Abstract
With optimized technique, the water-soluble contrast challenge is effective at triaging patients for operative vs non-operative management of suspected small bowel obstruction. Standardized study structure and interpretation guidelines aid in clinical efficacy and ease of use. Many tips and tricks exist regarding technique and interpretation, and their understanding may assist the interpreting radiologist. In the future, a CT-based water-soluble contrast challenge, utilizing oral contrast given as part of the initial CT examination, might allow for a more streamlined algorithm and provide more rapid results.
Background
Small bowel obstruction (SBO) results in significant morbidity and mortality as well as demand on healthcare resources.1,2 Intestinal obstruction represents a common cause of emergency general surgery admissions and often presents acutely to the emergency department.3–5 SBO is also a leading cause of emergency general surgery procedures, due to exploration and subsequent small bowel resection or adhesiolysis.6 For example, according to the most recent emergency laparotomy audit, 47% of patients who undergo emergency laparotomy in the UK are diagnosed with bowel obstruction intraoperatively.5
While sometimes the necessity for emergent surgery is determined based primarily on clinical factors, imaging plays an increasingly significant role in identifying patients with signs of bowel ischemia, necrosis, perforation, or closed loop obstructions that would necessitate emergent intervention and/or bowel resection.7–9 However, of the patients admitted for suspected SBO, many will ultimately not have a mechanical process, and only a small percentage, estimated at 11% in one study, will require operative management.3 Thus, in the setting of suspected SBO the necessity and timing of operative management presents a difficult clinical challenge due to a desire to balance early operative management of strangulated obstructions and timely exploration of SBOs that will not resolve with non-operative management in an attempt to avoid unnecessary surgical explorations.10–13
Diagnostic performance
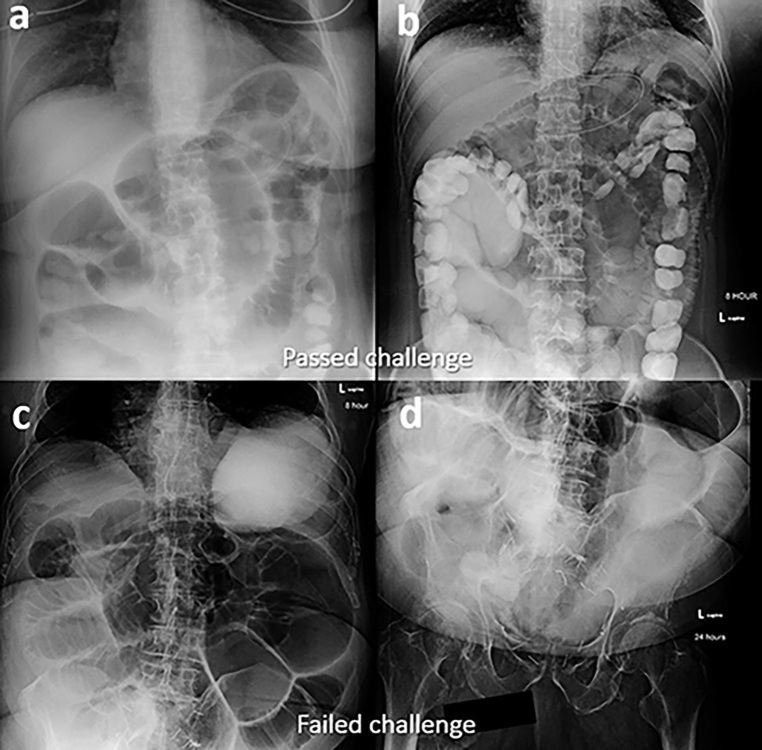
The water-soluble contrast (WSC) challenge has gained in popularity and has demonstrated its clinical utility in the setting of suspected SBO. Published use of WSC material in the setting of possible SBO dates back to the 1950s.14 Over time, a more formal ‘challenge’ protocol developed which, briefly, uses serial abdominal radiographs (AXR) obtained at set times after the administration of oral WSC to assess for successful transit of contrast into the colon. Challenges where contrast transits to the colon are considered passed or successful (Figure 1) while challenges where there is no colonic contrast are considered failed (Figures 2 and 3). Since its inception, multiple studies that mostly used Gastrografin have been published, demonstrating the diagnostic utility of a WSC challenge in the setting of suspected SBO.15–20 For example, a meta-analysis of 14 prospective studies reported by Branco et al demonstrated that the appearance of contrast in the colon by 24 h had a pooled sensitivity of 96% and positive-predictive value (PPV) of 99% for predicting successful non-operative SBO management.19 An additional meta-analysis by Ceresoli et al demonstrated that contrast passage into the colon on follow-up AXR had a sensitivity of 92% (95% CI 90 to 94%), specificity of 93% (95% CI 88 to 96%), PPV of 98% (95% CI 97 to 99%), and negative-predictive value of 75% (95% CI 70 to 81%).20 Certainly, it makes sense from a pathophysiological standpoint that contrast transit, which is dependent upon luminal patency, would be significantly correlated with surgical intervention, which is more likely to be required in cases of high-grade or complete obstruction. It is possible that the diagnostic performance results reported by many of these studies, some of which reported a sensitivity or specificity of 100%, may not be truly representative of the study performance in a more real-world heterogeneous clinical practice due to selection and confirmation bias. However, a recent retrospective study that utilized iohexol also demonstrated high sensitivity and PPV, 91.2 and 96.5% respectively, in a real-world setting where the necessity of surgical intervention was determined by the consulting surgeon using the WSC challenge results along with other imaging and clinical factors.21 Khasawneh et al also investigated the value of the WSC challenge in the post-operative patient (i.e. differentiating SBO vs post-operative ileus) with a calculated sensitivity of 98%, PPV of 94%, specificity of 63%, and negative-predictive value of 83%.22
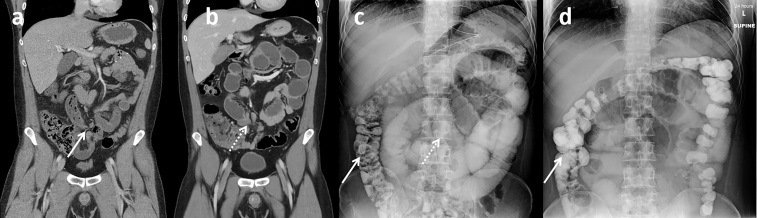
Figure 1.
