Abstract
BACKGROUND
Guidelines recommend nonstatin lipid-lowering agents in patients at very high risk for major adverse cardiovascular events (MACE) if low-density lipoprotein cholesterol (LDL-C) remains ≥70 mg/dL on maximum tolerated statin treatment. It is uncertain if this approach benefits patients with LDL-C near 70 mg/dL. Lipoprotein(a) levels may influence residual risk.
OBJECTIVES
In a post hoc analysis of the ODYSSEY Outcomes (Evaluation of Cardiovascular Outcomes After an Acute Coronary Syndrome During Treatment With Alirocumab) trial, the authors evaluated the benefit of adding the proprotein subtilisin/kexin type 9 inhibitor alirocumab to optimized statin treatment in patients with LDL-C levels near 70 mg/dL. Effects were evaluated according to concurrent lipoprotein(a) levels.
METHODS
ODYSSEY Outcomes compared alirocumab with placebo in 18,924 patients with recent acute coronary syndromes receiving optimized statin treatment. In 4,351 patients (23.0%), screening or randomization LDL-C was <70 mg/dL (median 69.4 mg/dL; interquartile range: 64.3–74.0 mg/dL); in 14,573 patients (77.0%), both determinations were ≥70 mg/dL (median 94.0 mg/dL; interquartile range: 83.2–111.0 mg/dL).
RESULTS
In the lower LDL-C subgroup, MACE rates were 4.2 and 3.1 per 100 patient-years among placebo-treated patients with baseline lipoprotein(a) greater than or less than or equal to the median (13.7 mg/dL). Corresponding adjusted treatment hazard ratios were 0.68 (95% confidence interval [Cl]: 0.52–0.90) and 1.11 (95% Cl: 0.83–1.49), with treatment-lipoprotein(a) interaction on MACE (Pinteraction = 0.017). In the higher LDL-C subgroup, MACE rates were 4.7 and 3.8 per 100 patient-years among placebo-treated patients with lipoprotein(a) >13.7 mg/dL or ≤13.7 mg/dL; corresponding adjusted treatment hazard ratios were 0.82 (95% Cl: 0.72–0.92) and 0.89 (95% Cl: 0.75–1.06), with Pinteraction = 0.43.
CONCLUSIONS
In patients with recent acute coronary syndromes and LDL-C near 70 mg/dL on optimized statin therapy, proprotein subtilisin/kexin type 9 inhibition provides incremental clinical benefit only when lipoprotein(a) concentration is at least mildly elevated. (ODYSSEY Outcomes: Evaluation of Cardiovascular Outcomes After an Acute Coronary Syndrome During Treatment With Alirocumab; NCT01663402)
Keywords: acute coronary syndrome, lipoprotein(a), low-density lipoprotein cholesterol, PCSK9 inhibitor
Current guidelines for cholesterol lowering of the American College of Cardiology and American Heart Association recommend the addition of nonstatin lipid-lowering therapies for patients at very high risk for major adverse cardiovascular events (MACE) when low-density lipoprotein cholesterol (LDL-C) levels remain ≥70 mg/dL on optimized statin treatment (1). This recommendation is based in part on the results of large clinical trials that demonstrated the clinical benefit of this approach (2–4).
Among patients with coronary heart disease receiving moderate to intensive statin therapy (eg, simvastatin 40–80 mg/d or atorvastatin 80 mg/d), approximately 50% to 60% achieve LDL-C levels <70 mg/dL (2,5). Of these, approximately 30% have LDL-C levels of 60 to 70 mg/dL (5). Whether patients with LDL-C levels close to 70 mg/dL on statin treatment derive incremental benefit from further lipid-lowering therapy is therefore a question that is frequently encountered in clinical practice.
Inhibitors of proprotein convertase subtilisin/kexin type 9 (PCSK9) produce large reductions of LDL-C as monotherapy or when added to statins. An additional effect of PCSK9 inhibitors is to reduce lipoprotein(a) concentration by 20% to 25% (3,4). Lipoprotein(a) is a type of low-density lipoprotein particle whose concentration is determined primarily genetically. It is believed to have atherogenic, proinflammatory, and prothrombotic properties (6). Epidemiologic and genetic studies associate lipoprotein(a) concentration with the risk for incident coronary, peripheral artery, and cerebrovascular disease (7–9).
The FOURIER (Further Outcomes Research With PCSK9 Inhibition in Subjects With Elevated Risk) and ODYSSEY Outcomes (Evaluation of Cardiovascular Outcomes After an Acute Coronary Syndrome During Treatment With Alirocumab) trials demonstrated reductions in the risk for MACE with PCSK9 inhibitors in statin-treated patients with established cardiovascular disease or acute coronary syndrome (ACS) and elevated levels of LDL-C, non-high-density lipoprotein cholesterol (non-HDL-C), or apolipoprotein B (3,4). Secondary analyses of these trials indicated that risks for coronary heart disease, peripheral artery disease, and venous thromboembolic events in the placebo groups and reductions in those risks with PCSK9 inhibition were associated with lipoprotein(a) concentration (10–14).
Most patients in the FOURIER and ODYSSEY Outcomes trials had qualifying LDL-C levels ≥70 mg/dL. However, some had LDL-C levels <70 mg/dL or that fluctuated around 70 mg/dL. In FOURIER, patients with baseline LDL-C <70 mg/dL derived similar benefit of treatment with evolocumab to those with baseline LDL-C ≥70 mg/dL, but the interaction of lipoprotein(a) levels in each of these categories was not ascertained (15). In this analysis of the ODYSSEY Outcomes trial, we determined if the PCSK9 inhibitor alirocumab influenced the risk for MACE after ACS when LDL-C levels were less than or around 70 mg/dL and whether any benefit of treatment was modified by concurrent levels of lipoprotein(a).
METHODS
PATIENTS AND TREATMENTS.
The ODYSSEY Outcomes trial (NCT01663402) (4,16) compared the effects of the PCSK9 inhibitor alirocumab with placebo in 18,924 patients with recent ACS. The protocol was approved by the Institutional Review Board at each site, and all patients provided informed consent. To qualify, patients had to meet at least 1 of the following lipoprotein criteria on intensive or maximum tolerated statin treatment: LDL-C ≥70 mg/dL, non-HDL-C ≥100 mg/dL, or apolipoprotein B ≥80 mg/dL. An additional measurement of lipoproteins was performed at the randomization visit, which occurred 2 to 16 weeks after the qualification visit on stable background lipid-lowering therapy. Lipoprotein(a) concentration was measured at randomization but was not considered in qualification. Qualifying patients were randomized in a 1:1 ratio to receive alirocumab 75 mg or matching placebo, administered subcutaneously every 2 weeks. In patients assigned to alirocumab, the dose was blindly titrated between 75 and 150 mg to maximize the number of patients who achieved LDL-C levels of 25 to 50 mg/dL or blindly replaced by placebo for LDL-C levels that remained <15 mg/dL on the lower dose (16). The primary outcome comprised death from coronary heart disease, nonfatal myocardial infarction, fatal or nonfatal ischemic stroke, or hospitalization for unstable angina.
MEASUREMENT OF LIPOPROTEINS AND CATEGORIZATION OF PATIENTS ACCORDING TO LIPOPROTEIN LEVELS.
LDL-C was calculated using the Friedewald formula unless levels were <15 mg/dL or the accompanying triglyceride concentration was >400 mg/dL; in those cases, LDL-C was measured using beta quantification. Lipoprotein(a) mass was measured using an automated immunoturbidimetric assay on a Siemens BNII nephelometric analyzer (Siemens Healthcare Diagnostics) with a lower limit of detection of 2 mg/dL and an interassay coefficient of variation of 3.1% to 4.8% depending on the lipoprotein(a) concentration. Heterogeneity in apolipoprotein(a) size has only a moderate effect on lipoprotein(a) recovery with this assay (17).
Characteristics and outcomes of patients were assessed in 2 LDL-C subgroups: a lower LDL-C subgroup with qualification and/or randomization visit LDL-C <70 mg/dL and a higher LDL-C subgroup with both LDL-C measurements ≥70 mg/dL. Each subgroup was dichotomized at the median baseline lipoprotein(a) concentration of the low LDL-C subgroup.
