Abstract
Objective:
To provide a semi-quantitative assessment of the effect of different analgesics (celecoxib, ketorolac, and paracetamol) on tooth movement and bone resorption using immunohistochemical staining of matrix metalloproteinase-13 (MMP-13).
Materials and Methods:
Forty white male rats (12-weeks old; body weight: 230–250 g) were divided into four groups (10 rats each) and were given the treatment once a day for 2 consecutive months. Group A (control group) rats were given the reverse osmosis water; group B rats were given 10 mg/kg celecoxib; group C rats were given 3 mg/kg ketorolac; and group D rats were given 150 mg/kg paracetamol. A precalibrated closed Sentalloy coil spring was placed inside each rat mouth to deliver a constant force of 50 cN. The magnitude of tooth movement was measured intraorally. After 2 months, the rats were sacrificed, and the sections were mounted on L-polylysine–coated glass slides. Slides from each specimen were stained with hematoxylin and eosin, and others were stained with MMP-13. Data were analyzed with the one-way analysis of variance (ANOVA).
Results:
Celecoxib, ketorolac, and paracetamol groups showed tooth movement of 1.81 ± 0.43 mm, 1.13 ± 0.28 mm, and 1.08 ± 0.27 mm, respectively. The mean number of MMP-13–positive osteoclasts was highest in celecoxib-treated group followed by the control group and was decreased in the ketorolac and paracetamol groups. Comparing all groups to the control revealed significant differences (P < .05).
Conclusion:
Administration of celecoxib did not reduce bone resorption or interfere with tooth movement in rats compared to other analgesics tested (ketorolac and paracetamol).
Keywords: NSAID, Tooth movement, MMP-13, Bone resorption
INTRODUCTION
Orthodontic movements occur by the application of continued and controlled mechanical forces on the tooth, resulting in areas of pressure and tension in the periodontal ligament. The sort of bone remodelling that promotes tooth movement is the result of cellular stress and occasionally inflammation involving clast cells, namely osteoblasts, osteocytes, cementoblasts, and fibroblasts. Mediators such as various cytokines, growth factors, and arachidonic acid products (prostaglandins and leukotrienes) participate in the inflammation process during bone remodelling.1,2
Prostaglandins (PGs), lipid mediators derived from arachidonic acid (AA), play central roles in the pathogenesis of inflammation, fever, and pain. PGs are generated by the oxygenation of AA to the unstable intermediate prostaglandin H2 (PGH2) by prostaglandin H synthetase (PGHS), of which there are two major isoforms: the constitutive PGHS-1 and the (generally) inducible PGHS-2. These enzymes are also commonly referred to as cyclo-oxygenase (COX)-1 and COX-2, respectively, in reference to the specific enzymatic active site that catalyzes AA oxygenation and provides the target for the majority of pharmacologic inhibitors of these enzymes.3
Pain and discomfort are frequent undesirable side effects of orthodontic treatment. It begins a few hours after the application of an orthodontic force and lasts for approximately 5 days.4 Nonsteroidal anti-inflammatory drugs (NSAIDs) are the most common medications taken worldwide for the treatment of pain, inflammation, and fever.5 Although chemically disparate, they produce their therapeutic effects by the common ability to inhibit the activity of COX enzymes.6 The use of preoperative analgesics provides blockage of afferent nerve impulses before they reach the central nervous system. Celecoxib and ketorolac are NSAIDs. They inhibit the synthesis of prostaglandin as ketorolac inhibits the enzyme COX, and celecoxib inhibits selective COX-2.7
Paracetamol is part of a class of drugs known as “aniline analgesics.”4 It is classified as an NSAID by some sources8,9; however, others do not consider it an NSAID.9,10 The main mechanism proposed is considered to be the inhibition of COX, and recent findings suggest that it is highly selective for COX-2.10 It was thought that it blocked a third isoform, COX-3, which is expressed only in the brain and the spinal cord. As a consequence, paracetamol has minimal effects on prostaglandin synthesis.11
Matrix metalloproteinases (MMPs) play a key role in the remodelling of the extracellular matrix. Experimental orthodontic tooth movement in animals, mostly rats, shows an increased expression of MMP-1, -2, -8, -9, and -13 and tissue inhibitor matrix metalloproteinase (TIMP)-1 and -3 in the periodontal ligament (PDL) and alveolar bone.12–14 MMP-2, MMP-8, and sometimes MMP-1 were reported to increase in the gingival crevicular fluid collected from orthodontic patients.15 Also, mRNA of MMP-13 levels has been detected both on the tension and compression sides in rodents, and MMP-13 gene expression seems to be time-dependent and differentially regulated.13
Reporting on the effect of drugs administered during induced tooth movement is still lacking standardized methodology in many aspects. Results reported are controversial and inconsistent leading to misinterpretation. The purpose of the present study is to provide a semi-quantitative assessment of the effect of different analgesics (celecoxib, ketorolac, and paracetamol) on tooth movement and bone resorption using immunohistochemical staining of MMP-13.
