Abstract
INTRODUCTION:
Advances in transjugular intrahepatic portosystemic shunt (TIPS) technology have led to expanded use. We sought to characterize contemporary outcomes of TIPS by common indications.
METHODS:
This was a multicenter, retrospective cohort study using data from the Advancing Liver Therapeutic Approaches study group among adults with cirrhosis who underwent TIPS for ascites/hepatic hydrothorax (ascites/HH) or variceal bleeding (2010–2015). Adjusted competing risk analysis was used to assess post-TIPS mortality or liver transplantation (LT).
RESULTS:
Among 1,129 TIPS recipients, 58% received TIPS for ascites/HH and 42% for variceal bleeding. In patients who underwent TIPS for ascites/HH, the subdistribution hazard ratio (sHR) for death was similar across all Model for End-Stage Liver Disease Sodium (MELD-Na) categories with an increasing sHR with rising MELD-Na. In patients with TIPS for variceal bleeding, MELD-Na ≥20 was associated with increased hazard for death, whereas MELD-Na ≥22 was associated with LT. In a multivariate analysis, serum creatinine was most significantly associated with death (sHR 1.2 per mg/dL, 95% confidence interval [CI] 1.04–1.4 and 1.37, 95% CI 1.08–1.73 in ascites/HH and variceal bleeding, respectively). Bilirubin and international normalized ratio were most associated with LT in ascites/HH (sHR 1.23, 95% CI 1.15–1.3; sHR 2.99, 95% CI 1.76–5.1, respectively) compared with only bilirubin in variceal bleeding (sHR 1.06, 95% CI 1.00–1.13).
DISCUSSION:
MELD-Na has differing relationships with patient outcomes dependent on TIPS indication. These data provide new insights into contemporary predictors of outcomes after TIPS.
INTRODUCTION
The development of decompensated liver disease is accompanied by complications of clinically significant portal hypertension including ascites, hepatic hydrothorax, and variceal bleeding (1). Transjugular intrahepatic portosystemic shunt (TIPS) placement effectively reduces portal pressure and is indicated for the treatment of refractory ascites and secondary prevention of variceal bleeding (2–4). The advent of expanded polytetrafluoroethylene-covered stents has resulted in improved stent patency rates and decreased need for subsequent revisions compared with noncovered or bare metal stents (5,6). Recent data also suggest improved transplant-free survival after TIPS in patients with high ($18) Model for End-Stage Liver Disease (MELD) scores (7,8). Despite these advances, reports of contemporary outcomes of TIPS with covered stents have been limited to small or single-center studies with very few large multicenter reports (9–11). Furthermore, these reports have been limited to studies that investigate single indications for TIPS among highly selected recipients. We sought to conduct a large multicenter investigation to better understand the contemporary outcomes of TIPS among patients with cirrhosis across the most common TIPS indications of emergent and nonemergent variceal bleeding and ascites or hepatic hydrothorax (ascites/HH) across all MELD Sodium (MELD-Na) scores.
METHODS
This was a retrospective study across 9 US academic medical centers participating in the Advancing Liver Therapeutic Approaches (ALTA) study group. The primary data set was comprised of adults older than 18 years with cirrhosis who underwent a first TIPS procedure from January 1, 2010, through December 31, 2016. Exclusion criteria for the primary data set included previous liver transplantation (LT), noncirrhosis etiology of portal hypertension, or TIPS for any other indication (Budd-Chiari, portal vein thrombosis, nodular regenerative hyperplasia, TIPS before abdominal surgery, etc.). For purposes of this study, indications for TIPS were limited to variceal bleeding and ascites/HH. Primary outcomes were LT or death. Secondary outcomes included complications of cirrhosis including hepatic encephalopathy (HE), paracentesis, and repeat endoscopy for continued variceal bleeding. HE was recorded if this appeared in a provider’s documentation or a patient was admitted to the hospital with HE as a primary diagnosis. Data were collected through a combination of electronic data queries and manual review of each center’s health record. Study data were collected and managed using research electronic data capture hosted at the organizing center, Northwestern University (12). The study was approved by the Institutional Review Boards at each of the 9 participating centers.
Clinical data
Demographic data, medical comorbidities, medications, liver ultrasound imaging, endoscopy reports, echocardiograms, and rates of HE within 6 months of TIPS and laboratory data were collected from the medical charts. Etiologies for cirrhosis were categorized as alcohol-associated, hepatitis C, nonalcoholic fatty liver disease, and other (hepatitis B, autoimmune, primary biliary cholangitis, primary sclerosing cholangitis, etc.). Laboratory values for MELD-Na were obtained using the values closest to 48 hours before TIPS. Values older than 28 days before the procedure date were not used. The MELD-Na score was calculated using the standard formula from the OPTN without an upper limit (13). The MELD-Na score was used over traditional MELD, given its improved discrimination for death or transplant particularly among lower MELD-Na scores (<18) (14,15). Proximate indication for the TIPS placement was obtained from the TIPS procedure reports and catalogued as either refractory ascites/HH or variceal/gastrointestinal bleeding. Variceal bleeding was defined as either esophageal, gastric, duodenal, rectal, or peristomal varices. A sensitivity analysis was performed, which stratified patients with variceal bleeding into emergent variceal bleeding (EVB) and non-EVB (nEVB). EVB was defined as TIPS that was placed within 4 days of endoscopy that identified a variceal bleeding source; nEVB included patients who received a TIPS for variceal bleeding >4 days after the index endoscopy confirming a variceal bleeding source. Pre-TIPS portosystemic gradient (PSG) was reported in the procedure report and defined as the pressure gradient (mm Hg) between the wedged hepatic vein pressure minus the systemic venous pressure of inferior vena cava (IVC) or the right atrium if free hepatic vein or IVC values were not available. Post-TIPS PSG was the difference between direct portal pressure measurement and the hepatic venous pressure. Because this was a retrospective study, centers were not directed or guided in their goal post-TIPS PSG or stent diameter, instead this was at the discretion of the operator at the time of TIPS.
Statistical analyses
Owing to inherent differences in the clinical context that culminated in TIPS placement, the cohort was stratified by indication (e.g., variceal bleeding or ascites/HH). For 52 (4%) patients who had both variceal bleeding and ascites/HH as indications per the TIPS report, we assigned a proximate indication of variceal bleeding category with appropriate substratification based on the timing of index endoscopy as defined above when appropriate.
Covariates were compared using χ2 and t-tests for categorical and continuous variables, respectively. The primary outcome was all-cause mortality with a competing risk of liver transplant. Time at risk was defined as time from TIPS placement to death, end of the study period (December 31, 2016), or liver transplant. Cumulative incidence plots were generated for the outcome of interest and competing risks. Univariate and multivariable competing risk analysis was performed using the Fine-Gray methodology adjusting for age, etiology of liver disease, and MELD-Na score (16,17). A sensitivity analysis was performed, which assessed the effect of each component of the MELD-Na score individually. In addition, MELD-Na was binned into small ranges to detect nonlinear, threshold effects on the outcome of interest. A P-value of <0.05 was considered statistically significant. Data processing and analysis were performed using SAS version 9.4 (SAS Institute, Cary, NC) and RStudio version 1.2.1578 with R packages international classification of disease, tableone, comorbidity, and cmprsk (16,18,19).
