Abstract
A 24-year-old woman visited the Ear Nose Throat (ENT) outpatient department with complaints of hoarseness for 2 months not responding to conservative management. Laryngoscopic examination revealed a whitish ulceroproliferative lesion in the anterior commissure and anterior two-thirds of bilateral true vocal cords with surrounding necrosis. In view of the above findings, the patient was planned for biopsy under general anaesthesia. Intraoperative findings showed multiple whitish necrotic friable tissue involving anterior two-thirds of bilateral false vocal cords, ventricle, bilateral true vocal cords, both aryepiglottic folds and laryngeal surface of epiglottis. Postoperative histopathology was consistent with tuberculosis. A pulmonology consultation was taken, and the patient was started on anti-tuberculosis chemotherapy. One month post therapy, the voice was symptomatically better. A flexible fibreoptic laryngoscopic examination was done, which revealed almost complete resolution of the lesion with minimal ulceration at the anterior one-third of right true vocal cord.
Keywords: TB and other respiratory infections, otolaryngology / ENT
Background
The clinical picture of laryngeal tuberculosis (TB) has tremendously changed over the last few years due to the advent of antibiotics.1 In the pre-antibiotic era, laryngeal TB was seen in 37% of pulmonary TB cases, but currently due to the miraculous antitubercular therapy (ATT), the incidence has come down to <1%.2
In the present era, owing to ATT, the clinical presentation of laryngeal TB has changed from being coexist with advanced pulmonary TB disease to isolated laryngeal TB with features like hoarseness, and odynophagia more common in fifth to sixth decade.1
We report a case of laryngeal TB with incidental pulmonary TB in a young adult. Laryngeal TB though rare must always be kept in mind as a differential diagnosis for benign lesions of larynx especially in a country like India where TB is rampant.
Case presentation
A 24-year-old woman presented to the ENT outpatient department with complaints of hoarseness followed by odynophagia for 2 months. She had previously sought consultation from another medical practitioner who had advised conservative management with proton-pump inhibitors and voice rest, but she had no relief of symptoms. She had no complaints of fever, cough, difficulty in breathing, night sweats, loss of appetite, loss of weight and no palpable neck nodes. Indirect laryngoscopy was performed which showed whitish ulcerative lesion in the anterior commissure, and anterior two-thirds of bilateral true vocal cords not impairing the vocal cord mobility. For a definitive diagnosis, she was planned for laryngoscopic examination and biopsy under general anaesthesia.
Investigations
A videostroboscopy was done preoperatively in which mucosal waves of bilateral vocal cords could not be appreciated.
An experienced voice pathologist did a preliminary voice evaluation. The maximum phonation duration was 3 s with phonatory arrest, and reduced respiratory support for speech. The GRBAS rating scale3 was scored as given in table 1.
Table 1.
Preliminary voice evaluation as GRBAS rating scale prior to starting ATT
| Component | Rating |
| (G)rade | 3 |
| (R)oughness | 3 |
| (B)reathiness | 3 |
| (A)sthenia | 3 |
| (S)train | 2 |
The patient’s voice quality was diagnosed as breathy. The recorded voice sample could not be analysed using the multi dimensional voice profile (MDVP) (Kay Pentax, USA) due to excessive voice breaks.
A laryngoscopic examination was performed under general anaesthesia which revealed multiple whitish necrotic tissue with underlying friable tissue involving anterior two-thirds of bilateral false vocal cords, ventricle and bilateral true vocal cords (figure 1). It was also seen involving both the aryepiglottic folds, and laryngeal surface of epiglottis from which tissue sample was sent for histopathological examination. Narrow band imaging was also done intraoperatively which showed no significant intraepithelial papillary capillary loops, or any abnormal vascular network, but areas of necrosis could be appreciated (figure 2).
Figure 1.