MATERIALS AND METHODS
Forty white male rats (12 weeks old; body weight: 230–250 g) were used in this study. The study was conducted under approval from the Animal Welfare Committee of Mansoura University. All animals were housed individually in plastic cages in a colony room inside the Medical Experimental Research Centre and fed standard pellet diet and water ad libitum. Pharmaceutical dosage forms containing the drugs were purchased from a local pharmacy and prepared by direct dissolution in reverse osmosis water. The rats were divided into four groups (10 rats each); in the three experimental groups, analgesics were given once a day for 2 consecutive months via a gastric tube:
Group A (control group) rats were given the reverse osmosis water;
Group B rats were given 10 mg/kg celecoxib;
Group C rats were given 3 mg/kg ketorolac; and
Group D rats were given 150 mg/kg paracetamol.
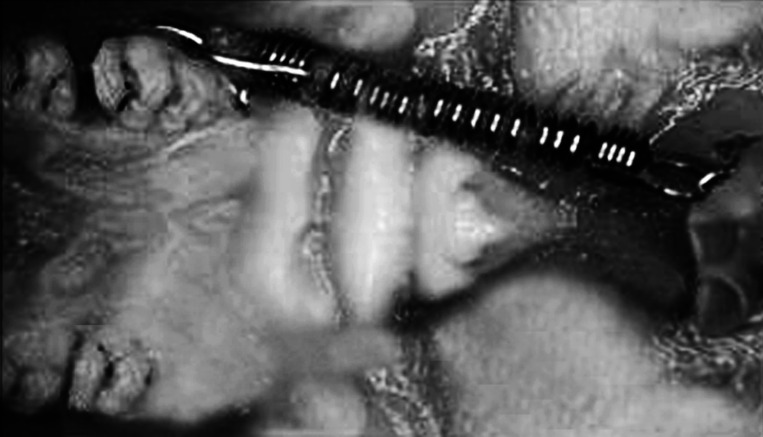
A precalibrated closed Sentalloy (GAC International, Bohemia, NY) coil spring was placed inside each rat mouth to deliver a constant force of 50 cN. The springs were tied to the upper left first molar by a steel ligature around the collum and fixed to the two upper incisors with a double-figure-eight-shaped ligature. An undercut on the maxillary incisors was created by a diamond bur to intensively ensure better stability of the ligature (Figure 1). No-mix orthodontic adhesive, Relay-a-Bond (Reliance Orthodontic Inc, Itasca, Ill) was applied on the end of the ligature to prevent irritation. The magnitude of tooth movement was determined by measuring the relative separation between the first and second maxillary molar using vernier calipers with sharpened tips inserted into occlusal pits. The distance between the mesial occlusal pits on the first and second molars was measured intraorally before appliance insertion and immediately after sacrifice. Measurements were performed by the same operator and were repeated five times for each rat.16 The positioning of the appliance was performed under general anesthesia produced by an intramuscular injection of a mixture of 30 mg/kg body weight of ketamine (Ketavet, Farmaceutici Gellini, Aprilia Lt, Italy), 0.75 mg/kg of acepromazine (Prequillan, Fatro, Bologna, Italy), and 4 mg/kg of xylazine (Rompun, Bayer, Leverkusen, Germany).17
Figure 1.
Intraoral picture of the appliance.
