Abstract
Objective:
To test the effect of alcohol on force decay of elastomeric chains in vitro in order to determine if increasing alcohol concentrations results in an increased amount of elastomeric chain force decay.
Materials and Methods:
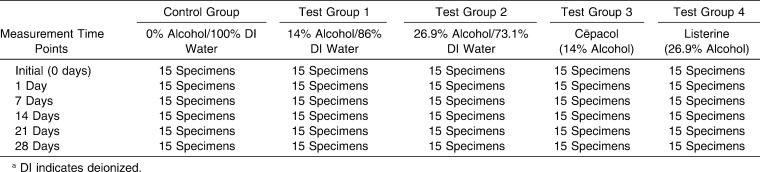
A prospective laboratory study was completed to test the effect of alcohol exposure on orthodontic elastomeric chain. A total of 450 specimens were divided into five test groups. Two test groups were each exposed to different alcohol concentrations (14% and 26.9%) and the other two test groups were exposed to different commercially available mouth rinses (Cēpacol -14% alcohol and Listerine - 26.9% alcohol) for 60 seconds twice a day. The control group followed all of the same procedures but was only exposed to deionized (DI) water. Force measurements were taken at six time points (initial, 1 day, 7 days, 14 days, 21 days, and 28 days).
Results:
There were no significant differences among groups at the initial time point (P = .52). Statistically significant effects of time on force decay were seen in all groups. All test groups showed significantly more force decay than the control group. Only a few statistically significant differences were observed when comparing force decay among the test groups.
Conclusions:
Alcohol causes an increase in force decay of elastomeric chain over time. A concentration dependence of alcohol on force decay of elastomeric chain was not observed.
Keywords: Force decay, Elastomeric chain, Alcohol, Mouth rinse
INTRODUCTION
Elastomeric power chain is commonly used in orthodontics to facilitate tooth movement and consolidate space. These polyurethane materials are manufactured as a spool of linked elastic chain, which can be cut to the specific number of modules needed. Due to the viscoelastic properties of power chain, however, the loss of force over time is inevitable and several studies have been done to illustrate this force decay.1–8 The orthodontic literature is currently lacking an evidence-based force level for optimal tooth movement.9 However, the rapid loss of an orthodontically applied force results in inefficient tooth movement and the need for an increased number of appointments to reactivate the appliances.
Various factors have been shown to impact the amount of force decay observed with elastomeric chains. In dry air, the percentage of force decay after 21 days was illustrated to be 42%–63%, depending on the product brand.9 In addition, force decay occurred more quickly when elastomers were submerged in a water bath. The greatest degree of force loss occurred in the first 3 hours with the remainder of force decay staying relatively constant over the next 21 days. Another factor affecting the degree of force decay is the amount of initial load applied to the elastomers.6 As the initial load increased, the percentage of force decay also increased. Although the percentage of force decay increased, the total remaining force at each time interval over 21 days was maintained at higher levels with increased initial loads.
In order to better simulate the oral environment, thermal cycling of the water bath in which elastomers were submerged was tested.2 A decreased percentage force decay was demonstrated at 30 minutes (23%–37%) and after 21 days (39%–61%). Additionally, tooth movement was simulated by decreasing the stretch distance of the elastomeric chain by 0.5 mm/week, while following the same thermal cycling protocol.2 Compared to the thermal cycled control, this resulted in a significantly higher amount of force decay at each time interval ending with (51%–68%) decay at 21 days.
Elastomeric chains tested in vivo showed more rapid and more extensive force decay than did those tested in vitro, although no specific explanation could be given.5 In an attempt to study possible intraoral environmental factors impressed on elastomeric chain, the effects of Coke were tested demonstrating greater force decay of elastomers stored in Coke compared with those in either air or water.7 However, it cannot be concluded that the acidic nature of Coke caused an increase in force decay because another study showed that acidic fluoride actually decreased the force decay of elastomeric chains.4
While the consensus of various studies1,5–7 is that force decay of elastomeric materials occurs more rapidly in vivo than in vitro, even when attempts to simulate the oral environment were made, no conclusions have been established as to what causes this difference. The effects of factors such as temperature and pH have been studied but remain controversial.2,7 Many factors that could be easily controlled by oral health care providers and patients, however, have not been tested.