RESULTS
Cohort characteristics
During the study period, 1,260 patients with cirrhosis received a TIPS, of whom 1,129 patients met our inclusion criteria, comprising 2,665 patient years of time at risk (median of 1.86 years interquartile range 0.52–3.82 years). The most common indication for TIPS was ascites/HH (N = 656), followed by nEVB (N = 325), and EVB (N = 148). Patient demographics stratified by indication are presented in Table 1. Most patients (>87%) received covered (polytetrafluoroethylene) TIPS endoprostheses, and this was similar across all indications. Patient characteristics and outcomes were similar for covered TIPS stents and bare metal stents (see Table, Supplementary Digital Content 1, http://links.lww.com/AJG/C89). The EVB group had the highest prevalence of alcohol-associated liver disease (47%) compared with nEVB (32.7%) and ascites/HH (34.8%; P < 0.002). The EVB group also had the highest prevalence of gastric varices (55.4%) compared with the nEVB (39%) and ascites/HH (7.3%) groups (P < 0.001). The ascites/HH group had the highest prevalence of spontaneous bacterial peritonitis (12.7% vs 6.7% for nEVB and 2.8% for EVB, P < 0.001) and higher prevalence of pre-TIPS HE (49.8% vs 34.7% for nEVB and 34.5% for EVB, P < 0.001). All groups had similar age, sex, and prevalence of portal vein thrombosis, baseline left ventricular ejection fraction, and pre-TIPS and post-TIPS PSG measurements. The cumulative incidence of death at 5 years after TIPS was statistically similar among the ascites/HH group and the combined variceal bleeding groups (N = 209, 29.8% vs N = 141, 31.9%, P = 0.54); however, the ascites/HH group was more likely to undergo LT (n = 136, 20.7% vs n = 50, 12.7%, P < 0.001) (Figure 1).
Table 1.
Characteristics of adults with cirrhosis who underwent tips for emergent and nonemergent variceal bleeding or ascites/hepatic hydrothorax
| Nonemergent variceal bleeding, N = 325 | Emergent variceal bleeding, N = 148 | Ascites/HH, N = 656 | P Value | |
|---|---|---|---|---|
| Age, mean (SD) | 56.9 (10.1) | 56.0 (10.1) | 57.5 (10.1) | 0.272 |
| Days of follow-up median (IQR) | 817 (382–1,640) | 709 (48–1,435) | 640 (161–1,218) | 0.003 |
| Women (%) | 133 (40.9) | 49 (33.1) | 258 (39.3) | 0.260 |
| Race (%) | ||||
| White | 216 (75.5) | 94 (71.2) | 515 (86.6) | <0.001 |
| Black/African American | 16 (5.6) | 13 (9.8) | 16 (2.7) | |
| Other | 48 (16.8) | 22 (16.7) | 64 (10.7) | |
| Primary cirrhosis etiology (%) | 0.002 | |||
| Alcohol | 106 (32.7) | 70 (47.3) | 228 (34.8) | |
| Hepatitis C | 98 (30.2) | 38 (25.7) | 212 (32.3) | |
| NAFLD | 55 (17.0) | 20 (13.5) | 131 (20.0) | |
| Othera | 66 (20.1) | 20 (13.5) | 85 (13.0) | |
| Complications of cirrhosis (%) | ||||
| History of ascites | 146 (46.8) | 58 (40.3) | 656 (100) | <0.001 |
| History of hepatic encephalopathy | 109 (34.7) | 50 (34.5) | 319 (49.8) | <0.001 |
| History of variceal bleeding | 325 (100) | 148 (100) | 76 (11.9) | <0.001 |
| Upper endoscopy before TIPS (%) | 246 (76.4) | 148 (100) | 363 (55.8) | <0.001 |
| Esophageal varices | 206 (82.7) | 126 (85.1) | 271 (73.4) | 0.002 |
| Gastric varices | 97 (39.0) | 82 (55.4) | 27 (7.3) | <0.001 |
| Esophageal and gastric varices | 71 (28.5) | 63 (42.6) | 20 (5.4) | <0.001 |
| History of hepatocellular carcinoma | 25 (8.0) | 4 (2.8) | 25 (3.9) | 0.010 |
| History of portal vein thrombosis | 25 (8.0) | 8 (5.6) | 39 (6.1) | 0.464 |
| History of spontaneous bacterial peritonitis | 21 (6.7) | 4 (2.8) | 81 (12.7) | <0.001 |
| Covered PTFE endoprosthesis | 281 (87) | 132 (89) | 586 (89) | 0.079 |
| Baseline laboratory values | ||||
| MELD-Na score, mean (SD) | 15.6 (6.6) | 17.1 (7.5) | 18.1 (6.0) | <0.001 |
| Bilirubin mg/dL, mean (SD) | 2.78 (3.3) | 3.9 (4.5) | 2.35 (1.8) | <0.001 |
| INR, mean (SD) | 1.54 (0.4) | 1.62 (0.4) | 1.52 (0.3) | 0.016 |
| Creatinine mg/dL, mean (SD) | 1.00 (0.55) | 1.09 (0.7) | 1.30 (0.8) | <0.001 |
| Sodium mg/dL, mean (SD) | 137 (4.3) | 138 (3.8) | 134 (6.2) | <0.001 |
| Hemodialysis (%) | 11 (3.4) | 6 (4.1) | 26 (4.0) | 0.894 |
| Baseline echocardiogram performed | 475 (72.4) | |||
| Left ventricular EF categorization | ||||
| EF <60% | 37.2% | 38.2% | 33.6% | 0.604 |
| EF 60%–70% | 52.1% | 46.1% | 54% | |
| EF >70% | 10.6% | 15.8% | 12.3% | |
| TIPS parameters | ||||
| Pre-TIPS PSG, mm Hg, mean (SD) | 16.7 (6.3) | 17.4 (6.1) | 17.2 (5.2) | 0.485 |
| Post-TIPS PSG, mm Hg, mean (SD) | 6.6 (3.8) | 6.4 (3.1) | 6.2 (3.0) | 0.316 |
| PSG decrement, mm Hg, mean (SD) | 10.38 (5.8) | 11.13 (5.3) | 11.0 (5.0) | 0.233 |
| Embolization of varices/shunts | 136 (43.3) | 64 (44.1) | 76 (12.2) | <0.001 |
| Outcome | <0.001 | |||
| Death (%) | 86 (27) | 55 (37) | 209 (32) | |
| Liver transplantation (%) | 46 (14) | 14 (10) | 136 (21) |
Ascites/HH, ascites and/or hepatic hydrothorax indication; EF, ejection fraction; INR, international normalized ratio; IQR, interquartile range; MELD-Na, model for end-stage liver disease with sodium; PSG, portosystemic gradient; PTFE, polytetrafluoroethylene; TIPS, transjugular intrahepatic portosystemic shunt.
Other, includes hepatitis B, hemochromatosis, primary sclerosing cholangitis, primary biliary cholangitis, cryptogenic cirrhosis, and alpha 1 antitrypsin.
Figure 1.