At the end of the experiments, the rats were sacrificed by an overdose of CO2 and decapitated; the heads were dried. Each upper jaw was placed in 10% neutral formalin for 24 hours and then rinsed with water because of high calcium content; the tissue specimens were decalcified in 10% ethylene diaminetetra-acetic acid, pH 7.2, for 6–8 weeks. The decalcified tissue specimens were dehydrated through ascending concentrations of ethanol. Subsequently, the specimens were processed for embedding in paraffin wax following routine protocol. The sections were mounted on L-polylysine–coated glass slides. Slides from each specimen were stained with hematoxylin and eosin, and others stained with MMP-13. Tooth-tissue sections were dewaxed and rehydrated in graded ethanol series to double-distilled water. Slides were subjected to antigen retrieval by microwave heating in 10 mM citrate buffer pH 6 at 90°C for 20 minutes. To inhibit endogenous peroxidase activity, they were incubated with 3% H2O2 in methanol for 15 minutes, and then rinsed for 20 minutes with phosphate-buffered saline (PBS), pH 7.4. Slides were incubated with mouse monoclonal anti-MMP-13 (Ab-1, clone VIIIA2, Neomarkers, Lab Vision, Fremont, Calif) at a 1∶20 working dilution in PBS, overnight at 4°C. Subsequent procedures were performed at room temperature. We used a highly sensitive detection kit, Envision (DakoCytomation code K4061, Carpinteria, Calif), with diaminobenzidine as a chromogen. Thorough washes were performed using PBS; then sections were counterstained with hematoxylin, mounted in aqueous mounting medium, and examined by light microscopy (Zeiss, Munich, Germany). Positive controls of breast carcinoma tissue sections were used. Negative controls were treated as described above except for the incubation with the primary antibody, PBS was used alone instead.15
Five random images from each cut section (stained with MMP-13) were captured using 20× and 40× magnification with a digital camera mounted on a light microscopy and monitored on a 17-inch computer screen using an imaging software program (Soft Imaging System, Munster, Germany). Counting of osteoclasts on the alveolar bone surface at the pressure side was performed at 20× magnification by three independent observers. Osteoclasts were identified as MMP-13–positive cells with more than three nuclei lying on the bone surface. The mean number of cells from the five captured images from each specimen was calculated and intraoperator error determined following repeated blind measurements.18,19 Data were analyzed with the statistical software SPSS, version 11.0J (SPSS Japan, Tokyo, Japan), with the one-way analysis of variance (ANOVA) and post hoc test. All values are expressed as mean ± standard deviation.
RESULTS
Orthodontic Tooth Movement
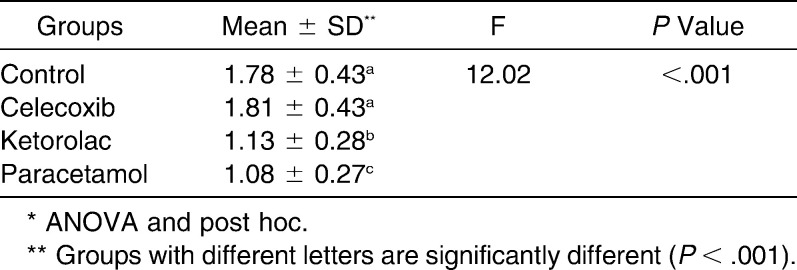
Mesial tooth displacement in the control animals was 1.78 ± 0.43 mm. The rats treated with celecoxib, ketorolac, and paracetamol showed tooth movement of 1.81 ± 0.43 mm, 1.13 ± 0.28 mm, and 1.08 ± 0.27 mm, respectively. The differences were statistically significant (P < .001) (Table 1).
Table 1.
The Mean ± Standard Deviation of Orthodontic Tooth Movement Among the Studied Groups Compared to Control*
Histologic Examination by Hematoxylin and Eosin Stain
Tooth movement was associated with areas of compression and tension. In the experimental sections, the periodontal ligament fibers were disoriented and osteoclasts appeared in the compression side. The celecoxib group showed a higher number of osteoclasts when compared to other groups, followed by the control group, and the ketorolac, and paracetamol groups (Figure 2A through D).
Figure 2.
