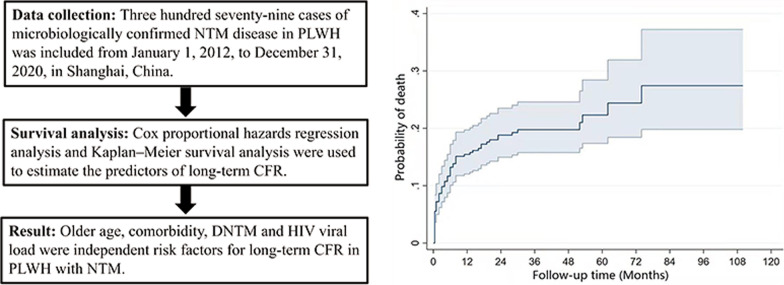
Abstract
Background
Few data are available regarding the long-term case-fatality rate (CFR) among people living with HIV (PLWH) with nontuberculous mycobacteria (NTM) disease. The aim of this study is to analyze the long-term CFR in patients with NTM disease and to identify risk factors for their death.
Methods
A retrospective cohort study of 379 cases of microbiologically confirmed NTM disease in PLWH was conducted from January 1, 2012, to December 31, 2020, in Shanghai, China. We used Kaplan–Meier survival analysis and the log-rank test to compare the long-term CFR in patients with disseminated NTM (DNTM) and localized NTM disease. Univariate Cox proportional hazards regression analysis and a stepwise Cox proportional hazards regression model were used to estimate the predictors of long-term CFR.
Results
The cohort was followed up for a median of 26 months. The total CFR was 15.7% by one year and increased to 22.6% at 5 years after the diagnosis of NTM disease. The 5-year CFR of PLWH with DNTM was significantly higher than that of PLWH with localized NTM (26.7% vs 19.6% for DNTM and localized NTM disease, respectively). Older age [hazard ratio (HR) = 1.04, 95% confidence interval (CI): 1.02–1.06, P < 0.001], comorbidity (HR = 2.05, 95% CI: 1.21–3.49, P < 0.01), DNTM (HR = 2.08, 95% CI: 1.17–3.68, P < 0.05), and HIV viral load (HR = 1.32, 95% CI: 1.12–1.55, P < 0.001) were all independent risk factors for long-term CFR. In the subgroup analysis, time to culture positivity was negatively correlated with CFR in patients with DNTM (HR = 0.90, 95% CI: 0.82–0.98, P < 0.05).
Conclusions
NTM was associated with a high long-term CFR in PLWH. Further approaches to prevent NTM disease in PLWH are urgently needed.
Graphical Abstract
Keywords: HIV/AIDS, Nontuberculous mycobacteria, Case-fatality rate, Risk factor
Background
Nontuberculous mycobacteria (NTM) disease is one of the leading opportunistic infections in people living with HIV (PLWH). Over the past decades, the number of cases of PLWH with NTM has increased with advances in screening techniques, the AIDS epidemic and the increase in the number of immunocompromised patients [1, 2]. Recently, data from the United States showed that the overall prevalence of NTM (among PLWH admitted to the hospital for pneumonia) was 49% (96/196) [3]. In another study, 37 cases of disseminated NTM (DNTM) were identified in 7,349 patients in Oregon, USA, between 2007 and 2012, with a median annual incidence of 110/100,000 HIV person-years and the highest incidence in those with CD4+ T cell count < 50 cells/mm3 (5,300/100,000 person-years) [4].
In the preantiretroviral therapy era, case fatality rates (CFRs) were high for NTM and even higher for DNTM, with an annual CFR of 71% [5]. Since the antiretroviral therapy (ART) era, AIDS has become a chronic disease, which has led to a significant increase in life expectancy in PLWH, and the incidence of disseminated Mycobacterium avium complex (DMAC) has declined significantly from 65.3/1,000 in 1992 to 2.0/1,000 in 2015 [6]. However, despite the availability of effective ART, the CFR for NTM remains high, with a CFR of 69% at 1 year and 27% at 3 years after the diagnosis of DMAC [7].
Modern population-based estimates of the long-term survival of HIV-infected patients with NTM are lacking. Studies have indicated that the long-term survival of PLWH with tuberculosis (TB) is lower than that of non-TB patients [8]. We hypothesized that long-term CFR would also be elevated in PLWH with NTM. Therefore, we conducted this study to analyze the long-term CFR in PLWH with NTM and to identify risk factors for death.
Methods
Study subjects
A retrospective analysis was performed on the clinical data of PLWH with NTM in Shanghai Public Health Clinical Center from January 1, 2012, to December 31, 2020. The inclusion criteria were as follows: (1) HIV-1 infection confirmed by Western blotting; (2) patients had at least one specimen with positive mycobacterial culture and negative for MPB64, or the mycobacterial sequencing results were NTM; (3) physicians deemed NTM to be an etiology of the diseases but not colonization. The exclusion criteria were patients younger than 18 years.