Passed challenge in a 31-year-old patient with prior history of right lower quadrant pancreas transplant a few years earlier presented with new abdominal pain. (a) Initial presentation CT with minimally dilated small bowel including ‘small bowel feces’ sign (arrow) suspicious for possible developing partial small bowel obstruction. Patient was initially managed non-operatively at an outside facility but was transferred due to non-resolution. (b) Repeat CT with i.v. contrast, completed 4 days after the first scan, demonstrates increasingly dilated and fluid-filled small bowel, as well as possible transition point in the right lower abdomen (dashed arrow). WSC challenge was initiated and 8-h AXR (c) demonstrated successful contrast transit into the colon (arrow) with persistent mildly dilated small bowel (dashed arrow). Subsequent 24-h AXR (d) demonstrated additional contrast transit to the colon (arrow) and improving small bowel dilatation. Given the success of the WSC challenge, an initial nasogastric (NG) tube clamp trial and subsequent diet advancement was completed. Non-operative management was successful with return of bowel function and patient discharge the next day. AXR, abdominal radiographs; WSC, water-soluble contrast.
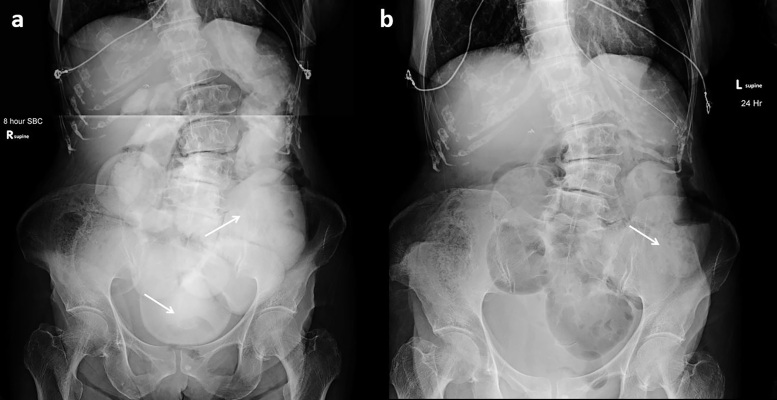
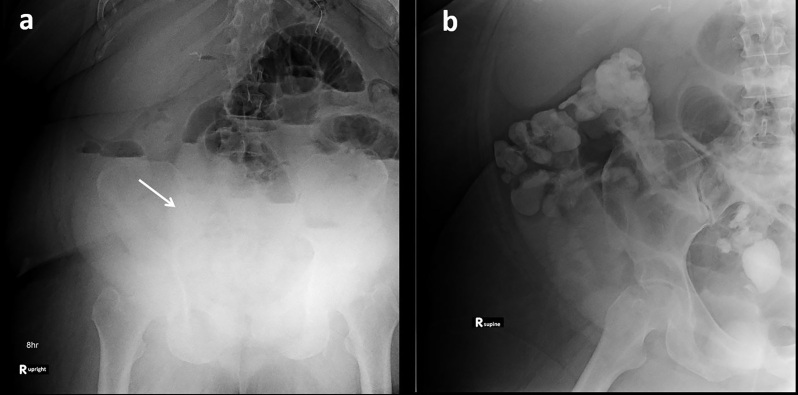
Figure 2.
Failed challenge in a 78-year-old with history of Crohn’s disease and prior ileocecetomy presenting with concern for small bowel obstruction. (a) 8-h AXR demonstrated oral contrast in multiple dilated loops of small bowel (arrows) and no evidence of colonic contrast. (b) 24-h AXR demonstrates further dilution of the small bowel contrast (arrow) and no colonic contrast passage identified. The patient required surgical management with lysis of adhesions. AXR, abdominal radiographs.
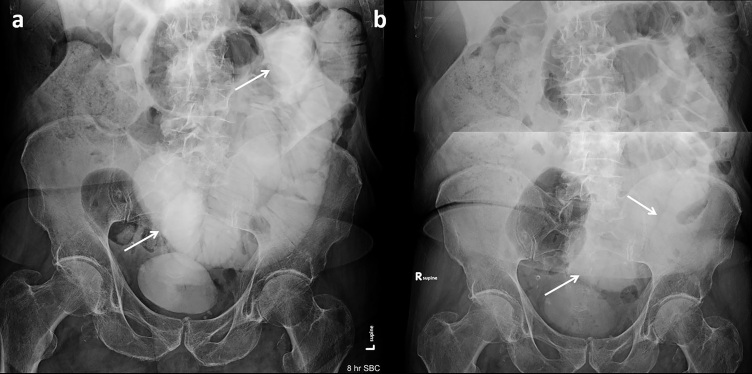
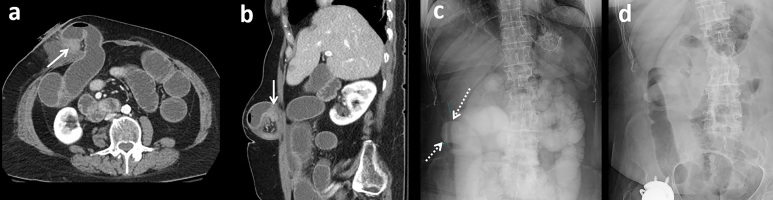
Figure 3.
