Abstract
Objectives
Accurate assessment of tobacco smoke exposure is key to evaluate its effects. We sought to validate and establish cut-offs for self-reported smoking and secondhand smoke (SHS) exposure during pregnancy using urinary cotinine and 4-(methylnitrosamino)-1-(-3-pyridyl)-1-butanol (NNAL) in a large contemporary prospective study from the USA, with lower smoking prevalence than has previously been evaluated.
Design
Prospective birth cohort.
Setting
Pregnancy clinics in New Hampshire and Vermont, USA.
Participants
1396 women enrolled in the New Hampshire Birth Cohort Study with self-reported smoking, urinary cotinine, NNAL and pregnancy outcomes.
Primary and secondary outcome measures
Cut-offs for urinary cotinine and NNAL concentrations were estimated from logistic regression models using Youden’s method to predict SHS and active smoking. Cotinine and NNAL were each used as the exposure in separate multifactorial models for pregnancy outcomes.
Results
Self-reported maternal smoking was: 72% non-smokers, 5.7% ex-smokers, 6.4% SHS exposure, 6.2% currently smoked, 10% unreported. Cotinine and NNAL levels were low and highly intercorrelated (r=0.91). Geometric mean cotinine, NNAL were 0.99 ng/mL, 0.05 pmol/mL, respectively. Cotinine cut-offs for SHS, current smoking were 1.2 ng/mL and 1.8 ng/mL (area under curve (AUC) 95% CI: 0.52 (0.47 to 0.57), 0.90 (0.85 to 0.94)). NNAL cut-off for current smoking was 0.09 pmol/mL (AUC=0.82 (95% CI 0.77 to 0.87)). Using cotinine and NNAL cut-offs combined gave similar AUC to cotinine alone, 0.87 (95% CI 0.82 to 0.91). Cotinine and NNAL gave almost identical effect estimates when modelling pregnancy outcomes.
Conclusions
In this population, we observed high concordance between self-complete questionnaire smoking data and urinary cotinine and NNAL. With respect to biomarkers, either cotinine or NNAL can be used as a measure of tobacco smoke exposure overall but only cotinine can be used to detect SHS.
Keywords: epidemiology, perinatology, statistics & research methods
Strengths and limitations of this study.
Compares the utility of two tobacco biomarkers, urinary cotinine and 4-(methylnitrosamino)-1-(-3-pyridyl)-1-butanol to identify smoking in pregnant women.
Set within a large contemporary birth cohort in rural USA with low prevalence of smoking.
Relies on the availability of both urinary biomarker values and self-reported smoking.
Introduction
The adverse effects of maternal smoking on birth weight and other pregnancy outcomes have been known for over 40 years with reports consistently showing that women who smoke cigarettes in pregnancy have smaller babies and are at greater risk of preterm delivery and other adverse outcomes.1 2 In more recent years, reports have focused on the effects of secondhand smoke (SHS) exposure in pregnancy and have shown evidence for small but statistically significant adverse effects on birth weight, stillbirth and congenital anomalies.3
Alongside these outcome-focused studies there has been a growing interest in the accuracy of the assessment of the exposure, tobacco smoke intake. Many epidemiological studies have used interviews or self-complete questionnaires to ascertain smoking in pregnancy. Questionnaires continue to be widely used since they are relatively easy and inexpensive to administer but biomarkers are increasingly used to validate self-reported smoking. Cotinine, a metabolite of nicotine, can be measured in urine, saliva and plasma samples and was shown in the late 1980s to discriminate well between smokers and non-smokers with high sensitivity and specificity for data-derived cut-offs.4 Since then many studies have derived cotinine cut-offs to discriminate between smokers and non-smokers for a range of patient groups including pregnant women,5 and these cut-offs have been used to identify participants whose reported smoking was inconsistent with their cotinine level that is, they were ‘misclassified’.6 Thus, the advantage of cotinine over questionnaire measures has been demonstrated in the presence of smoking misclassification.
