(Immunity 54, 1578–1593.e1–e5; July 13, 2021)
In our original article, the FlowSOM-generated CD3+CD56+CD4−CD8− T cell cluster was named NKT cells. CD3+CD56+ cells do not exclusively describe NKT cells, but rather a heterogeneous collection of unconventional T cells. As we had not included iNKT-specific CD1d tetramers loaded with α-galactosylceramide in our panels, to be more precise, we corrected the term NKT cells with CD56+ T cells throughout the text and in the listed figures, as well as Tables S1, S3, and S4 and the graphical abstract. This error does not affect the overall conclusions of the paper. Additionally, to more appropriately reflect this change, reference Zhang et al., 2020a has been replaced with Notarbartolo et al., 2021.The authors apologize for any confusion caused.
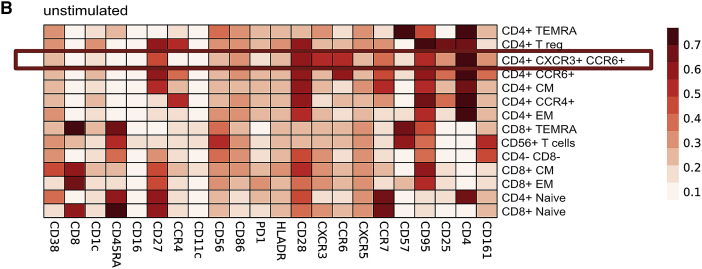
Figure 2B.
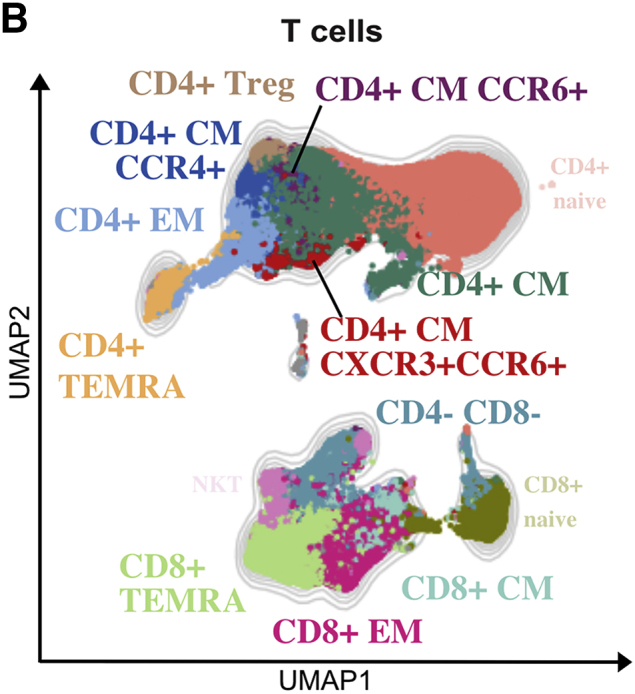
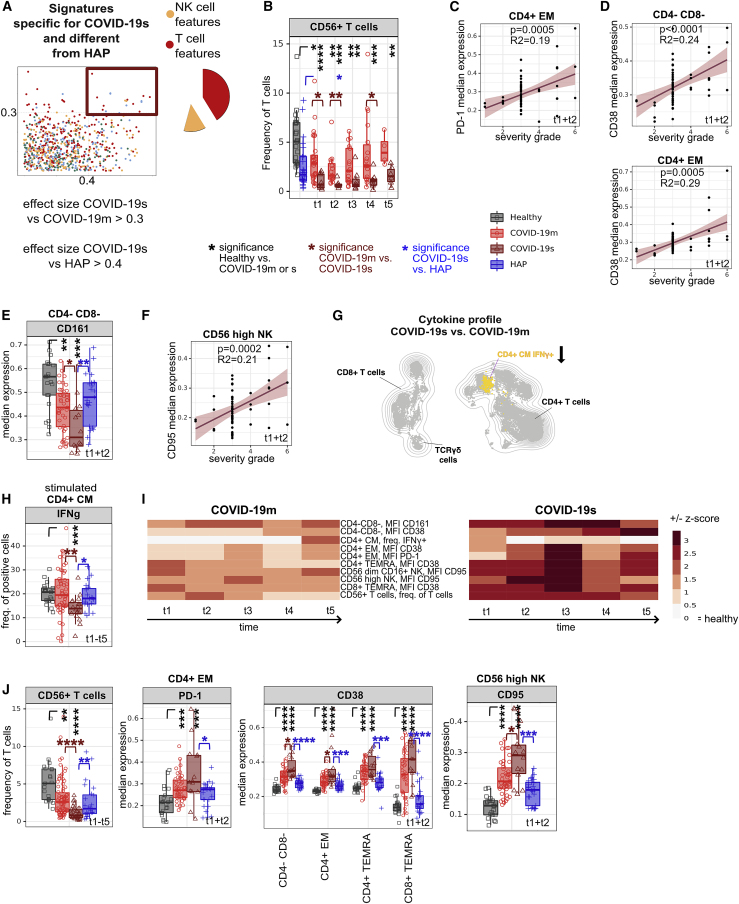
Shared T cell features between severe pathogen-induced RSs highlight the emergence of hyperinflammatory and exhausted subsets in COVID-19s (original)
Figure 2B.
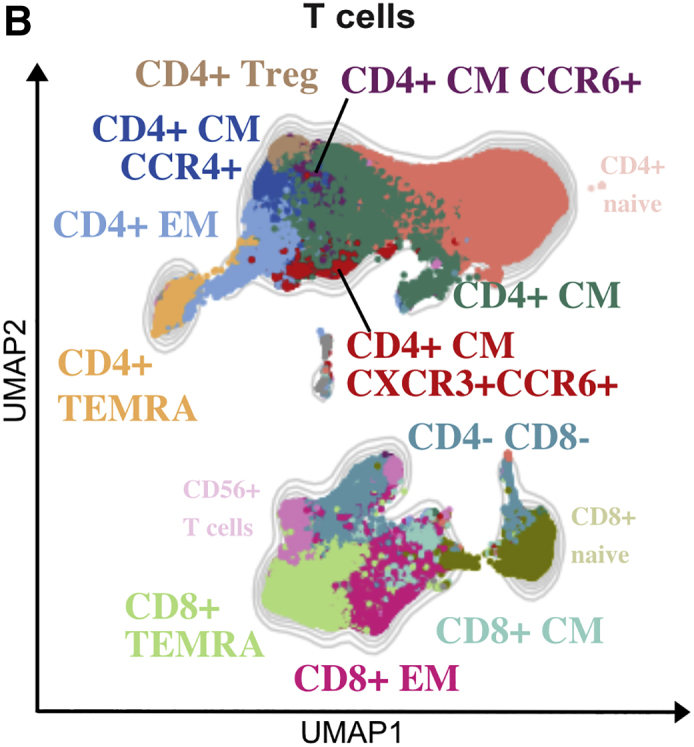