Study design
The following characteristics were recorded: sex, age, behavioral risk factors (such as current or former smoking, alcohol abuse), comorbidities, opportunistic infections, CD4+ T cell count, HIV viral load, ART regimen, NTM treatment, symptoms and the time from specimen culture initiation to positive report. Furthermore, we registered the high-resolution computed tomography (CT) scan. All abnormal CT scans were reported and divided into seven categories: (1) lymphadenopathy only; (2) lymphadenopathy with nodules; (3) lymphadenopathy with cavities; (4) lymphadenopathy with cavities and nodules; (5) nodules only; (6) cavities only; (7) and consolidation only. We recorded the laboratory data of all patients, including T-SPOT results, blood cell counts, hemoglobin levels, erythrocyte sedimentation rates, C-reactive protein levels and renal and liver function.
The diagnosis of DNTM was defined as a positive culture for mycobacterium from blood, cerebrospinal fluid, bone marrow, or biopsy of a sterile site or infection involving two or more noncontiguous body sites. Comorbidities were defined based on the Charlson Comorbidity Index [9, 10], which includes 19 major disease categories, including risk factors and potential prognostic factors for PLWH with NTM. Comorbidity data included diabetes, hypertension, cancer, chronic lung disease and other diseases. AIDS-defining opportunistic infections other than NTM infection were also recorded. Baseline CD4+ T cell count and HIV viral load were defined using the test results at admission or the closest record at admission. Anti-NTM medication use was defined as drug use for at least 2 weeks.
For the prognosis analysis, survival time was defined as the time from the beginning of definitive diagnosis of NTM to death, loss to follow-up, or the end of follow-up (December 31, 2020). Patients were followed up by telephone after discharge from the hospital. The outcome was all-cause mortality during the follow-up period.
The study was approved by the Ethics Committee of Shanghai Public Health Clinical Center (Ethics approval number: 2020-Y112-01). Informed consent was waived because of the retrospective design of the study.
Statistical analysis
SPSS statistics 25.0 (IBM, Armonk, NY, USA) and Stata 16.0 (StataCorp LP, College Station, TX, USA) were used for statistical analysis. The Shapiro–Wilk test was used to test whether the data conformed to a normal distribution. Normally distributed data are reported as the means and standard deviation (mean ± SD). Nonnormally distributed data are presented as the medians and interquartile range (IQR). Categorical variables were summarized with frequency counts and presented as a rate (%). The χ2 test, t test and Fisher’s exact test were used to test for statistically significant differences. Kaplan–Meier survival analysis and log-rank test were used to compare long-term CFR in patients with DNTM and localized NTM disease. Univariate Cox proportional hazards regression analysis and stepwise Cox proportional hazards regression models were used to estimate the predictors of long-term CFR. P < 0.05 indicated statistical significance. In these analyses, hazard ratios were combined with 95% confidence intervals.
Results
Clinical characteristics of the study population
Three hundred seventy-nine patients were included. Of these, 93.7% were male, and the median age was 38.0 (IQR: 30.0–50.0) years. In total, 7.4% of the patients were current or former smokers, and 2.6% consumed alcohol. One hundred thirteen patients (29.8%) had comorbidities, and 136 patients (35.9%) had opportunistic infections. In addition, the median CD4+ T cell count was 23.0 (IQR: 6.0–73.8) cells/μl, and the median HIV viral load was 4.84 (IQR: 1.9–5.5) log10 copies/ml. Two hundred ninety-four patients (77.6%) received ART prior to anti-NTM therapy, and the median time from initiation of ART to initiation of anti-NTM therapy was 31.0 (IQR: 4.0–127.0) months (Table 1).
Table 1.
Baseline characteristics of PLWH with NTM infection
| n (%) | |
|---|---|
| General information | |
| Total number of patients | 379 |
| Male sex | 355 (93.7%) |
| Age [years], Median (IQR) | 38.0 (30.0–50.0) |