STATISTICAL ANALYSIS.
The mean of qualification and randomization LDL-C values was calculated for each patient. Patient-level mean values were used to calculate median (interquartile range [IQR]) baseline LDL-C levels in each subgroup. Median (IQR) lipoprotein(a) was calculated from randomization visit values. The incidence of MACE in each LDL-C subgroup, lipoprotein(a) category, and treatment group was estimated by the number of first events per 100 patient-years of follow-up. Within each baseline LDL-C subgroup, the treatment effects on time to first MACE were assessed using a Cox proportional hazards model, stratified according to geographic region, with terms for treatment, baseline lipoprotein(a) category, and their interaction and summarized by hazard ratio (HR) with associated Wald 95% confidence interval (Cl) and the P value for the interaction term. These effects were also estimated in multivariable Cox regression models with adjustment for baseline demographics and clinical characteristics (candidate variables are listed in Supplemental Table l) that were related to risk for MACE as determined by stepwise selection, with P < 0.05 for model entry or exit (treatment, baseline lipoprotein[a] category, and their interaction were included in the adjusted models regardless of their statistical significance). Kaplan-Meier curves were constructed to depict the cumulative incidence of MACE in each LDL-C subgroup and lipoprotein(a) category. Analyses with restricted cubic splines were performed to assess the risk for MACE in each LDL-C subgroup as a function of continuous baseline lipoprotein(a) (18). A sensitivity analysis was performed excluding patients who had blinded, protocol-specified substitution of placebo for alirocumab for consecutive very low achieved LDL-C levels. For all analyses, 2-tailed P values <0.05 were considered to indicate statistical significance. All analyses were conducted according to the intention-to-treat principle, including all patients and events from randomization to the common study end date. Analyses were performed in SAS version 9.4 (IBM).
RESULTS
PATIENT CHARACTERISTICS.
A total of 18,924 patients qualified for the trial and underwent randomization at 1,315 sites in 57 countries. Equal numbers were assigned to alirocumab or placebo. The lower LDL-C subgroup (LDL-C <70 mg/dL at qualification or randomization visit) comprised 4,351 patients (23.0%). Among them, a measurement of LDL-C <70 mg/dL was obtained at the qualification visit only in 600 (13.8%), at the randomization visit only in 2,731 (62.8%), and at both visits in 1,020 (23.4%). After assigning each patient the mean value of qualification and randomization visit LDL-C determinations, median baseline LDL-C in the lower LDL-C subgroup was 69.4 mg/dL (IQR: 64.3–74.0 mg/dL). Median baseline lipoprotein(a) in the lower LDL-C subgroup was 13.7 mg/dL (IQR: 4.9–40.3 mg/dL).
The higher LDL-C subgroup (LDL-C >70 mg/dL at qualification and randomization visits) comprised 14,573 patients. Median baseline LDL-C was 94.0 mg/dL (IQR: 83.2–111.0 mg/dL), and median baseline lipoprotein(a) was 24.3 mg/dL (IQR: 7.464.7 mg/dL). For analytical purposes, both LDL-C subgroups were dichotomized at the same lipoprotein(a) level, 13.7 mg/dL. Baseline and selected postrandomization characteristics of each LDL-C subgroup according to lipoprotein(a) ≤ 13.7 or >13.7 mg/dL are shown in Table 1. Characteristics were well balanced between the alirocumab and placebo groups in each of the subgroups and lipoprotein(a) categories (data not shown).
Table 1.
Baseline and Postrandomlzation Characteristics According to LDL-C Subgroup and Lp(a) Category
| Lower LDL-C Subgroup: <70 mg/dL at Qualification or Randomization | Higher LDL-C Subgroup: ≥70 mg/dL at Qualification and Randomization | |||
|---|---|---|---|---|
|
|
|
|||
| Lp(a) ≤13.7 mg/dL (n = 2,181) | Lp(a) >13.7 mg/dL (n = 2,170) | Lp(a) ≤13.7 mg/dL (n = 5,454) | Lp(a) >13.7 mg/dL (n = 9,119) | |
|
| ||||
| Baseline characteristics | ||||
| Age, y | 57 (51 to 64) | 58 (51 to 65) | 58 (52 to 65) | 58 (52 to 65) |
| Femalea | 438 (20.1) | 453 (20.9) | 1,200 (22.0) | 2,671 (29.3) |
| Racea,b | ||||
| White | 1,721 (78.9) | 1,576 (72.6) | 4,612 (84.6) | 7,115 (78.0) |
| Black | 12 (0.6) | 70 (3.2) | 34 (0.6) | 357 (3.9) |
| Asian | 311 (14.3) | 399 (18.4) | 577 (10.6) | 1,211 (13.3) |
| Other | 137 (6.3) | 125 (5.8) | 231 (4.2) | 436 (4.8) |
| Geographic regiona,b | ||||
| Western Europe | 397 (18.2) | 445 (20.5) | 1,214 (22.3) | 2,119 (23.2) |
| Eastern Europe | 654 (30.0) | 460 (21.2) | 1,968 (36.1) | 2,355 (25.8) |
| North America | 291 (13.3) | 328 (15.1) | 667 (12.2) | 1,585 (17.4) |
| South America | 404 (18.5) | 346 (15.9) | 669 (12.3) | 1,169 (12.8) |
| Asia | 293 (13.4) | 376 (17.3) | 534 (9.8) | 1,090 (12.0) |
| Rest of world | 142 (6.5) | 215 (9.9) | 402 (7.4) | 801 (8.8) |
| Medical history | ||||
| History of hypertensionb | 1,444 (66.2) | 1,362 (62.8) | 3,556 (65.2) | 5,887 (64.6) |
| History of diabetesa | 778 (35.7) | 732 (33.7) | 1,534 (28.1) | 2,400 (26.3) |
| Current smokinga | 502 (23.0) | 466 (21.5) | 1,481 (27.2) | 2,111 (23.1) |
| History of polyvascular diseasea,c | 140 (6.4) | 142 (6.5) | 428 (7.8) | 844 (9.3) |
| Myocardial infarction prior to index event | 360 (16.5) | 361 (16.6) | 1,049 (19.2) | 1,869 (20.5) |
| CABG or PCI prior to index eventa | 353 (16.2) | 376 (17.3) | 1,049 (19.2) | 1,957 (21.5) |