One example of a controllable factor is the use of mouth rinses, which are commonly recommended to dental patients by orthodontists and other oral healthcare providers to assist in maintaining oral health during treatment. Many of these mouth rinses contain alcohol at various concentrations ranging from 0%–26.9%, with the majority around 14%. Ethanol is included in many mouth rinses as a dissolvent and carrier for the active ingredients. Is it possible that our recommendations may be contributing to the force decay of our materials and subsequently to less efficient orthodontic treatment? When analyzed microscopically, emersion of polyurethane orthodontic elastomeric modules in a 75% ethanol/water mixture caused structural and molecular modifications leading to decay of the specimen.3 However, initial and remaining force was not tested. Could the demonstrated structural and molecular changes due to exposure to ethanol result in a more rapid loss of force?
The purpose of this study was to test the effect of alcohol on force decay of elastomeric chains in vitro in order to determine if increasing alcohol concentrations results in an increased amount of elastomeric chain force decay. If clinically significant effects are found, it could result in modifications in the use of elastomeric chains during orthodontic therapy, as well as future studies that investigate the effects of other oral health products on force decay of elastomeric chain.
MATERIALS AND METHODS
A prospective laboratory study was completed to test the effect of alcohol exposure on orthodontic elastomeric chain. Five specimen groups were tested with a total sample size of 450 specimens. A specimen is described as a three-link, reduced module, clear elastomeric chain (Energy Chain, Rocky Mountain Orthodontics; Denver, Colo). Specimens were mounted on custom test jigs (Marquette University School of Dentistry). Five jigs, each with a series of pins set 23.5 mm apart, were used to hold stretched elastomeric chains at a constant length (Figure 1). These jigs allowed for complete submersion of the elastomeric chain in a water bath throughout the test period, as well as the dipping of elastomeric chains in respective control and test solutions. For 60 seconds, twice daily, control and test groups were each exposed to the respective solutions, and force measurements were taken at six time points during the experiment (Table 1).
Figure 1.
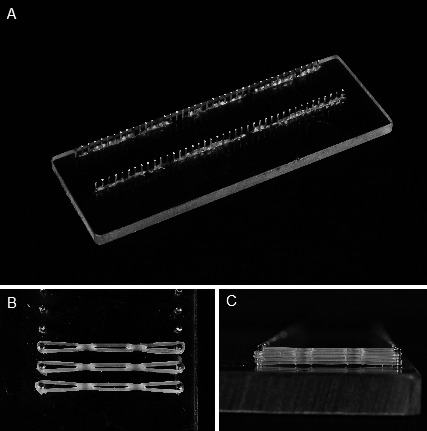
(A) Jig board with 25 sets of pins set 23.5 mm apart. (B) Elastomeric chains on jig board. (C) Four chains were placed on each pin set.
Table 1.