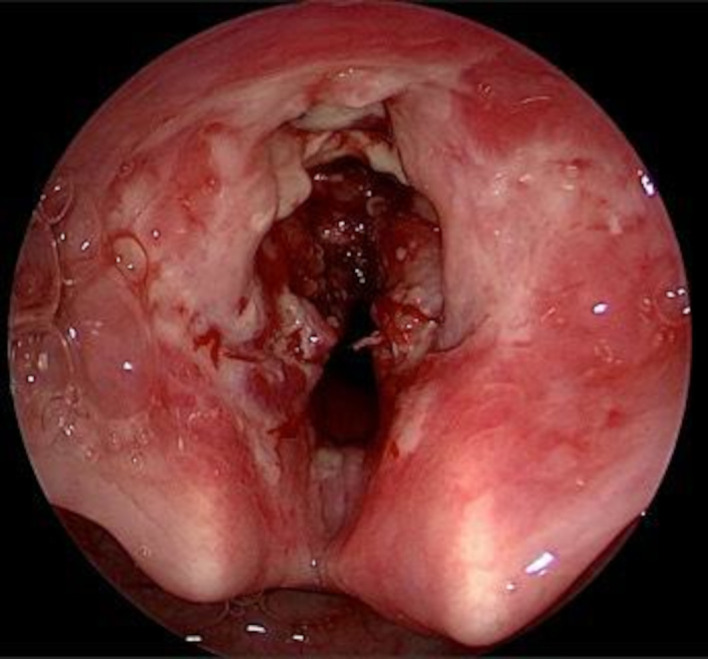
Multiple whitish necrotic tissue with underlying friable tissue involving anterior half of larynx.
Figure 2.
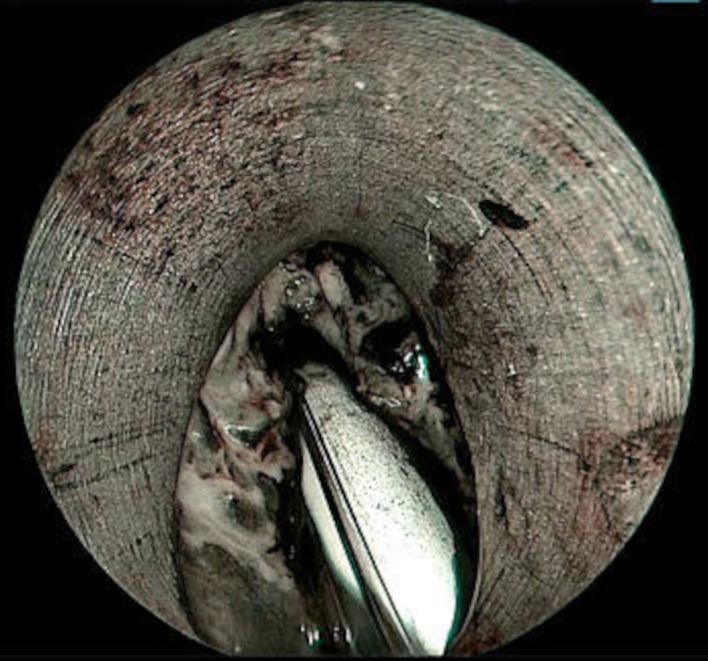
Narrow band imaging showed no significant intraepithelial papillary capillary loops seen.
The histopathological examination revealed foreign body granulomas with multinucleated giant cells, with some granulomas having central caseous necrosis with surrounding area showing mixed inflammation comprising of neutrophils, lymphocytes and plasma cells (figure 3). The Ziehl-Neelson stain for acid-fast bacilli was positive (figure 4). These features were consistent with that of TB. A pulmonologist opinion was taken to rule out pulmonary TB. A contrast-enhanced CT of chest was done which revealed multiple fine nodules, and few large nodular opacities in right upper lobe of lung suggestive of Koch’s disease.
Figure 3.