| Index CABG or PCIa | 1,553 (71.2) | 1,587 (73.1) | 3,873 (71.0) | 6,664 (73.1) |
| History of heart failurea | 318 (14.6) | 275 (12.7) | 905 (16.6) | 1,317 (14.4) |
| History of COPD | 82 (3.8) | 70 (3.2) | 215 (3.9) | 379 (4.2) |
| Medications | ||||
| High-intensity statina,b | 1,992 (91.3) | 2,036 (93.8) | 4,729 (86.7) | 8,054 (88.3) |
| ACE inhibitor or ARBa | 1,691 (77.5) | 1,685 (77.6) | 4,304 (78.9) | 7,036 (77.2) |
| Beta-blocker | 1,884 (86.4) | 1,849 (85.2) | 4,588 (84.1) | 7,674 (84.2) |
| Biometric and laboratory data | ||||
| Body mass index, kg/m2 a,b | 28.4 (25.8 to 31.5) | 27.9 (25.2 to 31.3) | 28.1 (25.5 to 31.2) | 27.7 (25.0 to 30.8) |
| Glycated hemoglobin, % | 5.9 (5.6 to 6.7) | 5.9 (5.6 to 6.5) | 5.8 (5.5 to 6.3) | 5.8 (5.5 to 6.2) |
| eGFR <60 mL/min/1.73 m2 a,b | 267 (12.2) | 313 (14.4) | 684 (12.5) | 1,275 (14.0) |
| LDL-C, mg/dLa,b | 69.0 (63.5 to 73.9) | 69.5 (65.0 to 74.3) | 93.0 (82.5 to 110.5) | 94.5(83.5 to 111.5) |
| LDL-Ccorrected, mg/dLa,b | 61.6 (53.9 to 66.4) | 49.7 (39.7 to 57.5) | 90.1 (78.9 to 108.4) | 75.9 (63.6 to 94.0) |
| Non-HDL-C, mg/dL | 95.4 (83.0 to 111.2) | 91.5 (81.5 to 107.0) | 121.6 (106.2 to 145.9) | 120.8 (105.4 to 142.5) |
| Apolipoprotein B, mg/dL | 68 (59 to 78) | 66 (58 to 75) | 83 (73 to 97) | 83 (73 to 97) |
| HDL-C, mg/dL | 39.4 (34.0 to 46.7) | 39.0 (33.2 to 46.0) | 43.2 (37.1 to 51.0) | 43.6 (37.5 to 51.4) |
| Triglycerides, mg/dLa,b | 159.3 (106.0 to 230.0) | 134.5 (92.0 to 197.3) | 133.6 (98.2 to 185.8) | 121.0 (90.3 to 165.5) |
| Lp(a), mg/dLa,b | 4.9 (2.0 to 8.4) | 40.4 (23.5 to 67.5) | 5.2 (2.0 to 9.0) | 53.3 (28.0 to 88.1) |
| High-sensitivity C-reactive protein, mg/La | 1.6 (0.7 to 3.6) | 1.5 (0.7 to 3.8) | 1.6 (0.8 to 3.7) | 1.7 (0.8 to 4.1) |
| Postrandomization characteristics | ||||
| Follow-up for MACE, y | 2.7 (2.2 to 3.4) | 2.6 (2.2 to 3.2) | 2.8 (2.2 to 3.4) | 2.7 (2.2 to 3.3) |
| Change in LDL-C to month 4, mg/dL | ||||
| Alirocumab | −39.0 (−49.0 to −22.0) | −37.0 (−47.0 to −23.2) | −61.0 (−75.7 to−45.6) − | 59.5 (−75.0 to −43.6) |
| Placebo | 7.3 (−4.2 to 19.0) | 6.6 (−4.6 to 18.9) | −2.0 (−16.2 to 12.0) | −1.0 (−13.5 to 12.7) |
| Change in LOL-Ccorrected to month 4, mg/dL | ||||
| Alirocumab | −38.2 (−48.5 to −22.0) − | −33.3 (−43.0 to −20.0) | −60.3 (−74.9 to −45.2) − | 54.9 (−70.3 to −39.4) |
| Placebo | 7.1 (−4.3 to 19.3) | 7.5 (−3.7 to 19.6) | −2.3 (−16.3 to 11.7) | −0.5 (−12.7 to 13.3) |
| Change in Lp(a) to month 4, mg/dL | ||||
| Alirocumab | 0 (−3.8 to 0) | −10.3 (−18.2 to −3.9) | −0.9 (−3.8 to 0) | −11.4 (−21.9 to −4.2) |
| Placebo | 0 (−0.8 to 0.9) | −1.9 (−9.9 to 3.9) | 0 (−0.7 to 1.0) | −2.3 (−9.9 to 5.5) |
| Protocol-specified substitution of placebo (alirocumab group only) | 234/1,059 (22.1) | 164/1,125 (14.6) | 197/2,726 (7.2) | 135/4,552 (3.0) |
Values are median (interquartile range), n (%), or n/N (%). The lower LDL-C subgroup was defined by a qualification or randomization visit LDL-C level <70 mg/dL. The higher LDL-C subgroup was defined by qualification and randomization LDL-C levels ≥70 mg/dL. The median baseline Lp(a) level in the lower LDL-C subgroup (13.7 mg/dL) was used to dichotomize both LDL-C subgroups. Postrandomization variables are presented descriptively, without statistical inference.
P < 0.05 for baseline characteristic comparison of Lp(a) categories within the higher LDL-C subgroup.
P < 0.05 for baseline characteristic comparison of Lp(a) categories within the lower LDL-C subgroup.
History of cerebrovascular or peripheral artery disease in addition to coronary heart disease.
ACE = angiotensin-converting enzyme; ARB = angiotensin receptor blocker; CABG = coronary artery bypass grafting; COPD = chronic obstructive pulmonary disease; eGFR = estimated glomerular filtration rate; HDL-C = high-density lipoprotein cholesterol; LDL-C = low-density lipoprotein cholesterol; LDL-Ccorrected = LDL-C corrected for the cholesterol content of lipoprotein(a) (calculated according to Kinpara et al [30] as LDL-Ccorrected = LDL-C - (lipoprotein(a) × 0.3); Lp(a) = lipoprotein(a); PCI = percutaneous coronary intervention.
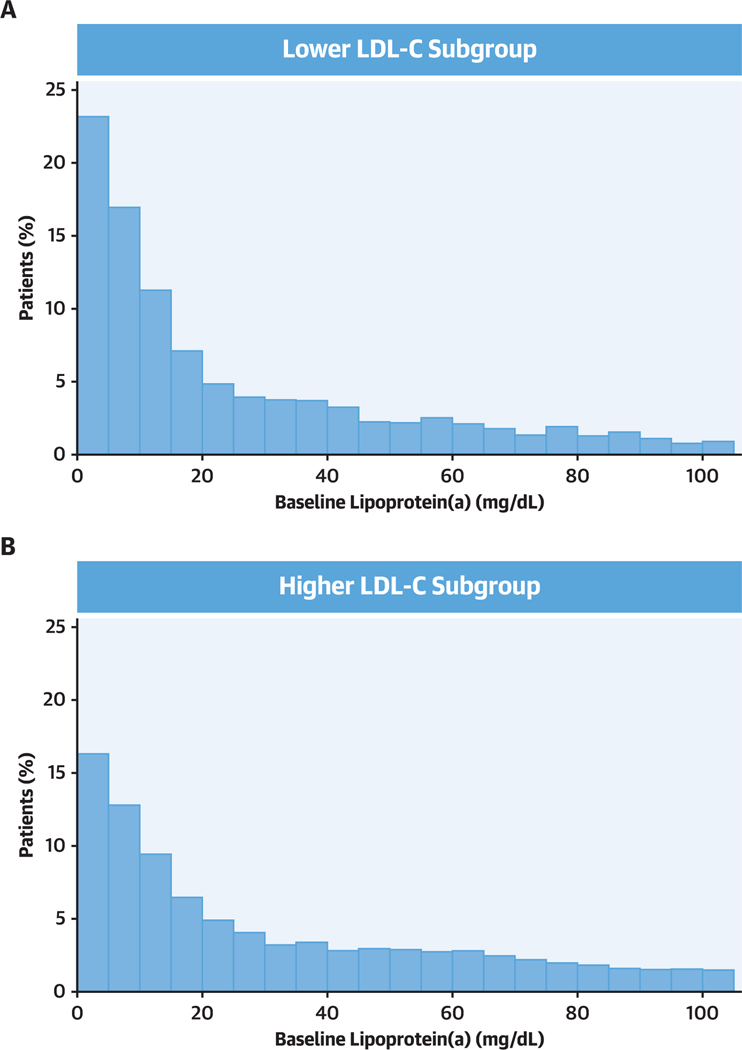
Compared with patients in the higher LDL-C subgroup, patients in the lower LDL-C subgroup were less likely to be female, white, European, or smokers and to have histories of prior myocardial infarction, percutaneous coronary intervention, coronary artery bypass surgery, or heart failure. Patients in the lower LDL-C subgroup were more likely to be South American or Asian, to have diabetes, and to be treated with high-intensity statins and had higher triglyceride and lower lipoprotein(a) levels. The distribution of lipoprotein(a) in the lower and higher LDL-C subgroups is shown in Figure 1.
Figure 1. Baseline Lipoprotein (a) Distribution in Lower and Higher Baseline LDL-C Subgroups.
(A) Lower low-density lipoprotein cholesterol (LDL-C) subgroup (n = 4,351) defined by a qualification or randomization LDL-C level <70 mg/dL. In this subgroup, median LDL-C was 69.4 mg/dL (interquartile range: 64.3–74.0 mg/dL). (B) Higher LDL-C subgroup (n = 14,573) defined by qualification and randomization LDL-C levels ≥70 mg/dL. In this subgroup, median LDL-C was 94.0 mg/dL (interquartile range: 83.2–111.0 mg/dL). The median value of lipoprotein(a) in the tower LDL-C subgroup (13.7 mg/dL) was used to dichotomize both LDL-C subgroups.