| Smoking (current or former) | 28 (7.4%) |
| Alcoholism | 10 (2.6%) |
| Comorbidity | 113 (29.8%) |
| Opportunistic infection | 136 (35.9%) |
| Cryptococcosis | 35 (25.7%) |
| Cytomegalovirus | 28 (20.6%) |
| Digestive tract fungal infections | 23 (16.9%) |
| Pneumocystis pneumonia | 12 (8.8%) |
| Talaromyces marneffei | 11 (8.1%) |
| Kaposi’s Sarcoma | 8 (5.9%) |
| Herpes zoster | 5 (3.7%) |
| Mycobacterium tuberculosis | 4 (2.9%) |
| Salmonella infection | 3 (2.2%) |
| Pulmonary aspergillosis | 2 (1.5%) |
| Bacterial pneumonia | 2 (1.5%) |
| Cerebral toxoplasmosis | 2 (1.5%) |
| Progressive multifocal leukoencephalopathy | 1 (0.7%) |
| Clinical manifestations | |
| Fever | 236 (63.4%) |
| Cough | 164 (44.1%) |
| HIV wasting syndrome | 81 (22.8%) |
| Abdominal pain and diarrhea | 68 (18.3%) |
| Central nervous system symptoms | 48 (12.9%) |
| Rash | 43 (11.6%) |
| HIV-related indicators | |
| CD4+ T cell count [cells/μl], Median (IQR) | 23 (6.0–73.8) |
| HIV viral load [log10 copies/ml], Median (IQR) | 4.8 (1.9–5.5) |
| ART before NTM treatment | 294 (77.6%) |
| ART to anti-NTM time, Median (IQR) | 31 (4.0–127.0) |
| NTM treatment | |
| Macrolides | 280 (73.9%) |
| Levofloxacin/Moxifloxacin | 248 (65.4%) |
| Ethambutol | 317 (83.6%) |
| Rifampicin/Rifabutin | 248 (65.4%) |
| Linezolid | 23 (6.1%) |
PLWH people living with HIV, NTM nontuberculous mycobacteria, IQR interquartile range, HIV human immunodeficiency virus, ART antiretroviral therapy
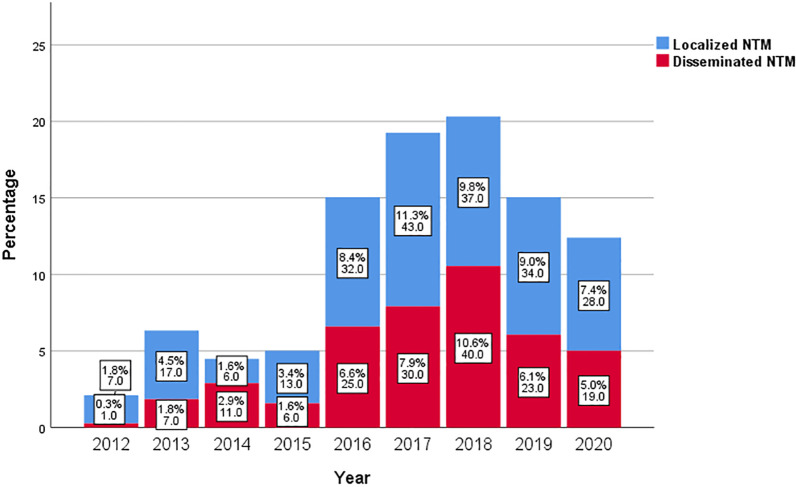
The most frequently reported symptom was fever (63.4%). Cough was reported in 44.1% of all cases. A total of 22.8% reported HIV wasting syndrome, 18.3% reported abdominal pain and/or diarrhea, 12.9% reported central nervous system symptoms such as headache and dizziness, and half of these patients had a combination of cryptococcal meningitis. The remaining 11.6% of patients had skin manifestations, such as rashes (Table 1). For each year from 2013 to 2020, DNTM accounted for almost half of the total number of NTM in PLWH (Fig. 1). Among the first reported positive specimens, sputum accounted for 60.7%; blood accounted for 23.5%; and stool accounted for 6.9%; while the rest were puncture fluid (4.0%), bronchoalveolar lavage or bronchial lavage fluid (1.3%), pleural effusion (0.8%), cerebrospinal fluid (0.8%), bone marrow (0.8%), urine (0.5%), hydroperitoneum (0.3%), abdominal abscess (0.3%) and secretions from ruptured skin (0.3%). The median time to culture positivity was 13.9 (IQR: 9.5–23.5) days.
Fig. 1.
Stacked bar charts of PLWH with disseminated NTM and with localized NTM disease. (The data showed in the charts is percentage and number). PLWH people living with HIV, NTM nontuberculous mycobacteria
Three hundred twenty-six (86.0%) patients received CT scans. Of the 326 patients, 148 (45.4%) were described as having lymphadenopathy. The most common morphology was mediastinal lymphadenopathy (128/148, 86.5%) (Table 2).
Table 2.
Radiology findings of PLWH with NTM infection
| n (%) | ||
|---|---|---|
| Lymphadenopathy | 148 (45.4%) | |
| Morphology | ||
| Mediastinal Lymphadenopathy | 128 (86.5%) | |
| Hilar lymphadenopathy | 42 (28.4%) | |
| Retroperitoneal lymph | 40 (27.0%) | |
| Axillary lymph nodes | 20 (13.5%) | |
| Supraclavicular/infraclavicular lymph nodes | 8 (5.4%) | |
| Celiac lymph node | 8 (5.4%) | |
| Pelvic lymph nodes | 1 (0.7%) | |
| Categories | ||
| Lymphadenopathy only | 111 (75.0%) | |
| Lymphadenopathy with nodules | 23 (15.5%) | |
| Lymphadenopathy with cavities | 10 (6.8%) | |
| Lymphadenopathy with cavities and nodules | 4 (2.7%) | |
| Nodules | 33 (10.1%) | |
| Cavities | 14 (4.3%) | |
| Consolidation | 3 (0.9%) | |
| Other infectious lesions | 107 (32.8%) | |
| No obvious abnormalities | 21 (6.4%) | |
Total of 326 patients received CT scans. PLWH people living with HIV, NTM nontuberculous mycobacteria, HIV human immunodeficiency virus
Treatment for NTM diseases consists of a multidrug regimen and a long course of therapy. Anti-NTM medication for patients included macrolides, levofloxacin/moxifloxacin, ethambutol, rifampicin/rifabutin, and linezolid (Table 1), which lasted for 9–12 months. Almost all of the enrolled patients received ART.
