Abstract
Objective:
To investigate stress distribution in the roots of maxillary central incisors bearing various types of root morphologies with regard to application of different types of orthodontic forces using the finite element model (FEM).
Materials and Methods:
FEMs of maxillary central incisors with different root morphologies (normal, short, blunt, dilacerated, and pipette) were constructed, and orthodontic forces in various directions (intrusion, extrusion, tipping, and rotational) were applied to the tooth axis at the bracket level.
Result:
On application of various forces, significantly increased stress was seen at the apex of the root with dilacerated morphology and at the cervical one-third region of the tooth with the short root. Increased stress was observed at the middle one-third region in the tooth with the pipette-shaped root during intrusion and extrusion.
Conclusions:
In the present study, the stress distribution pattern indicates that the maxillary central incisors with deviated root morphology are at higher risk of root resorption.
Keywords: Orthodontic force, Root resorption, Finite element model
INTRODUCTION
Root resorption is a biological response to an orthodontic force. Massler and Malone1 stated that root resorption occurs in 100% of orthodontic patients. It is suggested that the RANKL to OPG ratio in periodontal ligament (PDL) cells also contributes to root resorption during orthodontic tooth movement.
In cases of severe external apical root resorption, the compressed PDL cells may produce a large amount of RANKL and up-regulate osteoclastogenesis. This explains the greater increase of RANKL and decrease of OPG in cases of severe root resorption.2
In a comparative study using radiographs taken before and after treatment, Sameshima and Sinclair3 reported that teeth with abnormal root morphologies frequently show external root resorption, compared to normal root morphology. Mirabella and Årtun4 found that with an abnormal root shape, if orthodontic force is concentrated at a particular region of the deviated root shape root resorption may occur. Lee et al.5 stated that it is possible that patients with dental anomalies are at increased risk for apical resorption during orthodontic treatment. The mechanism may be that cementum and dentin are affected during root formation in such patients, thus reducing the ability of the cementum and dentin to resist resorption in situations involving excess of pressure. According to the deviated root shape, if an orthodontic force is concentrated at a particular region of the root, root resorption may occur.