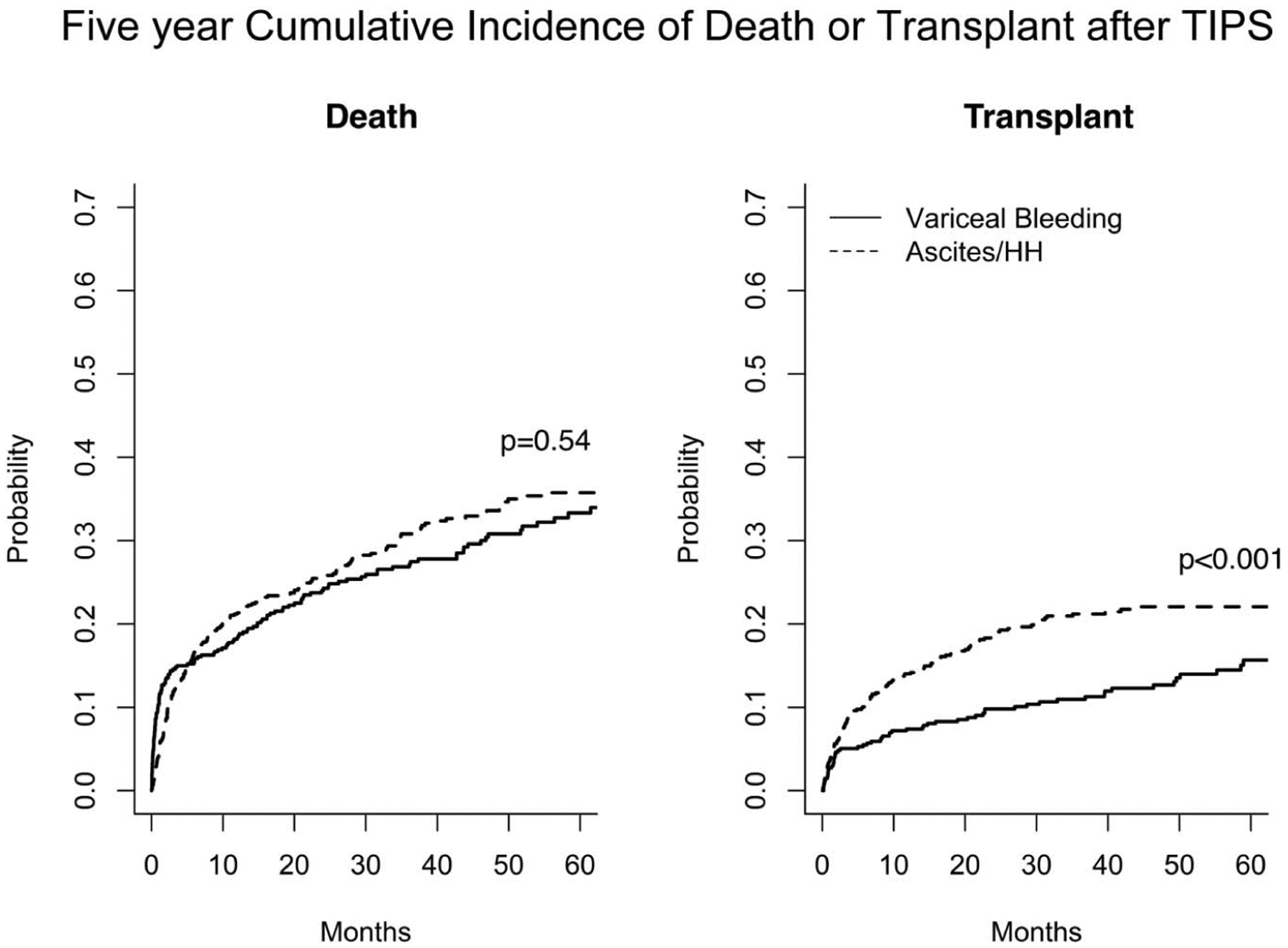
Unadjusted 5-year cumulative incidence of death or liver transplant after TIPS among ascites/HH and variceal bleeding indications. Ascites/HH, ascites/hepatic hydrothorax; TIPS, transjugular intrahepatic portosystemic shunt.
Impact of MELD-Na among patients undergoing TIPS for Ascites/HH
The unadjusted subdistribution hazard ratio (sHR) of death for patients who underwent TIPS for ascites/HH was similar across all MELD-Na ranges (Figure 2a). This result did not change whether MELD-Na was recast as a continuous variable (sHR 1.06 per 5 points of MELD-Na, 95% confidence interval [CI 0.94–1.19] P = 0.32). In contrast, the sHR of transplant increased with increasing MELD-Na (Figure 2b). This result did not change when controlling for etiology of liver disease.
Figure 2.
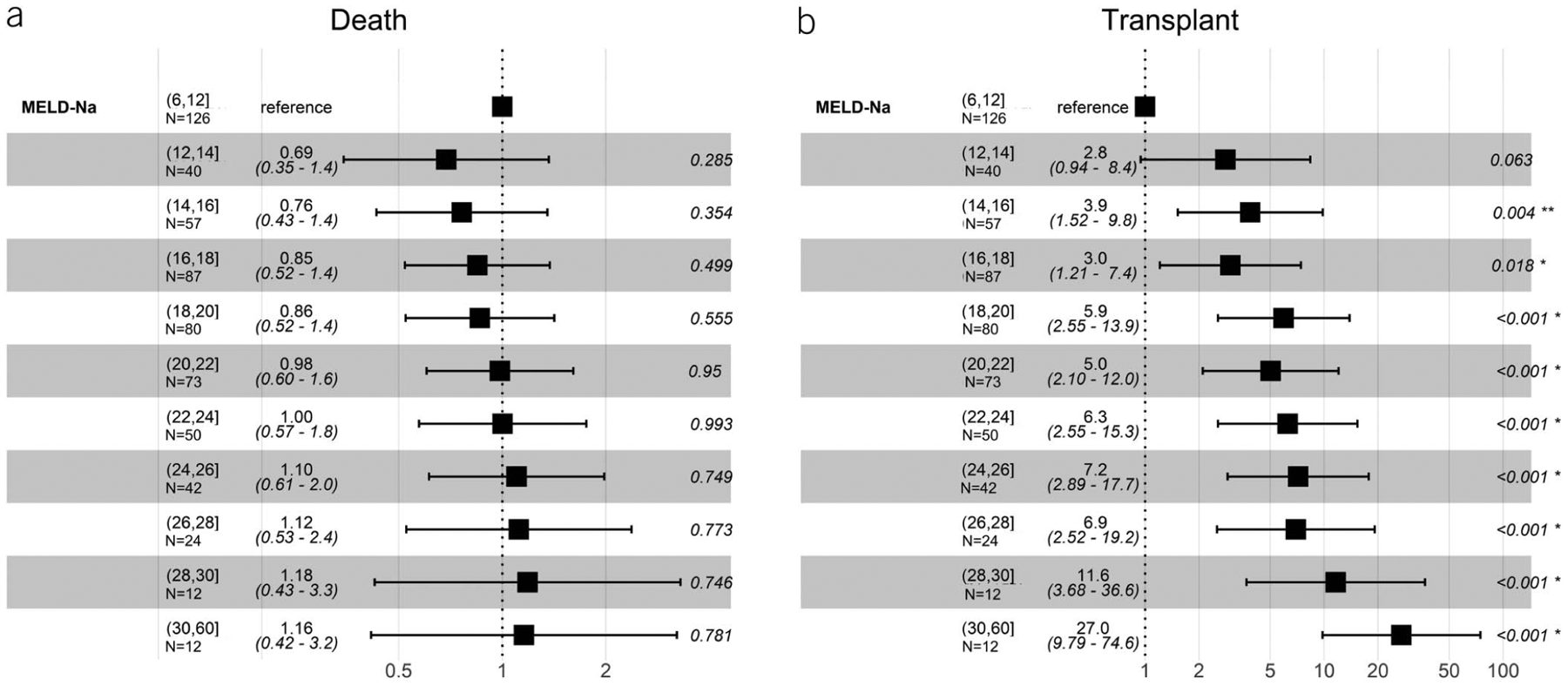
Unadjusted subdistribution hazard ratios for death and liver transplant among indication of ascites/HH after TIPS across all MELD-Na ranges. Ascites/HH, ascites/hepatic hydrothorax; MELD-Na, Model for End-Stage Liver Disease Sodium; TIPS, transjugular intrahepatic portosystemic shunt.
After adjustment for age and etiology of liver disease, serum creatinine was the only parameter that was significantly associated with an increased sHR for death (sHR 1.20 per mg/dL, 95% CI 1.04–1.4) among patients who underwent TIPS for ascites/HH (Figure 3a). Conversely, international normalized ratio (INR) (sHR 2.99 per mg/dL, 95% CI 1.76–5.1) and bilirubin (sHR 1.23 per mg/dL, 95% CI 1.15–1.33) were associated with the highest hazard of LT among this group (Figure 3b). Other factors that were not statistically significant were post-TIPS PSG measurement, the change in PSG after TIPS, and the etiology of liver disease.
