Nonalcoholic fatty liver disease (NAFLD), the most common cause of chronic liver disease,1 is independently associated with increased risk of cardiovascular disease (CVD), which is the leading cause of mortality in patients with NAFLD.2 This is likely caused by the centrality of the liver in lipid homeostasis. Prior cross-sectional studies have shown that NAFLD is associated with perturbations in lipid profile and atherogenic lipoprotein subparticles.3 Although statins improve lipid profile and CVD-associated mortality, residual CVD risk has been demonstrated in major statin trials.4,5 A key contributor to this residual risk is the limited ability of the standard lipid profile to precisely quantify atherogenic lipoprotein subparticles, such as small dense low-density lipoprotein (sdLDL), which might confer higher atherogenic risk. There are currently no studies evaluating the longitudinal impact of sdLDL on atherosclerotic events in NAFLD. Thus, we conducted a prospective study in patients with histologically confirmed NAFLD to better define the relationship among NAFLD, residual CVD risk, and sdLDL.
Methods
The study is an ancillary analysis of the natural history study of patients with NAFLD (N = 210), who had histologic confirmation of NAFLD and detailed lipoprotein analysis. Data were collected prospectively from 2010 to 2018, and all patients were followed until they either had an atherosclerotic event, death, or last follow-up. The primary endpoint was major adverse cardiovascular events consisting of acute coronary syndrome, stroke, or cardiac death (MACE-3).
Results
The study cohort consisted largely of females (n = 139; 66.2%), mean age was 54.9 ± 11.3 years, and mean body mass index of 33.6 ± 6.5 kg/m2. Histologically, 97 (46.2%) patients had nonalcoholic fatty liver and 113 (53.8%) had nonalcoholic steatohepatitis (NASH). Although low-density lipoprotein cholesterol (LDL-C) and total cholesterol levels were similar in patients with nonalcoholic fatty liver and NASH, patients with NASH were more likely to have lower serum high-density lipoprotein cholesterol and higher triglycerides.
Cardiovascular Events
MACE-3 occurred in 19 patients after median follow-up of 264 weeks (interquartile range, 132–398 weeks). The most common atherosclerotic event was acute coronary syndrome (n = 15), followed by stroke (n = 2), and cardiac death (n = 2). The patients experiencing atherosclerotic events were older (60 ± 8 vs 54 ± 11 years; P = .03). The incidence of MACE-3 was similar in patients with NASH and nonalcoholic fatty liver. The mean LDL-C did not differ between patients who had a MACE-3 and those who did not (120.7 ± 50.6 mg/dL vs 111.3 ± 40.8 mg/dL; P = .4). In contrast, sdLDL cholesterol (sdLDL-C) was significantly higher among patients who had MACE-3 compared with those who did not (45.5 ± 17.9 vs 35.5 ± 16.7 mg/dL; P = .02). LDL-C threshold of 100 mg/dL was not associated with future risk of MACE-3 (Figure 1A). Using previously published cutoff value of 25 mg/dL,6 we observed that patients with sdLDL-C ≥25 mg/dL were associated with higher risk of MACE-3 (Figure 1B). In multivariate Cox regression models, age (hazard ratio per standard deviation, 1.90; 95% confidence interval, 1.18– 3.07; P = .009), sdLDL-C (hazard ratio per standard deviation, 1.64; 95% confidence interval, 1.07–2.51; P = .02), and high-density lipoprotein (hazard ratio per standard deviation, 0.49; 95% confidence interval, 0.27–0.90; P = .02) were associated with higher risk of MACE-3 after adjusting for gender, body mass index, history of CVD, smoking exposure, hypertension, diabetes, and statin use.
Figure 1.