The tobacco-specific nitrosamine metabolite 4-(methylnitrosamino)-1-(-3-pyridyl)-1-butanol (NNAL) can be measured in urine samples and has been associated with smoking-related cancers.7 Urinary NNAL has been compared with cotinine to assess SHS exposure in adolescents by Benowitz and colleagues who reported that both biomarkers detected high percentages with SHS exposure among adolescents.8 Postpartum urinary NNAL was reported to be correlated with cotinine (rho=0.78), and associated with neonatal NNAL level (rho=0.71).9 A few studies have used both questionnaires and biomarkers to assess exposure to tobacco smoke in pregnancy: a cohort study from Korea explored the use of NNAL to assess tobacco smoke exposure and concluded that it added to the information provided by self-report or cotinine10; a mother–child cohort from Greece found that cotinine did not fully summarise exposure to 4-(methylnitrosamino)−1-(3-pyridyl)−1-butanol uptake11; a study from Poland explored relationships between maternal NNAL and cotinine in women reporting SHS or active smoking, and concluded that NNAL was a useful biomarker of prenatal exposure to carcinogens in newborns.12 Optimal cut-off points for detecting active and passive smoke exposure in pregnancy have been reported from the INMA Spanish cohort (18% active smokers)6 and from the Hokkaido Japanese cohort (19% active smokers).13
The New Hampshire Birth Cohort Study is a large ongoing prospective study from USA with lower smoking prevalence than has been evaluated historically and that has obtained detailed self-reported smoking data and urinary cotinine/NNAL levels. We sought to establish biomarker cut-offs for smoking and SHS exposure and to validate the use of self-reported smoking against the biomarkers to extend the knowledge base for the utility of NNAL. We hypothesised that NNAL would be strongly positively correlated with cotinine and that the two biomarkers would be similarly predictive of smoking and SHS, and that self-reported smoking would be shown to be reliable.
Methods
Study population
The New Hampshire Birth Cohort Study (NHBCS) is a prospective study that aims to examine the associations between environmental exposures and other factors, and maternal–child health outcomes.14 Beginning in January 2009, pregnant women between 24 and 28 weeks of gestation were recruited from prenatal clinics in New Hampshire. Criteria for eligibility included: age 18 to 45 years, English literacy, use a private, unregulated water system at home (eg, private well), not planning to move residence, and a singleton pregnancy. The current analyses includes all women recruited until January 2017.
Patients and public involvement
There was no patient involvement in this specific study but the New Hampshire Birth Cohort Study has an active dissemination programme for the community (https://geiselmed.dartmouth.edu/childrenshealth/quick-links/).
Data obtained
Demographic and lifestyle data including educational attainment and tobacco smoke exposure were obtained using NHBCS questionnaires administered at enrolment. Smoking status and number of cigarettes smoked per day were assessed during the 3 months prior to pregnancy, as well as during the first and second trimesters of pregnancy. Additionally, exposure to SHS was assessed through the number of hours per day and days per week while the pregnant mother was in areas where others were smoking during the 3 months prior to pregnancy and during the first and second trimesters of pregnancy. Maternal smoking status was categorised from participants’ reports in five groups: (1) current smoker (2) ex-smoker (3) non-smoker, SHS exposure (4) non-smoker, no SHS exposure (5) not reported. Number of cigarettes smoked per day was asked for current smokers. See online supplemental methods—additional information’ for details of how smoking was classified.
bmjopen-2021-054535supp001.pdf (1.4MB, pdf)
Maternal and infant anthropometry and birth outcome data were ascertained from prenatal and delivery medical records and included: mother’s height, preconception weight, infant sex, birth weight, gestational age, head circumference and crown-heel length. Infant measurements were normalised using z-scores to adjust for sex and gestation.
Biospecimens
Spot urine samples were collected by the subject at the time of enrolment, at approximately 24–28 weeks gestation and transported on ice packs and stored in a 4°C refrigerator. Processing of urines into aliquots occurred within 24 hours of collection. During processing 10 mL aliquots were transferred into 15 mL trace-free metal tubes and immediately stored at −80°C. 10 mL aliquots were thawed once to obtain aliquots for trace metal analysis and thawed again to obtain a 2 mL aliquot for cotinine and NNAL analysis. 2 mL aliquots were frozen at −80°C and referred to the Minnesota Children’s Health Exposure Analysis Resource Exposure Assessment Hub at the Masonic Cancer Center at the University of Minnesota. To assess urinary dilution, specific gravity was measured using digital refractometer.
Cotinine and NNAL
Two biomarkers of exposure to tobacco smoke, total cotinine and total NNAL were quantified in maternal urine samples at 24–28 weeks gestation. ‘Total’ refers to the sum of the compound and its glucuronide conjugates. The analysis was by LC-MS/MS as described previously.15 16 Lab reported limit of quantitation values for cotinine and NNAL were 0.5 ng/mL and 0.05 pmol/mL, respectively, with interassay coefficients of variation, 5% and 12%.
Statistics
The study population included all NHBCS women who provided a 24–28 gestational week urine sample from which cotinine and NNAL levels could be obtained. Since some women did not provide a urine sample, we compared maternal characteristics in those with and without these urines (included/excluded) using the t-test and chi-squared or Fisher’s exact test. We summarised the characteristics of the study population mothers and their babies using means and SD for continuous data and frequencies and percentages for categorical data. Some variables are reported as both continuous and categorical (age, body mass index, BMI) to aid interpretation and comparison with other studies. We cross-classified reported smoking by cotinine and NNAL levels in groups to explore the inter-relationships, and calculated the correlation between cotinine, NNAL and number of cigarettes smoked using Pearson’s coefficient. A participant’s reported smoking was defined as ‘misclassified’ (yes/no) if they reported not being a current smoker but had a urinary cotinine level at or above 30 ng/mL, the lowest cotinine cut-off value for active smoking reported in a recent review.5
We used logistic regression to model the relationship between being a current smoker and both cotinine and NNAL concentrations to determine the cotinine and NNAL level cut-offs that best identified current smoking. Youden’s method17 was used with the receiver operating characteristic curve to choose the cut-off that gave the best combination of sensitivity and specificity. A similar analysis was conducted to determine the best cut-off for each of cotinine and NNAL to identify SHS. These cut-offs were used to categorise the participant’s smoking status into groups for each of cotinine and NNAL: unexposed/exposed to SHS only/smoker.