Figure 3.
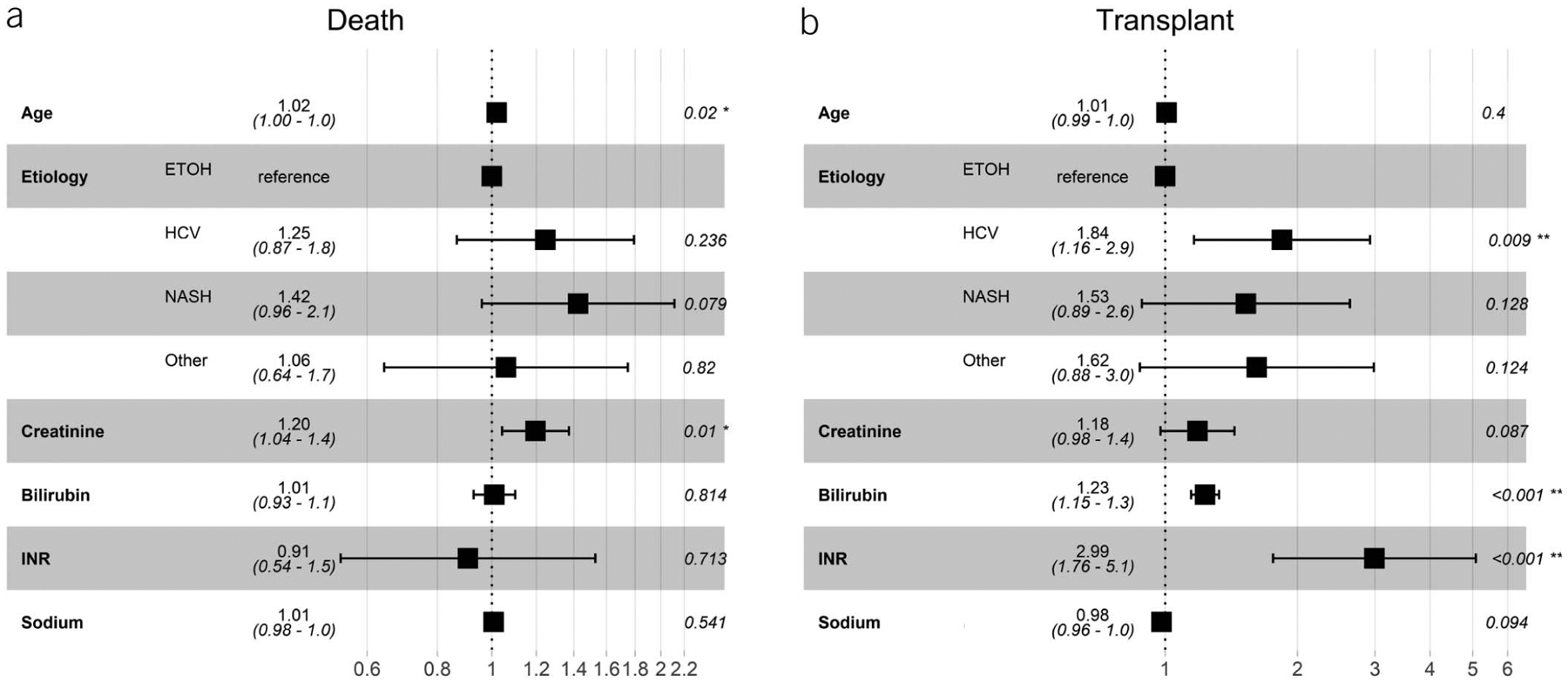
Adjusted subdistribution hazard ratios for death and liver transplant among indication of ascites/HH after TIPS controlling for age, etiology of liver disease, and components of MELD-Na score. Ascites/HH, ascites/hepatic hydrothorax; ETOH, alcohol; HCV, Hepatitis C; MELD-Na, Model for End-Stage Liver Disease Sodium; NASH, nonalcoholic steatohepatitis; TIPS, transjugular intrahepatic portosystemic shunt.
Impact of MELD-Na among patients undergoing TIPS for variceal bleeding
Figure 4 shows the unadjusted sHRs of death and transplant by MELD-Na score for patients who underwent TIPS for any variceal bleeding indication. MELD-Na score seemed to have a threshold effect with a MELD-Na score of ≥20 that was associated with a higher hazard of death and liver transplant (Figure 4a). Similar to the ascites/HH indication, these results were robust to adjustment for etiology of cirrhosis.
Figure 4.
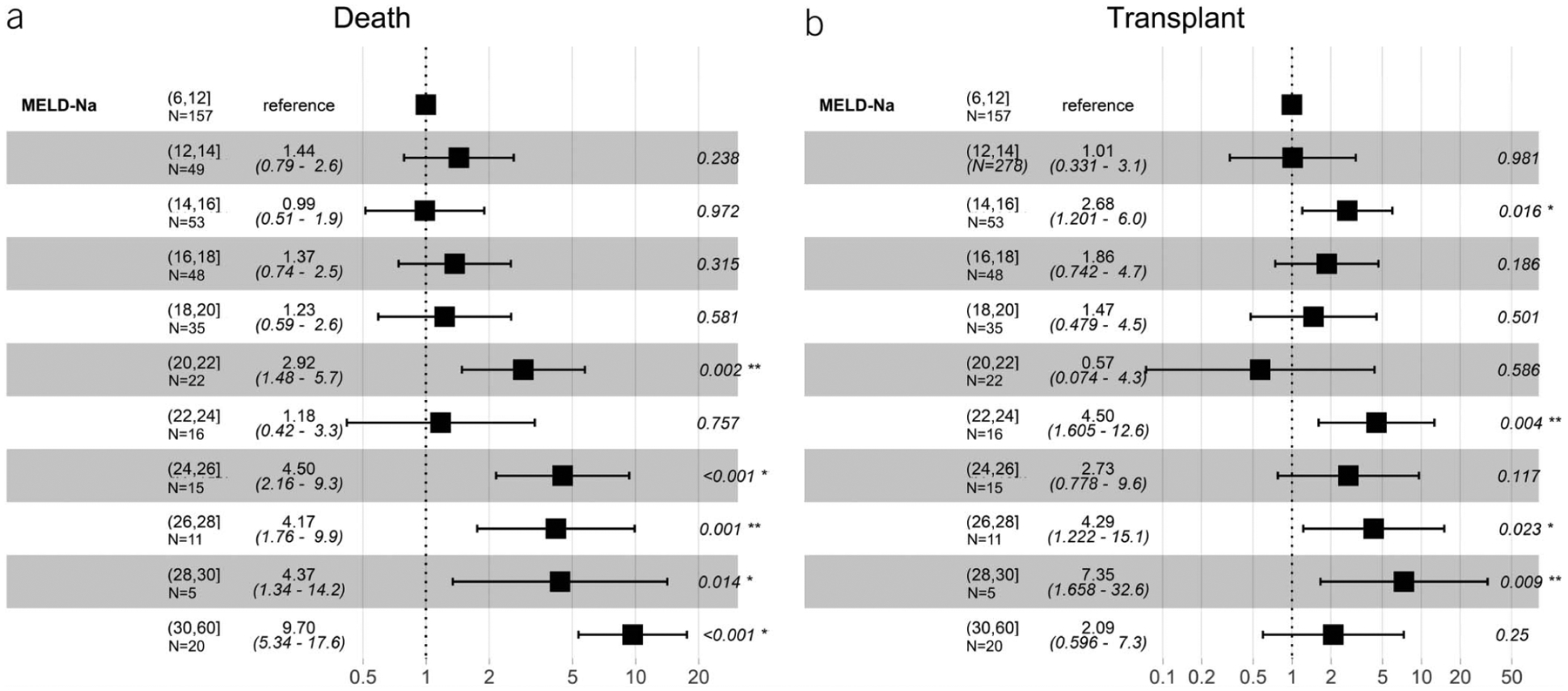
Unadjusted subdistribution hazard ratios for death and liver transplant among indication of variceal bleeding after TIPS across all MELD-Na ranges. MELD-Na, Model for End-Stage Liver Disease Sodium; TIPS, transjugular intrahepatic portosystemic shunt.
