D-CRT plan was designed to prescribe 70 Gy to PTV1, 60 Gy to PTV2, 40 Gy to PTV3, and 60 Gy to PTV1 and PTV2 in post-operative cases. In IMRT, 95% of the prescribed dose was set to cover 95% of 70 Gy/35 fr for high-risk PTV1, 63 Gy/35 fr for intermediate-risk PTV2, and 56 Gy/35 fr for low-risk PTV3. In the planning of IMRT, the mean prescription dose for the parotid gland excluding the PTV was constrained to below 26 Gy. The irradiated volume of WNRT covered Level II–IV lymph node volumes according to Radiation Therapy Oncology Group guidelines.2
Combined therapy
In the chemoradiotherapy (CRT) treatment, cisplatin (100 mg/m2) was administered every 3 weeks in combination with RT, followed by cisplatin for up to three cycles. When the glomerular filtration rate was less than 60 ml/min/1.73 m2, the cisplatin dose was reduced to 80% for patients. In the bioradiotherapy (BRT) treatment, cetuximab (400 mg/m2) was administered 1 week before the initiation of RT, and the dose was reduced to 250 mg/m2 every week during RT, followed by cetuximab for up to seven cycles. Patients with severe renal dysfunction ( glomerular filtration rate<40 ml/min/1.73 m2) or those older than 75 years underwent RT alone.
Salivary gland scintigraphy
Dynamic scintigraphy imaging was performed using a gamma camera with a low-energy, high-resolution parallel-hole collimator (Symbia T16; Siemens, Erlangen, Germany). Salivary gland images were sequentially obtained every 30 s for 25 min after intravenous injection of 370 MBq Tc-99m pertechnetate (99mTcO4-) following 1 h of fasting. 15 min after the injection, freshly squeezed lemons were administered orally to each patient as a stimulation substance. Time–activity curves were generated for the salivary glands during scintigraphy. Parameters including the maximum counts, minimum counts, time at maximum counts (Tmax), and the washout rate were calculated from the time–activity curve. The washout rate was determined using the formula: [(maximum count - minimum count after stimulation) / maximum count]×100%.3 These examinations were performed immediately before the start of RT, 3 months post-RT (early phase), and 12 months post-RT (late phase). Recovery rates were defined as when washout rates in the acute or late phase could be divided by the washout rate before RT. These procedures were performed in each of the bilateral parotid and submandibular glands.
Dosimetric parameters and statistic analysis
Dose–volume histograms were generated for all patients. Mean doses and relative volumes receiving ≥10 Gy (V10), ≥20 Gy (V20), ≥26 Gy (V26), ≥30 Gy (V30), and ≥40 (V40) were also determined. The receiver operating characteristic curve was plotted using these parameters, and the area under the curve was calculated. Scatter plots showing the correlation between the dose parameters and recovery rates were created.
Assessment of the model in predicting the recovery rate of salivary glands was performed using 95% confidence intervals (CIs). A p-value of <0.05 was considered statistically significant. Multivariate regression analyses using the Cox proportional hazards model were performed to identify the risk factors. Data were analyzed using R statistical software (R software v. 3.1.0, R Foundation for Statistical Computing, Vienna, Austria).
Results
Between June 2017 and January 2019, 57 patients were enrolled in this study, with patient characteristics summarized in Table 1. However, some patients had a relapse or metastasis (n = 6), deteriorating general condition (n = 3), treatment for other cancers (n = 3), death due to other diseases (n = 1), or were lost to follow-up (n = 13). Thus, patients may have received salivary gland scintigraphy once (n = 18), twice (n = 8), or thrice (n = 31). A total of 62 parotid glands in 31 patients underwent salivary gland scintigraphy three times. These 31 patients, with a total of 62 parotid glands, underwent RT methods (IMRT, n = 11; 3D-CRT, n = 20) with a corresponding combination therapy (CRT, n = 15; BRT, n = 6; RT alone, n = 10).
Table 1.