Additional failed challenge in a 91-year-old with remote history of prostate cancer and prostatectomy. (a) Diluted contrast seen in dilated loops of small bowel (arrows) on the 8-h AXR. (b) Subsequent 24-h AXR demonstrates further dilution of SB contrast in persistently dilated loops (arrows) and no evidence of colonic contrast. The patient required surgical management with lysis of adhesions.AXR, abdominal radiographs; SB, small bowel.
Therapeutic benefit
While there is strong consensus regarding the diagnostic utility of the WSC challenge, there is more uncertainty regarding the possible therapeutic benefit of the WSC challenge. Prior studies, including two meta-analyses and a multi-institutional prospective observational trial, concluded that the WSC challenge significantly decreased operative intervention or the length of hospital stay.18–20 For example, the meta-analysis by Ceresoli et al demonstrated that the administration of oral WSC in the setting of a WSC challenge reduced the need for surgery (odds ratio, 0.55), length of stay (weighted mean difference, 22.18 days), and time to resolution (weighted mean difference, 228.25 h).20 However, the randomized trial by Scotte et al found contrary results and did not find an additive benefit of the WSC challenge regarding clinical outcomes for SBO treatment.23 Two small studies have also evaluated a possible therapeutic benefit in the setting of suspected post-operative ileus; failing to find a significant difference in overall recovery time but with some possible benefit regarding earlier symptomatic relief.24,25 Further research, ideally in the setting of randomized controlled trials, may be helpful for better defining the possible therapeutic benefit.
WSC challenge technique
The initial step in the WSC challenge is regarding the appropriate patient selection and determination of whether there are features, either clinically or imaging-based, that might necessitate more emergent surgical management. Examples include clinical instability, rising serum lactic acid, occluded mesenteric vessels, closed loop obstruction or volvulus, hypoenhancing bowel, pneumatosis, pneumoperitoneum, or portal venous gas. Any of these features would point towards emergent surgical management. Assuming that none is present, a WSC challenge can be considered. Indeed, there are some features on CT such as the small bowel feces sign, which has been shown to correlate with a more subacute partial SBO that might be amenable to non-operative management.9
For all patients planning to undergo the WSC challenge, a NG tube is first placed by the ordering clinical service. Gastric decompression for at least 2 h is required to allow for adequate decompression of the stomach prior to contrast administration. Subsequently, undiluted WSC (we utilize 100 ml of iohexol (Omnipaque), concentration 300 mg iodine/mL) is administered at the patient’s bedside through the NG tube. Contrast type and amount varies somewhat in the published literature, although most protocols utilize from 40 to 150 ml of undiluted contrast (either diatrizoate meglumine/diatrizoate sodium or iohexol).20,21 If desired, 30 ml of water can be used to flush the NG tube prior to clamping for 2 h. Early in our experience, the set time for decompression prior to contrast administration, as well as the time for clamping following contrast administration was less rigid. However, we found this resulted in cases where the contrast was largely removed by the NG tube, resulting in a non-diagnostic study (Figure 4).
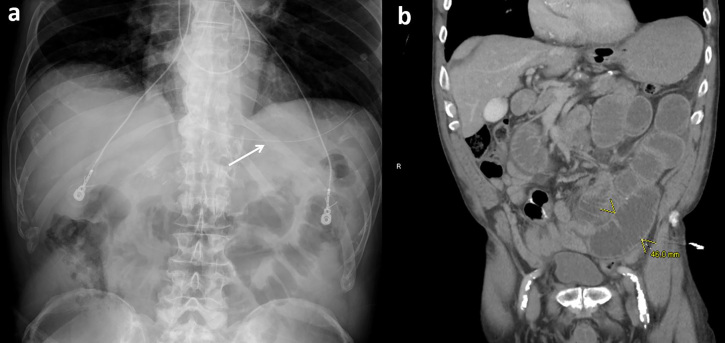
Figure 4.
No visible oral contrast. (a) No visible contrast on initial AXR. Decompressed stomach with nasogastric tube (arrow) in place. (b) Follow-up CT at 24 h confirms continued SBO and no visible oral contrast. Administered contrast was probably removed by early gastric tube decompression. The authors recommend allowing at least 2 h of NG tube clamping after contrast administration prior to restarting decompression. AXR, abdominal radiographs; NG, nasogastric; SBO, small bowel obstruction.
Abdominal radiographs are then obtained at set times following the administration of contrast. Evaluation of the literature demonstrates a variety of AXR times that have been utilized. Some studies, and institutions, obtain the first radiograph 2–6 h after contrast and while there is no definitive evidence regarding best practices, Ceresoli et al did find a significant increase in accuracy for AXR taken at 8–12 h relative to tests taken at 2–6 h, 82% (95% CI 77 to 86%) compared to 97% (95% CI 95 to 100%).20 At our institution (University of Wisconsin Hospitals and Clinics), we obtain the first AXR at 8 h after contrast administration. Ideally, the AXR is obtained in the radiology department, and a bedside portable AXR is only performed if required due to patient status. A two-view study (upright and supine) can be completed if desired by the ordering service. A 24-h AXR, using the same protocol, is also obtained as standard practice, unless specifically cancelled by the radiologist or ordering provider (e.g. for successful challenge at 8-h AXR). There appears to be greater consensus in the literature for using a 24-h AXR end point, which matches those proposed in the Bologna guidelines for management of adhesive SBO.26
One key feature that has proved vital for successful challenge administration and ease of use is having a dedicated order set available to providers through the electronic medical record. The order set is easily searchable and contains the specific order instructions for the necessary contrast and abdominal radiographs. The individual orders also include administration notes regarding NG clamping, radiograph type, etc. In our experience, we have found this communication significantly improves protocol consistency and ease of ordering.
