Abstract
Objectives
To provide a consensus statement describing best practices and evidence regarding head and neck cancer survivorship.
Methods
Key topics regarding head and neck cancer survivorship were identified by the multidisciplinary membership of the American Head and Neck Society Survivorship, Supportive Care & Rehabilitation Service. Guidelines were generated by combining expert opinion and a review of the literature and categorized by level of evidence.
Results
Several areas regarding survivorship including dysphonia, dysphagia, fatigue, chronic pain, intimacy, the ability to return to work, financial toxicity, lymphedema, psycho‐oncology, physical activity, and substance abuse were identified and discussed. Additionally, the group identified and described the role of key clinicians in survivorship including surgical, medical and radiation oncologists; dentists; primary care physicians; psychotherapists; as well as physical, occupational, speech, and respiratory therapists.
Conclusion
Head and neck cancer survivorship is complex and requires a multidisciplinary approach centered around patients and their caregivers. As survival related to head and neck cancer treatment improves, addressing post‐treatment concerns appropriately is critically important to our patient's quality of life. There continues to be a need to define effective and efficient programs that can coordinate this multidisciplinary effort toward survivorship.
Keywords: consensus statement, head and neck cancer, survivorship
1. INTRODUCTION
In the modern era, cancer remains a major public health problem worldwide and remains the second leading cause of death in the United States. In 2020, it is estimated 65,630 new cases of head and neck cancer (HNC) will occur, which accounts for 3.6% of new oncologic cases nationwide. 1
Well‐described risk factors in the development of squamous cell carcinoma (SCC) include tobacco use and alcohol consumption, which traditionally accounted for 90% of HNC cases. 2 In recent years, human papillomavirus (HPV)‐associated oropharyngeal SCC, a biologically unique and clinically distinct disease process, has been shown to encompass as much as 70% of new oropharyngeal carcinoma diagnoses. 3
After completion of an appropriate diagnostic work‐up, which includes a complete head and neck physical examination, radiographic imaging, and surgical biopsy, management of HNC is largely driven by the primary anatomic site, TNM (tumor, lymph node, and metastasis) classification, and patient medical comorbidities. Single‐treatment modalities such as surgery or radiation are typically reserved for the 30%–40% HNC patients who present with early‐stage (I or II) disease. Multi‐modality treatment is reserved for the approximately 60% of HNC patients presenting with locally or regionally advanced disease. 2 While cure rates for advanced‐stage HNC remain low, with the increased incidence of HPV associated tumors, there is a rise in overall HNC survivors in the United States. 4
In 1985, Dr. Fitzhugh Mullan first described his own personal battle with cancer and fundamentally changed the concept of survivorship. He delineated three key phases of survival: “acute survival,” a period that encompasses diagnosis and treatment; “extended survival,” a period that begins at treatment completion and includes surveillance; and “permanent survival,” a period vaguely defined as cure. 5
During this journey, survivors may encounter substantial medical, psychosocial, interpersonal, financial, and functional consequences. 6 As a unique discipline, cancer survivorship has garnered much attention in recent years, with a renewed focus on continuing care for patients beyond their defined surgery, radiation therapy, and/or chemotherapy. Recognizing these changes, the American Head and Neck Society (AHNS) has convened the AHNS Survivorship, Supportive Care & Rehabilitation Service, a committee on HNC survivorship. In this article, the concept of survivorship is reviewed to identify current practice patterns for HNC patients and gaps in survivor care providing a roadmap for future initiatives in HNC survivorship. We expand upon the guidelines set forth by the American Cancer Society (ACS) and the American Society of Clinical Oncology (ASCO) with special emphasis placed on the importance of the multidisciplinary model. 7 , 8 The goal of this document is to serve as a guide for head and neck oncologists, primary care clinicians, and allied health care providers treating HNC survivors. This clinical advice (summarized in Table 1) has been annotated throughout this article with key recommendations categorized by level of evidence, as defined by the University of Oxford Center for Evidence‐based Medicine (Table 2). 9 Recommendations supported by the National Comprehensive Cancer Network (NCCN) are categorized differently to incorporate expert consensus (Table 3). 2
TABLE 1.