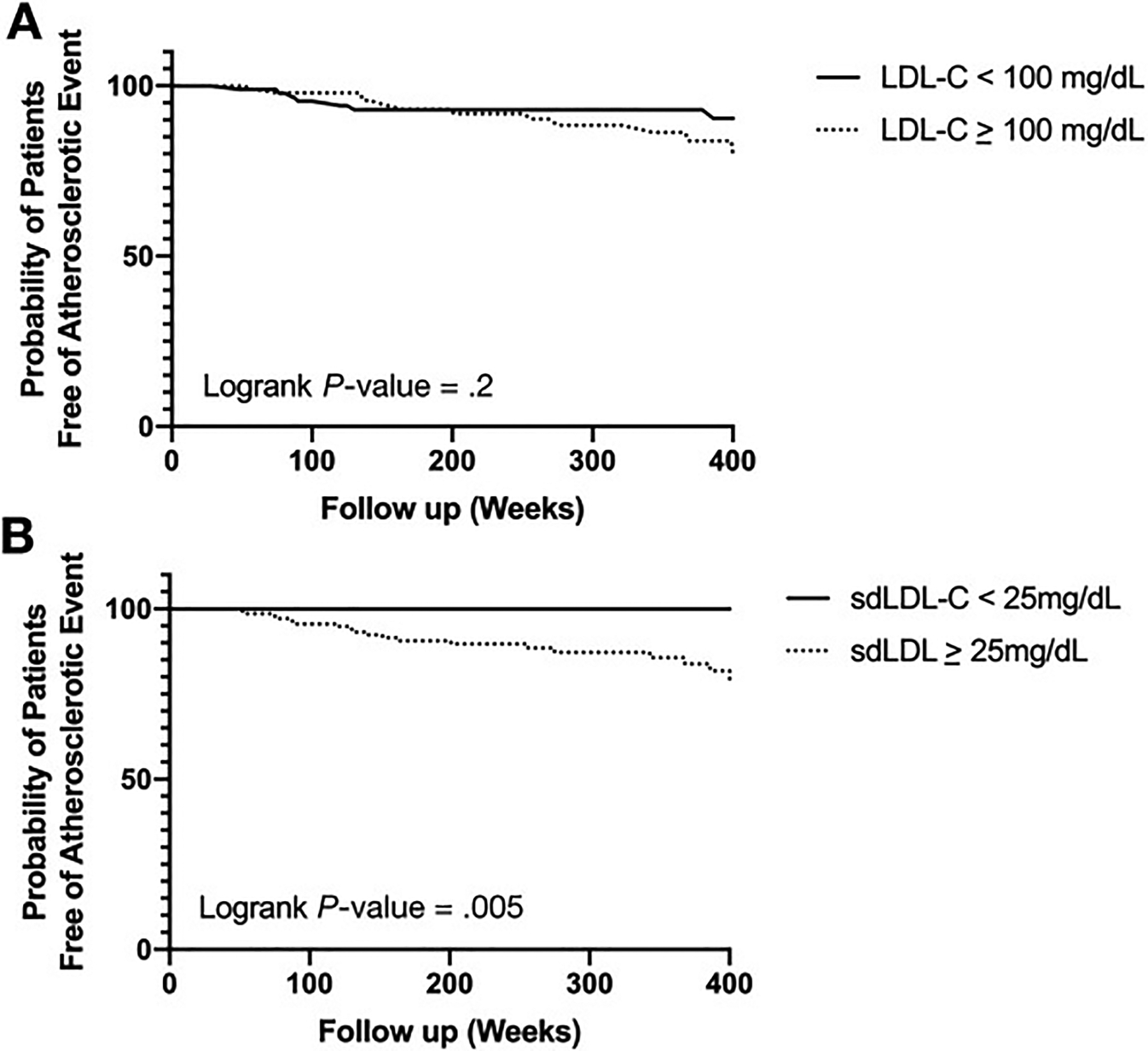
Kaplan-Meier plot showing cumulative probability of remaining atherosclerotic event free according to (A) LDL-C and (B) sdLDL-C.