We used cotinine and NNAL to assess the relationship between smoking and the outcome of pregnancy in multivariable regression models. We modelled each biomarker as a continuous variable, loge-transformed with values below the limit of detection (LOD) replaced by LOD/sqrt(2), that is, 0.3536 for cotinine and 0.0354 for NNAL. The following outcomes of pregnancy were analysed: birth weight, birth weight z-score, gestational age, small-for-gestational age (<10th percentile), preterm birth, head circumference z-score, and crown-heel length z-score. Results are given as regression coefficients for continuous outcomes and ORs for binary outcomes scaled to a one SD change in cotinine or NNAL as appropriate (with 95% CIs) to aid interpretation. All birth outcome models were adjusted for the following covariates: maternal age (continuous), BMI (loge-transformed), maternal education (beyond high school, yes/no) and parity (0 vs 1+).
In a sensitivity analysis, we separately modelled the effects of cotinine and NNAL on pregnancy outcome using the cut-offs derived previously to define smoking. In a post hoc change for this sensitivity analysis only, we re-categorised women who reported being smokers but had low urinary cotinine level when assessed (ie, below the data derived cut-off value) as active current smokers. We did this since we judged it likely that they were generally smoking in pregnancy.
Factors associated with self-reported smoking status during pregnancy (categorised as current smoker, ex-smoker, non-smoker) and being misclassified (yes/no) were explored using unifactorial and multivariable multinomial logistic regression. The following factors were included as possible predictors: maternal age at enrolment (continuous), BMI (loge-transformed), education (education beyond high school, yes/no), and parity (0 vs 1+). Statistical analyses were conducted using SAS V9.4, R V3.6.3.
Power calculations
An indicative power calculation was conducted according to the available cohort size, approximately 1300, varying slightly for different analyses due to missing data. Assuming two-sided significance 5%, mean birthweights 3500 g, 3470 g, 3300 g in the unexposed, SHS exposed only and current smokers, and SD 500,18 power is over 90% both for a one-way analysis of variance and for testing the trend across groups (Stata ‘power oneway’).
Results
A total 1739 women were enrolled in the NHBCS as of January 2017, of whom 1396 have cotinine and NNAL data and so comprise the study population for the current analyses. Online supplemental table S1 compares the characteristics for the study population with the 494 excluded (no cotinine/NNAL data) and indicates that the study population had a slightly lower mean BMI, were more likely to be primiparous, had more education, were less likely to smoke and were more likely to be of white race than those not included. Other characteristics were not appreciably different.
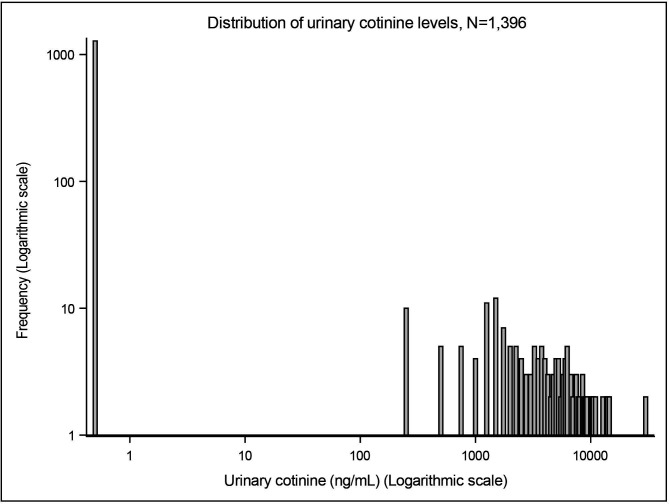
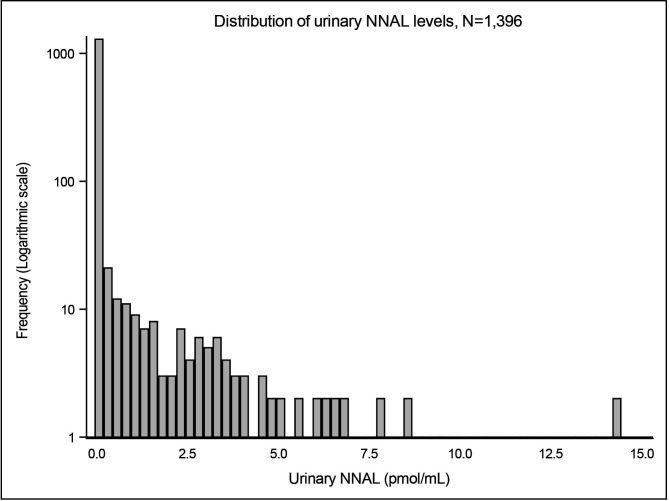