Patient and tumor characteristics
| Factors | No | |
|---|---|---|
| Sex | Male | 49 |
| Female | 8 | |
| Age | Median | 67 |
| Range | 27–85 | |
| Primary sites | Nasopharynx | 6 |
| Oropharynx | 21 | |
| Hypopharynx | 17 | |
| Larynx | 7 | |
| Oral | 3 | |
| Primary unknown | 1 | |
| Double primary | 1 | |
| Treatment methods | Chemoradiotherapy | 27 |
| Bioradiotherapy | 10 | |
| Radiotherapy alone | 20 | |
| Total doseose | 50.4 Gy | 2 |
| 60 Gy | 9 | |
| 70 Gy | 45 | |
| 70.2 Gy | 1 | |
| Number of scintigraphy examination | 1 | 18 |
| 2 | 8 | |
| 3 | 31 |
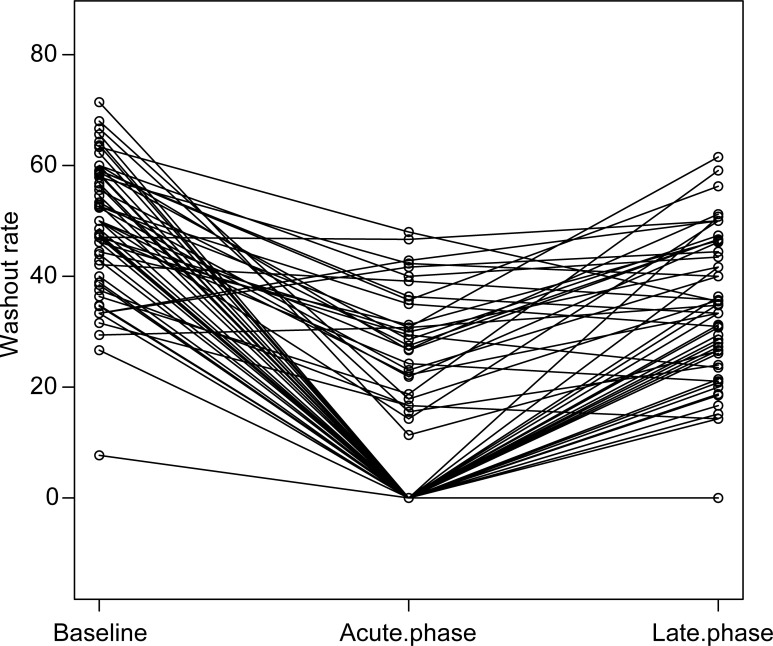
The median volume of the 62 parotid glands of the 31 patients was 23.4 ml. The mean dose for all patients was 35.8 Gy. Specifically, the mean dose was 32.3 Gy in the IMRT group and 39.0 Gy in the 3D-CRT group, with no significant difference (p = 0.24). The mean salivary washout rate was 49% (range: 8–71%) before RT, 14% (range: 0–48%) in the acute phase, and 29% (range: 0–62%) in the late phase. The changes in washout rate before and after RT are summarized in Figure 1. In many salivary glands (53/62), the washout rate improved in the late phase compared to that in the acute phase. The median washout rates were 0 and 58% in the acute and late phases, respectively. In the acute phase, the washout rates of 32 salivary glands reached zero, while the remaining 30 did not. The former received a median mean dose of 39.8 Gy while the latter received a mean dose of 25.5 Gy, with significant difference (p < 0.001) (Figure 1). Approximately, 42.9% of salivary glands whose washout rates reached zero in the acute phase recovered in the late phase.
Figure 1.
Changes in washout rates. Washout rates generally decreased in the acute phase and recovered in the late phase. Around 42.9% of salivary glands reached zero in the acute phase after a mean dose of 39.8 Gy and recovered in the late phase.
Regarding recovery rate in the acute phase, there was a significant difference observed between the RT alone and the non-RT alone groups (p < 0.01). In the late phase, the recovery rate was 72% in the group that received RT alone, 56% in the CRT group, and 44% in the BRT group. A significant difference was observed between the BRT and the RT alone group (p = 0.014), but not between the RT alone and CRT groups (p = 0.066). Recovery rates significantly improved in the IMRT group (p = 0.04).