Median follow-up for MACE was 2.6 years (IQR: 2.2–3.3 years) and 2.7 years (IQR: 2.2–3.3 years) in the lower and higher LDL-C subgroups, respectively, and did not differ according to lipoprotein(a) category in either subgroup. Changes in lipoprotein levels from baseline to month 4 are shown in Table 1. In the lower and higher LDL-C subgroups, median changes in LDL-C with alirocumab were −38 and −60 mg/dL, respectively, and did not differ according to baseline lipoprotein(a) category in either LDL-C subgroup. In patients with baseline lipoprotein(a) ≥13.7 mg/dL, median changes in lipoprotein(a) with alirocumab were similar in both lower and higher LDL-C subgroups (−10.3 and −11.4 mg/dL, respectively). In patients with baseline lipoprotein(a) <13.7 mg/dL, changes in lipoprotein(a) with alirocumab were minimal in both LDL-C subgroups. In patients assigned to placebo, there were minimal changes from baseline to month 4 in LDL-C and lipoprotein(a) in both LDL-C subgroups and lipoprotein(a) categories.
MACE ACCORDING TO LDL-C SUBGROUP AND lipoprotein (A) CATEGORY.
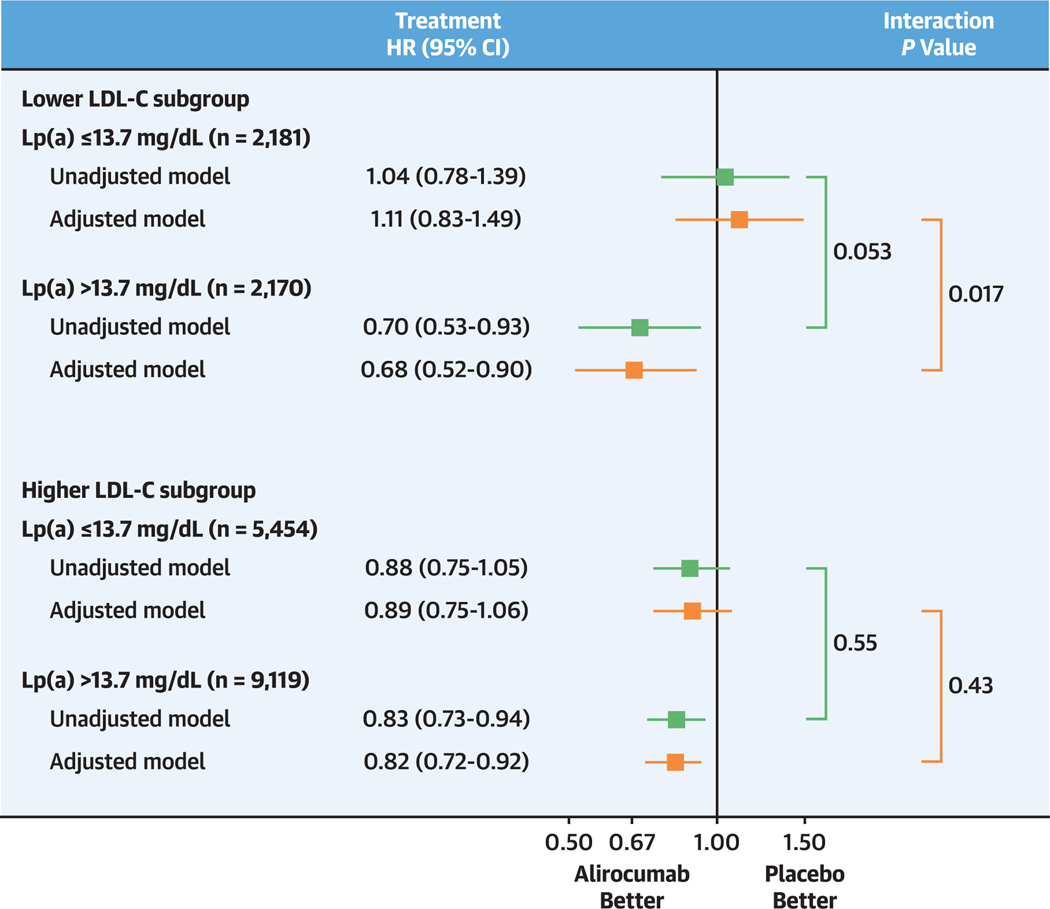
The Central Illustration shows the incidence rates for MACE and treatment HR (95% Cl) for each LDL-C subgroup and baseline lipoprotein(a) category. The cumulative incidence of MACE in each LDL-C subgroup and lipoprotein(a) category is depicted in Kaplan-Meier plots in Figure 2.
CENTRAL ILLUSTRATION. Effect of Alirocumab on Major Adverse Cardiovascular Events by Baseline Low-Density Lipoprotein Cholesterol Subgroup and Lipoprotein(a) Category.
The lower low-density lipoprotein cholesterol (LDL-C) subgroup (top) was defined by a qualification or randomization LDL-C level <70 mg/dL. The higher LDL-C subgroup (bottom) was defined by qualification and randomization LDL-C levels ≥70 mg/dL. Both LDL-C subgroups were dichotomized at the median baseline lipoprotein(a) concentration in the lower LDL-C subgroup (13.7 mg/dL). Unadjusted models were stratified by geographic region. Selection of variables in adjusted models is described in the text. In the lower LDL-C subgroup, adjustment variables were myocardial infarction prior to index event, history of diabetes, estimated glomerular filtration rate <60 mL/min/1.73 m2, history of heart failure, history of hypertension, and coronary artery bypass grafting or percutaneous coronary intervention prior to the qualifying acute coronary syndrome (ACS). In the higher LDL-C subgroup, adjustment variables were those in the lower LDL-C model plus history of chronic obstructive pulmonary disease, revascularization for the qualifying ACS, current smoking, and age.