Overall, the study population had a mean age 31 years, mean BMI 25, 43% were nulliparous and 88% had been educated beyond high school. Seventy-two per cent of women self-reported as non-smokers, a further 6% were not active smokers but exposed to SHS preconception or prenatal, 6% were ex-smokers and 6% were current smokers. Ten per cent of women did not report smoking status. Twenty-seven women (2%) reported not currently smoking but had cotinine levels consistent with active smoking and so their reported smoking was assumed to be ‘misclassified’. Among smokers, the number of cigarettes smoked was relatively low with most women reporting to smoke less than 10 cigarettes per day. Geometric mean cotinine and NNAL levels were 0.99 ng/mL and 0.05 pmol/mL, respectively, and both distributions were positively skewed (figures 1 and 2). For the newborns: mean birth weight was 3421 g, 52% male, 9.2% were preterm and 9.8% were small-for-gestational age (table 1). The full table is given in online supplemental table S2.
Figure 1.
Distribution of urinary cotinine level (ng/mL).
Figure 2.
Distribution of urinary NNAL level (pmol/mL). NNAL, 4-(methylnitrosamino)-1-(-3-pyridyl)-1-butanol.
Table 1.
Characteristics of the women and their babies (full version in online supplemental table S2)
| Total | 1396 |
| Maternal characteristics | |
| Maternal age, years mean (SD) | 31.3 (4.9) |
| Prepregnancy maternal weight (lb) mean (SD) | 153.9 (34.1) |
| Prepregnancy maternal height (in) mean (SD) | 64.9 (2.7) |
| Prepregnancy maternal BMI mean (SD) | 25.6 (5.6) |
| Parity: primiparous | 43% (586) |
| Mother’s race: white | 97% (1357) |
| Maternal level of education | |
| High school or less | 12% (140) |
| Junior college/college | 57% (696) |
| Postgraduate | 31% (377) |
| Maternal tobacco exposure | |
| Reported smoking at 24 weeks | |
| Non-smoker, no SHS | 72% (999) |
| SHS exposure preconception/prenatal | 6.4% (90) |
| Ex-smoker | 5.7% (79) |
| Current smoker | 6.2% (86) |
| Not reported | 10% (142) |
| Urinary cotinine, ng/mL mean (SD) | 339.54 (1621.98) |
| Geometric mean (geometric SD) | 0.99 (12.43) |
| NNAL, pmol/mL mean (SD) | 0.19 (0.81) |
| Geometric mean (geometric SD) | 0.05 (2.75) |
| Infant characteristics | |
| Gestational age, weeks mean (SD) | 38.96 (1.82) |
| Birth weight, grams mean (SD) | 3421.2 (552.3) |
| Birth weight z-score mean (SD) | −0.05 (1.03) |
| Small-for-gestational age (below 10th centile for age) % (n) | 9.8% (132) |
| Preterm birth (gestational age <37 wks) % (n) | 9.2% (129) |
| Infant sex—male % (n) | 52% (716) |
BMI, body mass index; NNAL, 4-(methylnitrosamino)-1-(-3-pyridyl)-1-butanol; SHS, secondhand smoke.
There was generally good agreement between reported smoking and urinary cotinine level (table 2). The majority of self-reported non-smokers had very low (undetectable) cotinine levels and the majority of current smokers had cotinine above 30 ng/mL, although some current smokers had very low cotinine levels (online supplemental table S3). NNAL levels were undetectable in almost all women; only 8% (107/1396) overall had NNAL at or above 0.1 pmol/mL (online supplemental table S3). Cotinine and NNAL levels were very similar among ex-smokers who reported smoking in the 3 months prior to conception compared with those who reported smoking earlier (online supplemental table S3). There were positive intercorrelations between the two tobacco biomarkers and the reported number of cigarettes smoked (online supplemental table S4). In particular, we noted a very strong correlation, r=0.91, between loge cotinine and loge NNAL (online supplemental table S1).
Table 2.