Head and neck cancer survivorship: AHNS Committee Guidelines
| Level of evidence | Topic | Recommendation |
|---|---|---|
| 2A a | Surgical oncology evaluation | Evaluate every 1–3 months in the 1st year after treatment; every 2–6 months in the 2nd year after treatment; every 4–8 months in the 3rd–5th year after treatment; and every 12 months thereafter |
| 2A a | Follow closely for a complete head and neck examination, indirect mirror and/or flexible fiberoptic laryngoscopy, and nasal endoscopy for patients with sinonasal malignancies | |
| 2B a | Monitor with EBV DNA in the setting of nasopharyngeal carcinoma surveillance | |
| 2A a | Radiation oncology evaluation | Evaluate for thyroid dysfunction (TSH/T4) every 6–12 months, particularly with prior neck irradiation |
| 2A a | Refer for regular dental examinations, especially if the patient received intraoral or salivary gland irradiation | |
| 2A a | Follow in close, alternating intervals with surgical and medical oncology | |
| 2A a | Medical oncology evaluation | Follow in close, alternating intervals with surgical and radiation oncology with attention to adverse effects related to chemotherapy and immunotherapy |
| 2A a | Radiographic imaging | Follow with radiographic imaging (CT, MRI, and/or PET/CT) based on worrisome signs or symptoms and areas inaccessible to clinical examination (i.e., salivary glands, nasopharynx, and skull base) |
| 2A a | Consider chest CT with or without contrast as clinically indicated for those with a smoking history | |
| 2A a | Survivorship clinic | Incorporate a multidisciplinary team including nurses, advanced practice providers, oncologists (medical, radiation, surgical) in close coordination with the patient's primary care provider on at least an annual basis |
| 2A a | Dysphonia and dysphagia | Incorporate speech‐language pathology evaluation and management for at risk patients |
| 5 b | Fatigue | Evaluate for underlying etiologies of fatigue |
| 2A b | Sexual dysfunction and intimacy | Screen for sexual and intimacy dysfunction |
| 2A b | Offer supportive care services for sexual function and intimacy issues | |
| 2A b | Consider pituitary dysfunction if patients have received skull base irradiation | |
| 2A b | Counsel regarding the risk of HPV transmission after treatment for HPV‐related OPSCC | |
| 2A a | Chronic pain | Screen for chronic pain at routine intervals |
| 2A a | Assess for the quality and severity of their pain using pain assessment tools | |
| 2A a | Evaluate for depressive symptoms in the presence of chronic pain | |
| 3B b | Consider ruling out recurrent disease as a cause of pain | |
| 2A a | Screen for opioid abuse | |
| 2C b , 2A a , 1A b | Offer non‐opioid analgesics including nonsteroidal anti‐inflammatory agents, acetaminophen, neuromodulators, and acupuncture | |
| 5 b | Refer to palliative and/or pain management specialists for refractory or opioid‐dependent pain | |
| 5 b | Physical therapy | Screen for physical rehabilitation needs early in the post‐treatment phase with objective assessments of upper limb dysfunction at regular intervals |
| 2A a | Assess for objective measures of trismus at regular intervals prior to and following therapy | |
| 5 b | Refer to exercise and physiotherapy professionals for structured rehabilitation after HNC treatment | |
| 1B b | Incorporate shoulder physiotherapy after completion of neck dissection | |
| 2A a | Lymphedema | Evaluate for lymphedema after HNC treatment |
| 2A a | Refer to certified lymphedema therapists for CDT | |
| 2A a | Psycho‐oncology | Screen for BID concerns |
| 2A a | Refer to psychology or psychiatry for the management of BID as indicated | |
| 1A b | Assess for distress, depression, and/or anxiety at regular intervals using a validated questionnaire | |
| 1B b | Consider referral to psychology or psychiatry if distress, depression, and/or anxiety is present | |
| 2A a | Hearing loss | Evaluate yearly, for first 2 years, for hearing loss via pure tone audiometry in at risk patients |
| 5 b | Respiratory therapy | Multidisciplinary team should be aware and versed in tracheostomy and laryngectomy stoma care |
| 5 b | Caregivers | Include caregivers in all aspects of HNC care provided by the oncology team and PCP |