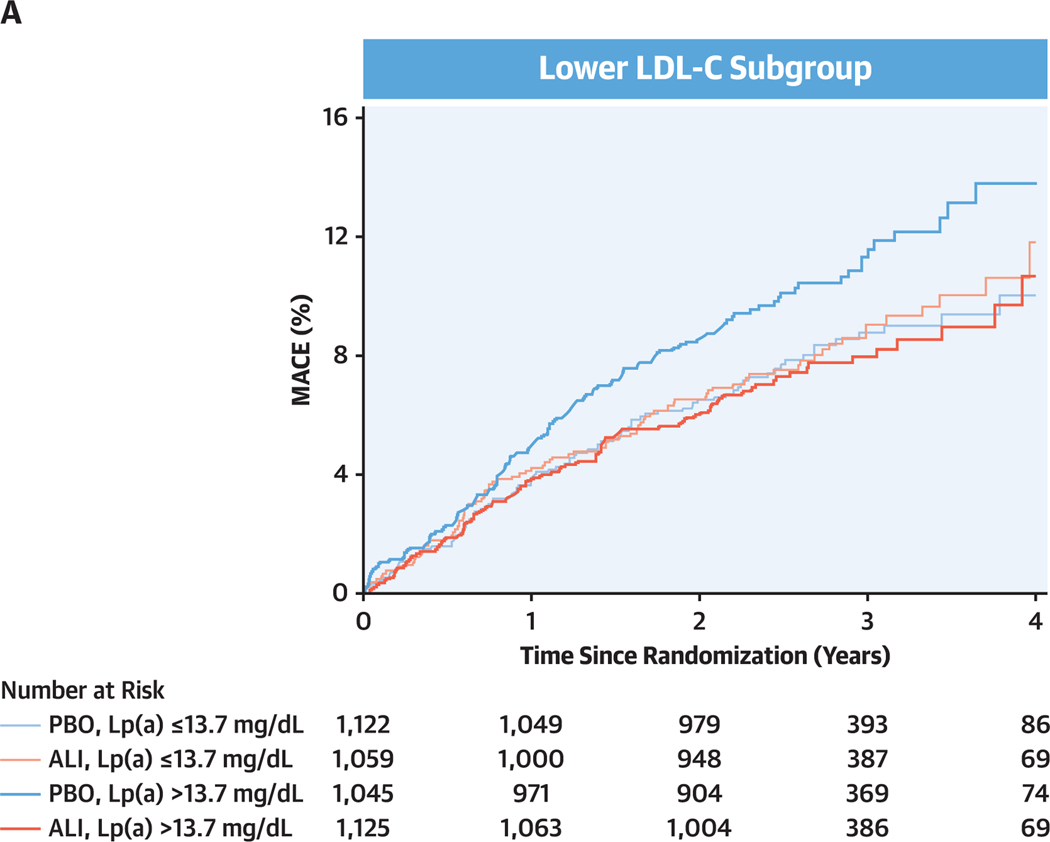
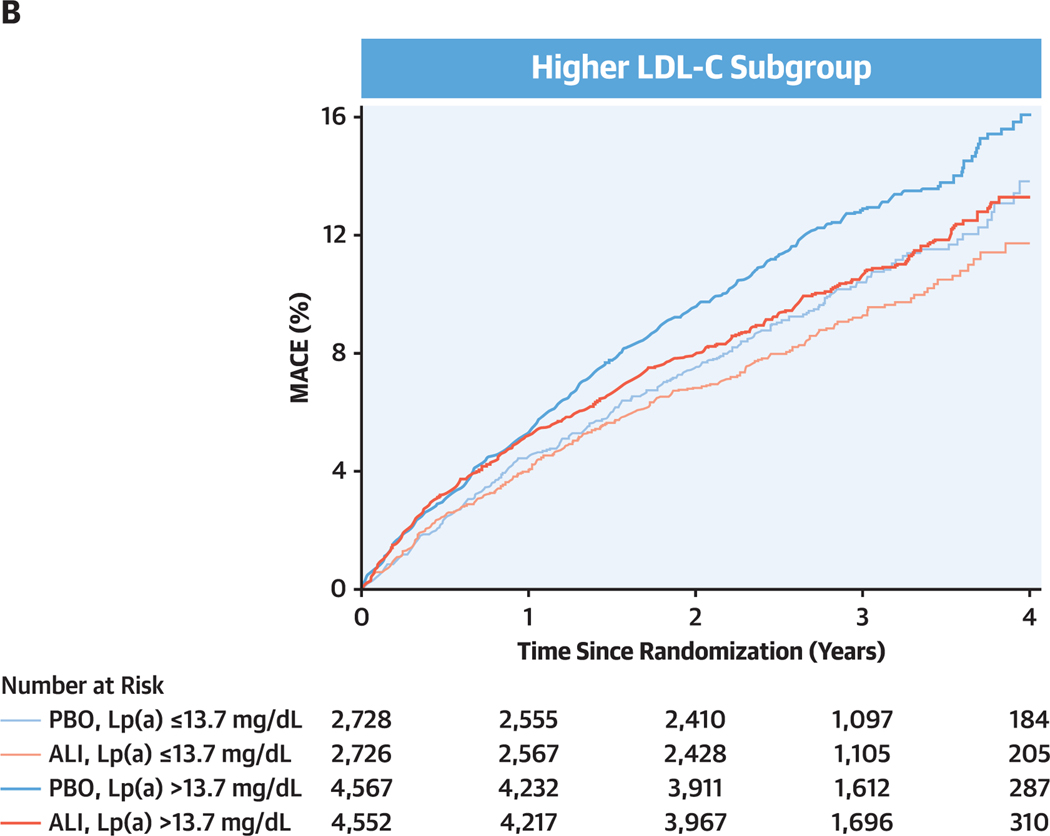
Figure 2. Cumulative Incidence of MACE by Baseline LDL-C Subgroup, Lipoprotein (a) Category, and Study Treatment.
(A) Lower low-density lipoprotein cholesterol (LDL-C) subgroup. (B) Higher LDL-C subgroup. Subgroups and dichotomization of baseline lipoprotein(a) (Lp[a]) as defined in Figure 1. ALI = alirocumab; MACE = major adverse cardiovascular events; PBO = placebo.
In patients assigned to placebo in the lower LDL-C subgroup, the incidence rate for MACE was higher in those with baseline lipoprotein(a) >13.7 mg/dL (4.2 per 100 patient-years) than in those with baseline lipoprotein(a) ≤13.7 mg/dL (3.1 per 100 patient-years; unadjusted HR: 1.31; 95% Cl: 0.99–1.72; P = 0.057). In an unadjusted model, alirocumab reduced the risk for MACE in those with baseline lipoprotein(a) >13.7 mg/dL (HR: 0.70; 95% Cl: 0.53–0.93) but not in those with baseline lipoprotein(a) ≤13.7 mg/dL (HR: 1.04; 95% Cl: 0.78–1.39). Results were similar in the adjusted model, with treatment HRs of 0.68 (95% Cl: 0.52–0.90) in those with baseline lipoprotein(a) >13.7 mg/dL and 1.11 (95% Cl: 0.83–1.49) in those with baseline lipoprotein(a) ≤13.7 mg/dL. There was interaction of treatment and baseline lipoprotein(a) category on risk for MACE, with unadjusted Pinteraction = 0.053 and adjusted Pinteraction = 0.017. Results were similar when examined by quartile of baseline lipoprotein(a) in the lower LDL-C subgroup. In quartiles l to 4, unadjusted treatment HRs were 1.11 (95% Cl: 0.74–1.67), 0.98 (95% Cl: 0.65–1.48), 0.72 (95% Cl: 0.48–1.08), and 0.69 (95% Cl: 0.47–1.01), respectively, with unadjusted Ptrend = 0.054 and adjusted Ptrend = 0.029.
In patients assigned to placebo in the higher LDL-C subgroup, the incidence rate for MACE was also higher in those with baseline lipoprotein(a) >13.7 mg/dL (4.7 per 100 patient-years) than in those with baseline lipoprotein(a) ≤13.7 mg/dL (3.8 per 100 patient-years). However, treatment HRs were favorable for alirocumab in both higher and lower lipoprotein(a) categories (unadjusted HRs: 0.83 [95% Cl: 0.73–0.94] and 0.88 [95% Cl: 0.75–1.05], respectively; adjusted HRs: 0.82 [95% CI: 0.72–0.92] and 0.89 [95% Cl: 0.75–1.06], respectively), without interaction of treatment and baseline lipoprotein(a) category (unadjusted Pinteraction = 0.55; adjusted Pinteraction = 0.43). Considering both LDL-C subgroups, the P value for 3-factor interaction of treatment, LDL-C subgroup, and lipoprotein(a) category on MACE was 0.19 in the adjusted model.