One analytical approach to studying stress during tooth movement, one that allows for reasonable approximation of the biological tissues, is the finite element model (FEM). The FEM is a noninvasive, accurate method that permits detailed analyses of tooth movement. In addition, it provides one with quantitative data that increase understanding of the physiologic reactions that occur after force application and that may yield an improved understanding of the reactions and interactions of individual tissues. Such detailed information and accurate analysis of the stresses and strain in the tissues are difficult to obtain using any other experimental technique because of the interaction of the surrounding investing tissues, which may then distort the data obtained for any individual material response.6
However, no biomechanical study has been done that clarifies the influence of different orthodontic forces (intrusion, extrusion, tipping, and rotational) on various root morphologies (normal, short, blunt, dilacerated, and pipette) during orthodontic force application. Thus, the present study aims to study the effects of different types of orthodontic forces on various types of root morphology using the FEM model.
MATERIALS AND METHODS
Preparation of FEM
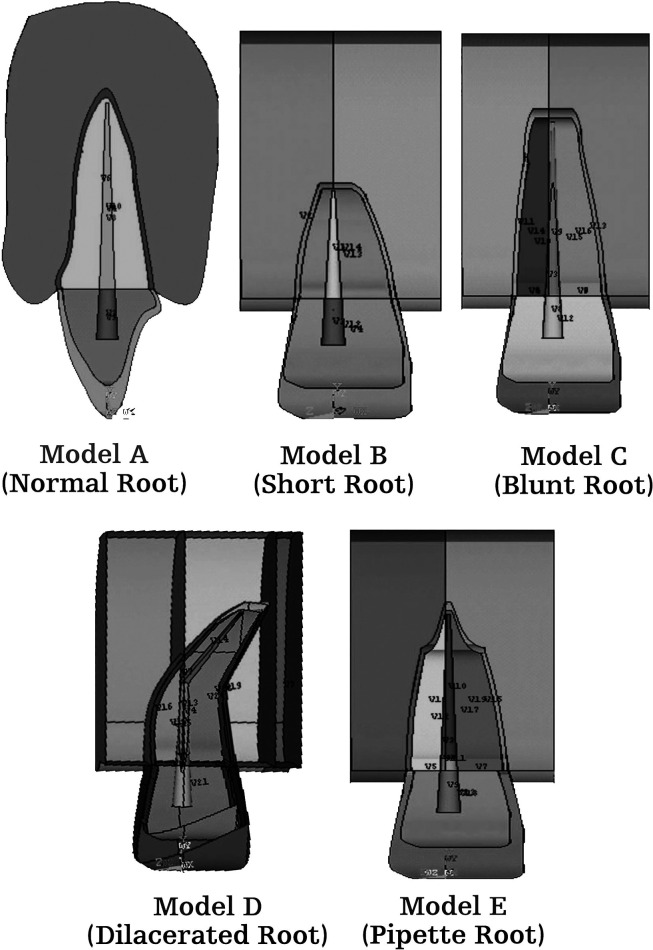
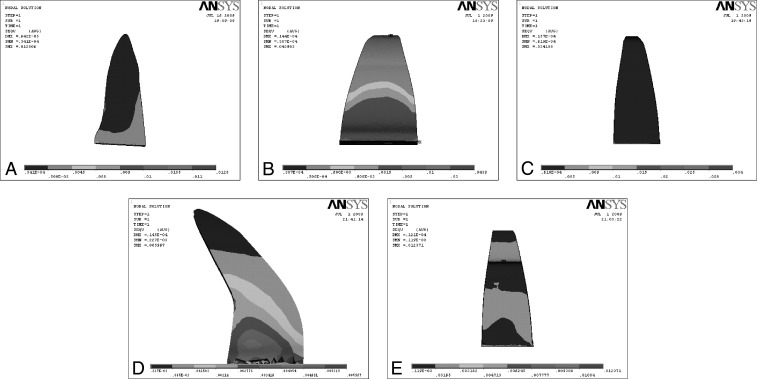
Three-dimensional FEMs of permanent maxillary central incisor, periodontal ligament, and alveolar bone (cancellous bone) with normal root morphology, and their four variations, as classified by Levander and Malmgren,7 were prepared using Ansys version 10 software (Figure 1A–E).
Figure 1.
Various root morphologies. (A) Normal root morphology. (B) Short root morphology. (C) Blunt root morphology. (D) Dilacerated root morphology. (E) Pipette root morphology.
Model A was constructed with a normal root shape with a crown length of 10.5 mm and a root length of 13.0 mm based on the data derived from Wheeler's Dental Anatomy, Physiology and Occlusion.8 Four other models (B, C, D, and E) with various root shapes were constructed. Model B had a short root length (8 mm), model C a blunt root, and model D a distally bent root, while in model E the root was pipette shaped, with constriction at the middle one-third root. The periodontal membrane that surrounded the root surface was 200 µm thick, and the alveolar bone was represented by cancellous bone of 2.0-mm thickness.
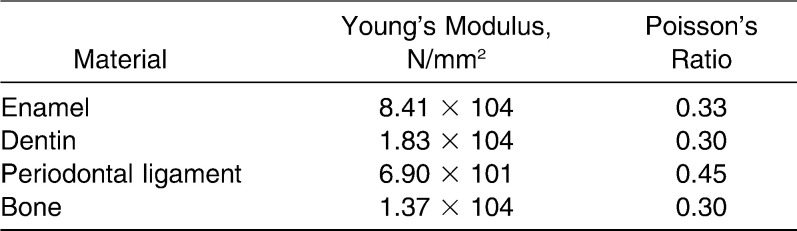
Material Properties
Each structure of the central incisor (ie, the enamel, dentine, and pulp; periodontal ligament; and cancellous bone) was meshed using an auto-meshing routine in the finite element analysis program. The material properties for all models were defined as linear and are shown in Table 1.
Table 1.