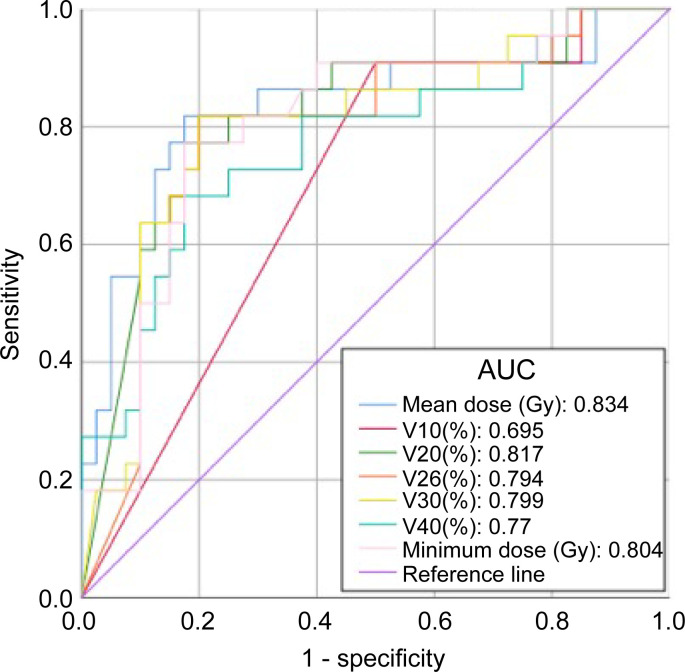
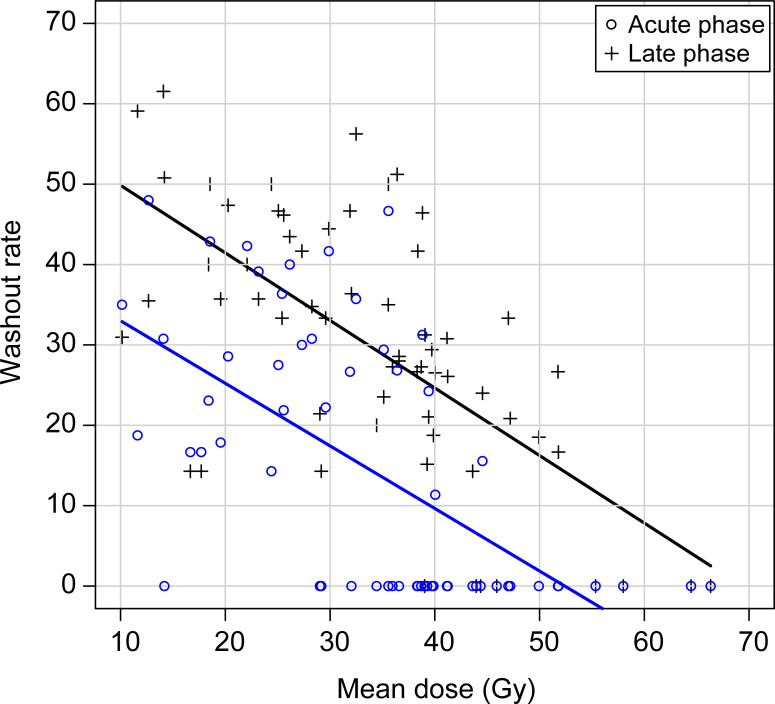
Recovery rates significantly decreased when the parameters of mean dose, V20, V26, V30, and V40 increased. According to the receiver operating characteristic data, the area under the curve of V20, mean dose, and minimum doses were greater than 0.8. The mean dose was also found to be a reliable indicator for determining salivary gland function (Figure 2, Table 2). The scatter plot shows that washout rates decreased as the mean dose increased, both in the acute and late phases (Figure 3). Washout rates in the late phase reached zero when a mean dose of more than 54 Gy was administered. Notably, only 5% of salivary gland functions reached zero washout rates in the late phase when less than 46 Gy was administered. Therefore, a mean dose of 46 Gy was derived as the tolerance dose of the salivary glands.
Figure 2.