Historically, the WSC challenge has been completed with Gastrografin (diatrizoate meglumine and diatrizoate sodium) and much of the research to date has focused on this contrast medium. It is thought that some of its therapeutic benefit may stem from its hyperosmolarity (1900 mOsm/l) which is theorized to possibly stimulate bowel peristalsis, increase the pressure gradient across the site of obstruction, and reduce bowel wall edema by shifting fluid into the intraluminal space. Other related ionic diatrizoate agents include Gastroview and Hypaque. However, it is important to note that the underlying evidence to support the exclusive use of diatrizoate-based agents is limited. Indeed, the only prior head-to-head comparison that we are aware of demonstrated no difference between iohexol and Gastrografin regarding bowel transit.27 Studies have also shown that the oral ingestion of iohexol was also better tolerated by the patient than Gastrografin.27,28 While iohexol is considered non-ionic and overall low osmolar, it remains hyperosmolar relative to plasma (672 mOsm/l for iohexol 300 mg/ml vs 285 mOsm/l for plasma). Certainly, the hyperosmolarity difference is greater for Gastrografin than iohexol, but as previously described, it is unclear if this leads to a true functional or therapeutic difference. Cost is often another consideration. While historically iohexol was a more expensive option, with the increased use of non-ionic, iodine-based contrast agents for CT, there has been an associated decrease in the overall cost and, at least at our institution, the cost of iohexol is now less than the cost of Gastrografin.29 Finally, while the overall incidence is probably low, there is also less concern for chemical pneumonitis or pulmonary edema from aspiration of iohexol.30 A recent retrospective study utilizing iohexol for the WSC challenge demonstrated high technical adequacy as well as high sensitivity and PPV in predicting non-operative management.21 Further research comparing the two agents would be beneficial, but for now local practice patterns, comfort with use, and availability might lead certain institutions to choose one over the other.
WSC challenge image interpretation
Interpretation of the obtained radiographs for the WSC challenge follows basic principles with a few additional tips and tricks for difficult or challenging situations. Similar to the evaluation of radiographic bowel gas, features such as peripheral location and haustral folds can be utilized to confidently identify colonic contrast. The contrast will first appear in the cecum and right colon with subsequent filling/transit into the remaining colon. One relatively frequent concern surrounds the visibility of diluted luminal contrast on the radiograph and in certain cases may make the study technically inadequate. However, one important point is that in our experience what may seem to be faint and diluted small bowel contrast will become more concentrated and easier to identify, if it successfully transits to the colon and concentrates (Figure 5). Therefore, if there is only persistent diluted small bowel contrast seen on 24-h AXR then the study most likely represents a failed challenge. Similarly, while rare, there are infrequent cases where little or no contrast can be seen on the WSC challenge radiographs, possibly due to a combination of dilution in dilated fluid-filled proximal bowel or possibly some component of contrast removal through the NG tube even after 2 h of clamping due to stasis. In these cases, we have found it best to discuss the outcome with the ordering provider and, while a repeat trial is sometimes offered, in our experience, this is anecdotally suggestive of a higher grade obstruction and the possible need for surgical management.
Figure 5.
Increased contrast concentration and visibility after successful transit into colon. (a) Contrast is initially difficult to see on 8-h AXR for an obese patient, although there is suggestion of diluted contrast in dilated small bowel loops (arrow) and no colonic contrast. (b) On subsequent 24-h AXR, colonic contrast is present, which is easier to see due to its more condensed nature. The patient was managed non-operatively and discharged 2 days later. This case highlights that even if small bowel contrast is initially difficult to see, with successful transit, there often will be clear evidence of a passed trial given concentration of contrast in the colon. Conversely, if only diluted small bowel contrast is seen, then this would suggest a failed trial and inadequate contrast passage. AXR, abdominal radiographs.
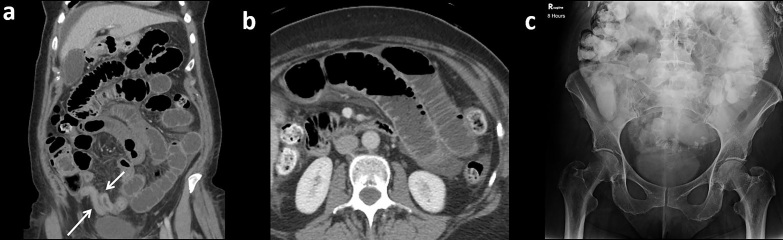
One challenging patient population is those with prior ostomy creation. In these patients, a trial might be considered successful if contrast is seen in ostomy bag or only a small residual amount remains present in the bowel (Figure 6). If contrast is seen extending to the ostomy, but not clearly in an ostomy bag, it may represent a successful challenge but recommendation for clinical correlation, including ostomy output, is prudent especially if the suspected site of obstruction was near the ostomy.
Figure 6.
Successful challenge in a 58-year-old with history of prior colectomy with end ileostomy and small bowel resection for incarcerated hernia. Presentation CT with i.v. and no oral contrast (a, axial; b, sagittal) demonstrates dilated fluid-filled small bowel extending to a transition point adjacent to the ileostomy stoma (arrows). (c) 8-h AXR from the WSC challenge with mildly dilated and contrast opacified small bowel extending to the ostomy site (dashed arrow). (d) 24-h AXR with complete contrast clearance consistent with patency. AXR, abdominal radiographs; WSC, water-soluble contrast.
Even in the setting of a successful challenge, some patients may still require additional time for return of normal bowel function. A study by Mulder et al found that 29% (60/208) of patients with eventually successful non-operative management required >48 h of either NGT decompression or to pass flatus (Figure 7).31 In general, we recommend obtaining the 24-h radiograph unless there is complete contrast transit at 8 h or if there are findings suspicious of persistent obstruction at 8 h that needs surgical management. Anecdotally, we have found in our practice possible correlation between the speed of contrast transit and degree of partial obstruction, if present. For example, we have found that a patient who has complete or near complete contrast transit into the colon on the 8-h radiograph is highly unlikely to have a partial SBO and, in the absence of an intermittent hernia or other predisposing factor, is unlikely to have short-term recurrence. In contrast, a patient who maintains a degree of contrast filled and dilated small bowel, even after passage of some contrast into the colon, may suggest a more significant degree of partial obstruction and, in our experience, is probably more likely to have eventual failure of conservative management and may ultimately require surgical intervention. However, it is important to note the paucity of literature or evidence in this area and further research is warranted.