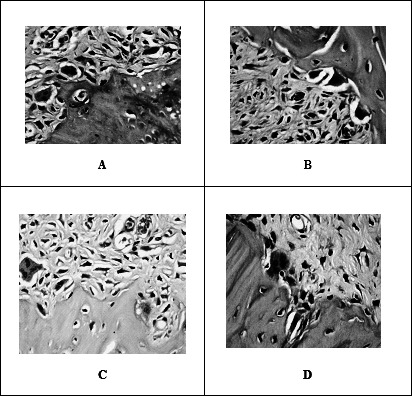
Photomicrographs of the alveolar bone at compression side show: the numbers of osteoclasts in their Howship's lacunae are more in control (A) and celecoxib (B) groups compared to ketorolac (C) and paracetamol (D) groups (hematoxylin and eosin 400×).
Immuonohistochemical Examination
On the compression side, the extracellular matrix of the PDL, osteoclasts and PDL cells showed positive immunoreactions of MMP-13. Some osteocytes scattered in the alveolar bone adjacent to resorbing surfaces were also immunoreactive to MMP-13 antibody. The mean number of MMP-13 positive osteoclasts was highest in the celecoxib group followed by the control group and was decreased in the ketorolac and paracetamol groups. There was mild positive immunoreaction for MMP-13 in the celecoxib group, a strong reaction in the control group, and a weak reaction in the ketorolac and paracetamol groups (Figure 3a through d) (Figure 4).
Figure 3.
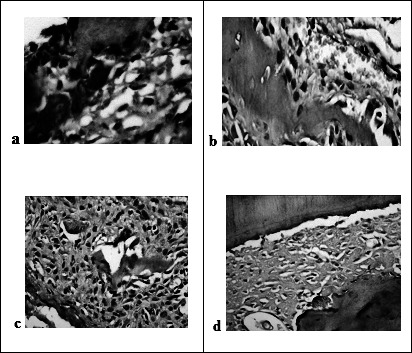
Photomicrographs of the alveolar bone at compression side show: strong positive immunoreactions for MMP-13 in the control group (a) (diaminobenzidine 400×), mild reaction in the celecoxib (b) group, while weak reaction in both the ketorolac (c) and paracetamol (d) groups (diaminobenzidine 200×).
Figure 4.
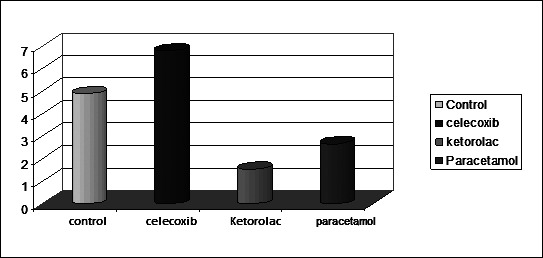
Means of osteoclast cell number in all groups.
DISCUSSION
Orthodontic tooth movement requires extensive remodelling of the periodontium. Remodelling is thought to be initiated in the PDL.20 MMPs play a key role in remodelling of the extracellular matrix.21 Most previous studies have examined the expression of different types of MMPs at the mRNA level. But, Leonardi et al.14 reported the immunohistochemical localization of MMP-13 within the PDL during experimental tooth movement.
As orthodontic tooth movement is considered to involve an inflammation process, many studies regarding the effect of anti-inflammatory drugs on tooth movement and bone resorption have been reported. However, controversy still exists.22 In this study, we used immunohistochemical staining of MMP-13 to provide a semi-quantitative assessment of the effect of celecoxib, ketorolac, and paracetamol on bone resorption.