Shared T cell features between severe pathogen-induced RSs highlight the emergence of hyperinflammatory and exhausted subsets in COVID-19s (corrected)
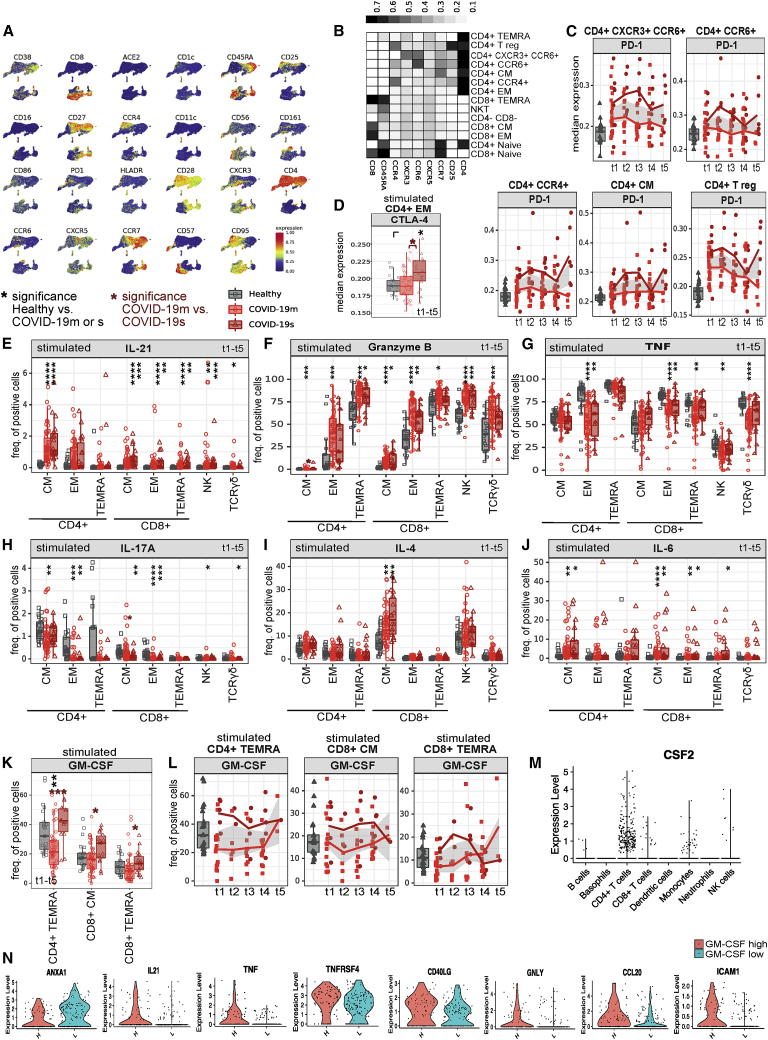
Figure 5.
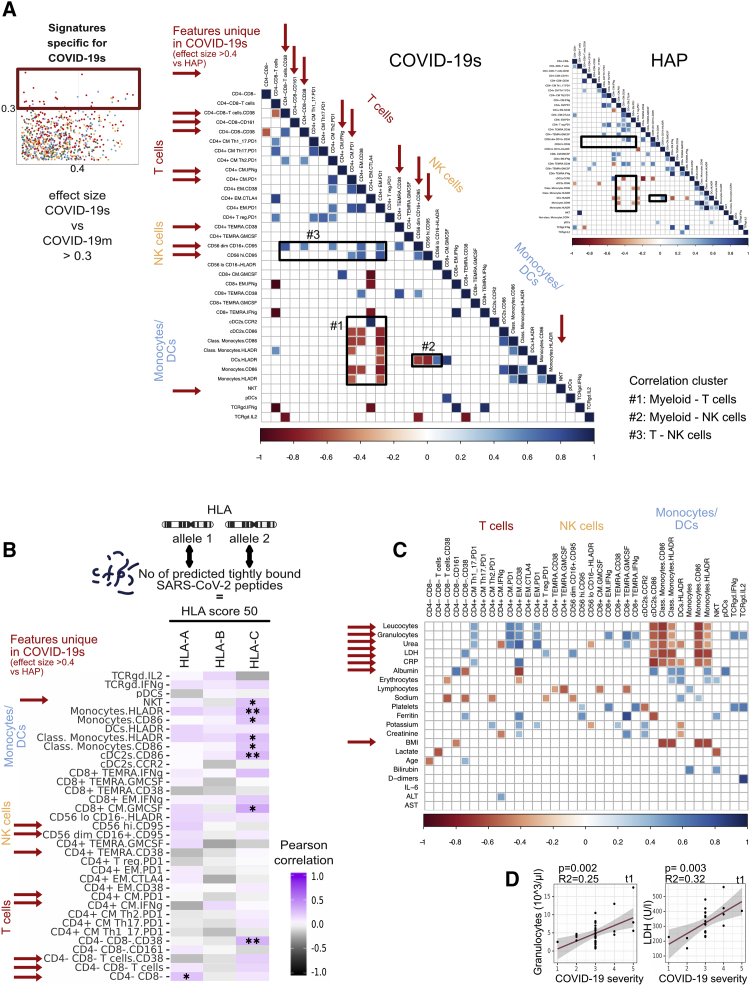
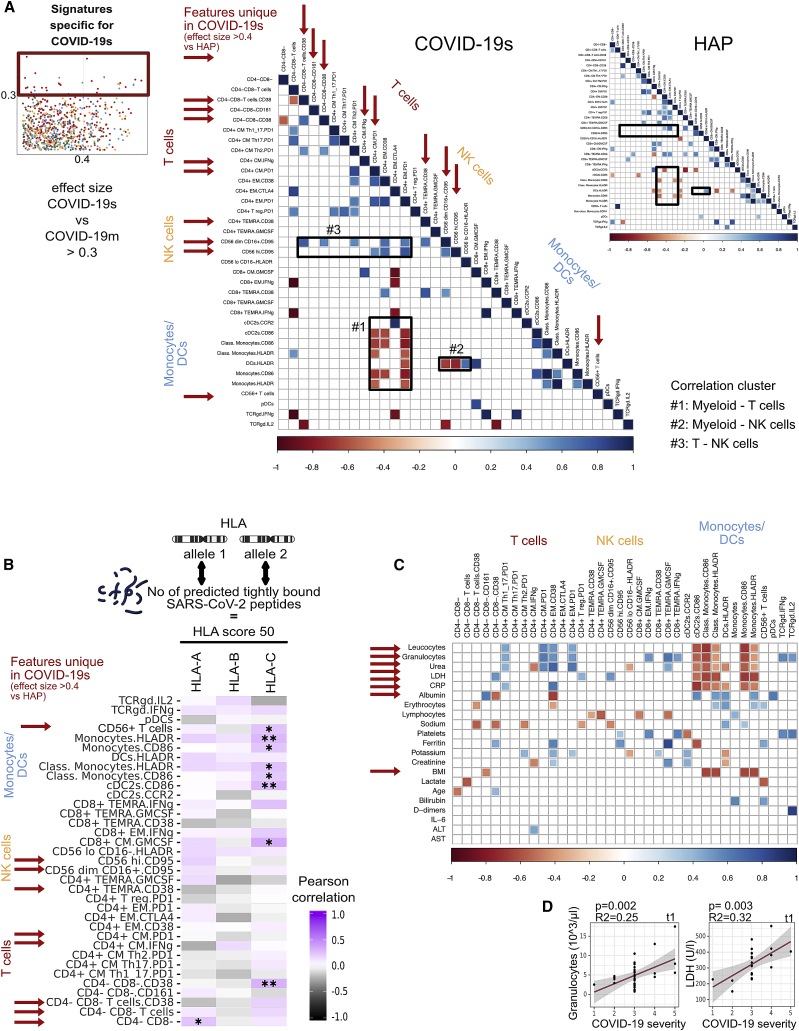
Distinct signatures of COVID-19s are exclusive to the lymphocyte compartment (original)
Figure 5.