Discussion
Atherosclerotic event among patients with NAFLD in the study was higher than the general population and represents a high-risk population. In the present study, no association between LDL-C and future risk of atherosclerotic events was noted and likely represents statin optimization. However, despite widespread use of statin therapy, atherosclerotic events were noted. This is representative of residual risk, which has been demonstrated repeatedly in major statin trials.5 A significant association exists between MACE-3 and sdLDL-C, thus underscoring the relationship between atherogenic lipoprotein subparticles and atherosclerosis, which may require direct measurements of these atherogenic lipoproteins to provide a more accurate risk-assessment profile. These findings are in line with a similar study performed in liver transplant recipients, many of whom had NAFLD, which demonstrated a similar association between sdLDL-C and CVD.6
The strengths of the current study include the prospective design, histologically established NAFLD with granular cardiovascular endpoint data. The findings of the study must be interpreted within the context of its limitations. There is ascertainment bias and results from patient recruitment at a tertiary care center with a higher prevalence of NASH and fibrosis. Although the sample size and atherosclerotic events are relatively modest, it provides valuable insight regarding residual risk, lipoprotein particles, and atherogenic risk.
In conclusion, the study findings identify sdLDL as a potentially modifiable lipoprotein parameter that can supplement the current clinical practice of optimizing atherosclerosis risk in patients with NAFLD. Additional studies are necessary to build on the current findings to provide a more precise way of mitigating CVD risk in patients with NAFLD.
Funding
The project [publication] described was supported by CTSA award No. UL1TR002649 from the National Center for Advancing Translational Sciences.
Conflicts of interest
These authors disclose the following: Arun J. Sanyal is President of Sanyal Biotechnology; has stock options in Genfit, Akarna, Tiziana, Indalo, Durect Inversago, and Galmed; served as a consultant to Astra Zeneca, Nitto Denko, Conatus, Nimbus, Salix, Tobira, Takeda, Jannsen, Gilead, Terns, Birdrock, Merck, Valeant, Boehringer-Ingelheim, Bristol Myers Squibb, Lilly, Hemoshear, Zafgen, Novartis, Novo Nordisk, Pfizer, Exhalenz, and Genfit; and has been an unpaid consultant to Intercept, Echosens, Immuron, Galectin, Fractyl, Syntlogic, Affimune, Chemomab, Zydus, Nordic Bioscience, Albireo, Prosciento, and Surrozen. His institution has received grant support from Gilead, Salix, Tobira, Bristol Myers, Shire, Intercept, Merck, Astra Zeneca, Malinckrodt, Cumberland, and Novartis. He receives royalties from Elsevier and UptoDate. The other authors disclose no conflicts.
References
- 1.Younossi ZM, Koenig AB, Abdelatif D, et al. Global epidemiology of nonalcoholic fatty liver disease: meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology 2016;64:73–84. [DOI] [PubMed] [Google Scholar]
- 2.Ekstedt M, Hagström H, Nasr P, et al. Fibrosis stage is the strongest predictor for disease-specific mortality in NAFLD after up to 33 years of follow-up. Hepatology 2015;61:1547–1554. [DOI] [PubMed] [Google Scholar]
- 3.Siddiqui MS, Fuchs M, Idowu MO, et al. Severity of nonalcoholic fatty liver disease and progression to cirrhosis are associated with atherogenic lipoprotein profile. Clin Gastroenterol Hepatol 2015;13:1000–1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Choi CU, Seo HS, Lee EM, et al. Statins do not decrease small, dense low-density lipoprotein. Tex Heart Inst J 2010; 37:421–428. [PMC free article] [PubMed] [Google Scholar]
- 5.Heart Protection Study Collaborative Group. MRC/BHF Heart Protection Study of cholesterol lowering with simvastatin in 20, 536 high-risk individuals: a randomised placebo-controlled trial. Lancet 2002;360:7–22.12114036 [Google Scholar]
- 6.Siddiqui MB, Arshad T, Patel S, et al. Small dense low-density lipoprotein cholesterol predicts cardiovascular events in liver transplant recipients. Hepatology 2019; 70:98–107. [DOI] [PMC free article] [PubMed] [Google Scholar]