Figure 5 shows the adjusted sHRs of death and transplant for age, etiology of liver disease, components of the MELD-Na score, and varices location for patients who underwent TIPS for bleeding. Gastric varices were associated with a reduced hazard of death (sHR 0.64, 95% CI 0.44–0.94, P = 0.022). When components of the MELD-Na score were examined, creatinine (sHR 1.37, 95% CI 1.08–1.7, P = 0.009) and INR (sHR 2.2 95% CI 1.17–4.14, P < 0.014) were associated with an increased hazard of death (Figure 5a). In comparison, only total bilirubin (sHR 1.06, 95% CI 1.00–1.13, P = 0.035) was associated with a significant hazard of transplant among patients undergoing TIPS for variceal bleeding (Figure 5b). Similar to ascites/HH, the post-TIPS PSG and decrement in PSG after TIPS was not statistically significant and ultimately not included in the adjusted model. Subgroup inferences between the EVB and nEVB group were limited because of low numbers of primary outcomes in the EVB group. Accordingly, sensitivity analysis excluding the EVB group did not change the effect size or direction of the above results.
Figure 5.
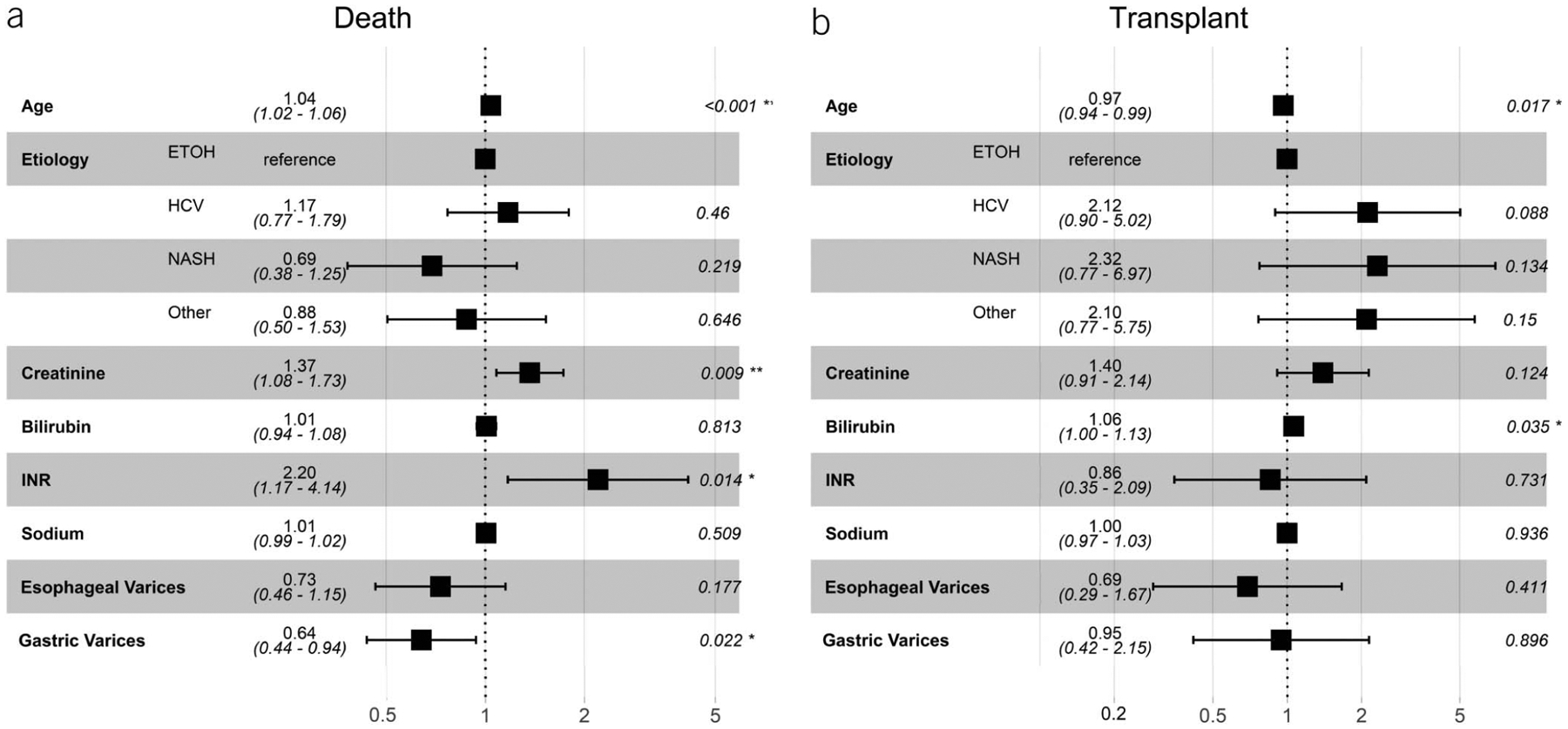
Adjusted subdistribution hazard ratios for death and liver transplant among indication of variceal bleeding after TIPS controlling for age, etiology of liver disease, and components of MELD-Na score. ETOH, alcohol; HCV, Hepatitis C; MELD-Na, Model for End-Stage Liver Disease Sodium; NASH, nonalcoholic steatohepatitis; TIPS, transjugular intrahepatic portosystemic shunt.
Impact of creatinine on death
A more detailed analysis of serum creatinine was performed, given its impact on death in both patients undergoing TIPS for variceal bleeding and ascites/HH. Despite adjustment for age, etiology of cirrhosis, and other MELD-Na components, a 52% increase in hazard of death was seen among patients whose serum creatinine was in the top quartile (≥1.1 mg/dL) in the 48 hours leading up to TIPS (sHR 1.52, 95% CI [1.01–2.3], P = 0.043) among patients who underwent TIPS for bleeding. In contrast, although serum creatinine during the same period was significantly associated with death among patients who underwent ascites/HH, no such threshold value was found (sHR 1.20 per mg/dL, 95% CI [1.05, 1.4], P < 0.05).
Cirrhosis-related complications after TIPS
A history of HE of any grade 1 year before TIPS was significantly higher among the ascites/HH indication (49.8%, P < 0.001) compared with nEVB (34.7%) and EVB (34.5%). After TIPS, persons with ascites/HH indication for TIPS were more likely to experience continued HE (50.3%, P < 0.001) and experience an episode of HE that required hospitalization (28%, P < 0.001) (Table 2). The rates of new HE after TIPS, however, were higher among the variceal bleeding indications. The need for a paracentesis greater than 90-day after TIPS was also similar across all indications.
Table 2.
Complications of cirrhosis after TIPS
| Complications of cirrhosis | Nonemergent variceal bleeding, N = 325 | Emergent variceal bleeding, N = 148 | Ascites/HH, N = 656 | P Value |
|---|---|---|---|---|
| Pre-TIPS hepatic encephalopathy: n (%)a | 109 (34.7) | 50 (34.5) | 319 (49.8) | <0.001 |
| Post-TIPS hepatic encephalopathy: n (%) | 164 (70.1) | 93 (73.8) | 408 (79.7) | 0.013 |
| Continued hepatic encephalopathy: n (%) | 73 (31.7) | 42 (33.3) | 257 (50.3) | <0.001 |
| New hepatic encephalopathy: n (%) | 87 (34.7) | 51 (39.8) | 150 (27.8) | 0.013 |
| Hospitalized for hepatic encephalopathy: n (%) | 58 (17.8) | 31 (20.9) | 184 (28.0) | <0.001 |
| Pre-TIPS therapeutic paracentesis: median (IQR) | 0 (0–0) | 0 (0–1) | 4 (1–8) | <0.001 |
| Post-TIPS therapeutic paracentesis ≥90 d, n (%) | 16 (37.2) | 3 (16.7) | 93 (36.0) | 0.238 |
| Post-TIPS variceal bleedingb: n (%) | 16 (4.9) | 10 (6.7) | 9 (1.2) | <0.001 |
Ascites/HH, ascites and/or hepatic hydrothorax indication; IQR, interquartile range; TIPS, transjugular intrahepatic portosystemic shunt.