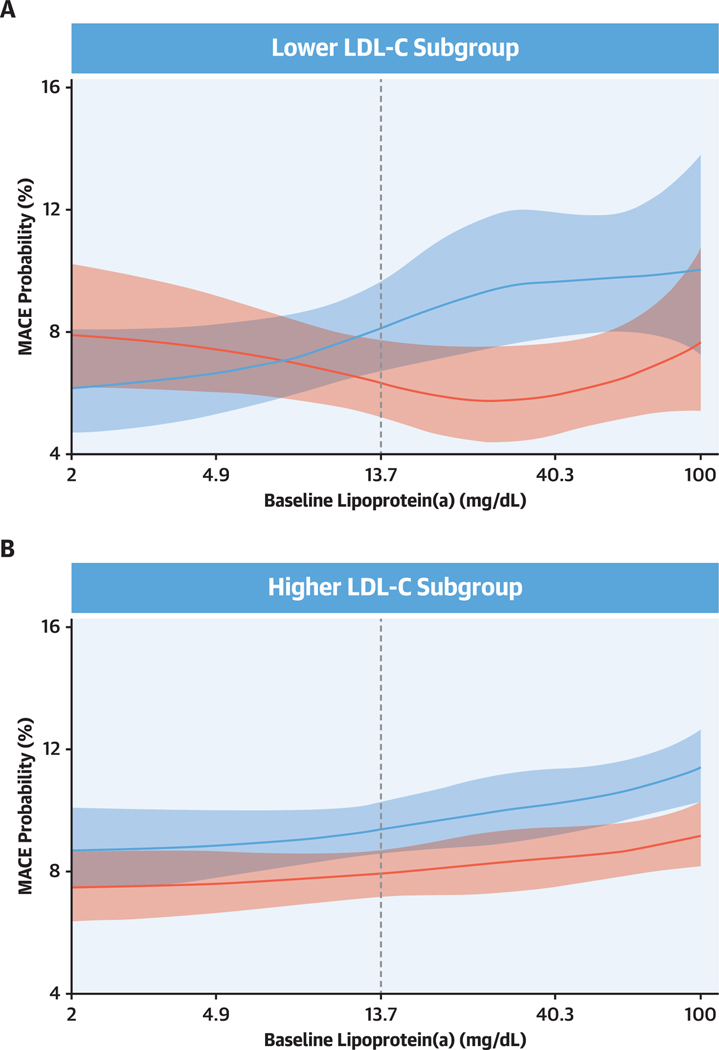
Figure 3 shows the probability of MACE as a function of continuous lipoprotein(a) in the placebo and alirocumab treatment groups, for the lower and higher LDL-C subgroups. Findings were consistent with the dichotomous analysis of lipoprotein(a) levels depicted in the Central Illustration. In the lower LDL-C subgroup, alirocumab reduced the risk for MACE only in patients with higher baseline lipoprotein(a), whereas in the higher LDL-C subgroup, alirocumab reduced the risk for MACE across the full range of baseline lipoprotein(a) concentrations. There was a significant interaction between treatment and baseline lipoprotein(a) spline effect in the lower LDL-C subgroup (Pinteraction = 0.031) but not in the higher LDL-C Subgroup (Pinteraction = 0.84).
Figure 3. Spline Analysis of Probability of MACE by Baseline Lipoprotein(a) and Treatment Group.
(A) Lower LDL-C subgroup. (B) Higher LDL-C subgroup. Red indicates alirocumab group; blue indicates placebo group. Shaded areas indicate 95% confidence intervals. Dashed lines indicate the median baseline lipoprotein(a) value (13.7 mg/dL) in the lower LDL-C subgroup. In each subgroup, the probability of MACE at the overall median follow-up of 2.7 years was estimated from a logistic regression model with a logit link function; logarithm of follow-up time was an offset variable. Models included adjustment for the baseline characteristics indicated in Figure 2, plus geographic region. Spline effect reflects a restricted cubic spline basis with knots at 4.9,13.6, and 40.3 mg/dL (ie, the spline was required to be linear for lipoprotein[a] <4.9 mg/dL and >40.3 mg/dL). Interaction of treatment and baseline lipoprotein(a) spline effect: Pinteraction = 0.031 and Pinteraction = 0.84 for lower and higher LDL-C subgroups, respectively. Subgroup definitions as in Figure 1.
SENSITIVITY ANALYSIS.
A total of 730 patients had blinded, protocol-specified substitution of placebo for alirocumab because of consecutive very low achieved LDL-C levels. These patients were distributed non-uniformly across baseline LDL-C and lipoprotein(a) categories (Table 1). To determine whether these patients influenced the results of the primary analysis depicted in the Central Illustration, a sensitivity analysis was performed that excluded them. The results of the sensitivity analysis, shown in Supplemental Figure l, are qualitatively and quantitatively similar to those in the Central Illustration. Among patients in the lower LDL-C subgroup, adjusted treatment HR with lipoprotein(a) >13.7 mg/dL was 0.68 (95% CI: O.51–0.91), adjusted treatment HR with lipoprotein(a) ≤13.7 mg/dL was 1.26 (95% Cl: 0.93–1.70), and adjusted Pinteraction was 0.004.
DISCUSSION
The central finding of this analysis of the ODYSSEY Outcomes trial is that patients with recent ACS, LDL-C near 70 mg/dL on optimized statin treatment, and lipoprotein(a) levels that were at least mildly elevated (≥13.7 mg/dL) derived substantial clinical benefit (reduced risk for MACE) from treatment with the PCSK9 inhibitor alirocumab. In contrast, patients with LDL-C near 70 mg/dL and lipoprotein(a) <13.7 mg/dL had no reduction in MACE with alirocumab. Patients with higher LDL-C levels derived consistent clinical benefit from alirocumab treatment irrespective of the level of lipoprotein(a). Findings were similar in a sensitivity analysis that excluded patients with blinded, protocol-specified substitution of placebo for alirocumab. Thus, in patients with recent ACS and LDL-C levels considered “controlled” on statin therapy, levels of lipoprotein(a) that are at least mildly elevated may identify an additional group to be considered for PCSK9 inhibitor therapy. Notably, a lipoprotein(a) concentration of 13.7 mg/dL is only slightly greater than the median level observed in cohorts without prior cardiovascular events (19,20). Because the present findings were derived from a post hoc analysis, a prospective evaluation of the clinical efficacy of PCSK9 inhibitor therapy in patients with nominally controlled LDL-C but elevated lipoprotein(a) is indicated.
In patients with baseline LDL-C near 70 mg/dL and lipoprotein(a) at or higher than the median of 13.7 mg/dL, alirocumab produced moderate absolute reductions in both LDL-C and lipoprotein(a) (median 37 and 10.3 mg/dL, respectively), accompanied by an approximately 30% reduction in the risk for MACE. In the Cholesterol Treatment Trialists analysis of statin trials (21), a 1 mmol/L (38.7 mg/dL) reduction in LDL-C with statin treatment was associated with a 21% reduction in the incidence of major vascular events, and a 37 mg/dL reduction in LDL-C would have been expected to produce a 20% reduction in events. Importantly, however, few patients in the trials that constituted the Cholesterol Treatment Trialists analysis had baseline LDL-C levels as low as 70 mg/dL. As LDL-C and lipoprotein(a) usually change in parallel under treatment with alirocumab, it is difficult to apportion the benefit of alirocumab on MACE to individual lipoprotein effects or their interaction. Nonetheless, the magnitude of the clinical treatment benefit observed in patients with lower levels of LDL-C but elevated levels of lipoprotein(a) suggests that lipoprotein(a) reduction contributed to the benefit and/or that elevated levels of lipoprotein(a) identify patients with atherosclerotic plaques amenable to stabilization with PCSK9 inhibition. In patients with baseline LDL-C near 70 mg/dL and lipoprotein(a) <13.7 mg/dL, alirocumab produced a similar reduction in LDL-C (median 3g mg/dL), but with minimal change in lipoprotein(a) and no clinical benefit. Moreover, in both the lower and higher LDL-C subgroups, LDL-C levels were similar in the higher versus lower lipoprotein(a) categories. Consequently, corrected LDL-C was lower in the higher lipoprotein(a) category and vice versa. Thus, treatment HRs for alirocumab were more favorable in patients with higher lipoprotein(a) despite lower levels of corrected or “true” LDL-C.
Together, these observations suggest that in statin-treated patients with recent ACS and LDL-C levels near 70 mg/dL, lipoprotein(a) levels that are at least mildly elevated are required to achieve a clinical benefit from PCSK9 inhibition. In these patients, the coordinated reduction of both LDL-C and lipoprotein(a) levels may be necessary to reduce the risk for MACE. Lipoprotein(a) is believed to have proinflammatory and prothrombotic properties (6). ACS is associated with heightened inflammation and usually reflects an atherothrombotic event (22). Modest reductions in lipoprotein(a) concentration with PCSK9 inhibition in the ODYSSEY Outcomes and FOURIER trials were associated with substantial reductions in the risk for MACE (10,12). A link among these observations may be that lipoprotein(a) particles are the principal carriers of oxidized phospholipids that are proinflammatory and may play a central role in atherothrombotic events (23). In this regard, the relationship of lipoprotein(a) concentration to risk for MACE and reduction of that risk with a PCSK9 monoclonal antibody may be stronger in a secondary prevention population with recent ACS than would be predicted from analyses relating lipoprotein(a) concentration to incident cardiovascular disease events in healthy cohorts (24).
STUDY LIMITATIONS.