Receiver operating characteristic curves for dose–volume analysis. AUC data revealed that the mean dose is the most reliable method for predicting salivary gland preservation. AUC, area under the curve.
Table 2.
ROC curve analysis as a predictor of recovery of salivary gland function
| Factor | AUC | 95% confidence interval | |
|---|---|---|---|
| Lower | Upper | ||
| V10 | 0.695 | 0.589 | 0.801 |
| V20 | 0.817 | 0.7 | 0.934 |
| V26 | 0.794 | 0.669 | 0.919 |
| V30 | 0.799 | 0.676 | 0.922 |
| V40 | 0.77 | 0.641 | 0.9 |
| Mean dose | 0.834 | 0.714 | 0.954 |
| Minimum dose | 0.804 | 0.685 | 0.923 |
AUC, area under the curve; ROC, receiver operating characteristic.
Figure 3.
Scatter plot demonstrating dispersion relationship between mean doses and recovery rates. Both the acute and late recovery rates decreased as the mean dose increased. The tolerance dose was determined to be 46 Gy, with a 5% risk of salivary gland destruction in the late phase.
Discussion
In this study, a total of 62 salivary glands in 31 patients were analyzed using scintigraphy immediately before, 3 months after, and 12 months after RT. The mean dose for RT was found to be the most reliable parameter for detecting the deterioration of salivary functions by scintigraphy. Upon analysis, the tolerance dose of salivary glands was determined to be 46 Gy, with only 5% of salivary gland functions reaching zero washout rates. Further, the salivary gland washout rate was found to decrease in the late phase as the mean dose increased with BRT.
Excess radiation to the salivary gland results in its hypofunction, which causes xerostomia. WNRT using 3D-CRT provides radiation to the neck lymph node area and provides considerable levels of radiation to the salivary gland, resulting in salivary gland loss of function. The IMRT technique has made it possible to irradiate tumoricidal doses to the neck lymph nodes while reducing the doses to the risk organs of the spinal cord and salivary glands. Dose–volume restriction is recommended for treatment planning in IMRT. However, investigation on the exact dosimetric values able to minimize adverse events is insufficient.
Sialometry and the Late Effects Normal Tissues–Subjective, Objective, Management, Analytic scales have been used to measure salivary gland functions. However, the former has poor reproducibility and the latter does not provide quantitative data.4,5 In this study, scintigraphy was used to quantitatively evaluate and objectively measure salivary function.6
Several authors have shown that salivary gland functions decrease when the irradiation dose exceeds 20–40 Gy.7–9 Dijkema et al reported that the parotid flow rate of 50% of patients decreased to less than 25% of the baseline when a mean dose of 39.9 Gy was administered.8 Eisbruch et al reported that salivary gland flow was maintained in the parotid mean dose of 24–26 Gy after 1 year.10 Hey et al reported that salivary gland function in patients treated with 3D-CRT or IMRT with mean parotid doses of 26 Gy or less reached a recovery rate of about 74% after 3 years.11 Several other studies also demonstrated a rapid decline in salivary gland function when the mean dose of the parotid gland approached 30 Gy.12,13 In our study, 42.9% of salivary glands reached zero washout rate in the acute phase after a median dose of 39.8 Gy was administered but 42.9% of salivary glands recovered in the late phase. A mean dose of 46 Gy was determined to have a 5% risk of salivary gland function loss after 1 year. A comparison among available studies (including the present study) on the relationship between the salivary gland recovery rate and the mean RT dose to the parotid gland is shown in Table 3.3,9,14–16 Our data were comparable to those of other studies.
Table 3.