Optimal cut-off points for urinary cotinine and urinary NNAL to define active smoking and SHS
| Cut-point | Sensitivity (95% CI) | Specificity (95% CI) | AUC (95% CI) | Youden index | |
| Cotinine (ng/mL) | |||||
| To detect SHS | 1.2 | 11% (6.7% to 17%) | 95% (86% to 99%) | 0.52 (0.47 to 0.57) | 0.06 |
| To detect active current smoking | 1.8 | 80% (70% to 88%) | 93% (91% to 94%) | 0.90 (0.85 to 0.94) | 0.73 |
| NNAL (pmol/mL) | |||||
| To detect current active smoking | 0.09 | 66% (55% to 76%) | 98% (97% to 99%) | 0.82 (0.77 to 0.87) | 0.64 |
| Cotinine and NNAL combined | |||||
| To detect current active smoking | Cotinine >1.8 or NNAL >0.09 | 81% (72% to 89%) | 89% (87% to 91%) | 0.87 (0.82 to 0.91) | Not applicable |
AUC, area under curve; NNAL, 4-(methylnitrosamino)-1-(-3-pyridyl)-1-butanol; SHS, secondhand smoke.
The data-derived cotinine cut-offs for SHS and current active smoking were 1.2 ng/mL and 1.8 ng/mL, respectively. The cotinine cut-off for SHS had high specificity but very low sensitivity whereas the cut-off for active current smoking had high sensitivity and high specificity (table 2). NNAL levels could not be used to detect SHS but an NNAL cut-off of 0.09 detected active current smoking in this population with very high specificity and moderate sensitivity (table 2). A post hoc analysis was conducted to determine whether cotinine plus NNAL improved the separation between smokers and non-smokers. This showed that using the criterion that either cotinine or NNAL were above their respective previously derived cut-offs, produced similar sensitivity and specificity and area under curve (AUC) to using cotinine alone (table 2).
Non-smokers without SHS exposure tended to be older and nearly all educated beyond high school (online supplemental table S5). In contrast, current smokers were younger and less than one half were educated beyond high school (online supplemental table S5). The associations between age, BMI, parity and education were weak (online supplemental table S6), allowing mutual adjustment in multivariable analyses. While the associations between smoking group and age, parity and BMI were weaker after mutual adjustment and not statistically significant, the association with education remained strong (online supplemental table S7).
The estimated effects of cotinine level and NNAL level on outcome of pregnancy were adjusted for maternal age, BMI, parity and education, and scaled to a SD increase in cotinine or NNAL. The scaled estimates for birth outcomes are very similar for the two biomarkers (table 3). Statistically significant inverse associations were observed for: birth weight (cotinine: −55.5 g, NNAL: −57.8 g), birth weight z-score (cotinine: −0.11, NNAL: −0.11), crown-heel length z-score (cotinine: −0.11, NNAL: −0.10). In the sensitivity analysis using the previously derived cotinine and NNAL cut-offs to define smoking groups, (non-smokers/SHS/smokers for cotinine; smokers/non-smokers for NNAL), there was a mean reduction in birth weight of 43 g in those with SHS exposure, and 128 g in active smokers compared with non-smokers. P values tended to be bigger (less significant) in the analyses with smoking biomarkers modelled in categories compared with as continuous (online supplemental table S8, table 3).
Table 3.
Outcome of pregnancy by loge transformed urinary cotinine and loge transformed NNAL level in pregnancy N=1396
| Outcome | Regression coefficient/OR (95% CI) P value |
|
| Cotinine, ng/mL (loge) | NNAL, pmol/mL (loge) | |
| Birthweight*, gram | −55.5 (−93.2 to –17.8) 0.0040 |
−57.8 (−96.6 to –18.5) 0.0039 |
| Birth weight z-score* | −0.11 (−0.18 to –0.04) 0.0027 |
−0.11 (−0.18 to –0.04) 0.0035 |
| Gestational age*, weeks | −0.11 (−0.24 to 0.01) 0.0795 |
−0.06 (−0.19 to 0.07) 0.3904 |
| Small-for-gestational age (< 10th centile) |
OR=1.22 (0.98 to1.52) 0.0884 |
OR=1.15 (0.92 to 1.44) 0.2326 |
| Preterm birth (<37 wks) |
OR=1.21 (0.98 to 1.50) 0.1002 |
OR=1.07 (0.84 to 1.36) 0.6066 |
| Crown-heel length z-score* | −0.11 (−0.22 to –0.003) 0.0433 |
−0.10 (−0.21 to 0.02) 0.0991 |
| Head circumference z-score* | −0.04 (−0.12 to 0.04) 0.2915 |
−0.03 (−0.11 to 0.05) 0.5055 |
*Regression coefficients and ORs scaled to 1 SD increase in loge cotinine (2.520) or loge NNAL (1.012) as appropriate.
†All models include the following covariates: maternal age, loge BMI, maternal education (high school vs beyond high school), parity (0 vs 1+).
‡Totals vary due to missing or unreported data.
BMI, body mass index; NNAL, 4-(methylnitrosamino)-1-(-3-pyridyl)-1-butanol.