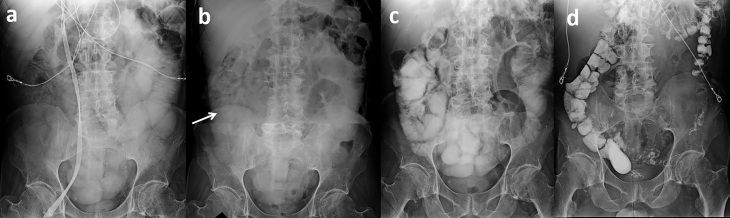
Figure 7.
Improved contrast transit between two closely spaced WSC challenges. Initial WSC challenge radiographs (a, 8 h; b, 24 h) demonstrate partial contrast transit into the colon (arrow) by 24 h but with persistent mildly dilated small bowel. The patient left against medical advice before a clamp trial and progression of diet could be attempted but returned the next day. Images from a repeat WSC challenge obtained 2 days after the initial challenge (c, 8 h; d, 24 h) demonstrate improved contrast transit with some colonic contrast seen at 8 h and complete transit into the colon by 24 h. Following the repeat challenge, the patient’s diet was successfully advanced with resolution of symptoms. WSC, water-soluble contrast
Utilizing the WSC challenge in atypical or heterogeneous patient populations
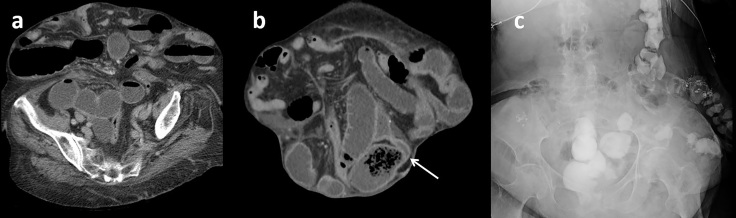
It is important to note that much of the research investigating the WSC challenge has focused solely on patients with suspected adhesive SBO. Additionally, many studies excluded patients with a history of cancer and/or recent surgery. However, prior work has supported the safety and possible utility of using the WSC challenge in the setting of recent surgery or history of malignancy.22,32 Oncologic patients present a particularly difficult challenge (especially those with active malignancy).32 Some possible complicating factors are increased in-hospital mortality, difficult goals of care situation, concern for tumor spread secondary to operative management, and high risk for recurrent SBO. However, often there may still be benefits of the WSC challenge, such as predicting non-operative management success (similar to a non-oncologic patient) and assistance with goals of care discussions (Figure 8). Khasawneh et al showed decreased exploratory operations in a set of patients with malignant history compared to a historical control group from before the initiation of the WSC challenge (26% vs 41%).32 Discussion with the ordering team may help to understand the aim of the study (i.e. attempt to avoid surgery, determine severity to assist with goals of care discussion) (Figure 9). Similarly, there was initially some concern about performing the WSC challenge in the early post-operative patient. However, Khasawneh et al found that the WSC challenge could be completed safely in this patient population22 and at our institution, this is not a contraindication to attempting a WSC challenge (Figure 10).
Figure 8.
Failed challenge in an oncologic patient with malignant obstruction from metastatic ovarian cancer who decompensated on therapy. (a) Prior CT with fluid-filled dilated SB from malignant SB tethering to an adjacent metastatic deposit (solid arrows). (b) 24-h WSC challenge radiograph demonstrates persistent dilated small bowel distended with air (solid arrow) or contrast (dashed arrow) without evidence of contrast transit into the colon. After goals of care discussion, the patient was transferred to comfort cares. WSC, water-soluble contrast; SB, small bowel.
Figure 9.
Additional examples of WSC challenge in patients with history of cancer Top patient (a, b) with known metastatic high-grade serous carcinoma complicated by recurrent SBO. Admission AXR (a) with evidence of SBO. Partial transit of contrast into colon at 8 h (b) consistent with a successful WSC challenge. Managed non-operatively. Bottom patient (c, d) with history of bladder and prostate cancer. Prior cystoprostatectomy, radiation therapy, and ileal conduit creation. 8-h AXR (a) with severely dilated SB and contrast only in stomach. 24-h AXR (b) with diluted contrast in dilated SB. The patient was subsequently taken for surgical management and lysis of adhesions. AXR, abdominal radiographs; SBO, small bowel obstruction; WSC, water-soluble contrast.
Figure 10.
Passed challenge in an immediate post-operative patient who initially presented with rapidly progressive SB obstruction due to a focal closed loop component secondary to adhesions. Underwent lysis of adhesions but no resection. Presented again 5 days later with increasing abdominal pain, nausea, and emesis. (a, b) CT with i.v. and no oral contrast from repeat admission demonstrated diffusely dilated small bowel and focally thickened loops (arrows in a) in the right lower quadrant which corresponded to the site of prior closed loop. (c) WSC challenge was initiated and demonstrated successful transit into the colon on 8-h AXR with some persistent contrast in mildly dilated SB. Overall, this was felt to be most suggestive of post-operative ileus. Patient was treated successfully with non-operative management. AXR, abdominal radiographs; SB, small bowel; WSC, water-soluble contrast.