Survival analysis
After a median of 26 months of follow-up, 69 patients (18.2%) died, and 48 (12.7%) were lost to follow-up. In 52.2% of patients, the follow-up period exceeded 2 years. The life table method showed an overall CFR of 15.7% at 1 year, 19.0% at 2 years, 20.0% at 3 years, 22.6% at 5 years, and 27.9% at 7 years. Univariate Cox regression analysis indicated that the following parameters were statistically significant for survival: older age, HIV viral load, ART before NTM treatment, comorbidity, linezolid and DNTM (Table 3). The probability of death in PLWH with NTM increased with time (Fig. 2).
Table 3.
Hazard ratio in univariate analysis and multivariate analysis
| Univariate analysis | Multivariate analysis | |||||
|---|---|---|---|---|---|---|
| Hazard ratio | 95% CI | P | Hazard ratio | 95% CI | P | |
| Age | 1.02 | 1.01–1.04 | 0.015 | 1.04 | 1.02–1.06 | 0.001 |
| Comorbidity | 2.01 | 1.24–3.23 | 0.005 | 2.05 | 1.21–3.49 | 0.008 |
| DNTM | 1.81 | 1.12–2.90 | 0.015 | 2.08 | 1.17–3.68 | 0.012 |
| HIV viral load | 1.25 | 1.07–1.46 | 0.006 | 1.32 | 1.12–1.55 | 0.001 |
| Linezolid | 3.64 | 1.85–7.15 | 0.001 | 4.71 | 2.25–9.83 | 0.001 |
| Sex | 1.59 | 0.50–5.04 | 0.435 | – | – | 0.697 |
| Smoking | 0.86 | 0.31–2.35 | 0.764 | – | – | 0.411 |
| Alcoholism | 1.30 | 0.32–5.29 | 0.719 | – | – | 0.592 |
| Opportunistic infection | 0.98 | 0.60–1.61 | 0.946 | – | – | 0.411 |
| CD4+ T cell count | 1.00 | 1.00–1.00a | 0.243 | – | – | 0.795 |
| ART before NTM treatment | 0.53 | 0.32–0.89 | 0.015 | – | – | 0.221 |
| Time to culture positivity | 0.98 | 0.96–1.01 | 0.125 | – | – | 0.232 |
Sex, age, smoking, alcoholism, comorbidity, opportunistic infection, CD4+ T cell count, HIV viral load, ART before NTM treatment, linezolid, DNTM and time to culture positivity were added to the model using stepwise procedures
–: blank (In the multivariate analysis, SPSS 25.0 did not show 95% CI and hazard ratio for variables that were not statistically significant); a: 95% CI for hazard ratio of CD4+ T cell count: 0.996–1.001
HIV human immunodeficiency virus, NTM nontuberculous mycobacteria, DNTM disseminated nontuberculous mycobacteria, ART antiretroviral therapy
Fig. 2.
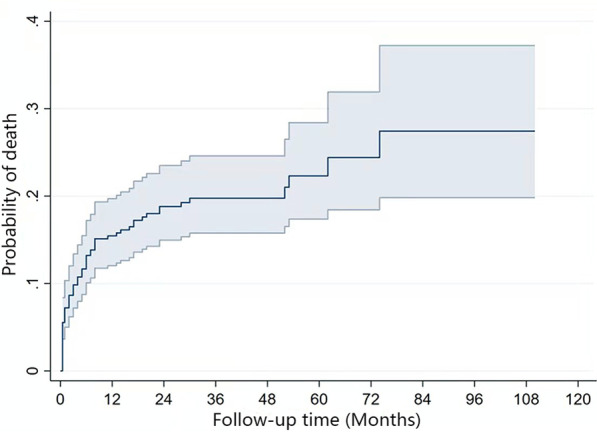
Probability of death (95% confidence interval) among PLWH with NTM. PLWH people living with HIV, NTM nontuberculous mycobacteria
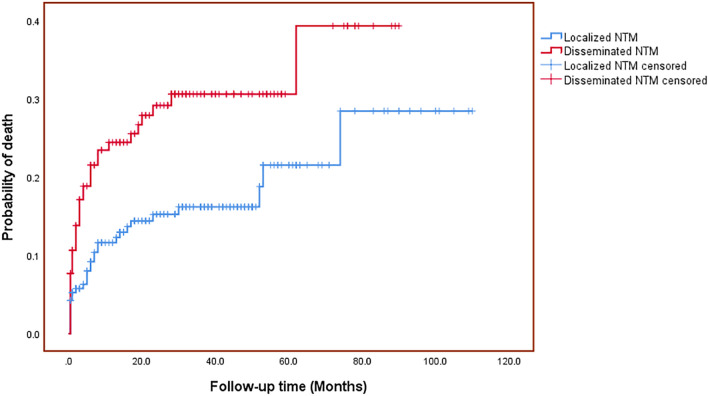
Considering that sex, smoking, alcoholism, opportunistic infection, CD4+ T cell count, and time to culture positivity were also important risk factors, these factors and all parameters that were statistically significant in the univariate analysis were included in a multivariate Cox proportional hazards model. The results showed a hazard ratio of 2.05 [95% confidence interval (CI): 1.21–3.49, P < 0.01] for patients with comorbidities compared with those without comorbidities. The hazard ratio caused by older age was 1.04 (95% CI: 1.02–1.06, P < 0.001). High levels of HIV viral load were statistically significant, with a hazard ratio of 1.32 (95% CI: 1.12–1.55, P < 0.001). DNTM was significantly correlated with poor survival outcomes (HR = 2.08, 95% CI: 1.17–3.68, P < 0.05) (Table 3). Kaplan–Meier analysis also revealed that the long-term CFR of the DNTM group was significantly higher than that of the localized infection group (Fig. 3). Surprisingly, patients not treated with linezolid had a significantly longer survival time than those treated with linezolid (HR = 4.71, 95% CI: 2.25–9.83, P < 0.001).