Comparison of relationship between the mean parotid gland doses and washout rates after radiotherapy with previous studies
| Authors | Evaluation methods | Number of subjects | Radiation methods | Mean parotid dose | Washout rates |
|---|---|---|---|---|---|
| Gupta et al.14 | Scintigraphy | 82 | IMRT 3D-CRT |
Ipsilateral: 50.5 Gy Contralateral: 35.4 Gy |
3 M: 26%, 12 M: 38%, 24 M: 59%, 36M:65.3% |
| Maes et al.9 | Scintigraphy | 78 | 3D-CRT | 21 Gy | 4 W: 31% 28 W: 79% |
| Roesink et al. (2004)3 | Scintigraphy | 192 | 3D-CRT | 33.1 Gy | 6 W: 42% 12 M: 72% |
| Braam et al.15 | Questionnaire Sialometry |
44 | 3D-CRT | Rt: 28.3 Gy Lt: 27.9 Gy |
6 W: 35%, 6 M: 47%, 12 M: 69% |
| Roesink et al. (2001)16 | Sialometry | 174 | 3D-CRT | 40 Gy | 6 W: 35%, 6 M: 50% 12 M: 58% |
| Itonaga et al. | Scintigraphy | 62 | IMRT 3D-CRT |
35.8 Gy | 3 M: 0% 12 M: 58% |
3D-CRT, three-dimensional conformal radiotherapy; IMRT, intensity-modulated radiotherapy.
Regarding recovery rates of salivary gland function, there was a significant difference between the BRT and the RT alone group, but not between the RT alone and the CRT group. Chemotherapy-induced xerostomia is reversible after completion of treatment as CRT does not generally correlate with the risk of xerostomia.17,18 However, the risk of xerostomia in cetuximab combination therapy is controversial. Bonner et al conducted the only randomized controlled study of RT vs BRT in patients with locally advanced head and neck cancers and reported that Grade 3 or 4 xerostomia was observed in 2.8% of patients in the RT group and 4.8% in the BRT group.19 Epidermal growth factor receptor expression in acinar and tubular elements of the parotid gland may be correlated with salivary gland hypofunction.20 To the best of our knowledge, no studies have reported a decrease in the recovery rate of salivary gland function in the BRT group compared to the RT group.
In our study, 31 of the 57 patients enrolled underwent 3 scintigraphy procedures, and a considerable number of patients dropped out due to the natural course of the primary disease. Therefore, the observation time of our study was relatively short. Eisbruch et al suggested that salivary line function may be restored up to 2 years after RT.21 Chen et al showed that the recovery rates of 31 patients treated with IMRT were 70% after 1 year of RT and 68% after 2 years, with no significant difference.22 This study employed both 3D-CRT and IMRT techniques and analyzed a wide dosimetric range of radiation administered to the salivary gland. The use of a variety of RT techniques may be better in studying the tolerance threshold of the salivary gland than only IMRT as shown by the normal tissue complication probability analysis by Dijkema et al.23 Our data were comparable with other studies which reported 43 Gy and 43.6 Gy for 3D-CRT and IMRT, respectively, for the 1-year TD50 of salivary gland recovery rate.3,22
The limitation of this study is that it does not have data about quality of life of patients related to the salivary gland secretion function. A further prospective study with more patients is necessary to evaluate the benefit of IMRT concerning QOL.
Conclusion
Scintigraphy revealed that the salivary glands recovered from post-RT hypofunction when decreased doses were administered. The mean tolerance dose of the salivary gland was determined to be 46 Gy. Further, combined RT with cetuximab was shown to deteriorate salivary gland function, but this was not observed in combination therapy with cisplatin.
Contributor Information
Tomohiro Itonaga, Email: itonaga@tokyo-med.ac.jp.
Koichi Tokuuye, Email: ktokuue@tokyo-med.ac.jp.
Ryuji Mikami, Email: mikami-r@tokyo-med.ac.jp.
Akira Shimizu, Email: entakira@tokyo-med.ac.jp.
Hiroki Sato, Email: satohiro@tokyo-med.ac.jp.
Mana Yoshimura, Email: radiolmn@tokyo-med.ac.jp.
Kiyoaki Tsukahara, Email: tsuka@tokyo-med.ac.jp.
Kazuhiro Saito, Email: saito-k@tokyo-med.ac.jp.