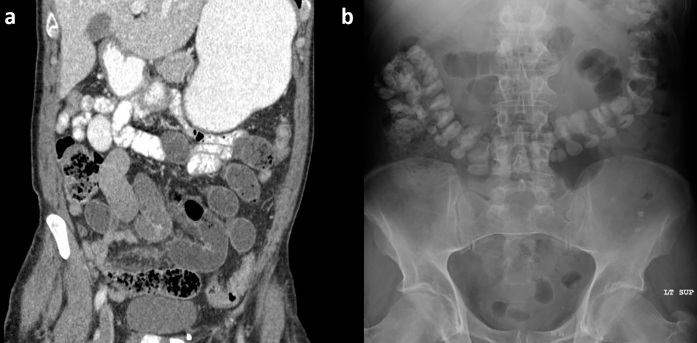
An additional group that was not included in the traditional WSC challenge studies were those with hernia-related obstructions, although previously there were at least two case reports of the use of a WSC challenge in the setting of an obturator33 or large ventral hernia.34 One group in particular that offers both a diagnostic and clinical management challenge is patients with complex ventral/incisional hernias as well as recurrent parastomal hernia, since it can be difficult to predict if the obstruction will resolve without surgery and even with operative management, the risk of recurrence might remain high. Again, a recent retrospective study looking at patients managed with an iohexol-based WSC challenge did allow for the inclusion of patients with hernia-related obstructions, if deemed appropriate by the ordering surgeon, without those patients experiencing any resultant complication (Figure 11).21
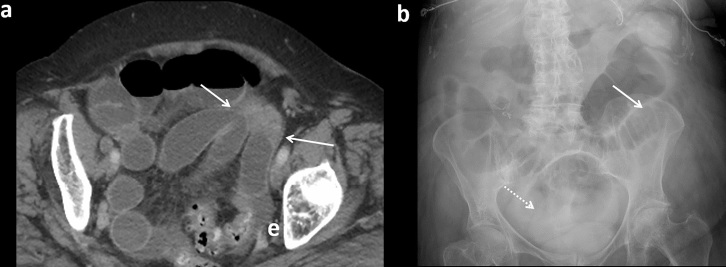
Figure 11.
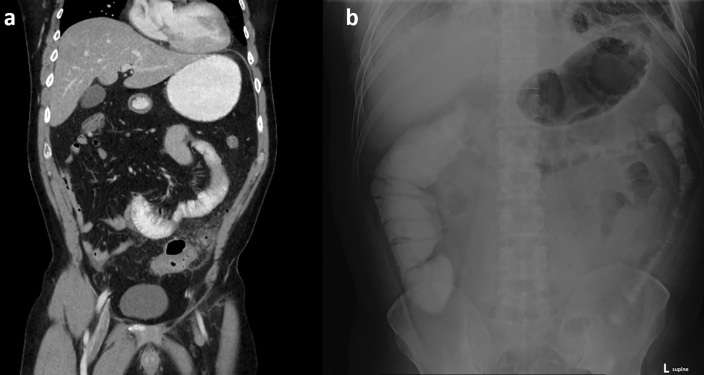
Passed challenge in setting of large ventral hernia in an 89-year-old with multiple prior abdominal surgeries. (a) Axial CT without oral contrast demonstrates dilated, mostly fluid-filled small bowel extending into an anterior ventral hernia. (b) Anterior coronal CT image with small bowel fecal sign at the suspected transition point. (c) Patient successfully passed the contrast challenge (8-h AXR) and was managed non-operatively. AXR, abdominal radiographs
It is the authors’ belief that while inclusion of challenging patient populations might affect the overall diagnostic performance results of the study, the WSC challenge offers a powerful tool to assist the surgical services regarding whether or not to undergo operative management and, indeed, it may be these difficult cases where the challenge can offer its greatest additive value. Discussion with the ordering service prior to challenge initiation and after the AXR results are available may further increase the utility and accuracy of the study.
CT-based WSC challenge
One consideration for the future is to formally combine a WSC challenge with the initial CT examination that is typically first obtained in the setting of suspected SBO. In general, the utilization of positive oral contrast material for abdominal CT varies widely. Beyond clinical indications where its use is generally indicated (e.g. suspected bowel leak or fistula, CT colonography) or best avoided (e.g. suspected mesenteric ischemia or GI bleed), there are a number of clinical scenarios where controversy exists.35 One such area of controversy is suspected SBO, where some might consider oral contrast administration prior to CT to be contraindicated. Although the contrast often fails to reach the transition point of a true mechanical bowel obstruction at the time of CT imaging, its transit can be followed on subsequent AXR or CT imaging, as indicated. In some cases, transit of the diluted CT oral contrast can be followed for evidence of transit into the colon (Figures 12 and 13). However, in many patients, the diluted contrast may be difficult to see, especially with increasing body habitus. One possible solution would be to reduce the degree of oral contrast dilution in this specific patient population and future research might investigate an optimized concentration allowing for consistent visibility radiographically without sacrificing the diagnostic capacity of the CT. A benefit of this protocol would be decreasing the time delay between patient presentation and initiation of a WSC challenge. In cases of non-obstructive functional/motility bowel issues or mild partial obstruction, the oral contrast bolus may also prove to be therapeutic. Overall, this hybrid CT approach to the WSC challenge could potentially streamline the evaluation of suspected SBO, and warrants further investigation.
Figure 12.
Example of successful CT-based WSC challenge. (a) Initial CT which demonstrates dilated fluid-filled distal small bowel. Oral contrast was not near the transition point. (b) Follow-up AXR demonstrating transit of CT oral contrast into colon. AXR, abdominal radiographs; WSC, water-soluble contrast.
Figure 13.
Additional CT-based WSC challenge in a 46-year-old male presenting with abdominal pain. (a) Presentation CT, including i.v. and oral contrast, demonstrates suspected diverticulitis in the left abdomen. Adjacent dilated proximal small bowel was favored to be reactive ileus. (b) Follow-up abdominal radiograph, obtained 24 h later, demonstrates complete transit of oral contrast into the colon and confirms small bowel patency. WSC, water-soluble contrast.