We found that an indirect proof of the inhibition of PGs was the reduced mesial movement of teeth in rats treated with ketorolac and paracetamol compared with teeth in control rats. On the other hand, when no significant difference was observed between the control group and the celecoxib group, the theory that celecoxib does not inhibit peripheral secretion of PGs is supported.23,24
Moreover, paracetamol and ketorolac showed a smaller number of osteoclasts and less intense expression of MMP-13. This may be explained by inhibiting the secretion of prostaglandins, thereby reducing orthodontic tooth movement. On the contrary, celecoxib did not seem to affect the number of osteoclasts; however, the intensity of MMP-13 expression was milder compared to the control group. It was hypothesized that cyclooxygenase inhibitors with differences in COX-1/COX-2 selectivity ratio could affect, in a different manner, the movement of teeth during application of force. This is compatible with the idea that factors depending on synthesis via COX-1 are involved in the bone remodeling process during orthodontic tooth movement.24,25
In concordance with our results, Jerome et al.23 and De Carlos et al.24 found that celecoxib did not interfere with tooth movement. Also, Sandy and Harris25 found that the NSAID inhibited the appearance of osteoclasts, but had no significant effect on tooth movement. Our results are also in agreement with Leonardi et al.14 who showed an increased expression of MMP-1, -2, -8, -9, and -13 in the PDL and alveolar bone during experimental orthodontic tooth movement in rats. Also, Bildt et al.21 reported an increased expression of MMPs at the resorption side as well as the apposition side. In this study, the intensity of MMP-13 expression was milder in the three groups compared to the control group. This may be due to inhibition of cyclo-oxygenase enzyme. Larkins et al.26 found that the expression and activation of MMPs may be directly proportional to the overexpression of COX-2 in breast cancer cells. Also, they confirmed that the biosynthesis of prostaglandin E2 (PGE2) requires three sequential enzymatic reactions: phospholipase A2, COX-1 or COX-2, and PGE2 synthesis.
So, our results confirmed that administration of celecoxib to rats did not result in the reduction of the extent of root resorption. However, other studies on rats should be interpreted with caution as no human trials on immunohistochemical localization of MMP-13 have been reported so far. Moreover, despite research findings, there is no standard of care for analgesic use in the pain management of orthodontic patients.27–29 Apparently, the prescription of analgesics after activation of the orthodontic appliance poses a paradox: analgesics suppress the patient's pain and discomfort, but on the other hand, they reduce the effectiveness of cellular stress and inflammation during bone resorption and induced tooth movement.30 Bone resorption induced by tooth movement is not mediated solely by prostaglandins but by a pool of mediators such as leukotrienes, cyclic adenosine monophosphate, collagenase, and many others that are generated by forces applied to periodontal tissues.31,32
When prescribing an analgesic to relieve the patient's pain after activation of orthodontic appliances, the orthodontist should take into account the patient's history of drugs side effects and the possibility of hypersensitivity. Medications might have an important influence on the rate of tooth movement, and information on their consumption is essential to adequately discuss treatment planning with patients. We recommend that such an inquiry should be part of every orthodontic diagnosis. Furthermore, there is a need for further well-designed studies to evaluate the effects of various medications on orthodontic tooth movement.
CONCLUSION
Administration of celecoxib did not reduce bone resorption or interfere with tooth movement in rats compared with other analgesics (ketorolac and paracetamol) tested in this study.