Documentation of hepatic encephalopathy of any grade 1 year before TIPS.
Variceal bleeding that was confirmed on endoscopy.
DISCUSSION
Historically, predictors of death after TIPS have been advancing age, elevated serum bilirubin, prolonged prothrombin time and traditional MELD scores >18 (20–22). The limitations of applying these clinical predictors to contemporary TIPS recipients is that these reports exclusively used bare metal TIPS stents, which are associated with high failure rates, and do not consider the potential influence of portal hypertension etiology. Instead of assuming a “one size fits all” to TIPS risk prediction, we sought to determine outcomes associated with the most common indications for TIPS, ascites, and variceal bleeding among patients with cirrhosis. We observed that in TIPS recipients in the modern era, the relationship between MELD-Na score and patient outcomes remains strong but is not straightforward and varies by TIPS indication and patient age. Specifically, among patients undergoing TIPS for variceal bleeding, we noted a nonlinear relationship between MELD-Na score and hazard of death, with similar outcomes among patients using a threshold MELD-Na <22. In contrast, patients undergoing TIPS for ascites/HH seemed to have a more linear relationship, with increasing hazard of death and transplant as MELD-Na increased. Delving further, we demonstrate that not all MELD-Na scores are created equal—the 4 components of the MELD-Na score have differing importance regarding the outcomes. Namely, we find that creatinine is highly associated with death for patients undergoing TIPS, whereas INR and bilirubin are more often associated with LT.
The historical association of serum creatinine with post-TIPS mortality led to its incorporation into the MELD score, and these findings have been reproduced with other models (9,20,23–25). Among patients with cirrhosis, renal failure is associated with a significant increase in mortality (26). The interplay between renal function and TIPS however is complex. It is suspected that in patients with significant portal hypertension, placing a TIPS results in increased venous return of splanchnic blood, leading to increased effective circulating blood volume and presumed subsequent improvement in serum creatinine and renal function. Previous studies have demonstrated that patients with more advanced renal dysfunction at the time of TIPS benefit most from TIPS with significant improvement in renal function after placement (27,28). What is less clear is whether improvement in renal function after TIPS translates to improved survival. In a retrospective, matched study of TIPS compared with large volume paracentesis for ascites, controlled analyses of TIPS recipients demonstrated similar survival compared with serial paracentesis, regardless of the baseline renal function (28). In a meta-analysis of 4 randomized controlled trials comparing noncovered TIPS to large volume paracentesis, serum creatinine was not associated with increased mortality in multivariate analyses (11). These studies however do not differentiate elevated serum creatinine from acute kidney injury vs chronic kidney disease. Patients with chronic kidney disease would be less likely to have improvements in renal function from increased effective circulating blood volume after TIPS when compared with patients with transient acute kidney insufficiency. In small single-center studies, chronic kidney disease has been shown to be an independent risk factor for increased mortality after TIPS (29–31). Our findings are limited by not having accurate knowledge of duration or severity of underlying chronic kidney disease or information about the trajectory of renal function at that time of TIPS in this retrospective cohort. Of note, data published separately by Ge et al. (32) using a similar data set demonstrated that persistent renal dysfunction after TIPS was more common in persons with nonalcoholic steatohepatitis (NASH) (33% vs 17%, P = 0.01) and comorbid diabetes (42% vs 24%, P = 0.001), suggesting higher rates of more advanced chronic kidney disease at the time of TIPS in the NASH population. This analysis, however, excluded patients who died or were transplanted before 30-day laboratory follow-up was available. In our fully adjusted model, etiology of cirrhosis (including NASH) did not alter the association between higher creatinine and increased risk of death (33).
These findings raise the important question of whether there is a creatinine threshold in which risk of death with TIPS substantially increases. We found that a creatinine value of 1.1 mg/dL was associated with an increased sHR for death among recipients for EVB only. It is important to highlight that the mean serum creatinine for the entire cohort was 1.1 mg/dL, and in many instances, this is considered a normal value. In clinical practice, a cutoff whereby over half of the patients would be considered high risk unfortunately does not aid in the discrimination of patients who would be considered high risk for mortality. We caution the reader in interpreting that a cutoff serum creatinine would preclude a patient from receiving TIPS; rather, this finding highlights the need for close clinical attention to the patient with an elevated creatinine at the time of TIPS. This finding emphasizes the need for future study in the assessment of renal function beyond serum creatinine in TIPS candidates, which has well-established limitations of accurate estimation of renal function in patients with end-stage-liver disease (34,35).
These findings must be interpreted in the context of the approach used in this study. We took into account liver transplant as a competing risk for death. Hence, the finding of similar survival across all MELD-Na ranges cannot be refuted by the assertion that patients with higher MELD-Na scores simply received liver transplant instead of dying. We did not compare TIPS with non-TIPS medical care in ascites, so we cannot assert that TIPS itself improves survival in the ascites/HH indication. The finding of similar survival across all MELD-Na ranges compared with those with a MELD-Na of 6–12 may be interpreted as MELD-Na not discriminating or being associated with death after TIPS. Conversely, increasing MELD-Na score was in fact associated with a higher hazard of liver transplant. There seems to be a group of patients with ascites/HH who, if they did not receive a liver transplant, ultimately had a similar risk of death compared with low MELD-Na score TIPS recipients. Limitations to this interpretation are that these retrospective data represent patients who were selected in clinical practice to undergo TIPS and may be simultaneously listed or considered for liver transplant, or liver transplant was the ultimate intent of the provider. In an adjusted analysis, patients with more advanced hepatic dysfunction as measured by INR and total bilirubin were strongly associated with an increased hazard for liver transplant when controlling for serum creatinine and sodium. This suggests that patients receiving a TIPS for ascites/HH may need to still be considered for liver transplant evaluation despite a low MELD-Na score and particularly if there are elevations in serum INR and bilirubin suggesting more advanced hepatic dysfunction.
In comparison to the ascites/HH group, we observed that those who underwent TIPS for variceal bleeding had increasing hazard for death or transplant at MELD-Na scores above 20. This may be related to the heterogeneity of patients with variceal bleeding because it relates to liver transplant. For example, in clinical practice, patients who present with variceal bleeding receive a TIPS either for bleeding gastric varices or failed endoscopic intervention and are subsequently referred for liver transplant only if there is ongoing hepatic dysfunction. Hence, the MELD-Na score at the time of TIPS does not necessarily predict the future need for liver transplant. This likely explains why serum bilirubin was the only component of MELD-Na that was predictive of liver transplant in the adjusted analysis. These findings raise the question of the accuracy of MELD-Na in predicting death after TIPS in a contemporary era. This has been highlighted by recent data suggesting alternative scoring systems, such as the Freiburg index of post-TIPS survival, which incorporate age, bilirubin, albumin, and creatinine into a score that has significantly improved discrimination for survival compared to MELD-Na (36).