Conclusion
In conclusion, successful transit of contrast to the colon during the WSC challenge can be utilized effectively to predict which SBO patients are likely to be successfully managed non-operatively. After an initial triage regarding the appropriateness of the WSC challenge in an individual patient, many tips and tricks exist regarding study implementation and interpretation that may assist the interpreting radiologist. While the WSC challenge is most often utilized in the setting of uncomplicated, adhesive SBO there are other situations where it can be safely utilized and might offer value to the surgical services, such as in the immediate post-operative period or in patients with an oncologic history. In the future a CT-based WSC challenge, utilizing oral contrast given as part of the initial CT examination, might allow for a streamlined algorithm and alleviate the need for additional contrast administration.
Footnotes
Disclosure: P.J.P. has served as an consultant to Bracco, Zebra, and GE Healthcare; and is shareholder in SHINE & Elucent.
Contributor Information
Edward M. Lawrence, Email: ELawrence@uwhealth.org.
Perry J. Pickhardt, Email: ppickhardt2@uwhealth.org.
REFERENCES
- 1.Irvin TT. Abdominal pain: a surgical audit of 1190 emergency admissions. Br J Surg 1989; 76: 1121–5. doi: 10.1002/bjs.1800761105 [DOI] [PubMed] [Google Scholar]
- 2.Ray NF, Denton WG, Thamer M, Henderson SC, Perry S. Abdominal adhesiolysis: inpatient care and expenditures in the United States in 1994. J Am Coll Surg 1998; 186: 1–9. doi: 10.1016/S1072-7515(97)00127-0 [DOI] [PubMed] [Google Scholar]
- 3.Gale SC, Shafi S, Dombrovskiy VY, Arumugam D, Crystal JS. The public health burden of emergency general surgery in the United States: A 10-year analysis of the Nationwide Inpatient Sample--2001 to 2010. J Trauma Acute Care Surg 2014; 77: 202–8. doi: 10.1097/TA.0000000000000362 [DOI] [PubMed] [Google Scholar]
- 4.Ozturk E, van Iersel M, Stommel MM, Schoon Y, Ten Broek RR, van Goor H. Small bowel obstruction in the elderly: a plea for comprehensive acute geriatric care. World J Emerg Surg 2018; 13: 48. doi: 10.1186/s13017-018-0208-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.NELA Project Team . Sixth patient report of the National emergency laparotomy audit. RCoA London; 2020. [Google Scholar]
- 6.Scott JW, Olufajo OA, Brat GA, Rose JA, Zogg CK, Haider AH, et al. Use of national burden to define operative emergency general surgery. JAMA Surg 2016; 151: e160480. doi: 10.1001/jamasurg.2016.0480 [DOI] [PubMed] [Google Scholar]
- 7.Paulson EK, Thompson WM. Review of small-bowel obstruction: the diagnosis and when to worry. Radiology 2015; 275: 332–42. doi: 10.1148/radiol.15131519 [DOI] [PubMed] [Google Scholar]
- 8.Diamond M, Lee J, LeBedis CA. Small bowel obstruction and ischemia. Radiol Clin North Am 2019; 57: 689–703. doi: 10.1016/j.rcl.2019.02.002 [DOI] [PubMed] [Google Scholar]
- 9.Scrima A, Lubner MG, King S, Pankratz J, Kennedy G, Pickhardt PJ. Value of MDCT and clinical and laboratory data for predicting the need for surgical intervention in suspected small-bowel obstruction. AJR Am J Roentgenol 2017; 208: 785–93. doi: 10.2214/AJR.16.16946 [DOI] [PubMed] [Google Scholar]
- 10.Hwang U, Aufses AH, Bickell NA. Factors associated with delays to emergency care for bowel obstruction. Am J Surg 2011; 202: 1–7. doi: 10.1016/j.amjsurg.2010.05.003 [DOI] [PubMed] [Google Scholar]
- 11.Teixeira PG, Karamanos E, Talving P, Inaba K, Lam L, Demetriades D. Early operation is associated with a survival benefit for patients with adhesive bowel obstruction. Ann Surg 2013; 258: 459–65. doi: 10.1097/SLA.0b013e3182a1b100 [DOI] [PubMed] [Google Scholar]
- 12.Bickell NA, Federman AD, Aufses AH. Influence of time on risk of bowel resection in complete small bowel obstruction. J Am Coll Surg 2005; 201: 847–54. doi: 10.1016/j.jamcollsurg.2005.07.005 [DOI] [PubMed] [Google Scholar]
- 13.Zielinski MD, Eiken PW, Bannon MP, Heller SF, Lohse CM, Huebner M, et al. Small bowel obstruction-who needs an operation? A multivariate prediction model. World J Surg 2010; 34: 910–9. doi: 10.1007/s00268-010-0479-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Epstein B. Nonabsorbable water-soluble contrast mediums: their use in diagnosis of intestinal obstruction. JAMA 1955; 165: 2–4. [DOI] [PubMed] [Google Scholar]
- 15.Baghdadi YMK, Choudhry AJ, Goussous N, Khasawneh MA, Polites SF, Zielinski MD. Long-term outcomes of gastrografin in small bowel obstruction. J Surg Res 2016; 202: 43–8. doi: 10.1016/j.jss.2015.11.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goussous N, Eiken PW, Bannon MP, Zielinski MD. Enhancement of a small bowel obstruction model using the gastrografin® challenge test. J Gastrointest Surg 2013; 17: 110–7. doi: 10.1007/s11605-012-2011-6 [DOI] [PubMed] [Google Scholar]
- 17.Zielinski MD, Eiken PW, Heller SF, Lohse CM, Huebner M, Sarr MG, et al. Prospective, observational validation of a multivariate small-bowel obstruction model to predict the need for operative intervention. J Am Coll Surg 2011; 212: 1068–76. doi: 10.1016/j.jamcollsurg.2011.02.023 [DOI] [PubMed] [Google Scholar]