| 5 b | Social support groups | Educate on social support groups and their impact on understanding the wide‐ranging sequelae of oncologic treatment |
| 5 b | Return to work | Counsel on medical disability rights and protections afforded by federal law |
| 5 b | Financial burden | Refer to social workers and financial navigators to understand health care costs associated with HNC care |
| 2A b | Primary care physician | Include PCP involvement for age‐appropriate and gender‐appropriate screening of other neoplasms and general health as well as well‐being |
| 1B b | Dental care | Counsel to maintain close follow‐up with the dental professional |
| 5 b | Encourage to avoid tobacco and alcohol to minimize the risk of dental disease | |
| 1A b | Substance abuse | Refer for tobacco and alcohol cessation counseling and abstinence resources as needed |
| 1B b | Physical activity/exercise | Encourage regular physical activity and exercise |
| 5 b | HPV counseling | Counsel on the sequelae of HPV related oncologic disease and the potential value of vaccination |
Abbreviations: BID, body image disturbance; CDT, complete decongestive therapy; CT, computed tomography; EBV DNA, Epstein–Barr virus deoxyribonucleic acid; HNC, head and neck cancer; HPV, human papillomavirus; OPSCC, oropharyngeal squamous cell carcinoma; PCP, primary care physician; TSH, thyroid‐stimulating hormone.
NCCN categories of evidence and consensus.
Oxford level of evidence.
TABLE 2.
Oxford levels of evidence
| Level | Study design |
|---|---|
| 1A | Systematic review of randomized controlled trials |
| 1B | Individual randomized controlled trial |
| 1C | All or none case‐series |
| 2A | Systematic review of cohort studies |
| 2B | Individual cohort study |
| 2C | Outcomes research |
| 3A | Systematic review of case–control studies |
| 3B | Individual case–control study |
| 4 | Case‐series |
| 5 | Expert opinion |
TABLE 3.
NCCN categories of evidence and consensus
| Category 1: Based upon high‐level evidence, there is uniform NCCN consensus that the intervention is appropriate. |
| Category 2A: Based upon lower‐level evidence, there is uniform NCCN consensus that the intervention is appropriate. |
| Category 2B: Based upon lower‐level evidence, there is NCCN consensus that the intervention is appropriate. |
| Category 3: Based upon any level of evidence, there is major NCCN disagreement that the intervention is appropriate. |
2. CANCER SURVEILLANCE
A person is considered a cancer survivor from the time of diagnosis through the balance of his or her life. Although cancer surveillance and cancer survivorship are often described in an intermingled fashion, they represent distinct concepts. 7 Cancer surveillance generally refers to surveillance for disease recurrence, progression, or second primary cancers, often during the first 5 years after treatment completion. Cancer survivorship is a broader concept of life‐long care that also includes intervention for consequences of cancer and treatment including physical and psychosocial effects, coordination of care between primary care providers and specialists, prevention of new cancers and late effects, and survivorship care planning. 2 For HNC patients, the relationship between cancer surveillance and cancer survivorship is an area of active research and will be discussed in Section 2.5. However, most of the existing medical literature supports a transition beyond 5 years after treatment completion to focusing less on cancer surveillance and more on other aspects of survivorship care. This section will outline the integration of surveillance and survivorship concepts during the first 5 years after treatment. During this time, the oncologic treatment team provides close clinical surveillance, augmented by appropriate radiographic imaging and other ancillary testing designed to detect disease recurrence while also managing treatment‐related side effects and other aspects of survivorship. The use of a formal document to outline the details of the treatment and outline a survivorship care plan can be helpful and several documents published by AHNS and ASCO can help accomplish this. 6 , 7 Figure 1 illustrates a timeline of a proposed survivorship care plan based on the recommendations from this document.
FIGURE 1.