Fig. 3.
Probability of death among PLWH with disseminated NTM and localized NTM disease. PLWH people living with HIV, NTM nontuberculous mycobacteria
In addition, we performed a stratified analysis by baseline CD4+ T cell count. Patients with CD4+ T cell counts > 50 cells/μl had CFRs of 7.9%, 12.1%, and 17.9% at 1, 3, and 7 years, respectively. Older age (HR = 1.09, 95% CI: 1.04–1.14, P < 0.001) and DNTM (HR = 3.52, 95% CI: 1.01–12.28, P < 0.05) were independent prognostic factors. Patients with CD4+ T cell counts ≤ 50 cells/μl had CFRs of 19.9%, 24.4% and 32.7% at 1, 3 and 7 years, respectively. Comorbidities (HR = 2.14, 95% CI: 1.19–3.89, P < 0.05) and linezolid usage (HR = 2.97, 95% CI: 1.34–6.58, P < 0.01) were independent prognostic factors. ART before NTM treatment was more beneficial for patients (HR = 0.46, 95% CI: 0.25–0.84, P < 0.05).
In the subgroup analysis for patients with DNTM, time to culture positivity was negatively correlated with CFR (HR = 0.90, 95% CI: 0.84–0.96, P < 0.01). The longer the time to a positive culture of the specimen, the lower the number of NTMs, thus favoring survival. Older age (HR = 1.05, 95% CI: 1.02–1.08, P < 0.01), comorbidity (HR = 2.38, 95% CI: 1.14–4.96, P < 0.05) and linezolid usage (HR = 3.39, 95% CI: 1.43–8.02, P < 0.01) remained independent risk factors for long-term CFR (Table 4).
Table 4.
Hazard ratio in multivariate analysis of PLWH with DNTM
| Hazard ratio | 95% CI | P | |
|---|---|---|---|
| Age | 1.05 | 1.02–1.08 | 0.004 |
| Comorbidity | 2.38 | 1.14–4.96 | 0.021 |
| Linezolid | 3.39 | 1.43–8.02 | 0.006 |
| Time to culture positivity | 0.90 | 0.84–0.96 | 0.002 |
Sex, age, smoking, alcoholism, comorbidity, opportunistic infection, CD4+ T cell count, HIV viral load, ART before NTM treatment, linezolid, DNTM and time to culture positivity were added to the model using stepwise procedures
HIV human immunodeficiency virus, ART antiretroviral therapy, PLWH people living with HIV, DNTM disseminated nontuberculous mycobacteria
Discussion
Our study demonstrated that the long-term CFR of PLWH with NTM is high, despite these patients receiving treatment or even recovering. Further analysis revealed that the long-term CFR for disseminated infections was higher than that for those without disseminated infections. Older age, comorbidity, HIV viral load, DNTM and linezolid usage were independent prognostic factors for PLWH with NTM. Although the CD4+ T cell count was not significant in the multivariate analysis, we found that patients with CD4+ T cell counts ≤ 50 cells/μl had a higher CFR. These patients were more likely to acquire DNTM. For patients with DNTM, the time to culture positivity was negatively correlated with CFR.
Collins et al. studied patients with HIV/AIDS from 1992 to 2015 [7]. Despite effective ART, they found that DMAC infection was associated with a significant increase in CFR in the years following diagnosis. This is similar to the results in our study. Another Japanese study also showed that DNTM was significantly associated with CFR, and the median baseline CD4+ T cell count was significantly lower in nonsurvivors than in survivors [11]. However, their sample size of 24 was not very convincing, and further studies are needed.
Data on the impact of NTM on long-term CFR in PLWH are limited, but studies in HIV-negative cohorts have also found high long-term CFR in NTM survivors. Typically, factors such as older age and comorbidities have been reported to be associated with poor prognosis. A systematic review gave an overall estimate of 5-year CFR from NTM pulmonary disease studies. Despite the high heterogeneity of the enrolled studies, the pooled estimate of 5-year all-cause mortality of the 9035 patients was 27% (95% CI: 21.3–37.8) [12]. Predictors of CFR that were consistent across studies included male sex, presence of comorbidities and older age of patients [12]. Since the vast majority of PLWH in our cohort were male, there was a gender skew; thus, our data are not quite applicable to the general population.