- 18.Zielinski MD, Haddad NN, Cullinane DC, Inaba K, Yeh DD, Wydo S, et al. Multi-institutional, prospective, observational study comparing the Gastrografin challenge versus standard treatment in adhesive small bowel obstruction. J Trauma Acute Care Surg 2017; 83: 47–54. doi: 10.1097/TA.0000000000001499 [DOI] [PubMed] [Google Scholar]
- 19.Branco BC, Barmparas G, Schnüriger B, Inaba K, Chan LS, Demetriades D. Systematic review and meta-analysis of the diagnostic and therapeutic role of water-soluble contrast agent in adhesive small bowel obstruction. Br J Surg 2010; 97: 470–8. doi: 10.1002/bjs.7019 [DOI] [PubMed] [Google Scholar]
- 20.Ceresoli M, Coccolini F, Catena F, Montori G, Di Saverio S, Sartelli M, et al. Water-soluble contrast agent in adhesive small bowel obstruction: a systematic review and meta-analysis of diagnostic and therapeutic value. Am J Surg 2016; 211: 1114–25. doi: 10.1016/j.amjsurg.2015.06.012 [DOI] [PubMed] [Google Scholar]
- 21.Lawrence EM, Pickhardt PJ. Water-soluble contrast challenge for suspected small-bowel obstruction: technical success rate, accuracy, and clinical outcomes. AJR Am J Roentgenol 2021; 217: 1365–6. doi: 10.2214/AJR.21.26132 [DOI] [PubMed] [Google Scholar]
- 22.Khasawneh MA, Ugarte MLM, Srvantstian B, Dozois EJ, Bannon MP, Zielinski MD. Role of gastrografin challenge in early postoperative small bowel obstruction. J Gastrointest Surg 2014; 18: 363–8. doi: 10.1007/s11605-013-2347-6 [DOI] [PubMed] [Google Scholar]
- 23.Scotté M, Mauvais F, Bubenheim M, Cossé C, Suaud L, Savoye-Collet C, et al. Use of water-soluble contrast medium (gastrografin) does not decrease the need for operative intervention nor the duration of hospital stay in uncomplicated acute adhesive small bowel obstruction? A multicenter, randomized, clinical trial (adhesive small bowel obstruction study) and systematic review. Surgery 2017; 161: 1315–25. doi: 10.1016/j.surg.2016.11.026 [DOI] [PubMed] [Google Scholar]
- 24.Biondo S, Miquel J, Espin-Basany E, Sanchez JL, Golda T, Ferrer-Artola AM, et al. A double-blinded randomized clinical study on the therapeutic effect of Gastrografin in prolonged postoperative ileus after elective colorectal surgery. World J Surg 2016; 40: 206–14. doi: 10.1007/s00268-015-3260-9 [DOI] [PubMed] [Google Scholar]
- 25.Vather R, Josephson R, Jaung R, Kahokehr A, Sammour T, Bissett I. Gastrografin in prolonged postoperative ileus: a double-blinded randomized controlled trial. Ann Surg 2015; 262: 23–30. doi: 10.1097/SLA.0000000000001062 [DOI] [PubMed] [Google Scholar]
- 26.Di Saverio S, Coccolini F, Galati M, Smerieri N, Biffl WL, Ansaloni L, et al. Bologna guidelines for diagnosis and management of adhesive small bowel obstruction (ASBO): 2013 update of the evidence-based guidelines from the world society of emergency surgery ASBO working group. World J Emerg Surg 2013; 8: 42. doi: 10.1186/1749-7922-8-42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stordahl A, Laerum F, Gjølberg T, Enge I. Water-soluble contrast media in radiography of small bowel obstruction. Comparison of ionic and non-ionic contrast media. Acta Radiol 1988; 29: 53–6. [PubMed] [Google Scholar]
- 28.Pollentine A, Ngan-Soo E, McCoubrie P. Acceptability of oral iodinated contrast media: a head-to-head comparison of four media. Br J Radiol 2013; 86: 20120636. doi: 10.1259/bjr.20120636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Johnson B, Hinshaw JL, Robbins JB, Pickhardt PJ. Objective and subjective intrapatient comparison of iohexol versus diatrizoate for bowel preparation quality at CT colonography. AJR Am J Roentgenol 2016; 206: 1202–7. doi: 10.2214/AJR.15.15373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lovett I, Donchey S, Doust B, Branson J, Munro V. The effects of water soluble contrast agents on the respiratory tract. Australas Radiol 1989; 33: 124–7. doi: 10.1111/j.1440-1673.1989.tb03253.x [DOI] [PubMed] [Google Scholar]
- 31.Mulder MB, Hernandez M, Ray-Zack MD, Cullinane DC, Turay D, Wydo S, et al. A Significant Proportion of Small Bowel Obstructions Require >48 Hours to Resolve After Gastrografin. J Surg Res 2019; 233: 408–12. doi: 10.1016/j.jss.2018.08.019 [DOI] [PubMed] [Google Scholar]
- 32.Khasawneh MA, Eiken PW, Srvantstyan B, Bannon MP, Zielinski MD. Use of the Gastrografin challenge in patients with a history of abdominal or pelvic malignancy. Surgery 2013; 154: 769–76. doi: 10.1016/j.surg.2013.07.002 [DOI] [PubMed] [Google Scholar]
- 33.Leow JJ, How KY, Goh MH, Woon WWL, Low JK. Non-operative management of obturator hernia in an elderly female. Hernia 2014; 18: 431–3. doi: 10.1007/s10029-012-1036-9 [DOI] [PubMed] [Google Scholar]
- 34.D'Agostino R, Ali NS, Leshchinskiy S, Cherukuri AR, Tam JK. Small bowel obstruction and the gastrografin challenge. Abdom Radiol 2018; 43: 2945–54. doi: 10.1007/s00261-018-1591-3 [DOI] [PubMed] [Google Scholar]
- 35.Pickhardt PJ. Positive oral contrast material for abdominal CT: current clinical indications and areas of controversy. AJR Am J Roentgenol 2020; 215: 69–78. doi: 10.2214/AJR.19.21989 [DOI] [PubMed] [Google Scholar]