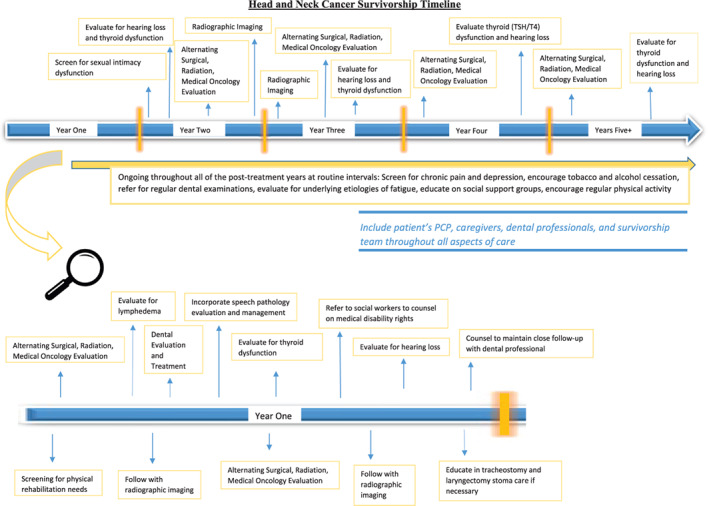
A proposed timeline depicting the timing of the different assessments suggested by these guidelines
2.1. Surgical oncology
During the cancer surveillance period, it is recommended that patients follow‐up with their head and neck surgeon at regular, established intervals after treatment completion. The head and neck surgeon may remain a part of the surveillance team even in those settings where the patient received non‐surgical treatment. The NCCN has established guidelines for surveillance, entitled “Follow‐up Recommendations” (FOLL‐A) under the current Treatment Guideline for Head and Neck Cancers, which are updated periodically (current v2.2020—June 2020) and summarized below:
Patients should be evaluated every 1–3 months in the 1st year after treatment; every 2–6 months in the 2nd year after treatment; every 4–8 months in the 3rd–5th year after treatment; and every 12 months thereafter (NCCN Category 2A).
Clinic visits should include a complete head and neck examination, indirect mirror and/or flexible fiberoptic laryngoscopy, and nasal endoscopy for patients with sinonasal malignancies (NCCN Category 2A).
In some cases, initial follow‐up after surgical treatment may be more frequent based on symptoms, risk factors, treatment sequelae, or physician preference (Oxford Category 5).
Consider EBV DNA monitoring for nasopharyngeal carcinoma surveillance (NCCN Category 2B).
Cancer surveillance visits should also include screening and evaluation for toxicities, quality of life (QOL) measures, and health maintenance (see Sections 3 and 4).
Establishment of survivorship care within 1 year of definitive HNC treatment (NCCN Guidelines: Survivorship).
Currently, no randomized clinical trials have compared outcomes in patients following recommended cancer surveillance to those not adhering to practice guidelines. A recent analysis of 3975 HNC patients in Ontario revealed that less than 50% met recommended follow‐up guidelines after 2 years of treatment. 10 Another study of esophageal cancer surveillance demonstrated a 9% rate of second primary with early detection made possible with periodic nasopharyngolaryngoscopy. 11 Detection of early lesions may also be aided by advanced optical technology such as high definition fiber optic imaging, video archiving, and narrow‐band imaging. 12 Education and training resources for flexible fiberoptic laryngoscopy can be found through the American Academy of Otolaryngology and OTOSOURCE. While “cure” has traditionally been defined as 5 years of disease‐free interval, most patients require at least annual follow‐up beyond 5 years for treatment‐related concerns (see Section 2.5). Similarly, screening for second primaries should also be considered based on risk factors, including the continuation of tobacco and alcohol use.
2.1.1. Recommendation
Patients who have undergone treatment for HNC should be:
Evaluated every 1–3 months in the 1st year after treatment; every 2–6 months in the 2nd year after treatment; every 4–8 months in the 3rd–5th year after treatment; and every 12 months thereafter (NCCN Category 2A).
Followed by a surgical oncologist for a complete head and neck examination, indirect mirror and/or flexible fiberoptic laryngoscopy, and nasal endoscopy in the setting of sinonasal malignancy (NCCN Category 2A).
Monitored with EBV DNA for nasopharyngeal carcinoma surveillance (NCCN Category 2B).