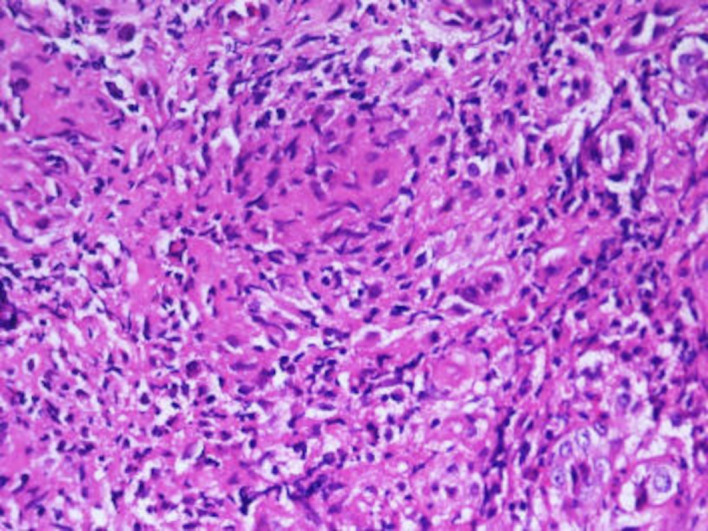
Microscopic examination shows multiple epithelioid cell granulomas (H&E, 400×).
Figure 4.
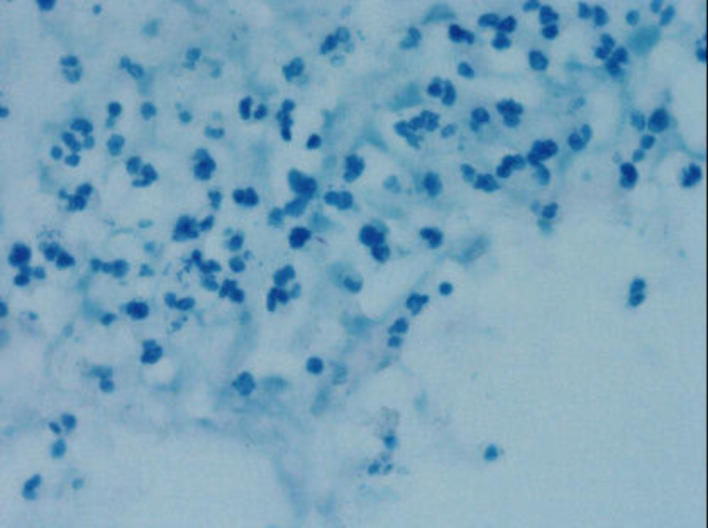
Scattered magenta coloured acid fast bacilli (Ziehl-Neelson stain, 1000× (oil immersion).
Treatment
The patient was advised to register in a nearby directly observed treatment short course clinic to start anti-TB chemotherapy. She was started on weight based daily treatment regimen of rifampicin, isoniazid, pyrazinamide and ethambutol hydrochloride.
Outcome and follow-up
After 4 weeks of ATT, the patient was followed up, and had symptomatic improvement in her voice quality. A repeat flexible fibreoptic laryngoscopic examination revealed almost complete resolution of the lesion with minimal ulceration on anterior one-third of right true vocal cord (figure 5). Also, the voice sample could be analysed in this visit using the MDVP. All parameters were affected as enumerated in table 2.
Figure 5.
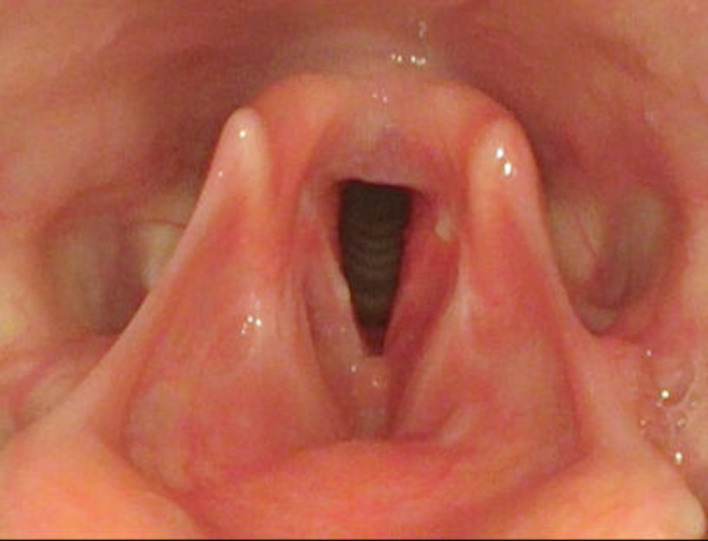
FFL examination revealed almost complete resolution of the lesion with minimal ulceration on anterior one-third of right true vocal cord. FFL, flexible fibreoptic laryngoscopic.