Discussion
Tobacco smoke contains many constituents including nicotine and carbon monoxide which are known to adversely affect the mother and fetus through vasoconstriction (nicotine) and hypoxia (carbon monoxide)19 and hence the accurate assessment of tobacco smoke exposure in pregnancy is critical. In this paper, we have reported on the validation of self-reported smoking in an ongoing cohort study of pregnant women in rural USA, the New Hampshire Birth Cohort Study (NHBCS), using two biomarkers, cotinine and NNAL. The prevalence of maternal smoking among NHBCS is 6.2% and among those who smoked, the number smoked is low with the majority of NHBCS smokers reporting smoking less than 10 cigarettes per day. This prevalence of maternal smoking is lower than the overall US average, 7.2%, and the New Hampshire prevalence, 11%, reported for 2016 from the National Vital Statistics System.20 These low levels of smoking in NHBCS were borne out by the cotinine and NNAL levels and contrast the higher prevalence of maternal smoking in other cohorts such as the Boston Cohort that reports that 10% women smoked in pregnancy,21 INMA study from Spain where 19% women self-reported smoking in pregnancy,6 the DEMOCOPHES study from Romania, Portugal and Poland with 25%, 30%, 19%, respectively,22 and the Hokkaido Japanese cohort (19%).13
Our study shows broad agreement between questionnaire reports and both biomarkers. The use of questionnaires is cheaper and easier to collect as can be done without invasive and expensive laboratory analyses and potentially more representative of a woman’s smoking as questions usually ask about smoking over a period of time. In contrast, as well as being objective and not subject to reporting bias, biomarker levels relate to recent tobacco smoke exposure—the half-life of cotinine in urine of pregnant smokers has been estimated to be about 8 hours,23 shorter than in non-pregnant women due to accelerated metabolism in pregnancy.24 The biomarker levels may be especially useful to determine effects of recent exposure.
Very few women misreported their smoking: under 2% of women (n=27) reported themselves as non-smoking but had tobacco biomarker levels consistent with active smoking. Just over 1% of women (n=17) reported being active smokers but their biomarker levels were very low. The low level of tobacco biomarker in these 17 self-reported smokers is likely due to infrequent smoking which resulted in abstinence in the period prior to collection of the urine sample. These observations show the value of having both questionnaire and biomarker data and alerts us to a limitation of using short-term biomarkers alone to quantify tobacco exposure. In our study, the low level of discordance between self-reported smoking and biomarker level provides reassurance that the choice of which to use in analyses may be taken according to the question and nature of the modelling required.
The derived cut-off for urinary cotinine to define active smoking was low, 1.8 ng/mL, reflecting the low number of cigarettes smoked by NHBCS women. This means that among NHBCS women and other similar populations where women smoke very little in pregnancy, cotinine levels are very reliable for predicting active smoking (sensitivity=80%, specificity=93%; AUC=0.90). However, cotinine levels are poor predictors of SHS (AUC: 0.52). A review article reported study-specific cut-offs for urinary cotinine varying between 31.5 and 550 ng/mL,5 which is substantially higher than ours. The INMA study also reports a higher cut-off than ours, 82 ng/mL,6 although the DEMOCOPHES study reported cut-offs of 4.4 ng/mL (Poland), 7.9 (Portugal) and 254.2 (Romania).22
NNAL was able to distinguish between active smoking and non-smoking or SHS exposure with high specificity (98%) and moderate sensitivity (66%). However, given the low urinary NNAL values among our women NNAL levels were not able to be used to define SHS. The NNAL cut-off derived using NHBCS data, 0.09 pmol/mL, was higher than that reported by Benowitz in adolescents, 0.058 (after conversion to SI units). There was a very high correlation between cotinine and NNAL (0.91) and so it is unsurprising that using cotinine plus NNAL gave no additional predictive ability beyond using cotinine alone, in our population.
When we used biomarker data to define tobacco exposure, we observed the expected relationships with pregnancy outcomes. Of particular note is that the estimated mean reductions in birth weight, birth weight z-score and crown-heel length were very similar using cotinine compared with using NNAL, reflecting the concordance of the two biomarkers as measures of maternal tobacco smoke intake. When we used the data-derived cut-offs to define smoking groups we were able to estimate the effect of SHS as a mean reduction of 43 g or 0.07 z-score units. The birth weight reduction falls within the 95% CI from the pooled value reported in the Nieuwenhuijsen review of the literature with a pooled mean reduction of 60 g and 95% CI of 39 to 803. This is reassuring given our data are from a relatively recent cohort with relatively low rates of current smoking.