2.2. Radiation oncology
Patients are recommended to follow‐up with their radiation oncologists, if they received radiation treatment, throughout the cancer surveillance period. Timely evaluation of possible persistent or recurrent locoregional disease after completion of radiation is crucial, as well as close communication with medical and surgical oncologists. In some cases, it can be quite challenging to distinguish radiation treatment effects from disease persistence or recurrence, which may require further evaluation. In addition to performing cancer surveillance, the radiation oncologist will manage acute, subacute, and chronic toxicities of radiation and evaluate QOL measures as well as health maintenance. Many head and neck radiation toxicities are discussed in Sections 3 and 4, and comprehensive reviews regarding these topics have recently been published. 13 , 14 These toxicities may be complex and interrelated; for example, xerostomia may cause secondary dental complications, dysphagia, dietary changes, and depression. A multidisciplinary team approach to manage these manifestations is critical; the radiation oncologist should coordinate care with speech‐language pathologists, dieticians, dentists, physical therapists, and other specialists as clinically indicated. At each visit, the radiation oncologist should provide patient education and emphasize the importance of routine neck and swallowing exercises.
2.2.1. Recommendation
Patients who have undergone radiation for HNC should be:
Evaluated for thyroid dysfunction (TSH/T4) every 6–12 months, particularly in the setting of neck irradiation (NCCN Category 2A).
Referred for regular dental examinations, especially if the patient received intraoral or salivary gland irradiation (NCCN Category 2A).
Followed in close, alternating intervals, in conjunction with surgical oncology and medical oncology as outlined in Section 2.1 (NCCN Category 2A).
2.3. Medical oncology
Medical oncologists are recommended to perform detailed cancer‐related histories and physical examinations at routine, established intervals throughout the care continuum, including post‐treatment surveillance, if the patient received systemic therapy (e.g., chemotherapy, immunotherapy). Specifically, recognizing and managing the side effects of commonly utilized chemotherapy agents such as platinum agents (i.e., cisplatin, carboplatin, and oxaliplatin), taxanes (paclitaxel and docetaxel), and vinca alkaloids (vincristine and vinblastine) remain challenging, including when patients are afflicted with chemotherapy‐induced peripheral neurotoxicity (CIPN). Although poorly understood, CIPN typically carries a dose‐dependent and cumulative relationship, though patients with pre‐existing neuropathy or those receiving doublet therapy may develop CIPN at lower doses. CIPN presents as a “glove and stocking” and its incidence decreases as one is further from treatment. 15 , 16 Treatment interventions can include duloxetine, topical menthol, topical 8% capsaicin, and physical therapy. 17 , 18 , 19 , 20
There are no well‐defined guidelines and limited medical literature regarding the timing or frequency of evaluation by medical oncology. 21 Nonetheless, these assessments should be incorporated into a shared responsibility surveillance plan with surgical and radiation oncologists that take into account NCCN guidelines. Establishment of clinical care pathways that incorporate evidence‐based guidelines, clinical expertise, and program infrastructure/feasibility to integrate multidisciplinary care, cancer surveillance, and care transitions should also be considered. 21 A shared surveillance plan may also serve to reduce patient burden on individual specialties, thus permitting improved patient access. In addition to cancer surveillance and toxicity monitoring, the role of medical oncology evaluations could also be viewed as a potential facilitator of improved health promotion and care coordination with primary care physicians (PCPs) and survivorship clinics to potentially minimize health care fragmentation. 22
2.3.1. Recommendation
Patients who have undergone treatment for HNC should be:
Followed in close, alternating intervals, in conjunction with surgical and radiation oncology as outlined in Section 2.1 (NCCN Category 2A).
2.4. Radiographic imaging
Recommendations for post‐treatment imaging for HNC include the following (adapted from NCCN Guidelines for Head and Neck Cancers v2.2020):
- For patients with locoregionally advanced disease:
- If there is clinical concern for residual disease or progression, CT of primary site and neck with contrast and/or MRI with contrast may be considered at 4–8 weeks after treatment.
- If there is no concern for residual disease, post‐treatment imaging may be performed with CT or MRI with contrast at 3–4 months after completion of treatment.
- For those patients in which PET/CT is used for follow‐up, PET/CT should be performed within 3–6 months after definitive treatment.
Post‐treatment, consider obtaining radiographic imaging of primary site (and neck, if treated) within 3–4 months to establish a new baseline for surveillance following surgery, definitive radiation, or chemoradiation (NCCN Category 2A).