Table 2.
Voice analysis using MDVP 4 weeks after taking ATT
| Parameter | Value |
| Average fundamental frequency | 243.31 Hz |
| Mean fundamental frequency | 170.75 Hz |
| Jitter percentage | 8.93% |
| Shimmer percentage | 15.68% |
| Voice turbulence index | 0.308 |
| Soft phonation index | 3.212 |
The GRBAS rating scale was readministered. An improvement in the voice quality (breathy component) was observed on perceptual rating by a scale of one, and the maximum phonation time (MPT) increased to 8 s. The patient has been enrolled for symptomatic voice therapy for the same.
Discussion
TB is one of the most common communicable diseases in India. India accounts for 27% of the world’s total TB cases.4 It is more common in the fifth to sixth decade with a male-to-female ratio of 3:1.1
Extrapulmonary TB constitutes 15%–20% of TB cases in general practice among HIV-negative adults in India. A study by Sharma et al was conducted in 520 cases of TB, out of which tuberculous cervical lymphadenopathy accounted for 91.35%, laryngeal TB for 4.3%, tuberculous otitis media for 1.4%, nasal TB for 1.4 and oral TB for 1.4%, making laryngeal TB the second most common form of extrapulmonary TB seen in ENT department.5
The most common symptom of laryngeal TB is hoarseness followed by odynophagia and foreign body sensation in throat. The symptoms of laryngeal TB are not different from any of the similar conditions causing hoarseness like laryngeal papilloma, chronic laryngitis and laryngeal carcinoma, which makes diagnosis based on just clinical features difficult.6 Thaller et al stated that the laryngoscopic findings in laryngeal TB depends on the site of lesion.7 If the mucosa is adherent to the underlying structure such as vocal cord, it manifests as an ulcer just like in our case. However, if the mucosa is loosely attached to the underlying structure such as aryepiglottic fold, it manifests as hyperemia or oedema which is usually the most common presentation. The textbook description of turban-shaped epiglottis, interarytenoid mamillations and mouse-nibbled appearance is rarely seen these days.
There are two theories that account for laryngeal TB. The bronchogenic theory which states posterior laryngeal involvement is more common due to infected sputum, and the patient usually presents with odynophagia, and the haematogenous spread theory states that the anterior laryngeal involvement especially laryngeal surface of epiglottis is more common. The second theory is in favour of our case.1 8
A case series reported by Nishiike et al in 15 patients of laryngeal TB showed that 12 patients also had pulmonary involvement. The epiglottis was the most common site to be involved with multiple ulceration as the common finding.1
In the current modern era, certain investigations like videostroboscopy help to narrow down the diagnosis. Though histopathological examination is the gold standard investigation for any laryngeal lesion, videostroboscopy supports a benign lesion based on presence of mucosal waves ruling out malignancy. In a study by Ling et al, videostroboscopy in 19 patients of laryngeal TB showed that the most common finding was markedly reduced mucosal wave and absent vocal cord vibration.6
Radiological examination of larynx complements clinical examination. In laryngeal TB, CT usually shows diffuse bilateral thickening of vocal cord and epiglottis in acute phase and in chronic phase lesion is usually localised and confused with carcinoma of larynx.9
As per the study by Thaller et al, carcinoma larynx and laryngeal TB may sometimes coexist.7 In the presence of active pulmonary TB, biopsy from the larynx can be delayed due to the risk of transmitting infection is high as laryngeal TB is highly contagious.
Thus laryngeal TB though rare, must be thought of as a differential diagnosis in benign lesions of larynx because it has excellent response to anti-tubercular drugs and the lesion almost disappears in 2–3 months.
Patient’s perspective.