Our population sample study data come from a large ongoing birth cohort from Northern New England where smoking data were carefully collected using detailed self-complete questionnaires supplemented by urinary tobacco biomarker data. This is one of only a few studies to examine NNAL in a pregnant population: Lee et al10 studied 251 pregnant women (8.4% smokers) in South Korea and reported that positive NNAL, defined as NNAL greater than the lowest LOD, 2.0 pg/mL and not urinary cotinine, was an independent predictor of spontaneous abortion, preterm birth and small-for-gestational age. Florek et al detected raised levels of cotinine and NNAL in newborn urine whose mothers had been exposed to tobacco in Poland (N=121),12 and Vardavas et al found that exposure to tobacco smoke correlated with cotinine and NNAL in Greece (N=1317).11
The limitations of our study are that urines were obtained at one time point only, 24–28 weeks gestation, and while identified misclassification of smoking was low, 10% of women did not report smoking. For these women, the biomarker data suggested that around a quarter were active smokers compared with 6.2% among those who responded to smoking questions and so using questionnaire data alone will underestimate the true prevalence of smoking. Most of our self-reported smoking questions were related to current habit and so were not subject to recall bias but we did enquire about SHS exposure preconception and so those responses may have been affected by errors in recall.
Overall, we observed good concordance between our self-complete questionnaire smoking data and tobacco biomarker levels, suggesting that the percentage of misclassified non-smokers is small. Further we have found that an NNAL data-derived cut-off can be used to separate smokers from non-smokers with high specificity and moderate sensitivity, although in our population cotinine was a better predictor of reported smoking overall, with high sensitivity and specificity. We suggest on the basis of this relatively recent pregnancy cohort of USA women from rural Northern New England that either detailed self-completed questionnaire smoking data or biomarker data may be used in analyses of the effects of tobacco smoke on health outcomes in children. We further suggest that cotinine levels rather than NNAL levels be used to detect SHS exposure.
Supplementary Material
Acknowledgments
We would like to extend our deepest appreciation of the participants and staff of the New Hampshire Birth Cohort and Center for Molecular Epidemiology. We gratefully acknowledge Alexandra Roy, an MPH candidate at the University of New England for her assistance with literature review for the analyses described in this paper. Finally we thank Karin Vevang for her key role in managing the logistics of the CHEAR project at the University of Minnesota.
Footnotes
JLP and TJP contributed equally.
Contributors: MRK, TJP, VS and EB conceptualised and designed this study, and assisted with methodology. SEM, LP and TJP conducted and quality-assured all laboratory assessments. CT conducted the literature review. JLP and YH conceived and conducted the statistical analyses. JLP drafted the manuscript including all tables and figures. All authors reviewed the manuscript and agreed the final submission.
Funding: Research supported in this publication was supported in part by the National Institutes of Health: P20GM104416, P42ES007373, P01 ES022832, UC2ES026533, UH30D023275, UG3OD023275 and US EPA RD83544201.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data may be obtained from a third party and are not publicly available. These data form part of the NIH HHEAR programme. The data will be uploaded to their portal at a future date. https://www.niehs.nih.gov/research/supported/exposure/hhear/index.cfm.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
This study was approved by Trustees of Dartmouth College Committee For The Protection Of Human Subjects #STUDY00020844. Participants gave informed consent to participate in the study before taking part.
References
- 1.Simpson WJ. A preliminary report on cigarette smoking and the incidence of prematurity. Am J Obstet Gynecol 1957;73:808–15. 10.1016/0002-9378(57)90391-5 [DOI] [PubMed] [Google Scholar]
- 2.The health consequences of smoking for women: a report of the Surgeon General. Washington: United States 1980.
- 3.Nieuwenhuijsen MJ, Dadvand P, Grellier J, et al. Environmental risk factors of pregnancy outcomes: a summary of recent meta-analyses of epidemiological studies. Environ Health 2013;12:6. 10.1186/1476-069X-12-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jarvis MJ, Tunstall-Pedoe H, Feyerabend C, et al. Comparison of tests used to distinguish smokers from nonsmokers. Am J Public Health 1987;77:1435–8. 10.2105/AJPH.77.11.1435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kim S. Overview of cotinine cutoff values for smoking status classification. Int J Environ Res Public Health 2016;13:ijerph13121236. 10.3390/ijerph13121236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aurrekoetxea JJ, Murcia M, Rebagliato M, et al. Determinants of self-reported smoking and misclassification during pregnancy, and analysis of optimal cut-off points for urinary cotinine: a cross-sectional study. BMJ Open 2013;3:34. 10.1136/bmjopen-2012-002034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hecht SS. Biochemistry, biology, and carcinogenicity of tobacco-specific N-nitrosamines. Chem Res Toxicol 1998;11:559–603. 10.1021/tx980005y [DOI] [PubMed] [Google Scholar]