REFERENCES
- 1. van Rij CM, Oughlane-Heemsbergen WD, Ackerstaff AH, Lamers EA, Balm AJM, Rasch CRN. Parotid gland sparing imrt for head and neck cancer improves xerostomia related quality of life. Radiat Oncol 2008; 3: 41. doi: 10.1186/1748-717X-3-41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Grégoire V, Ang K, Budach W, Grau C, Hamoir M, Langendijk JA, et al. Delineation of the neck node levels for head and neck tumors: a 2013 update. dahanca, eortc, hknpcsg, ncic ctg, ncri, rtog, trog consensus guidelines. Radiother Oncol 2014; 110: 172–81: S0167-8140(13)00514-8. doi: 10.1016/j.radonc.2013.10.010 [DOI] [PubMed] [Google Scholar]
- 3. Roesink JM, Moerland MA, Hoekstra A, Van Rijk PP, Terhaard CHJ. Scintigraphic assessment of early and late parotid gland function after radiotherapy for head-and-neck cancer: a prospective study of dose-volume response relationships. Int J Radiat Oncol Biol Phys 2004; 58: 1451–60. doi: 10.1016/j.ijrobp.2003.09.021 [DOI] [PubMed] [Google Scholar]
- 4. Eisbruch A, Rhodus N, Rosenthal D, Murphy B, Rasch C, Sonis S, et al. How should we measure and report radiotherapy-induced xerostomia? Semin Radiat Oncol 2003; 13: 226–34. doi: 10.1016/S1053-4296(03)00033-X [DOI] [PubMed] [Google Scholar]
- 5. Miah AB, Gulliford SL, Clark CH, Bhide SA, Zaidi SH, Newbold KL, et al. Dose-response analysis of parotid gland function: what is the best measure of xerostomia? Radiother Oncol 2013; 106: 341–45: S0167-8140(13)00126-6. doi: 10.1016/j.radonc.2013.03.009 [DOI] [PubMed] [Google Scholar]
- 6. Loutfi I, Nair MK, Ebrahim AK. Salivary gland scintigraphy: the use of semiquantitative analysis for uptake and clearance. J Nucl Med Technol 2003; 31: 81–85. [PubMed] [Google Scholar]
- 7. Blanco AI, Chao KSC, El Naqa I, Franklin GE, Zakarian K, Vicic M, et al. Dose-volume modeling of salivary function in patients with head-and-neck cancer receiving radiotherapy. Int J Radiat Oncol Biol Phys 2005; 62: 1055–69. doi: 10.1016/j.ijrobp.2004.12.076 [DOI] [PubMed] [Google Scholar]
- 8. Dijkema T, Raaijmakers CPJ, Ten Haken RK, Roesink JM, Braam PM, Houweling AC, et al. Parotid gland function after radiotherapy: the combined michigan and utrecht experience. Int J Radiat Oncol Biol Phys 2010; 78: 449–53. doi: 10.1016/j.ijrobp.2009.07.1708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Maes A, Weltens C, Flamen P, Lambin P, Bogaerts R, Liu X, et al. Preservation of parotid function with uncomplicated conformal radiotherapy. Radiother Oncol 2002; 63: 203–11. doi: 10.1016/S0167-8140(02)00013-0 [DOI] [PubMed] [Google Scholar]
- 10. Eisbruch A, Ten Haken RK, Kim HM, Marsh LH, Ship JA. Dose, volume, and function relationships in parotid salivary glands following conformal and intensity-modulated irradiation of head and neck cancer. Int J Radiat Oncol Biol Phys 1999; 45: 577–87. doi: 10.1016/s0360-3016(99)00247-3 [DOI] [PubMed] [Google Scholar]
- 11. Hey J, Setz J, Gerlach R, Janich M, Hildebrandt G, Vordermark D, et al. Parotid gland-recovery after radiotherapy in the head and neck region--36 months follow-up of a prospective clinical study. Radiat Oncol 2011; 6: 125. doi: 10.1186/1748-717X-6-125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Saarilahti K, Kouri M, Collan J, Hämäläinen T, Atula T, Joensuu H, et al. Intensity modulated radiotherapy for head and neck cancer: evidence for preserved salivary gland function. Radiother Oncol 2005; 74: 251–58. doi: 10.1016/j.radonc.2004.11.004 [DOI] [PubMed] [Google Scholar]