Material Parameters Used in the Finite Element Model
Boundary Conditions and Solution
The element shape described in the model was a solid 10-noded tetrahedral. The FEM approximately consisted of 125,891 elements and 21,732 nodes. Nodes at the mesiodistal and the bottom surface of the alveolar bone were restricted within 6 degrees of freedom.9
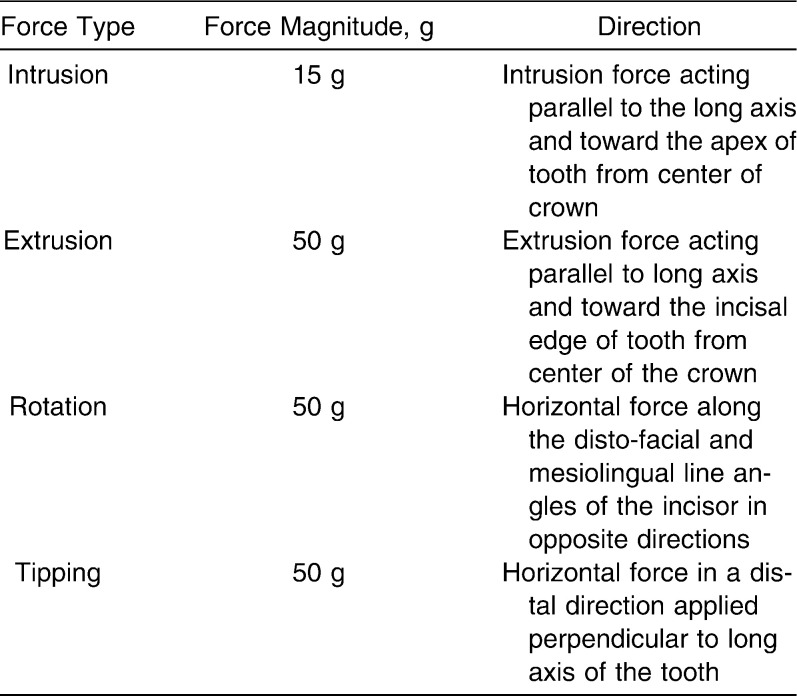
An area of 3.3 × 4.8 mm was fixed as the loaded portion, assuming a size reflected by the size of the bracket (Figure 2). Experimental orthodontic forces in various directions to the tooth axis were applied to the bracket base area, as stated in Table 2. Stresses and strains for each model on application of each force were calculated with Ansys 10 software using linear structural analysis.
Figure 2.
Area of force application.
Table 2.
Force System Applied
RESULTS
The stress distribution (compressive and tensile) on the central incisor under various conditions is shown in Figures 3 through 6. All models had a tendency to concentrate stress at the cervical area when intrusive, extrusive, tipping, and rotational forces were applied (Table 3). No significant stress concentration was observed in the root of model A (normal root shape) when various forces were applied (Table 3).
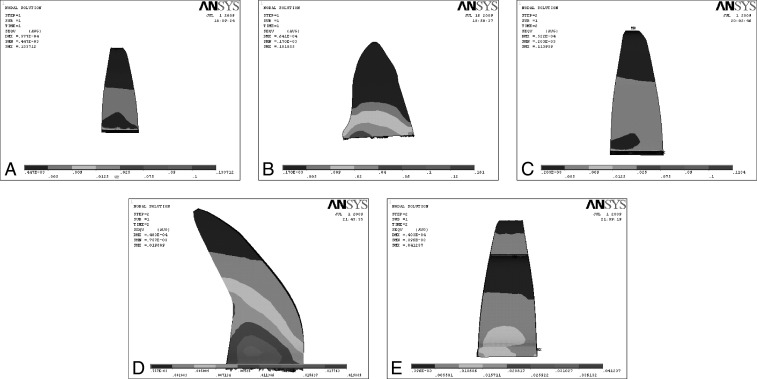
Figure 3.
Effect on application of intrusion force on various root morphologies. (A) Intrusion force on normal root. (B) Intrusion force on short root. (C) Intrusion force on blunt root. (D) Intrusion force on dilacerated root. (E) Intrusion force on pipette root.
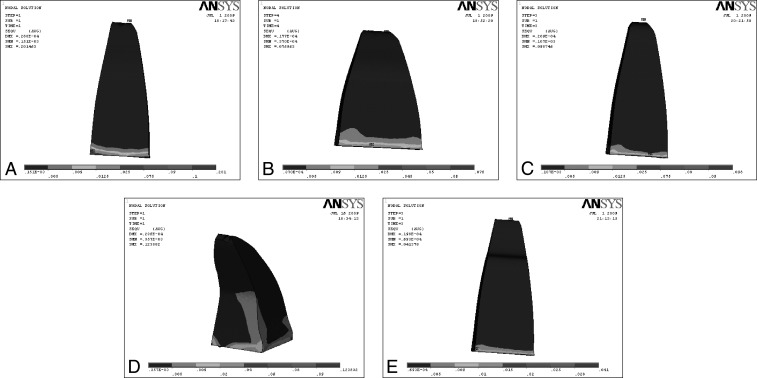
Figure 4.
Effect on application of extrusive force on various root morphologies. (A) Extrusion force on normal root. (B) Extrusion force on short root. (C) Extrusion force on blunt root. (D) Extrusion force on dilacerated root. (E) Extrusion force on pipette root.