Laboratory Experimental Designa
The control and test groups were independently submerged in separate, 37°C water baths, housed in an incubator (Precision Incubator, National Equipment Sales and Leasing; Easton, Mass) and monitored daily with a thermometer (Fisherbrand 76 mm immersion thermometer; Fisher Scientific, Pittsburgh, Pa). The pH of the water baths was maintained between 6.00 and 7.00 using a pH meter (Accumet AR25 dual channel pH/ion meter; Fisher Scientific, Pittsburgh, Pa) and adjusted as needed using sodium hydroxide and citric acid.6,7
To evaluate the effects of alcohol contained in commercially available mouth rinses on the force decay of elastomeric chain, two mouth rinses were used. We chose Cēpacol antibacterial mouthwash (Combe Inc, White Plain, NY) and Listerine antiseptic mouthwash (Johnson & Johnson, McNeil-PPC, Skillman, NJ), which have an alcohol content of 14% and 26.9% by volume, respectively. These mouth rinses were selected because their alcohol concentrations represent the mean and maximum values currently available in the US market. To test the effects of the alcohol content only and not any of the other ingredients found in mouth rinses, two deionized (DI) water/alcohol mixtures (reagent alcohol, absolute; Mallinckrodt Chemicals, Phillipsburg, NJ) corresponding to the alcohol concentrations of the selected mouth rinses (14% and 26.9%) were tested. Concentration mixtures were made based on volume percentage and stored in a container with a lid to avoid evaporation. The mixtures were checked periodically and adjusted when required using a hydrometer (Fisherbrand Alcohol Hydrometer and Borosillicate Cylindar with pouring lip; Fisher Scientific, Pittsburgh, Pa) to ensure that the concentrations remained constant throughout the experiment.
The manufacturers of the majority of available mouth rinses in the United States recommend using the product twice a day for 60 seconds at a time. Similarly, the test specimens were submerged in the respective water, alcohol mixture, or mouth rinse for 60 seconds, twice a day, for the entire 28-day test period. Each 60-second exposure was measured using a digital clock (model 810046; Sper Scientific, Scottsdale, Ariz), and the two daily exposures were separated by 9 hours. After being submerged in the respective solutions, specimens were dipped in separate, intermediate DI water baths for 10 seconds to simulate salivary rinsing of the alcohol from the oral cavity, and then placed back into their 37°C water bath. The control group underwent the same protocol; however, these elastics were only exposed to DI water.
Six test measurements of remaining force were made at the following time intervals: initial (0), 1, 7, 14, 21, and 28 days. Force measurements were obtained with a digital force tester (Lutron FG-5000; accuracy ± [0.4% + 1 digit]; Lutron Electronic Enterprise Co Ltd, Taipei, Taiwan) by a single, blinded examiner with the assistance of a second examiner. Prior to the initiation of the study, the force tester was calibrated by measuring the weight of known items, to ensure the reliability of the instrument. After each measurement the force tester was reset to a zero reading before taking the next measurement. During force measurement, the jigs were securely bound to a bench top using a vice clamp.
Measurements were made by leaving one end of the elastomeric chain secured on the pin and fixing the other to the force tester, allowing for the measurement of the tensile force (Figure 2). Measurement readings were taken with the elastomeric chain stretched to the same 23.5 mm length that the jig pins had previously maintained them. The elastics were positioned by one examiner and all readings from the force tester were read by a separate examiner. All chains were handled and measured in the same manner at the same vertical and horizontal distance on the jig board to ensure consistent measurements.
Figure 2.

(A) Side. (B) Top view of elastomeric chain force levels being measured with a digital force gauge.
Statistical Analysis
Based on a previous study,6 the within-group standard deviation was expected to be 4.6 cN. With a sample size of 15 specimens per treatment combination, the study was designed to have 80% power to detect a difference of 4.9 cN between any two treatment combinations, assuming a two-sided 5% significance level for each test. However, the actual within-group standard deviation during the study was 12.8 cN, leading to 80% power to detect differences of 13.6 cN. With five groups, each having 15 specimens at six time points, a total sample size of 450 specimens (90 specimens per group) was tested (Table 1). Specimens were discarded after measurements were made. In the case that any elastomeric chains were to break during testing, an additional 15 specimens were added to the control and test groups. Therefore, 525 specimens were used. The effects of group (DI water, 14% alcohol, 26.9% alcohol, Listerine, and Cēpacol) and time (initial, 1, 7, 14, 21, and 28 days) on force were analyzed with a two-way analysis of variance (ANOVA). Pair-wise comparisons were conducted using Fisher protected least significant differences method.