Distinct signatures of COVID-19s are exclusive to the lymphocyte compartment (corrected)
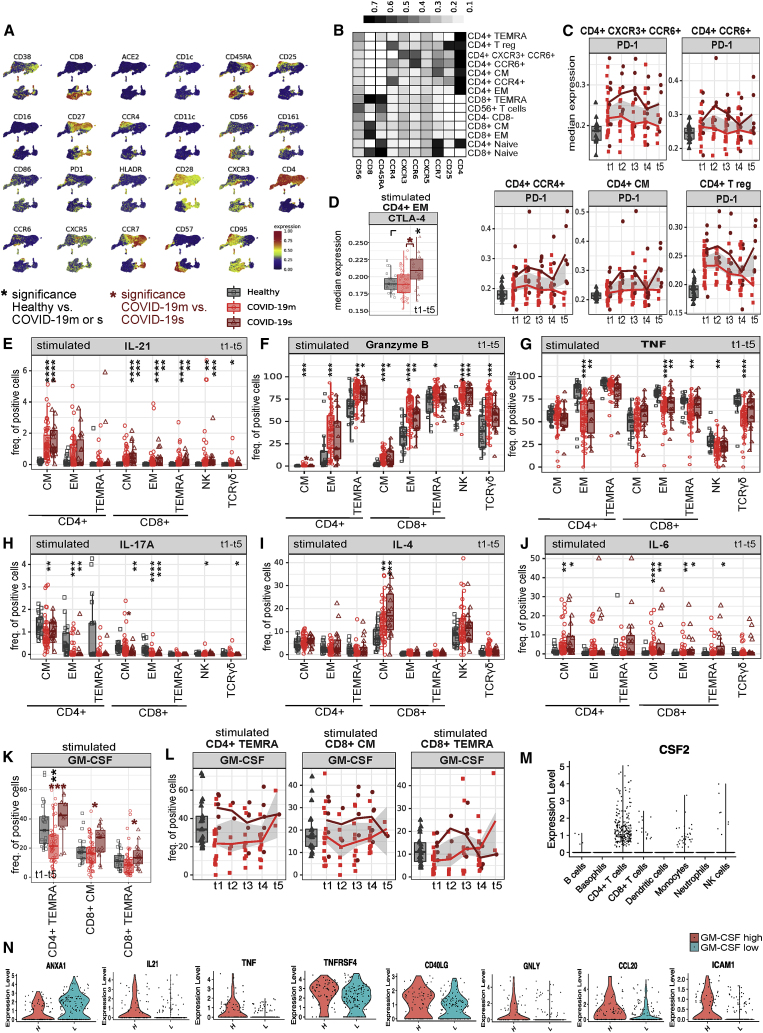
Figure 6.
HLA profile links COVID-19 immunopathology to impaired virus recognition (original)
Figure 6.
HLA profile links COVID-19 immunopathology to impaired virus recognition (corrected)
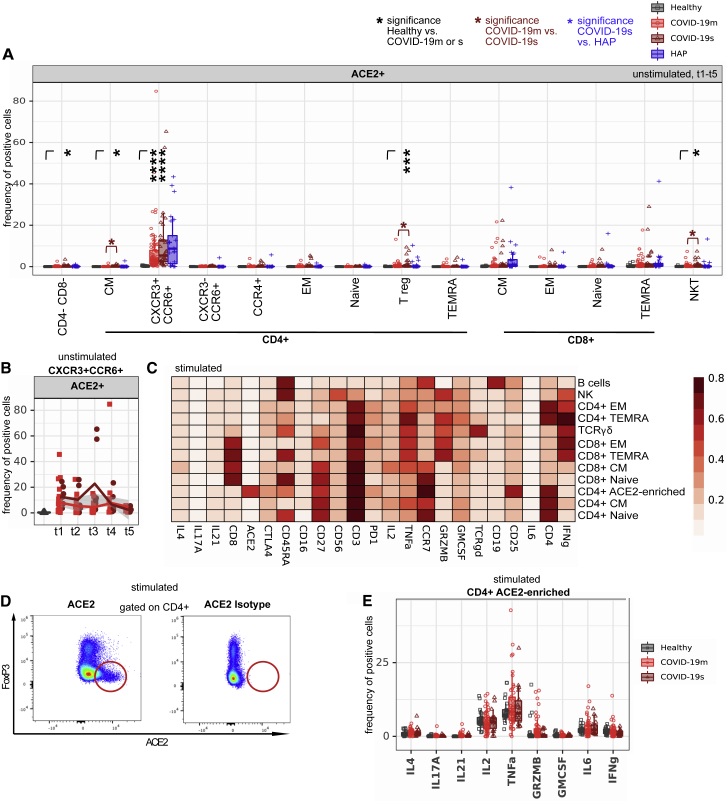
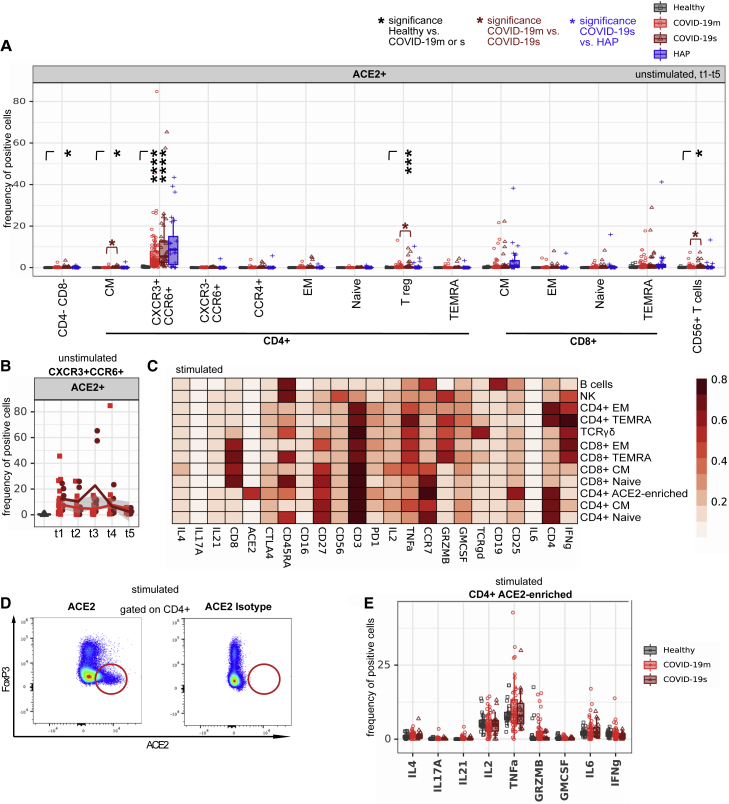
Figure 7B.
ACE2 expression in a CD4+ T cell subset increases after ex vivo stimulation (original)
Figure 7B.