Further radiographic imaging may be indicated based on worrisome signs or symptoms, smoking history, and areas inaccessible to clinical examination (i.e., salivary glands, nasopharynx, and skull base) (NCCN Category 2A).
Routine annual radiographic imaging is often employed for HNC difficult to visualize including salivary glands, nasopharynx, or skull base (NCCN Category 2A).
Chest CT with or without contrast as clinically indicated for patients with a smoking history (NCCN Guidelines for Lung Cancer Screening) (NCCN Category 2A).
Carotid ultrasound screening may also be considered every 2–5 years for patients who received neck irradiation.
Of note, a recent study at MD Anderson Cancer Center of 1508 HNC patients (62% T0–T2) treated with definitive radiation revealed a low yield and high cost for routine radiographic imaging beyond 2 years after treatment without clinically suspicious findings; a 12.6% rate of disease recurrence was appreciated, with 70% of recurrences occurring within the first 2 years. 23
2.4.1. Recommendation
Patients who have undergone treatment for HNC should undergo:
Post‐treatment baseline radiographic imaging of primary (and neck if treated) within 3–4 months (NCCN Category 2A).
For those patients in which PET/CT is used for follow‐up, PET/CT should be performed within 3–6 months after definitive treatment (NCCN Category 2A).
Further radiographic imaging based on worrisome signs or symptoms, smoking history, and areas inaccessible to clinical examination (i.e., salivary glands, nasopharynx, and skull base) (NCCN Category 2A).
Chest CT with or without contrast as clinically indicated for patients with a smoking history (NCCN Guidelines for Lung Cancer Screening) (NCCN Category 2A).
2.5. Survivorship clinic
The relationship between cancer surveillance and survivorship remains poorly defined for HNC patients. However, as noted above, most of the existing medical literature supports a transition toward non‐surveillance aspects of survivorship care at 5 years after treatment completion. This transition point is supported by a decreased risk of recurrence and an increase in the ratio of non‐cancer‐related mortality to cancer‐related mortality. 24 Increased non‐cancer‐related mortality in >5‐year survivors is multifactorial but appears to be largely driven by cardiovascular disease (i.e., exacerbation of hypertension, increased risk of stroke, and myocardial infarction). 25 A Surveillance, Epidemiology and End Results (SEER) population‐based study demonstrated a high comorbidity burden in HNC patients at the time of diagnosis which increased significantly at 5 years post‐diagnosis; this trend was measured in both HPV‐related and HPV‐unrelated disease variants. 26 In addition to a higher rate of medical problems, HNC survivors have also been shown to demonstrate a nearly twofold risk (adjusted rate ratio, 1.97) of suicide over time compared to patients with other types of cancers. 27
HNC survivorship care is extraordinarily complex and requires a robust understanding of the underlying cancer pathophysiology, the chronic sequelae of relevant treatment modalities, and the interaction between cancer, oncologic treatment, and patient comorbidities. This naturally raises the question of who should provide survivorship care. The majority of existing data supports a simple unitary answer: a multidisciplinary team. Existing data support an approach which generates algorithms designed to maximize care delivered while minimizing the burden placed on patients for multiple clinic visits and extensive traveling. 28 Multiple studies have demonstrated a role for registered nurses in survivorship care. A recent meta‐analysis demonstrated significant gains in some QOL domains for patients enrolled in nurse‐led survivorship care. 29 Data from other tumor types also support the involvement of advanced practice providers in survivorship care. 30 In part due to these findings, survivorship care is often transitioned to nurses or advanced practice providers at multiple centers throughout the United States. In lieu of organized survivorship care plans, primary care providers sometimes become de facto survivorship care providers. However, there is significant data that indicates that patients prefer specialist‐led care. 31 , 32 This represents a recognition on the part of patients that oncologists familiar with the survivor, cancer type, and treatment regimen are better equipped to understand late sequelae of disease and associated treatment. Ideally, survivorship care would be provided by a team consisting of nurses, advanced practice providers, oral health care providers, medical oncologists, radiation oncologists, and/or surgical oncologists working in close coordination with the patient's primary care provider. 33
2.5.1. Recommendation
Patients who are beyond 5 years of HNC treatment and are designated as survivors may be:
Followed by a multidisciplinary team consisting of nurses, advanced practice providers, oral health care providers, medical oncologists, and/or radiation oncologists, in conjunction with the annual follow‐up with surgical oncologists, working in close coordination with the patient's primary care provider on at least an annual basis (NCCN Category 2A).