RESULTS
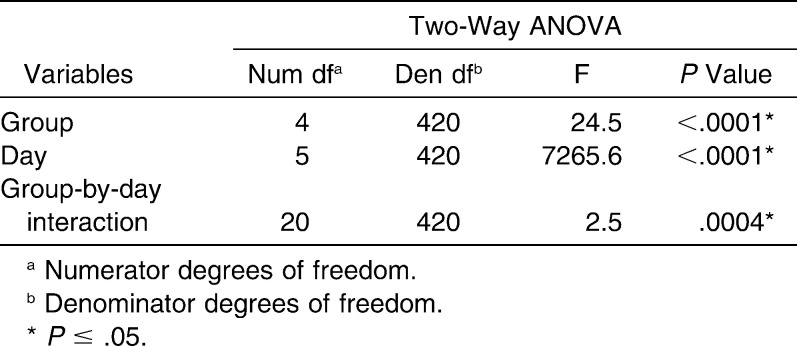
There were no significant differences among groups at the initial time point (P = .52). The results of the ANOVA (Table 2) illustrated that the group-by-time interaction was statistically significant (P = .0004), and therefore time comparisons were made by group and group comparisons were made by time.
Table 2.
Results of the Two-Way ANOVA of Elastomeric Chain Force Decay For Time, Groups, and the Interaction Between Variables
Statistically significant effects of time on force decay were seen in all groups (Figure 3; Table 3). Force decay increased in the DI water, 14% alcohol, and 26.9% alcohol groups between all time-points except 21 days and 28 days where a plateau occurred. Force decay increased in the Listerine group through 14 days and then reached a plateau. Force decay increased in the Cēpacol group through 14 days, reached a plateau between 14 days and 21 days, but then increased again between 21 days and 28 days.
Figure 3.
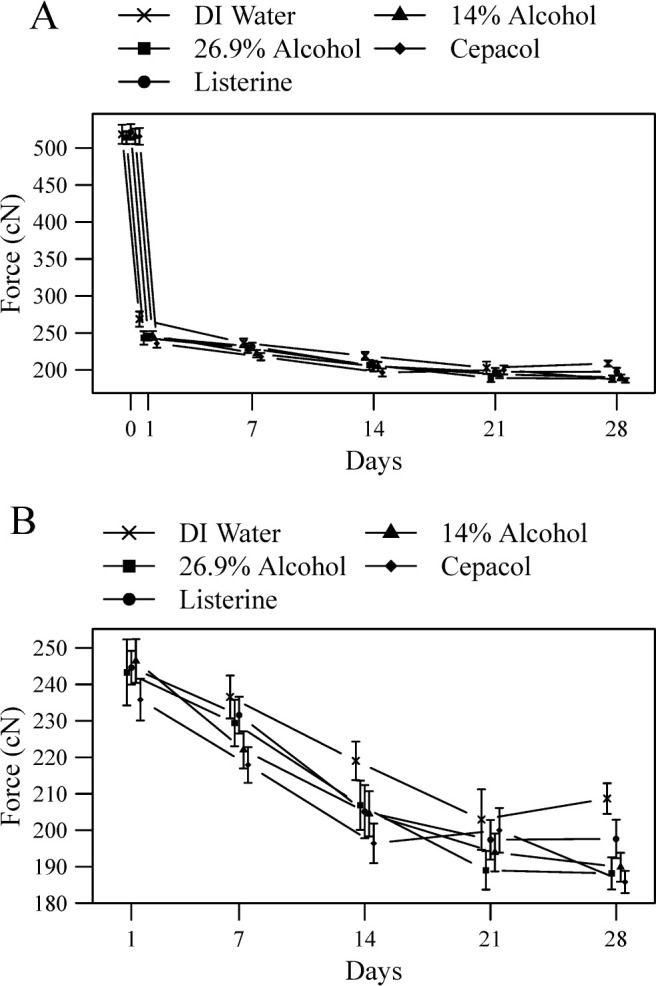
(A) Graphic representation of elastomeric chain force levels over the 28-day experiment. Each point represents group specimen means with 95% confidence intervals. (B) Same results showing only days 1–28.