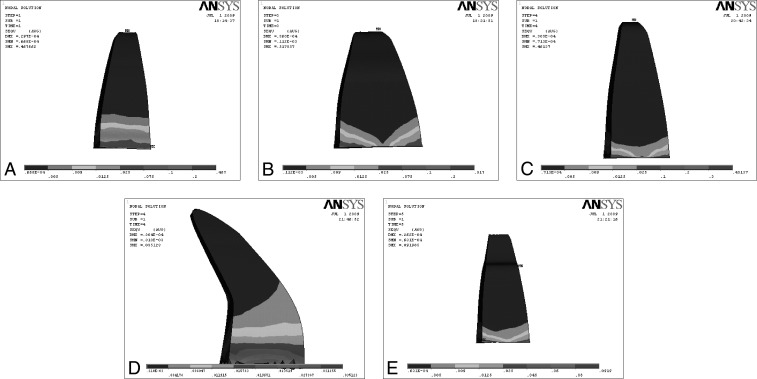
Figure 5.
Effect on application of tipping force on various root morphologies. (A) Tipping force on normal root. (B) Tipping force on short root. (C) Tipping force on blunt root. (D) Tipping force on dilacerated root. (E) Tipping force on pipette root.
Figure 6.
Effect on application of rotational force on various root morphologies. (A) Rotational force on normal root. (B) Rotational force on short root. (C) Rotational force on blunt root. (D) Rotational force on dilacerated root. (E) Rotational force on pipette root.
Table 3.
Statistical Analysisa
In model B (short root), when the experimental orthodontic forces were applied, an area of high-level stress was observed at the cervical of the root (intrusion, 0.01 N/mm2; extrusion, 0.04 N/mm2; tipping, 0.02 N/mm2; and rotation, 0.1 N/mm2).
Model C (blunt root apex) showed no significant stress concentration in the plot patterns (Table 3). When intrusive and extrusive forces were applied, the area of high-level stress increased in model C compared with model A, but when tipping and rotation forces were applied, it decreased in model C compared with model A.
During orthodontic force application, model D (dilacerated root apex) showed a significant stress concentration at the middle (intrusion, 0.002 N/mm2; extrusion, 0.009 N/mm2; tipping, 0.009 N/mm2; and rotation, 0.008 N/mm2) and apical (intrusion, 0.000141 N/mm2; extrusion, 0.000622 N/mm2; tipping, 0.000309 N/mm2; and rotation, 0.000805 N/mm2).
Model E (pipette shape) showed a stress concentration at the middle of the pipette-shaped root (Table 3).
DISCUSSION
In this study, experimental orthodontic force was applied to five FEMs with various root shapes, and their stress distribution on the root was evaluated. The forces were applied to the surface, assuming a bracket base, for the intrusion, extrusion, tipping, rotation, and bodily movement that are normally used in clinical practice.
For model A, which had a normal root shape when forces were applied, no significant stress concentration was observed at the root (Table 3). In model B (short root), significant stress was concentrated at the neck of the root. This finding is related to the alteration of the crown-root ratio. A decrease in the ratio of the root to the crown is thought to enhance loading on the root, resulting in significant stress. Taithongchai et al.10 and Thongudomporn and Freer11 reported that roots with a short apex enhanced root resorption, which supports the findings of the present study.
Model C (blunt-shaped root) showed no significant stress concentration at the root. The stress level at the root apex was increased during intrusion and extrusion and decreased during tipping and rotation compared with model A. The biomechanical burden on the root apex is likely to increase during intrusion and extrusion and to decrease during rotation and tipping in blunt-shaped compared with angular-shaped roots. These findings are partly in accordance with the results of Thongudomporn and Freer11 and Levander and Malmgren.7 In their radiographic studies they reported that blunt-shaped roots frequently showed root resorption when compared with normal roots. The reason for this difference may be related to genetic or other predispositions. Newman12 reported that a congenital blunt-shaped root results from physical defects and genetic factors during the root formation stage.
In model D (dilacerated root apex), stress was concentrated at the middle and apical regions of the root during intrusive, extrusive, tipping, and rotational force application. The results concerning stress distribution on the dilacerated root shape are in agreement with the findings of Levander and Malmgren7 and Mirabella and Årtun,4 who reported that a bent shape induced root resorption.
For model E (pipette shape), regardless of the direction of force application, stress was concentrated at the middle of the root. In their radiographic studies, Thongudomporn and Freer11 and Sameshima and Sinclair3 described that teeth with a pipette-shaped root apex enhanced root resorption. The findings of the present investigation support their results.
The present study clarified stress distribution at the root apex due to various root shapes. In the short, dilacerated, blunt, and pipette-shaped roots, stresses were significantly concentrated at the root, and, therefore, close attention must be paid to deviated root shape.