Acknowledgments
The authors would like to thank the Egyptian Ministry of Higher Education and Scientific Research for funding.
REFERENCES
- 1.Consolaro A. Reabsorções Dentárias Nas Especialidades Clínicas 2nd ed. Maringá, Brazil: Dental Press; 2005. pp. 353–400. [Google Scholar]
- 2.Consolaro A. Orthodontic treatment does not cause pulpal necrosis. Dental Press Endod. 2011;1:14–20. [Google Scholar]
- 3.Lee W. C. Experimental study of the effect of prostaglandin administration on tooth movement—with particular emphasis on the relationship to the method of PGE1 administration. Am J Orthod Dentofacial Orthop. 1990;98:231–241. doi: 10.1016/s0889-5406(05)81600-2. [DOI] [PubMed] [Google Scholar]
- 4.Aronoff D. M, Oates J. A, Boutaud O. New insights into the mechanism of action of acetaminophen: its clinical pharmacologic characteristics reflect its inhibition of the two prostaglandin H2 synthases. Clin Pharmacol Ther. 2006;79:9–19. doi: 10.1016/j.clpt.2005.09.009. [DOI] [PubMed] [Google Scholar]
- 5.Fernandes L. M, Ogaard B, Skoglund L. Pain and discomfort experienced after placement of a conventional or a superelastic NiTi aligning archwire. A randomized clinical trial. J Orofac Orthop. 1998;59:331–339. doi: 10.1007/BF01299769. [DOI] [PubMed] [Google Scholar]
- 6.Mitchell J. A, Warner T. D. COX isoforms in the cardiovascular system: understanding the activities of non-steroidal anti-inflammatory drugs. Nat Rev Drug Discov. 2006;5:75–86. doi: 10.1038/nrd1929. [DOI] [PubMed] [Google Scholar]
- 7.Shibazaki T, Yozgatian J. H, Zeredo J. L, Gonzales C, Hotokezaka H, Koga Y, Yoshida N. Effect of celecoxib on emotional stress and pain-related behaviors evoked by experimental tooth movement in the rat. Angle Orthod. 2009;79:1169–1174. doi: 10.2319/121108-629R.1. [DOI] [PubMed] [Google Scholar]
- 8.Wagner W, Khanna P, Furst D. E. Nonsteroidal anti-inflammatory drugs, disease-modifying antirheumatic drugs, nonopioid analgesics and drugs used in gout. In: Katzung B. G, editor. Basic and Clinical Pharmacology 9th ed. Vol. 586 New York, NY: McGraw-Hill; 2004. [Google Scholar]
- 9.Bertolini A, Ferrari A, Ottani A, Guerzoni S, Tacchi R, Leone S. Paracetamol: new vistas of an old drug. CNS Drug Rev. 2006;12:250–275. doi: 10.1111/j.1527-3458.2006.00250.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hinz B, Cheremina O, Brune K. Acetaminophen (paracetamol) is a selective cyclooxygenase-2 inhibitor in man. FASEB J. 2008;22:383–390. doi: 10.1096/fj.07-8506com. [DOI] [PubMed] [Google Scholar]
- 11.Roche J. J, Cisneros G. J, Acs G. The effect of acetaminophen on tooth movement in rabbits. Angle Orthod. 1997;67:231–236. doi: 10.1043/0003-3219(1997)067<0231:TEOAOT>2.3.CO;2. [DOI] [PubMed] [Google Scholar]
- 12.Domon S, Shimokawa H, Matsumoto Y, Yamaguchi S, Soma K. In situ hybridization for matrix metalloproteinase-1 and cathepsin K in rat root-resorbing tissue induced by tooth movement. Arch Oral Biol. 1999;44:907–915. doi: 10.1016/s0003-9969(99)00091-6. [DOI] [PubMed] [Google Scholar]
- 13.Takahashi I, Nishimura M, Onodera K, Bae J. W, Mitani H, Okazaki M, Sasano Y, Mitani H. Expression of MMP-8 and MMP-13 genes in the periodontal ligament during tooth movement in rats. J Dent Res. 2003;82:646–651. doi: 10.1177/154405910308200815. [DOI] [PubMed] [Google Scholar]
- 14.Leonardi R, Talic N. F, Loreto C. MMP-13 (collagenase 3) immunolocalisation during initial orthodontic tooth movement in rats. Acta Histochem. 2007;109:215–220. doi: 10.1016/j.acthis.2007.01.002. [DOI] [PubMed] [Google Scholar]
- 15.Cantarella G, Cantarella R, Caltabiano M, Risuglia N, Bernardini R, Leonardi R. Levels of matrix metalloproteinases 1 and 2 in human gingival crevicular fluid during initial tooth movement. Am J Orthod Dentofacial Orthop. 2006;130:568.e11–16. doi: 10.1016/j.ajodo.2006.04.020. [DOI] [PubMed] [Google Scholar]