Table 3.
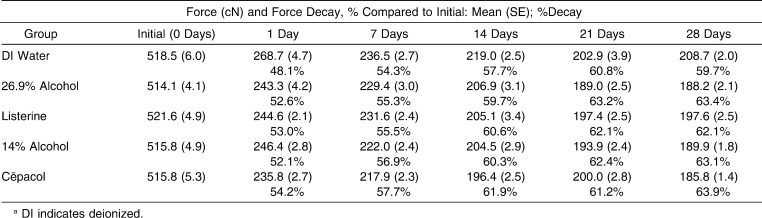
Force Measurements and Percent Force Decay of Each Group Over 28 Daysa
All test groups showed significantly increased force decay compared with the control group, and each test group compared to the control group in the following ways (Figure 3; Table 3). Increased force decay was demonstrated in the 26.9% alcohol group at each time point except 7 days (P = .13) and was demonstrated in the 14% alcohol group at each time point except 21 days (P = .05). Increased force decay was demonstrated in the Listerine group at each time point except 7 days (P = .29) and 21 days (P = .23) and was demonstrated in the Cēpacol group at each time point except 21 days (P = .53).
Few statistically significant differences were observed when comparing test groups (Figure 3; Table 3). No differences in force decay were shown between the 14% alcohol group and the 26.9% alcohol group at any time point. No differences were shown between the Listerine group and 26.9% alcohol group except at 28 days where increased force decay was demonstrated in the 26.9% alcohol group (P = .04). No differences were shown between the Cēpacol group and the 14% alcohol group except at 1 day where increased force decay was demonstrated in the Cēpacol group (P = .02). No differences were shown between the Listerine group and the Cēpacol group except at 7 days and 28 days where increased force decay was demonstrated in the Cēpacol group (P = .004 and P = .01, respectively).
DISCUSSION
The results of this study demonstrate that alcohol causes a statistically significant increase in the amount of force decay seen in elastomeric chains exposed to alcohol and commercial mouth rinse containing alcohol compared to those exposed only to water. The results also confirm previous studies1,2,4–8 showing force decay over time, which is high initially and then reaches a plateau (Figures 2 and 3). For all groups, substantial amounts of force decay occurred until 14 days. The Listerine group reached a plateau in 14–28 days. The Cēpacol group reached a plateau between 14 days and 21 days but then showed a significant increase in force decay again between 21 days and 28 days. The DI water, 14% alcohol, and 26.9% alcohol groups showed a plateau effect starting at 21 days. The differences in force decay found between the Listerine and Cēpacol groups, while not statistically significant, could be explained by the other ingredients found in these mouth rinses.
Inconsistencies were seen at 7 days and 21 days. At 7 days, nonsignificant differences were seen for the 26.9% alcohol and Listerine groups when compared with the control group. At 21 days, nonsignificant differences were seen for the 14% alcohol, Listerine, and Cēpacol groups when compared with the control group. Because pH, temperature, and exposure times were controlled, we hypothesize that the differences observed between the test groups are a function of elastomeric chain variation. While the elastics used in this study were all selected from the same manufacturer batch, there still seemed to be variation between individual elastics. Furthermore, it is possible that the elastomeric chains used in the study were not impacted at equal rates by the alcohol. The general trend observed in this study is similar to that presented in the literature; however, the inconsistent force decay seen in all groups could be contributed to this idea of unequal material exposure.