ACE2 expression in a CD4+ T cell subset increases after ex vivo stimulation (corrected)
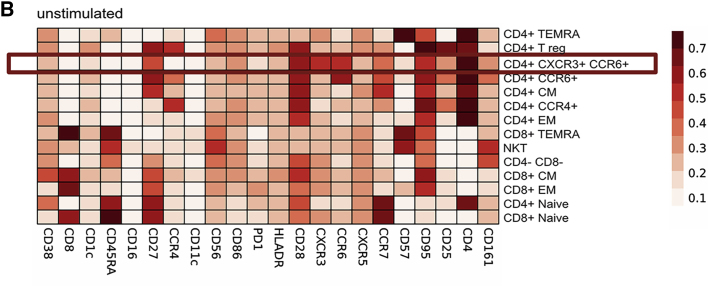
Figure S2.
(original)
Figure S2.
(corrected)
Figure S7.
(original)
Figure S7.
(corrected)
Reference
- Notarbartolo S., Ranzani V., Bandera A., Gruarin P., Bevilacqua V., Putignano A.R., Gobbini A., Galeota E., Manara C., Bombaci M., et al. Integrated longitudinal immunophenotypic, transcriptional and repertoire analyses delineate immune responses in COVID-19 patients. Sci Immunol. 2021;6:eabg5021. doi: 10.1126/sciimmunol.abg5021. [DOI] [PubMed] [Google Scholar]