First, the analysis was conducted on a post hoc basis. Second, patients in ODYSSEY Outcomes with LDL-C levels <70 mg/dL qualified for the trial on the basis of alternative criteria of elevated non-HDL-C or apolipoprotein B. The findings of the present analysis cannot necessarily be extended to patients with elevated lipoprotein(a) but levels of LDL-C, non-HDL-C, and apolipoprotein B less than trial thresholds.
Third, lipoprotein(a) concentration was determined using a mass assay. Overall, mass and molar concentrations of lipoprotein(a) are highly correlated, but their ratio varies according to isoform size (25). Although atherothrombotic risk may be more closely related to the molar concentration of lipoprotein(a) (26), molar concentration is underestimated at high mass concentration and overestimated at low mass concentration (25). To the extent such effects were present, they would have biased our study to the null.
Fourth, lipoprotein(a) may be a positive acute-phase reactant after ACS (27). However, patients in ODYSSEY Outcomes were randomized at a minimum of 1 and a median of 2.6 months after the qualifying ACS, and lipoprotein(a) levels did not change significantly between randomization and month 4 in the placebo group (10).
Fifth, the 3-factor interaction of treatment, LDL-C subgroup, and lipoprotein(a) category on MACE was nonsignificant. This is because patients with higher LDL-C levels, who constituted 77% of the study population, had a relatively consistent treatment benefit irrespective of lipoprotein(a) levels.
Finally, some studies have indicated that the relationship between lipoprotein(a) concentration and cardiovascular risk may vary across ethnic groups (28,29). The ODYSSEY Outcomes population was predominantly white, and generalizability of the findings to other populations is uncertain.
CONCLUSIONS
Many high-risk patients achieve LDL-C levels of approximately 70 mg/dL on intensive statin treatment with or without ezetimibe (2,5). These patients lie at the cusp of guideline criteria to augment lipid-lowering therapy, but physicians are uncertain whether to take further therapeutic steps. In current clinical practice, few such patients are treated with PCSK9 inhibitors. Determining the concentration of lipoprotein(a) might have utility in identifying a subset of these patients who could derive substantial benefit from PCSK9 inhibitor treatment and similarly identifying a subset for whom benefit is unlikely and added cost and complexity of treatment could be avoided. In patients whose LDL-C remained substantially >70 mg/dL (median g4.0 mg/dL), alirocumab treatment provided consistent benefit irrespective of lipoprotein(a) levels, and therefore determining lipoprotein(a) may not affect therapeutic decision making to the same extent.
Supplementary Material
PERSPECTIVES.
COMPETENCY IN PATIENT CARE AND PROCEDURAL SKILLS:
In patients with recent ACS and LDL-C levels near 70 mg/dL on intensive statin treatment, the PCSK9 inhibitor alirocumab further lowered LDL-C but reduced the risk for adverse cardiovascular events only in those with elevated lipoprotein(a) levels.
TRANSLATIONAL OUTLOOK:
Randomized trials are needed to evaluate the effect of PCSK9 inhibition in patients with LDL-C levels nominally controlled with statins who have elevated lipoprotein(a) levels.
ACKNOWLEDGMENTS
The authors thank the patients, study coordinators, and investigators who participated in this trial. Sophie Rushton-Smith, PhD (MedLink Healthcare Communications), provided editorial assistance in the preparation of the manuscript (limited to editing for style, referencing, and figure and table editing) and was funded by Sanofi.
FUNDING SUPPORT AND AUTHOR DISCLOSURES
ODYSSEY Outcomes (NCT01663402) was funded by Sanofi and Regeneron Pharmaceuticals. Dr Schwartz has received research support to the University of Colorado from AstraZeneca, Resverlogix, Roche, Sanofi, and The Medicines Company; and is a coinventor on pending US patent 62/806,313 (“Methods for Reducing Cardiovascular Risk”) assigned in full to the University of Colorado. Dr Szarek has served as a consultant or on advisory boards (or both) for CiVi, Resverlogix, Baxter, Esperion, Lexicon, Sanofi, and Regeneron Pharmaceuticals. Dr Bittner has received grant support from Sanofi, AstraZeneca, DalCor, Esperion, Bayer, The Medicines Company, and Amgen (all paid direct to her institution); and has received personal fees from Sanofi. Dr Diaz has received research grants from Sanofi, DalCor Pharmaceuticals, the Population Health Research Institute, the Duke Clinical Research Institute, the TIMI group, Amgen, Cirius, Montreal Health Innovations Coordinating Center, and Lepetit; and has received personal fees, as a member of the executive steering committee, from Amgen and Cirius. Dr Goodman has received research grant support (eg, steering committee or data and safety monitoring committee) and/or speaker and consulting honoraria (eg, advisory boards) from Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Bristol Myers Squibb, CSL Behring, Daiichi Sankyo/American Regent, Eli Lilly, Esperion, Ferring Pharmaceuticals, GlaxoSmithKline, HLS Therapeutics, Janssen/Johnson & Johnson, Merck, Novartis, Novo Nordisk, Pendopharm, Pfizer, Regeneron, Sanofi, Servier, and Valeo Pharma; and has received salary support and honoraria from the Heart and Stroke Foundation of Ontario/University of Toronto (Polo) Chair, the Canadian Heart Research Centre and MD Primer, the Canadian VIGOUR Centre, the Cleveland Clinic Coordinating Centre for Clinical Research, the Duke Clinical Research Institute, the New York University Clinical Coordinating Centre, and the PERFUSE Research Institute. Dr Jukema has received research grants from the Netherlands Heart Foundation, the Interuniversity Cardiology Institute of the Netherlands, and the European Commission Seventh Framework Programme; and has received research support from Amgen, Astellas, AstraZeneca, Daiichi Sankyo, Eli Lilly, Merck-Schering-Plough, Pfizer, Roche, and Sanofi. Dr Landmesser has received research grants from Novartis, Bayer, and Amgen; and has received lecture or advisory honorary fees from Amgen, Sanofi, Novartis, Bayer, Pfizer, and The Medicines Company. Dr López-Jaramillo has received honoraria for lectures from Menarini, Abbott, and Merck. Drs Manvelian and Pordy are employees of Regeneron Pharmaceuticals. Dr Scemama is an employee of Sanofi. Dr Sinnaeve has received institutional grants from the Flemish government, AstraZeneca, and Daiichi Sankyo; is a corecipient of a named chair supported by Bayer; and has received advisory and speaker fees (all institutional) from Sanofi, Amgen, Idorsia, Bristol Myers Squibb/Pfizer, AstraZeneca, and Abbott. Dr White has received grant support paid to the institution and fees for serving on a steering committee for the ODYSSEY Outcomes trial from Sanofi and Regeneron Pharmaceuticals, for the ACCELERATE study from Eli Lilly, for the STRENGTH trial from Omthera Pharmaceuticals, for the SPIRE trial from Pfizer, for the HEART-FID study from American Regent, for the CAMELLIA-TIMI study from Eisai, for the dal-GenE study from DalCor Pharma UK, for the AEGIS-II study from CSL Behring, for the SCORED trial and the SOLOIST-WHF trial from Sanofi Australia, and for the CLEAR Outcomes study from Esperion Therapeutics. Dr White has served on advisory boards for Actelion, Sirtex, and Genentech (an affiliate of F. Hoffmann-La Roche; Lytics Post-PCI Advisory Board at the European Society of Cardiology); and has received lecture fees from AstraZeneca. Dr. Steg has received grants and nonfinancial support (cochair of the ODYSSEY Outcomes trial; as such, he received no personal fees, but his institution has received funding for the time he has devoted to trial coordination, and he has received support for travel related to trial meetings) from Sanofi; has received research grants and personal fees from Bayer (steering committee, MARINER, grant for epidemiological study), Merck (speaker fees, grant for epidemiological studies), Sanofi (cochair of the ODYSSEY Outcomes trial; cochair of the SCORED trial; consulting and speaking), Servier (chair of the CLARIFY registry; grant for epidemiological research), and Amarin (executive steering committee for the REDUCE-IT trial; consulting); and has received personal fees from Amgen, Bristol Myers Squibb, Boehringer Ingelheim, Pfizer, Idorsia, Myokardia, Novo Nordisk, Novartis, Regeneron Pharmaceuticals, and AstraZeneca. Dr Steg also has a European application number/patent number, issued on October 26, 2016 (15712241.7), for a method for reducing cardiovascular risk (all royalties assigned to Sanofi).