- 16.Ong C. K, Joseph B. K, Waters M. J, Symons A. L. Growth hormone receptor and IGF-I receptor immunoreactivity during orthodontic tooth movement in the prednisolone-treated rat. Angle Orthod. 2001;71:486–493. doi: 10.1043/0003-3219(2001)071<0486:GHRAII>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 17.Gonzales C, Hotokezaka H, Yoshimatsu M, Yozgatian J. H, Darendeliler M. A, Yoshida N. Force magnitude and duration effects on amount of tooth movement and root resorption in the rat molar. Angle Orthod. 2008;78:502–509. doi: 10.2319/052007-240.1. [DOI] [PubMed] [Google Scholar]
- 18.Chang H. H, Wu C. B, Chen Y. J, et al. MMP-3 response to compressive forces in vitro and in vivo. J Dent Res. 2008;87:692–696. doi: 10.1177/154405910808700714. [DOI] [PubMed] [Google Scholar]
- 19.Andrade I, Jr, Taddei S. R, Garlet G. P, Garlet T. P, Teixeira A. L, Silva T. A, Teixeira M. M. CCR5 down-regulates osteoclast function in orthodontic tooth movement. J Dent Res. 2009;88:1037–1041. doi: 10.1177/0022034509346230. [DOI] [PubMed] [Google Scholar]
- 20.Kawarizadeh A, Bourauel C, Götz W, Jäger A. Early responses of periodontal ligament cells to mechanical stimulus in vivo. J Dent Res. 2005;84:902–906. doi: 10.1177/154405910508401006. [DOI] [PubMed] [Google Scholar]
- 21.Bildt M. M, Bloemen M, Kuijpers-Jagtman A. M, Von den Hoff J. W. Matrix metalloproteinases and tissue inhibitors of metalloproteinases in gingival crevicular fluid during orthodontic tooth movement. Eur J Orthod. 2009;31:529–535. doi: 10.1093/ejo/cjn127. [DOI] [PubMed] [Google Scholar]
- 22.Gonzales C, Hotokezaka H, Matsuo K, Shibazaki T, Yozgatian J. H, Darendeliler M. A. Effects of steroidal and nonsteroidal drugs on tooth movement and root resorption in the rat molar. Angle Orthod. 2009;79:715–726. doi: 10.2319/072108-381.1. [DOI] [PubMed] [Google Scholar]
- 23.Jerome J, Brunson T, Takeoka G, Foster C, Moon H. B, Graged E, Zeichner-David M. Celebrex offers a small protection from root resorption associated with orthodontic movement. J Calif Dent Assoc. 2005;33:951–959. [PubMed] [Google Scholar]
- 24.De Carlos F, Cobo J, Perillan C, Garcia M. A, Arguelles J, Vijande M, Costales M. Orthodontic tooth movement after different coxib therapies. Eur J Orthod. 2007;29:596–599. doi: 10.1093/ejo/cjm072. [DOI] [PubMed] [Google Scholar]
- 25.Sandy J. R, Harris M. Prostaglandins and tooth movement. Eur J Orthod. 1984;6:175–182. doi: 10.1093/ejo/6.3.175. [DOI] [PubMed] [Google Scholar]
- 26.Larkins T. L, Nowell M, Singh S, Sanford G. L. Inhibition of cyclooxygenase-2 decreases breast cancer cell motility, invasion and matrix metalloproteinase expression. BMC Cancer. 2006;6:181–187. doi: 10.1186/1471-2407-6-181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stabile A. C, Stuani M. B, Leite-Panissi C. R, Rocha M. J. Effects of short term acetaminophen and celecoxib treatment on orthodontic tooth movement and neuronal activation in rat. Brain Res Bull. 2009;79:396–401. doi: 10.1016/j.brainresbull.2009.05.014. [DOI] [PubMed] [Google Scholar]
- 28.Bartzela T, Türp J. C, Motschall E, Maltha J. C. Medication effects on the rate of orthodontic tooth movement: a systematic literature review. Am J Orthod Dentofacial Orthop. 2009;135:16–26. doi: 10.1016/j.ajodo.2008.08.016. [DOI] [PubMed] [Google Scholar]
- 29.Consolaro A, Maldonado V, Júnior M, Consolaro M. Sources of controversies over analgesics prescribed after activation of orthodontic appliances acetylsalicylic acid or acetaminophen. Dental Press J Orthod. 2010;15:16–24. [Google Scholar]
- 30.Walker J. B, Buring S. M. NSAID impairment of orthodontic tooth movement. Ann Pharmacother. 2001;35:113–115. doi: 10.1345/aph.10185. [DOI] [PubMed] [Google Scholar]
- 31.Yamasaki K. The role of cyclic AMP, calcium, and prostaglandins in the induction of osteoclastic bone resorption associated with experimental tooth movement. J Dent Res. 1983;62:877–881. doi: 10.1177/00220345830620080501. [DOI] [PubMed] [Google Scholar]
- 32.King G. J, Collier J. A bone resorptive agent extracted from orthodontically-treated tissues of the rat. Angle Orthod. 1986;56:299–308. doi: 10.1043/0003-3219(1986)056<0299:ABRAEF>2.0.CO;2. [DOI] [PubMed] [Google Scholar]