Several studies have compared long-term CFR among PLWH after completing TB treatment to those without TB [8]. The 5-year CFR for patients who completed TB treatment was 10.2% compared to patients without TB (5.6%) [8]. In our study, the 5-year CFR for NTM was 22.6%, which appears to be more than twice as high as that of TB patients. Chiang et al. also reported that PLWH with DMAC (n = 58) had a three times higher 1-year CFR than those with TB (n = 98) (48.3% vs 16.3%) [13]. This implies that the long-term prognosis of NTM in PLWH is of critical importance.
Behavioral factors (such as smoking and alcoholism) [14, 15] and various respiratory or nonrespiratory comorbidities [16] increase the risk of acquiring NTM and may partially account for the increased long-term CFR among NTM survivors. Multiple studies in largely HIV-negative populations have documented structural lung defects and impaired pulmonary function after NTM infection, and many studies have found a strong association with increased CFR between a history of NTM and chronic obstructive pulmonary disease and bronchiectasis [17–21]. Recently, Mourad et al. found that the expected survival was reduced by approximately 4 years for a diagnosis of NTM lung disease without comorbidity and by 8.6 years for a diagnosis of NTM lung disease with comorbidity [22]. Among HIV-negative patients with NTM with and without comorbidities, the 5-year CFR after diagnosis was 44.9% and 25.0%, respectively [22].
The high occurrence of disease relapse and increasing drug resistance may lead to an increased CFR. A multicenter study showed that 9.5% of patients with NTM pulmonary infection experienced multiple episodes, with 24.8% of them suffering from relapsing infections caused by the same NTM species [23]. An observational retrospective study from Italy revealed that 35.3% of patients had unsuccessful treatment outcomes, including discontinuation of therapy (13.5%), recurrence (11.2%), reinfection (5.3%), treatment failure (4.1%) and relapse (1.2%) [24]. The treatment of treatment-refractory NTM cases or patients with drug-resistant NTM isolates remains challenging. This may indicate a poor prognosis and high CFR. In clinical practice, patients with NTM infections caused by more resistant species may use linezolid, as their conditions are more severe. This may partially explain why using linezolid was associated with poor outcome in our study.
In addition, NTM may cause persistent inflammation and immune activation, which may increase susceptibility to HIV infection, promote HIV viral replication, and accelerate the progression of HIV disease [25]. As shown in a previous study, 79% (19/24) of PLWH with DNTM had immune reconstitution syndrome, suggesting difficulty in the management of DNTM [11]. Therefore, clinicians should pay high attention to DNTM in PLWH. However, the diagnosis of NTM infection in PLWH is difficult, as the available methods are limited. Notably, nearly half of the patients had abnormal CT imaging in our study, most commonly mediastinal lymphadenopathy. This may give the physician a clue in treating PLWH suspected of NTM infection.
There are some limitations in our study. First, this study was conducted at a single center, and our results may not be generalizable to other regions due to its retrospective nature. Second, we did not use the American Thoracic Society criteria for NTM lung disease. Third, no further species identification was available for most patients. Several studies have found that different mycobacterium species were not significant for CFR analysis [26, 27]. However, a 15-year follow-up study of 1445 patients with NTM pulmonary disease showed that the accurate identification of the species or subspecies of the NTM pathogen is very important in the prognosis [28]. Data from Canada also showed that NTM disease was associated with higher rates of death for all species combined and for most individual species [29]. Therefore, further research on species identification is needed. Furthermore, although the sensitivity of MPB64 is high, a very small percentage of patients may still have a false-negative result [30]. It is possible that a few patients enrolled were due to TB.
Conclusions
To our knowledge, this is the largest study to date that evaluates the long-term CFR and associated prognostic factors for NTM in PLWH in the modern ART era. Long-term CFR for NTM in PLWH is high. Older age, comorbidity and DNTM are independent prognostic factors for NTM. These findings highlight the critical importance of PLWH with NTM and suggest that PLWH with a history of NTM may require closer long-term follow-up.
Acknowledgements
Not applicable.
Abbreviations
- CFR
Case-fatality rate
- HIV
Human immunodeficiency virus
- PLWH
People living with HIV
- NTM
Nontuberculous mycobacteria
- DNTM
Disseminated nontuberculous mycobacteria
- MAC
Mycobacterium avium Complex
- DMAC
Disseminated Mycobacterium avium complex
- ART
Antiretroviral therapy
- TB
Tuberculosis
- CT
Computed tomography
- SD
Standard deviation
- IQR
Interquartile range
Authors’ contributions
HL, JC and LL conducted the study conception and design. JH and LG collected the data of patients. JH and JC analyzed and interpreted the data. JH wrote the manuscript. YS made grammatical revisions to the manuscript. JC and HL critically revised and finally approved the manuscript. RZ, TQ, JS, ZW, WS, YT, JW, SX, JY and YS supervised the project. All authors read and approved the final manuscript.