ABBREVIATIONS AND ACRONYMS
- ACS
acute coronary syndrome
- Cl
confidence interval
- HDL-C
high-density lipoprotein cholesterol
- HR
hazard ratio
- IQR
interquartile range
- LDL-C
low-density lipoprotein cholesterol
- MACE
major adverse cardiovascular events
- PCSK9
proprotein subtilisin/kexin type 9
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
APPENDIX For a supplemental table and figure and a complete list of the ODYSSEY Outcomes committee members, investigators, and contributors, please see the online version of this paper.
REFERENCES
- 1.Grundy SM, Stone NJ, Bailey AL, et a I. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2019;73(24):e285–350. [DOI] [PubMed] [Google Scholar]
- 2.Cannon CP, Blazing MA, Giugliano RP, et al. Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med. 2015;372: 2387–97. [DOI] [PubMed] [Google Scholar]
- 3.Sabatine MS, Giugliano RP, Keech AC, et al. Evolocumab and clinical outcomes in patients with cardiovascular disease. N Engl J Med. 2017;376: 1713–22. [DOI] [PubMed] [Google Scholar]
- 4.Schwartz GG.Steg PG,Szarek M,et al. Alirocumab and cardiovascular outcomes after acute coronary syndrome. N Engl J Med. 2018;379:2097–107. [DOI] [PubMed] [Google Scholar]
- 5.Wiviott SD, Cannon CP, Morrow DA, et al. Can low-density lipoprotein be too low? The safety and efficacy of achieving very low low-density lipoprotein with intensive statin therapy: a PROVE IT-TIMI 22 substudy. J Am Coll Cardiol. 2005;46: 1411–6. [DOI] [PubMed] [Google Scholar]
- 6.Tsimikas S. A test in context: lipoprotein(a): diagnosis, prognosis, controversies, and emerging therapies. J Am Coll Cardiol. 2017;69:692–711. [DOI] [PubMed] [Google Scholar]
- 7.Gurdasani D, Sjouke B, Tsimikas S, et al. Lipoprotein(a) and risk of coronary, cerebrovascular, and peripheral artery disease: the EPIC-Norfolk prospective population study. Arterioscler Thromb Vase Biol. 2012;32:3058–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Langsted A, Nordestgaard BG, Kamstrup PR. Elevated lipoprotein (a) and risk of ischemic stroke. J Am Coll Cardiol. 2019;74:54–66. [DOI] [PubMed] [Google Scholar]
- 9.Laschkolnig A, Kollerits B, Lamina C, et a I. Lipoprotein (a) concentrations, apolipoprotein (a) phenotypes, and peripheral arterial disease in three independent cohorts. Cardiovasc Res. 2014; 103:28–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bittner VA, Szarek M, Aylward PE, et a I. Effect of alirocumab on lipoprotein(a) and cardiovascular risk after acute coronary syndrome. J Am Coll Cardiol. 2020;75:133–44. [DOI] [PubMed] [Google Scholar]
- 11.Marston NA, Gurmu Y, Melloni GEM, et al. The effect of PCSK9 (proprotein convertase subtilisin/kexin type 9) inhibition on the risk of venous thromboembolism. Circulation. 2020;141:1600–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.O’Donoghue ML, Fazio S, Giugliano RP, et al. Lipoprotein(a), PCSK9 inhibition, and cardiovascular risk. Circulation. 2019;139: 1483–92. [DOI] [PubMed] [Google Scholar]
- 13.Schwartz GG, Steg PG, Szarek M, et al. Peripheral artery disease and venous thromboembolic events after acute coronary syndrome: role of lipoprotein(a) and modification by alirocumab: prespecified analysis of the ODYSSEY Outcomes randomized clinical trial. Circulation. 2020;141: 1608–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Szarek M, Bittner VA, Aylward P, et al. Lipoprotein(a) lowering by alirocumab reduces the total burden of cardiovascular events independent of low-density lipoprotein cholesterol lowering: ODYSSEY Outcomes trial. Eur Heart J. 2020;41: 4245–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Giugliano RP, Keech A, Murphy SA, et al. Clinical efficacy and safety of evolocumab in high-risk patients receiving a statin: secondary analysis of patients with low LDL cholesterol levels and in those already receiving a maximal-potency statin in a randomized clinical trial. JAMA Cardiol. 2017; 2:1385–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schwartz GG, Bessac L, Berdan LG, et a I. Effect of alirocumab, a monoclonal antibody to PCSK9, on long-term cardiovascular outcomes following acute coronary syndromes: rationale and design of the ODYSSEY outcomes trial. Am Heart J. 2014; 168:682–9. [DOI] [PubMed] [Google Scholar]
- 17.Scharnagl H, Stojakovic T, Dieplinger B, et al. Comparison of lipoprotein (a) serum concentrations measured by six commercially available immunoassays. Atherosclerosis. 2019;289:206–13. [DOI] [PubMed] [Google Scholar]
- 18.Hastie TJ, Tibshirani RJ, Friedman JH. The Elements of Statistical Learning: Data Mining, Inference, and Prediction. New York: SpringerVerlag, 2001. [Google Scholar]
- 19.Emerging Risk Factors Collaboration, Erqou S, Kaptoge S, et al. Lipoprotein(a) concentration and the risk of coronary heart disease, stroke, and nonvascular mortality. JAMA. 2009;302:412–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Waldeyer C, Makarova N, Zeller T, et al. Lipoprotein(a) and the risk of cardiovascular disease in the European population: results from the BiomarCaRE consortium. Eur Heart J. 2017;38: 2490–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Baigent C, Keech A, Kearney PM, et al. Efficacy and safety of cholesterol-lowering treatment: prospective meta-analysis of data from 90,056 participants in 14 randomised trials of statins. Lancet. 2005;366:1267–78. [DOI] [PubMed] [Google Scholar]
- 22.Schwartz GG, Do RQ. Special patient populations: acute coronary syndromes. In: Ballantyne CM, editor. Clinical Lipidology: A Companion to Braunwald’s Heart Disease. 2nd ed. Philadelphia: Elsevier Saunders, 2015:454–68. [Google Scholar]
- 23.Moriarty PM, Gorby LK, Stroes ES, Kastelein JP, Davidson M, Tsimikas S. Lipoprotein(a) and its potential association with thrombosis and inflammation in COVID-19: a testable hypothesis. Curr Atheroscler Rep. 2020;22:48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Burgess S, Ference BA, Staley JR, et a I. Association of LPA variants with risk of coronary disease and the implications for lipoprotein(a)- lowering therapies: a mendelian randomization analysis. JAMA Cardiol. 2018;3:619–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tsimikas S, Fazio S, Viney NJ, Xia S, Witztum JL, Marcovina SM. Relationship of lipoprotein(a) molar concentrations and mass according to lipoprotein (a) thresholds and apolipoprotein(a) isoform size. J Clin Lipidol. 2018;12:1313–23. [DOI] [PubMed] [Google Scholar]
- 26.Gudbjartsson DF, Thorgeirsson G, Sulem P, et al. Lipoprotein(a) concentration and risks of cardiovascular disease and diabetes. J Am Coll Cardiol. 2019;74:2982–94. [DOI] [PubMed] [Google Scholar]
- 27.Maeda S, Abe A, Seishima M, Makino K, Noma A, Kawade M. Transient changes of serum lipoprotein (a) as an acute phase protein. Atherosclerosis. 1989;78:145–50. [DOI] [PubMed] [Google Scholar]
- 28.Pare G, Caku A, McQueen M, et a I. Lipoprotein (a) levels and the risk of myocardial infarction among 7 ethnic groups. Circulation. 2019; 139:1472–82. [DOI] [PubMed] [Google Scholar]
- 29.Lee SR, Prasad A, Choi YS, et al. LPA gene, ethnidty, and cardiovascular events. Circulation. 2017;135:251–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kinpara K, Oka da H, Yoneyama A, Okubo M, Murase T. Lipoprotein(a)-cholesterol: a significant component of serum cholesterol. Clin Chim Acta. 2011;412:1783–7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.