The major strength of our study is that we have analyzed the outcomes in one of the largest collections of patients undergoing TIPS across multiple centers with various practice patterns for TIPS placement in a modern era. We also use advanced statistical techniques that account for the competing risk of LT on death in this patient population. However, several limitations warrant mention. First, this is a retrospective study incurring the usual caveats to this methodology. Selection bias is also a concern. Patients in this cohort received a TIPS at tertiary academic centers within the United States, and selection criteria for TIPS were not uniform across each site. We could not adjust for the care plan intended for the patient, specifically if they were planned for TIPS as a destination therapy or as a bridge to liver transplant. In anticipation of this, we used a competing risk analysis with carefully ascertained outcomes to properly adjust our analyses. We also could not infer factors related to the TIPS procedure that reduced risk of death or need for liver transplant. Specifically, the change and final PSG did not influence the sHRs for death or transplant and thus were not included in the final model. Of note, in personal communication with center investigators, we determined that practice patterns differ significantly across providers and centers regarding how the PSG is measured after TIPS. Although there are strong data to support using the free hepatic or IVC pressure, rather than the right atrial pressure, as the systemic venous pressure when calculated the PSG; this is not routine practice at all centers (37–39). Thus, interpretation of the absolute and change in PSG across practitioners and sites is limited by the retrospective data and lack of a standard TIPS protocol.
We also note that the EVB group in our cohort does not represent a preemptive TIPS strategy as initially described by Garcia-Pagan et al. (3) whereby patients admitted with variceal bleeding with Childs-C cirrhosis or Childs-B with active bleeding at endoscopy be considered for TIPS. This approach was published shortly after the beginning of our cohort period (2010–2016) and was not yet incorporated into practice guidelines. We also do not believe that this approach was used often among our centers because several observational reports of practice patterns within a similar time period demonstrated very low rates (7%–10%) of use of preemptive TIPS (40,41).
In conclusion, among a large multicenter contemporary population of adults with cirrhosis undergoing TIPS with covered stents, we found that MELD-Na does not necessarily discriminate the risk of death after TIPS in ascites/HH indications, whereas MELD-Na ≥20 does discriminate the risk for post-TIPS mortality when performed for a variceal bleeding indication. When controlling for the components of the MELD-Na score, serum creatinine was the strongest predictor of death after TIPS regardless of indication. These data challenge previously held assumptions of a one size fits all risk stratification approach to TIPS. Future prospective study across multiple centers is needed to fully investigate the impact of TIPS indication and underlying pathophysiology of renal dysfunction as unique predictors of adverse TIPS outcomes.
Supplementary Material
Study Highlights.
WHAT IS KNOWN
Transjugular intrahepatic portosystemic shunt (TIPS) stent technology has improved considerably over the past decade.
Patient outcomes after TIPS are limited to small or investigational studies of highly selected patients.
Limited knowledge exists about the outcomes of TIPS recipients in routine clinical practice.
WHAT IS NEW HERE
These results demonstrate the real-world outcomes of TIPS recipients in the modern era.
Model for End-Stage Liver Disease Sodium has a different relationship with patient outcomes that is dependent on the TIPS indication.
Serum creatinine, independent of Model for End-Stage Liver Disease Sodium, is the strongest predictor of death post-TIPS, whereas bilirubin and international normalized ratio predict liver transplantation.
ACKNOWLEDGEMENTS
The authors wish to acknowledge the American Society for Transplantation Liver and Intestinal Community of Practice (AST LICOP) Education Subcommittee for providing a forum for investigators to collaborate on the enclosed study. This manuscript has been reviewed by ALTA Study group for scientific content and consistency of data interpretation with previous ALTA publications.
CONFLICTS OF INTEREST
Guarantor of the article: Lisa B. VanWagner, MD, MSc.
Specific author contributions: J.R.B.: study concept and design, acquisition of data, analysis and interpretation of data, drafting of the manuscript, critical revision of the manuscript for important intellectual content, statistical analysis, and study supervision. N.R.M.: analysis and interpretation of data, drafting of the manuscript, critical revision of the manuscript for important intellectual content, and statistical analysis. K.P.K., J.G., M.G., N.J., G.M., E.S., A.S., J.C.L., A.P.D., T.C., S.P., C.F., E.C., U.R., B.T.: acquisition of data, drafting of the manuscript, and critical revision of the manuscript for important intellectual content. D.G.: study concept and design, acquisition of data, analysis and interpretation of data, statistical analysis, administrative, and technical. L.B.V.: study concept and design, acquisition of data, analysis and interpretation of data, drafting of the manuscript, critical revision of the manuscript for important intellectual content, statistical analysis, administrative, technical, material support, and study supervision.
Financial support:
This study did not receive direct financial support. The ALTA Study Group however is funded by an investigator-initiated grant from W.L. Gore and Associates. This secondary analysis was funded by the National Heart, Lung, and Blood Institute grant number, K23 HL136891. The Northwestern Medicine Enterprise Data Warehouse (NMEDW) and Research Electronic Data Capture (REDCap) are funded, in part, by the National Center for Advancing Translational Sciences (NCATS) of the NIH research grant UL1TR001422 to the Northwestern University Clinical and Translational Sciences (NUCATS) Institute. The sponsor (W.L. Gore and Associates) had no input into the overall design and conduct of the ATLA Study. The funding agencies for the authors (NIDDK and NIA) played no role in the analysis of the data or the preparation of this manuscript.
Potential competing interests:
L.B.V. receives investigator-initiated and educational grant support and is on the speaker’s bureau for W.L. Gore & Associates, the manufacturer of the TIPS Viatorr stent. J.R.B. receives investigator-initiated grant support from W.L. Gore & Associates.
Footnotes
SUPPLEMENTARY MATERIAL accompanies this paper at http://links.lww.com/AJG/C89
REFERENCES
- 1.D’Amico G, Garcia-Tsao G, Pagliaro L. Natural history and prognostic indicators of survival in cirrhosis: A systematic review of 118 studies. J Hepatol 2006;44(1):217–31. [DOI] [PubMed] [Google Scholar]
- 2.Garcia-Tsao G, Sanyal AJ, Grace ND, et al. ; Practice Guidelines Committee of the American Association for the Study of Liver Diseases; Practice Parameters Committee of the American College of Gastroenterology. Prevention and management of gastroesophageal varices and variceal hemorrhage in cirrhosis. Hepatology 2007;46(3):922–38. [DOI] [PubMed] [Google Scholar]
- 3.García-Pagán JC, Caca K, Bureau C, et al. Early use of TIPS in patients with cirrhosis and variceal bleeding. N Engl J Med 2010;362(25):2370–9. [DOI] [PubMed] [Google Scholar]
- 4.Gordon FD, Anastopoulos HT, Crenshaw W, et al. The successful treatment of symptomatic, refractory hepatic hydrothorax with transjugular intrahepatic portosystemic shunt. Hepatology 1997;25(6): 1366–9. [DOI] [PubMed] [Google Scholar]
- 5.Angermayr B, Cejna M, Koenig F, et al. Survival in patients undergoing transjugular intrahepatic portosystemic shunt: ePTFE-covered stentgrafts versus bare stents. Hepatology 2003;38(4):1043–50. [DOI] [PubMed] [Google Scholar]
- 6.Bureau C, Garcia-Pagan JC, Otal P, et al. Improved clinical outcome using polytetrafluoroethylene-coated stents for TIPS: Results of a randomized study. Gastroenterology 2004;126(2):469–75. [DOI] [PubMed] [Google Scholar]
- 7.Ascha M, Hanouneh M, S Ascha M, et al. Transjugular intrahepatic porto-systemic shunt in patients with liver cirrhosis and model for end-stage liver disease ≥15. Dig Dis Sci 2017;62(2):534–42. [DOI] [PubMed] [Google Scholar]
- 8.Spengler EK, Hunsicker LG, Zarei S, et al. Transjugular intrahepatic portosystemic shunt does not independently increase risk of death in high model for end stage liver disease patients. Hepatol Commun 2017;1(5):460–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Berry K, Lerrigo R, Liou IW, et al. Association between transjugular intrahepatic portosystemic shunt and survival in patients with cirrhosis. Clin Gastroenterol Hepatol 2016;14(1):118–23. [DOI] [PubMed] [Google Scholar]
- 10.D’Amico G, Luca A, Morabito A, et al. Uncovered transjugular intrahepatic portosystemic shunt for refractory ascites: A meta-analysis. Gastroenterology 2005;129(4):1282–93. [DOI] [PubMed] [Google Scholar]
- 11.Salerno F, Cammà C, Enea M, et al. Transjugular intrahepatic portosystemic shunt for refractory ascites: A meta-analysis of individual patient data. Gastroenterology 2007;133(3):825–34. [DOI] [PubMed] [Google Scholar]
- 12.Harris PA, Taylor R, Thielke R, et al. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009;42(2):377–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.MELD Calculator–OPTN (https://optn.transplant.hrsa.gov/resources/allocation-calculators/meld-calculator/). Accessed October 17, 2020.