Funding
This work was supported by the Shanghai Commission of Science and Technology (20MC1920100 and 21Y11901200), Shanghai key Infectious Disease Project (shslczdzk01102), Shanghai Municipal Health Commission (GWV-10.1-XK02), the development fund for Shanghai talents (2020089) and the Shanghai “Rising stars of Medical Talent” Youth Development Program [No. 2019-72].
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethical approval and consent to participate
The study was approved by the Ethics Committee of Shanghai Public Health Clinical Center. (Ethics approval number: 2020-Y112-01). Informed consent was waived because of the retrospective design of the study.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Jingjing Hu and Ling Gu contributed equally to this work
Contributor Information
Li Liu, Email: liuli@shphc.org.cn.
Jun Chen, Email: qtchenjun@163.com.
Hongzhou Lu, Email: luhongzhou@fudan.edu.cn.
References
- 1.Agizew T, Basotli J, Alexander H, Boyd R, Letsibogo G, Auld A, et al. Higher-than-expected prevalence of non-tuberculous mycobacteria in HIV setting in Botswana: implications for diagnostic algorithms using Xpert MTB/RIF assay. PLoS ONE. 2017;12(12):e189981. doi: 10.1371/journal.pone.0189981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rivero-Lezcano OM, Gonzalez-Cortes C, Mirsaeidi M. The unexplained increase of nontuberculous mycobacteriosis. Int J Mycobacteriol. 2019;8(1):1–6. doi: 10.4103/ijmy.ijmy_18_19. [DOI] [PubMed] [Google Scholar]
- 3.Lapinel NC, Jolley SE, Ali J, Welsh DA. Prevalence of non-tuberculous mycobacteria in HIV-infected patients admitted to hospital with pneumonia. Int J Tuberc Lung Dis. 2019;23(4):491–497. doi: 10.5588/ijtld.18.0336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Varley CD, Ku JH, Henkle E, Schafer SD, Winthrop KL. Disseminated nontuberculous mycobacteria in HIV-infected patients, Oregon, USA, 2007–2012. Emerg Infect Dis. 2017;23(3):533–535. doi: 10.3201/eid2303.161708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moore RD, Chaisson RE. Natural history of opportunistic disease in an HIV-infected urban clinical cohort. Ann Intern Med. 1996;124(7):633–642. doi: 10.7326/0003-4819-124-7-199604010-00003. [DOI] [PubMed] [Google Scholar]
- 6.Chen H, Clifford DB, Deng L, Wu K, Lee AJ, Bosch RJ, et al. Peripheral neuropathy in ART-experienced patients: prevalence and risk factors. J Neurovirol. 2013;19(6):557–564. doi: 10.1007/s13365-013-0216-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Collins LF, Clement ME, Stout JE. Incidence, long-term outcomes, and healthcare utilization of patients with human immunodeficiency virus/acquired immune deficiency syndrome and disseminated Mycobacterium avium complex from 1992–2015. Open Forum Infect Dis. 2017;4(3):x120. doi: 10.1093/ofid/ofx120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Koenig SP, Kim A, Shepherd BE, Cesar C, Veloso V, Cortes CP, et al. Increased mortality after tuberculosis treatment completion in persons living with human immunodeficiency virus in Latin America. Clin Infect Dis. 2020;71(1):215–217. doi: 10.1093/cid/ciz1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 10.Quan H, Li B, Couris CM, Fushimi K, Graham P, Hider P, et al. Updating and validating the Charlson comorbidity index and score for risk adjustment in hospital discharge abstracts using data from 6 countries. Am J Epidemiol. 2011;173(6):676–682. doi: 10.1093/aje/kwq433. [DOI] [PubMed] [Google Scholar]
- 11.Kobayashi T, Nishijima T, Teruya K, Aoki T, Kikuchi Y, Oka S, et al. High mortality of disseminated non-tuberculous mycobacterial infection in HIV-infected patients in the antiretroviral therapy era. PLoS ONE. 2016;11(3):e151682. doi: 10.1371/journal.pone.0151682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Diel R, Lipman M, Hoefsloot W. High mortality in patients with Mycobacterium avium complex lung disease: a systematic review. BMC Infect Dis. 2018;18(1):206. doi: 10.1186/s12879-018-3113-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chiang CH, Lee GH, Chiang TH, Tang PU, Fang CT. Disseminated Mycobacterium avium complex infection as a differential diagnosis of tuberculosis in HIV patients. Int J Tuberc Lung Dis. 2020;24(9):922–927. doi: 10.5588/ijtld.19.0602. [DOI] [PubMed] [Google Scholar]
- 14.Yeligar SM, Chen MM, Kovacs EJ, Sisson JH, Burnham EL, Brown LA. Alcohol and lung injury and immunity. Alcohol. 2016;55:51–59. doi: 10.1016/j.alcohol.2016.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Agusti A, Hogg JC. Update on the pathogenesis of chronic obstructive pulmonary disease. N Engl J Med. 2019;381(13):1248–1256. doi: 10.1056/NEJMra1900475. [DOI] [PubMed] [Google Scholar]