Although this study illustrates that alcohol has a statistically significant effect on the force decay of elastomeric chain in vitro, its impact in clinical situations is still inconclusive. After 28 days, the control group had 208.7 cN of force remaining and the average amount of force remaining for the test groups was 190.4 cN. The orthodontic community has yet to establish an agreed upon minimal level of force required to move a single tooth or group of teeth. Because alcohol does not result in total force decay of elastomeric chain over a 28-day period, it is unclear how detrimental elastomeric chain exposure to alcohol is in clinical settings. However, the research is clear that elastomeric chains studied in vivo have significantly more force decay that those studied in vitro.1,5–7 This compound effect could result in force levels lower than those seen in this study and perhaps below the threshold of optimal force for orthodontic tooth movement. Further studies should be conducted to ascertain the degree of elastomeric chain force decay observed when exposed to alcohol in vivo. Until research can demonstrate otherwise, the current practice of changing elastomeric chains every 21–28 days during certain forms of tooth movement, seems acceptable from the perspective of performance of dental materials.
CONCLUSIONS
Alcohol causes an increase in force decay of elastomeric chains over time.
A concentration dependence of alcohol on force decay of elastomeric chain was not observed.
Acknowledgments
The authors would like to thank the staff of the Indiana University School of Dentistry Department of Orthodontics and Oral Facial Genetics as well as Dr David Berzins of Marquette University for their support of this study. We would also like to thank Rocky Mountain Orthodontics for their contribution of elastomeric chains, Christine M. Buckley, Jennifer S. Eder, and Rolando Torres for their laboratory assistance, and George Eckert for his statistical support.
REFERENCES
- 1.Ash J. L, Nikolai R. J. Relaxation of orthodontic elastomeric chains and modules in vitro and in vivo. J Dent Res. 1978;57:685–690. doi: 10.1177/00220345780570050301. [DOI] [PubMed] [Google Scholar]
- 2.De Genova D. C, McInnes-Ledoux P, Weinberg R, Shaye R. Force degradation of orthodontic elastomeric chains—a product comparison study. Am J Orthod. 1985;87:377–384. doi: 10.1016/0002-9416(85)90197-6. [DOI] [PubMed] [Google Scholar]
- 3.Eliades T, Eliades G, Silikas N, Watts D. C. In vitro degradation of polyurethane orthodontic elastomeric modules. J Oral Rehabil. 2005;32:72–77. doi: 10.1111/j.1365-2842.2004.01366.x. [DOI] [PubMed] [Google Scholar]
- 4.Ferriter J. P, Meyers C. E, Jr, Lorton L. The effect of hydrogen ion concentration on the force-degradation rate of orthodontic polyurethane chain elastics. Am J Orthod Dentofacial Orthop. 1990;98:404–410. doi: 10.1016/S0889-5406(05)81648-8. [DOI] [PubMed] [Google Scholar]
- 5.Kuster R, Ingervall B, Burgin W. Laboratory and intra-oral tests of the degradation of elastic chains. Eur J Orthod. 1986;8:202–208. doi: 10.1093/ejo/8.3.202. [DOI] [PubMed] [Google Scholar]
- 6.Lu T. C, Wang W. N, Tarng T. H, Chen J. W. Force decay of elastomeric chain—a serial study. Part II. Am J Orthod Dentofacial Orthop. 1993;104:373–377. doi: 10.1016/S0889-5406(05)81336-8. [DOI] [PubMed] [Google Scholar]
- 7.Nattrass C, Ireland A. J, Sherriff M. The effect of environmental factors on elastomeric chain and nickel titanium coil springs. Eur J Orthod. 1998;20:169–176. doi: 10.1093/ejo/20.2.169. [DOI] [PubMed] [Google Scholar]
- 8.Wong A. K. Orthodontic elastic materials. Angle Orthod. 1976;46:196–205. doi: 10.1043/0003-3219(1976)046<0196:OEM>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 9.Ren Y, Maltha J. C, Kuijpers-Jagtman A. M. Optimum force magnitude for orthodontic tooth movement: a systematic literature review. Angle Orthod. 2003;73:86–92. doi: 10.1043/0003-3219(2003)073<0086:OFMFOT>2.0.CO;2. [DOI] [PubMed] [Google Scholar]