When I first developed change in voice, I thought it’s just a simple sore throat, and that it should resolve with some medications but when the problem was persisting even after 3 months of medications, I was quite frightened. That’s when I came to PGIMER and got a couple of tests done, one of which being endoscopy which showed a growth in my voice box. My family members and myself were quite stressed about the fact that it could be cancer. I was informed that the only way to get a diagnosis was to get a biopsy from the growth. Once the biopsy report came out to be tuberculosis, I was a little less worried. I was explained about the disease and the need for anti-tuberculosis therapy (ATT). After 1 month of ATT, I could personally feel a change in my voice and a repeat endoscopy was done. The doctors showed me the endoscopy images before and after the ATT, I was quite amazed how medicine can do such wonders. Though my voice is not yet completely normal, I’m hoping to get my voice back to normal and I’m receiving voice therapy for the same. I would really like my case to be reported because it’s not as frightening as it seems and the treatment is quite straightforward with wonderful results.
Learning points.
In the current era, diagnosis of laryngeal tuberculosis (TB) is difficult since it shares the same clinical features as other lesions of larynx like hoarseness and hence we would like to emphasise on the importance of biopsy.
Diagnosis of laryngeal TB by biopsy is crucial because it has excellent response to anti-tubercular drugs and the lesion almost disappears in 2–3 months.
Medical management is the treatment of choice in laryngeal TB than surgery.
Footnotes
Contributors: RR was involved in taking care of the patient when admitted in the ward and follow-up of the patient and has written the first draft. PS was the speech pathologist who analysed the patient’s voice and edited the first draft. NS was involved in analysing the histopathological examination of the biopsy. RSV is the consultant in charge for the patient, had operated on the patient, followed up the patient, has seen, edited and takes responsibility for the manuscript.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Case reports provide a valuable learning resource for the scientific community and can indicate areas of interest for future research. They should not be used in isolation to guide treatment choices or public health policy.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Ethics statements
Patient consent for publication
Consent obtained directly from patient(s).
References
- 1.Nishiike S, Irifune M, Doi K, Sawada T, et al. Laryngeal tuberculosis: a report of 15 cases. Ann Otol Rhinol Laryngol 2002;111:916–8. 10.1177/000348940211101010 [DOI] [PubMed] [Google Scholar]
- 2.Huon L-K, Huang S-H, Wang P-C, et al. Clinical photograph. laryngopharyngeal tuberculosis masquerading as chronic laryngopharyngitis. Otolaryngol Head Neck Surg 2009;141:537–8. 10.1016/j.otohns.2009.05.013 [DOI] [PubMed] [Google Scholar]
- 3.Hirano M. Clinical examination of voice. New York, NY: Springer-Verlag, 1981. [Google Scholar]
- 4.Sathiyamoorthy R, Kalaivani M, Aggarwal P, et al. Prevalence of pulmonary tuberculosis in India: a systematic review and meta-analysis. Lung India 2020;37:45–52. 10.4103/lungindia.lungindia_181_19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sharma S, Rana AK. Ent manifestations of tuberculosis: an important aspect of ENT practice. Pan Afr Med J 2020;36:295. 10.11604/pamj.2020.36.295.24823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ling L, Zhou S-H, Wang S-Q. Changing trends in the clinical features of laryngeal tuberculosis: a report of 19 cases. Int J Infect Dis 2010;14:e230–5. 10.1016/j.ijid.2009.05.002 [DOI] [PubMed] [Google Scholar]
- 7.Thaller SR, Gross JR, Pilch BZ, et al. Laryngeal tuberculosis as manifested in the decades 1963-1983. Laryngoscope 1987;97:848–50. [PubMed] [Google Scholar]
- 8.Kurokawa M, Nibu K-ichi, Ichimura K-ichi, et al. Laryngeal tuberculosis: a report of 17 cases. Auris Nasus Larynx 2015;42:305–10. 10.1016/j.anl.2015.02.012 [DOI] [PubMed] [Google Scholar]
- 9.Moon WK, Han MH, Chang KH, et al. Laryngeal tuberculosis: CT findings. AJR Am J Roentgenol 1996;166:445–9. 10.2214/ajr.166.2.8553964 [DOI] [PubMed] [Google Scholar]