- 8.Benowitz NL, Nardone N, Jain S, et al. Comparison of Urine 4-(Methylnitrosamino)-1-(3)Pyridyl-1-Butanol and Cotinine for Assessment of Active and Passive Smoke Exposure in Urban Adolescents. Cancer Epidemiol Biomarkers Prev 2018;27:254–61. 10.1158/1055-9965.EPI-17-0671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Benowitz NL, Flanagan CA, Thomas TK, et al. Urine 4-(methylnitrosamino)-1-(3) pyridyl-1-butanol and cotinine in Alaska native postpartum women and neonates comparing smokers and smokeless tobacco users. Int J Circumpolar Health 2018;77:1528125. 10.1080/22423982.2018.1528125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee SW, Han YJ, Cho DH, et al. Smoking Exposure in Early Pregnancy and Adverse Pregnancy Outcomes: Usefulness of Urinary Tobacco-Specific Nitrosamine Metabolite 4-(Methylnitrosamino)-1-(3-Pyridyl)-1-Butanol Levels. Gynecol Obstet Invest 2018;83:365–74. 10.1159/000485617 [DOI] [PubMed] [Google Scholar]
- 11.Vardavas CI, Fthenou E, Patelarou E, et al. Exposure to different sources of second-hand smoke during pregnancy and its effect on urinary cotinine and tobacco-specific nitrosamine (NNAL) concentrations. Tob Control 2013;22:194–200. 10.1136/tobaccocontrol-2011-050144 [DOI] [PubMed] [Google Scholar]
- 12.Florek E, Piekoszewski W, Basior A, et al. Effect of maternal tobacco smoking or exposure to second-hand smoke on the levels of 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol (NNAL) in urine of mother and the first urine of newborn. J Physiol Pharmacol 2011;62:377–83. [PubMed] [Google Scholar]
- 13.Kobayashi S, Sata F, Hanaoka T, et al. Association between maternal passive smoking and increased risk of delivering small-for-gestational-age infants at full-term using plasma cotinine levels from the Hokkaido study: a prospective birth cohort. BMJ Open 2019;9:e023200. 10.1136/bmjopen-2018-023200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gilbert-Diamond D, Cottingham KL, Gruber JF, et al. Rice consumption contributes to arsenic exposure in US women. Proc Natl Acad Sci U S A 2011;108:20656–60. 10.1073/pnas.1109127108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Murphy SE, Park S-SL, Thompson EF, et al. Nicotine N-glucuronidation relative to N-oxidation and C-oxidation and UGT2B10 genotype in five ethnic/racial groups. Carcinogenesis 2014;35:2526–33. 10.1093/carcin/bgu191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Carmella SG, Ming X, Olvera N, et al. High throughput liquid and gas chromatography-tandem mass spectrometry assays for tobacco-specific nitrosamine and polycyclic aromatic hydrocarbon metabolites associated with lung cancer in smokers. Chem Res Toxicol 2013;26:1209–17. 10.1021/tx400121n [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Youden WJ. Index for rating diagnostic tests. Cancer 1950;3:32–5. [DOI] [PubMed] [Google Scholar]
- 18.Peacock JL, Cook DG, Carey IM, et al. Maternal cotinine level during pregnancy and birthweight for gestational age. Int J Epidemiol 1998;27:647–56. 10.1093/ije/27.4.647 [DOI] [PubMed] [Google Scholar]
- 19.Larsen S, Haavaldsen C, Bjelland EK, et al. Placental weight and birthweight: the relations with number of daily cigarettes and smoking cessation in pregnancy. A population study. Int J Epidemiol 2018;47:1141–50. 10.1093/ije/dyy110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Drake P, Driscoll AK, Mathews TJ. Cigarette smoking during pregnancy: United States, 2016. NCHS Data Brief 2018;305:1–8. [PubMed] [Google Scholar]
- 21.Robison RG, Kumar R, Arguelles LM, et al. Maternal smoking during pregnancy, prematurity and recurrent wheezing in early childhood. Pediatr Pulmonol 2012;47:666–73. 10.1002/ppul.22501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lupsa I-R, Nunes B, Ligocka D, et al. Urinary cotinine levels and environmental tobacco smoke in mothers and children of Romania, Portugal and Poland within the European human biomonitoring pilot study. Environ Res 2015;141:106–17. 10.1016/j.envres.2015.03.018 [DOI] [PubMed] [Google Scholar]
- 23.Dempsey D, Jacob P, Benowitz NL. Accelerated metabolism of nicotine and cotinine in pregnant smokers. J Pharmacol Exp Ther 2002;301:594–8. 10.1124/jpet.301.2.594 [DOI] [PubMed] [Google Scholar]
- 24.The health consequences of involuntary exposure to tobacco smoke: a report of the surgeon General. Atlanta (GA), United States 2006. [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2021-054535supp001.pdf (1.4MB, pdf)
Data Availability Statement
Data may be obtained from a third party and are not publicly available. These data form part of the NIH HHEAR programme. The data will be uploaded to their portal at a future date. https://www.niehs.nih.gov/research/supported/exposure/hhear/index.cfm.