- 13. Chao KS, Deasy JO, Markman J, Haynie J, Perez CA, Purdy JA, et al. A prospective study of salivary function sparing in patients with head-and-neck cancers receiving intensity-modulated or three-dimensional radiation therapy: initial results. Int J Radiat Oncol Biol Phys 2001; 49: 907–16. doi: 10.1016/s0360-3016(00)01441-3 [DOI] [PubMed] [Google Scholar]
- 14. Gupta T, Hotwani C, Kannan S, Master Z, Rangarajan V, Murthy V, et al. Prospective longitudinal assessment of parotid gland function using dynamic quantitative pertechnate scintigraphy and estimation of dose-response relationship of parotid-sparing radiotherapy in head-neck cancers. Radiat Oncol 2015; 10: 67. doi: 10.1186/s13014-015-0371-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Braam PM, Roesink JM, Raaijmakers CPJ, Busschers WB, Terhaard CHJ. Quality of life and salivary output in patients with head-and-neck cancer five years after radiotherapy. Radiat Oncol 2007; 2: 3. doi: 10.1186/1748-717X-2-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Roesink JM, Moerland MA, Battermann JJ, Hordijk GJ, Terhaard CH. Quantitative dose-volume response analysis of changes in parotid gland function after radiotherapy in the head-and-neck region. Int J Radiat Oncol Biol Phys 2001; 51: 938–46. doi: 10.1016/s0360-3016(01)01717-5 [DOI] [PubMed] [Google Scholar]
- 17. Deasy JO, Moiseenko V, Marks L, Chao KSC, Nam J, Eisbruch A. Radiotherapy dose-volume effects on salivary gland function. Int J Radiat Oncol Biol Phys 2010; 76: S58-63. doi: 10.1016/j.ijrobp.2009.06.090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Jensen SB, Pedersen AML, Vissink A, Andersen E, Brown CG, Davies AN, et al. A systematic review of salivary gland hypofunction and xerostomia induced by cancer therapies: management strategies and economic impact. Support Care Cancer 2010; 18: 1061–79. doi: 10.1007/s00520-010-0837-6 [DOI] [PubMed] [Google Scholar]
- 19. Bonner JA, Harari PM, Giralt J, Cohen RB, Jones CU, Sur RK, et al. Radiotherapy plus cetuximab for locoregionally advanced head and neck cancer: 5-year survival data from a phase 3 randomised trial, and relation between cetuximab-induced rash and survival. Lancet Oncol 2010; 11: 21–28. doi: 10.1016/S1470-2045(09)70311-0 [DOI] [PubMed] [Google Scholar]
- 20. Lantini MS, Piludu M, Cossu M. Subcellular localization of epidermal growth factor in human parotid gland. Histochem J 2001; 33: 427–31. doi: 10.1023/a:1013780028887 [DOI] [PubMed] [Google Scholar]
- 21. Eisbruch A, Kim HM, Terrell JE, Marsh LH, Dawson LA, Ship JA. Xerostomia and its predictors following parotid-sparing irradiation of head-and-neck cancer. Int J Radiat Oncol Biol Phys 2001; 50: 695–704. doi: 10.1016/s0360-3016(01)01512-7 [DOI] [PubMed] [Google Scholar]
- 22. Chen W-C, Lai C-H, Lee T-F, Hung C-H, Liu K-C, Tsai M-F, et al. Scintigraphic assessment of salivary function after intensity-modulated radiotherapy for head and neck cancer: correlations with parotid dose and quality of life. Oral Oncol 2013; 49: 42–48: S1368-8375(12)00232-1. doi: 10.1016/j.oraloncology.2012.07.004 [DOI] [PubMed] [Google Scholar]
- 23. Dijkema T, Terhaard CHJ, Roesink JM, Braam PM, van Gils CH, Moerland MA, et al. Large cohort dose-volume response analysis of parotid gland function after radiotherapy: intensity-modulated versus conventional radiotherapy. Int J Radiat Oncol Biol Phys 2008; 72: 1101–9. doi: 10.1016/j.ijrobp.2008.02.059 [DOI] [PubMed] [Google Scholar]