- 16.Lee H, Myung W, Lee EM, Kim H, Jhun BW. Mortality and prognostic factors of nontuberculous mycobacterial infection in Korea: a population-based comparative study. Clin Infect Dis. 2021;72(10):e610–e619. doi: 10.1093/cid/ciaa1381. [DOI] [PubMed] [Google Scholar]
- 17.Balavoine C, Andrejak C, Marchand-Adam S, Blanc FX. Relationships between COPD and nontuberculous mycobacteria pulmonary infections. Rev Mal Respir. 2017;34(10):1091–1097. doi: 10.1016/j.rmr.2017.09.004. [DOI] [PubMed] [Google Scholar]
- 18.Flume PA, Chalmers JD, Olivier KN. Advances in bronchiectasis: endotyping, genetics, microbiome, and disease heterogeneity. Lancet. 2018;392(10150):880–890. doi: 10.1016/S0140-6736(18)31767-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Berra G, Plojoux J, Soccal PM, Janssens JP. Identification of non-tuberculous mycobacteria in COPD patients undergoing lung volume reduction: more frequent than expected? Respiration. 2019;98(3):279–280. doi: 10.1159/000501697. [DOI] [PubMed] [Google Scholar]
- 20.Pyarali FF, Schweitzer M, Bagley V, Salamo O, Guerrero A, Sharifi A, et al. Increasing non-tuberculous mycobacteria infections in veterans with COPD and association with increased risk of mortality. Front Med (Lausanne) 2018;5:311. doi: 10.3389/fmed.2018.00311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McShane PJ, Naureckas ET, Tino G, Strek ME. Non-cystic fibrosis bronchiectasis. Am J Respir Crit Care Med. 2013;188(6):647–656. doi: 10.1164/rccm.201303-0411CI. [DOI] [PubMed] [Google Scholar]
- 22.Mourad A, Baker AW, Stout JE. Reduction in expected survival associated with nontuberculous mycobacterial pulmonary disease. Clin Infect Dis. 2021;72(10):e552–e557. doi: 10.1093/cid/ciaa1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huang HL, Cheng MH, Lu PL, Shu CC, Wang JY, Wang JT, et al. Epidemiology and predictors of NTM pulmonary infection in Taiwan—a retrospective, five-year multicenter study. Sci Rep. 2017;7(1):16300. doi: 10.1038/s41598-017-16559-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Aliberti S, Sotgiu G, Castellotti P, Ferrarese M, Pancini L, Pasat A, et al. Real-life evaluation of clinical outcomes in patients undergoing treatment for non-tuberculous mycobacteria lung disease: a ten-year cohort study. Respir Med. 2020;164:105899. doi: 10.1016/j.rmed.2020.105899. [DOI] [PubMed] [Google Scholar]
- 25.Havlir DV, Torriani FJ, Schrier RD, Huang JY, Lederman MM, Chervenak KA, et al. Serum interleukin-6 (IL-6), IL-10, tumor necrosis factor (TNF) alpha, soluble type II TNF receptor, and transforming growth factor beta levels in human immunodeficiency virus type 1-infected individuals with Mycobacterium avium complex disease. J Clin Microbiol. 2001;39(1):298–303. doi: 10.1128/JCM.39.1.298-303.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Longworth SA, Blumberg EA, Barton TD, Vinnard C. Non-tuberculous mycobacterial infections after solid organ transplantation: a survival analysis. Clin Microbiol Infect. 2015;21(1):43–47. doi: 10.1016/j.cmi.2014.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wetzstein N, Hügel C, Wichelhaus TA, Hogardt M, Eickmeier O, Küpper-Tetzel CP, et al. Species distribution and clinical features of infection and colonisation with non-tuberculous mycobacteria in a tertiary care centre, central Germany, 2006–2016. Infection. 2019;47(5):817–825. doi: 10.1007/s15010-019-01317-2. [DOI] [PubMed] [Google Scholar]
- 28.Jhun BW, Moon SM, Jeon K, Kwon OJ, Yoo H, Carriere KC, et al. Prognostic factors associated with long-term mortality in 1445 patients with nontuberculous mycobacterial pulmonary disease: a 15-year follow-up study. Eur Respir J. 2020;55(1):1900798. doi: 10.1183/13993003.00798-2019. [DOI] [PubMed] [Google Scholar]
- 29.Marras TK, Campitelli MA, Lu H, Chung H, Brode SK, Marchand-Austin A, et al. Pulmonary nontuberculous mycobacteria-associated deaths, Ontario, Canada, 2001–2013. Emerg Infect Dis. 2017;23(3):468–476. doi: 10.3201/eid2303.161927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Raj A, Singh N, Gupta KB, Chaudhary D, Yadav A, Chaudhary A, et al. Comparative evaluation of several gene targets for designing a multiplex-PCR for an early diagnosis of extrapulmonary tuberculosis. Yonsei Med J. 2016;57(1):88–96. doi: 10.3349/ymj.2016.57.1.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.