Kook et al.13 reported that teeth with a deviated root shape (ie, peg shaped or with short roots) do not always suffer from root resorption. This is thought to be related to risk factors other than mechanical factors. Al-Qawasmi14 stated that genetic predisposition influenced external root resorption.
CONCLUSIONS
Deviated roots, such as short, dilacerated, blunt, or pipette-shaped roots, resulted in a greater loading of the root than did normal root shapes during orthodontic force application. These deviations may result in the patient being prone to root resorption. It is therefore important to evaluate the root shape at the beginning of orthodontic treatment.
Dilacerated root morphology was seen to be the most affected root morphology for stress concentration, followed by the pipette-shaped root.
REFERENCES
- 1.Massler M, Malone A. J. Root resorption in human permanent teeth. A roentgenographic study. Am J Orthod. 1954;40:619–633. [Google Scholar]
- 2.Yamaguchi M, Aihara N, Kojima T, Kasai K. RANKL increase in compressed periodontal ligament cells from root resorption. J Dent Res. 2006;85:751–756. doi: 10.1177/154405910608500812. [DOI] [PubMed] [Google Scholar]
- 3.Sameshima G, Sinclair P. Predicting and preventing root resorption: part I. Diagnostic factors. Am J Orthod Dentofacial Orthop. 2001;119:505–510. doi: 10.1067/mod.2001.113409. [DOI] [PubMed] [Google Scholar]
- 4.Mirabella A. D, Årtun J. Risk factors for apical root resorption of maxillary anterior teeth in adult orthodontic patients. Am J Orthod Dentofacial Orthop. 1995;108:48–55. doi: 10.1016/s0889-5406(95)70065-x. [DOI] [PubMed] [Google Scholar]
- 5.Lee R. Y, Årtun J, Alonzo T. A. Are dental anomalies risk factors for apical root resorption in orthodontic patients. Am J Orthod Dentofacial Orthop. 1999;116:187–195. doi: 10.1016/s0889-5406(99)70217-9. [DOI] [PubMed] [Google Scholar]
- 6.Middleton J, Jones M, Wilson A. The role of the periodontal ligament in bone modeling: the initial development of a time-dependent finite element model. Am J Orthod Dentofacial Orthop. 1996;109:155–162. doi: 10.1016/s0889-5406(96)70176-2. [DOI] [PubMed] [Google Scholar]
- 7.Levander E, Malmgren O. Evaluation of the risk of root resorption during orthodontic treatment. A study of upper incisors. Eur J Orthod. 1988;10:30–38. doi: 10.1093/ejo/10.1.30. [DOI] [PubMed] [Google Scholar]
- 8.Ash M, Stanley N. J, Nelson J. Wheeler's Dental Anatomy Physiology and Occlusion 8th ed. St Louis, MO: Saunders Publishers; 2004. [Google Scholar]
- 9.Boucher M. Understanding the 6 degrees of freedom: using the correct CMM alignment principles. Version 3. Available at: Knol. Accessed December 16, 2008. http://www.cmmquaterly.com/ezine/training/28-training/205-understanding-the-6-degrees-of-freedom. [Google Scholar]
- 10.Taithongchai R, Sookkorn K, Killiany D. M. Facial and dentoalveolar structure and the prediction of apical root shortening. Am J Orthod Dentofacial Orthop. 1996;110:296–302. doi: 10.1016/s0889-5406(96)80014-x. [DOI] [PubMed] [Google Scholar]
- 11.Thongudomporn U, Freer T. J. Anomalous dental morphology and root resorption during orthodontic treatment: a pilot study. Aust Orthod J. 1998;15:162–167. [PubMed] [Google Scholar]
- 12.Newman W. G. Possible etiologic factors in external root resorption. Am J Orthod. 1975;67:522–539. doi: 10.1016/0002-9416(75)90298-5. [DOI] [PubMed] [Google Scholar]
- 13.Kook Y-A, Park S, Sameshima G. T. Peg-shaped and small lateral incisors not at higher risk for root resorption. Am J Orthod Dentofacial Orthop. 2003;123:253–258. doi: 10.1067/mod.2003.81. [DOI] [PubMed] [Google Scholar]
- 14.Al-Qawasmi R. A. Genetic predisposition to external apical root resorption. Am J Orthod Dentofacial Orthop. 2003;123:242–252. doi: 10.1067/mod.2003.42. [DOI] [PubMed] [Google Scholar]