- 14.Guy J, Somsouk M, Shiboski S, et al. New model for end stage liver disease improves prognostic capability after transjugular intrahepatic portosystemic shunt. Clin Gastroenterol Hepatol 2009;7(11):1236–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ahmed R, Santhanam P, Rayyan Y. MELD-Na as a prognostic indicator of 30- and 90-day mortality in patients with end-stage liver disease after creation of transjugular intrahepatic portosystemic shunt. Eur J Gastroenterol Hepatol 2015;27(10):1226–7. [DOI] [PubMed] [Google Scholar]
- 16.Gray RJ. A class of K-sample tests for comparing the cumulative incidence of a competing risk. Ann Stat 1988;16(3):1141–54. [Google Scholar]
- 17.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc 1999;94(446):496–509. [Google Scholar]
- 18.Wasey JO, Team RC. ICD: Comorbidity Calculations and Tools for ICD-9 and ICD-10 Codes. Vienna, Austria. Published online 2018. (https//cranr-projectorg/web/packages/icd/indexhtml webcite). [Google Scholar]
- 19.Yoshida K, Bohn J. Tableone: Create “Table 1” to Describe Baseline Characteristics [R Package]. 2018. (https://cran.r-project.org/web/packages/tableone/tableone.pdf) [Google Scholar]
- 20.Wiesner R, Edwards E, Freeman R, et al. Model for end-stage liver disease (MELD) and allocation of donor livers. Gastroenterology 2003;124(1):91–6. [DOI] [PubMed] [Google Scholar]
- 21.Malinchoc M, Kamath PS, Gordon FD, et al. A model to predict poor survival in patients undergoing transjugular intrahepatic portosystemic shunts. Hepatology 2000;31(4):864–71. [DOI] [PubMed] [Google Scholar]
- 22.Pan JJ, Chen C, Caridi JG, et al. Factors predicting survival after transjugular intrahepatic portosystemic shunt creation: 15 years’ experience from a single tertiary medical center. J Vasc Interv Radiol 2008;19(11):1576–81. [DOI] [PubMed] [Google Scholar]
- 23.Alessandria C, Ozdogan O, Guevara M, et al. MELD score and clinical type predict prognosis in hepatorenal syndrome: Relevance to liver transplantation. Hepatology 2005;41(6):1282–9. [DOI] [PubMed] [Google Scholar]
- 24.Lee M, Lee JH, Oh S, et al. CLIF-SOFA scoring system accurately predicts short-term mortality in acutely decompensated patients with alcoholic cirrhosis: A retrospective analysis. Liver Int 2015;35(1):46–57. [DOI] [PubMed] [Google Scholar]
- 25.Ascha M, Abuqayyas S, Hanouneh I, et al. Predictors of mortality after transjugular portosystemic shunt. World J Hepatol 2016;8(11):520–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ginès P, Schrier RW. Renal failure in cirrhosis. N Engl J Med 2009;361(13): 1279–90. [DOI] [PubMed] [Google Scholar]
- 27.Anderson CL, Saad WE, Kalagher SD, et al. Effect of transjugular intrahepatic portosystemic shunt placement on renal function: A 7-year, single-center experience. J Vasc Interv Radiol 2010;21(9):1370–6. [DOI] [PubMed] [Google Scholar]
- 28.Allegretti AS, Ortiz G, Cui J, et al. Changes in kidney function after transjugular intrahepatic portosystemic shunts versus large-volume paracentesis in cirrhosis: A matched cohort analysis. Am J Kidney Dis 2016;68(3):381–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dhanasekaran R, Gonzales P, West J, et al. Predictors of early mortality post transjugular intrahepatic portosystemic shunts and the role of hepatic venous pressure gradient. Gastrointest Interv 2012;1(1):63–8. [Google Scholar]
- 30.Hingorani N, Catron T, Abdel Al AK, et al. Impact of chronicity of renal dysfunction on post-TIPS outcomes: 920. Am J Gastroenterol 2017;112: S515–18. [Google Scholar]
- 31.Kim HK, Kim YJ, Chung WJ, et al. Clinical outcomes of transjugular intrahepatic portosystemic shunt for portal hypertension: Korean multicenter real-practice data. Clin Mol Hepatol 2014;20(1):18–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ge J, Lai JC, Boike JR, et al. Nonalcoholic fatty liver disease and diabetes mellitus are associated with post–transjugular intrahepatic portosystemic shunt renal dysfunction: An advancing liver therapeutic Approaches group study. Liver Transpl 2021;27(3):329–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Byrne CD, Targher G. NAFLD as a driver of chronic kidney disease. J Hepatol 2020;72(4):785–801. [DOI] [PubMed] [Google Scholar]
- 34.Asrani SK, Jennings LW, Trotter JF, et al. A model for glomerular filtration rate assessment in liver disease (GRAIL) in the presence of renal dysfunction. Hepatology 2019;69(3):1219–30. [DOI] [PubMed] [Google Scholar]
- 35.Levitsky J, O’Leary JG, Asrani S, et al. Protecting the kidney in liver transplant recipients: Practice–based recommendations from the American Society of transplantation Liver and Intestine Community of Practice. Am J Transpl 2016;16(9):2532–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bettinger D, Sturm L, Pfaff L, et al. Refining prediction of survival after TIPS with the novel Freiburg index of post-TIPS survival. J Hepatol 2021; 74(6):1362–72. [DOI] [PubMed] [Google Scholar]
- 37.La Mura V, Abraldes JG, Berzigotti A, et al. Right atrial pressure is not adequate to calculate portal pressure gradient in cirrhosis: A clinical-hemodynamic correlation study. Hepatology 2010;51(6): 2108–16. [DOI] [PubMed] [Google Scholar]
- 38.Casado M, Bosch J, García-Pagán JC, et al. Clinical events after transjugular intrahepatic portosystemic shunt: Correlation with hemodynamic findings. Gastroenterology 1998;114(6):1296–303. [DOI] [PubMed] [Google Scholar]
- 39.Silva-Junior G, Turon F, Baiges A, et al. Timing affects measurement of portal pressure gradient after placement of transjugular intrahepatic portosystemic shunts in patients with portal hypertension. Gastroenterology 2017;152(6):1358–65. [DOI] [PubMed] [Google Scholar]
- 40.Thabut D, Pauwels A, Carbonell N, et al. Cirrhotic patients with portal hypertension-related bleeding and an indication for early-TIPS: A large multicentre audit with real-life results. J Hepatol 2018;68(1): 73–81. [DOI] [PubMed] [Google Scholar]
- 41.Hernández-Gea V, Procopet B, Giráldez Á, et al. Preemptive-TIPS improves outcome in high-risk variceal bleeding: An observational study. Hepatology 2018;69(1):30182. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