3. QUALITY OF LIFE
3.1. Dysphonia and dysphagia
Patients affected by HNC commonly experience symptoms related to compromised speech, voice, and swallowing function and thus experience diminished QOL. Speech and swallowing impairments may arise from the intrinsic neoplastic process or resultant sequelae of therapy including surgery, radiation, and/or chemoradiation therapy. 34 Factors such as anatomic alterations, dentition loss, xerostomia, trismus, mucositis, post‐treatment lymphedema, fibrosis, and neuropathy may detrimentally influence a person's ability for mastication, deglutition, and nutritional maintenance. 35 , 36
Symptoms of swallowing disturbance are common among patients with HNC. In one SEER based study, the prevalence of dysphagia, stricture, and aspiration pneumonia was 45.3%, 10.2%, and 8.7%, respectively. The prevalence of these signs and symptoms was modified by site (lower in oral cavity), stage (higher with more advanced cancers), and by treatment modality (higher in patients receiving non‐surgical or multiple treatment modalities). 37 Other studies suggest that nearly one in two patients experience poor QOL due to dysphagia and that up to 84% of patients may experience aspiration. 38 , 39 The addition of chemotherapy as a radiosensitizer may further exacerbate these symptoms. 40
Collaborative multidisciplinary efforts centered on the patient's ability to preserve and recoup functional speech and swallowing may produce improvements in QOL. 41 SLP providers may help identify baseline speech and swallowing disturbances and provide education and counseling that may help patients understand the implications of their disease and its impact on speech and swallowing. 42 This assessment of baseline function may help identify patients at risk of chronic dysfunction and those who may require additional assistance for nutritional needs (i.e., gastrostomy tube) or for phonatory rehabilitation (i.e., alaryngeal speech). In patients receiving radiotherapy or chemoradiation therapy, SLP providers may teach and encourage prophylactic swallowing exercises that may improve post‐treatment functional outcomes. 43 , 44 In such patients, swallowing exercises that help maintain oral intake through the treatment phase are associated with better long‐term swallowing outcomes and reduced gastrostomy tube dependence. 45
Patients with HNC also experience difficulties with speech and voice production that may include a spectrum of presentations: impaired intelligibility to poor quality of voice or loss of natural speech. Speech therapy may certainly improve speech quality and intelligibility. 46 SLP providers may also help identify optimal strategies for speech rehabilitation and restoration in alaryngeal patients via an electrolarynx, tracheoesophageal puncture, or esophageal speech.
As a result, early referral to SLP providers should be considered for patients with HNC as part of their evaluation and treatment planning. SLP professionals may play a role in ongoing survivorship care for patients through surveillance of functional outcomes related to speech and swallowing. Symptom driven assessment tools including modified barium swallow study, fiberoptic endoscopic evaluation of swallowing, and videostroboscopy provide insights into developing targeted interventions including the teaching of compensatory maneuvers and swallowing exercises when needed. 41 Given the potential for development of late toxicities in patients receiving radiation therapy, long‐term evaluation of cranial nerve function, speech, and swallowing are indicated.
3.1.1. Recommendation
Patients who have been diagnosed with HNC, particularly of the aerodigestive tract, should be:
Referred to SLP providers for evaluation and management of speech, voice, and swallowing symptoms (NCCN Category 2A).
Referred to SLP, if possible, prior to the initiation of therapy to optimize post‐treatment communication and swallowing outcomes (NCCN Category 2A).
3.2. Fatigue
Cancer‐related fatigue is a distressing, persistent, and subjective sense of physical, emotional, and/or cognitive tiredness or exhaustion related to cancer or oncologic treatment that is not proportional to recent activity and interferes with normal function. 47 Typically, cancer‐related fatigue is not relieved by rest or sleep. Most patients diagnosed with HNC experience cancer‐related fatigue, particularly when treatment includes radiation or chemoradiation. 48 , 49 Symptoms related to fatigue peak early (i.e., within 8 weeks) after initiation of radiation, with persistent or worsening of symptoms over the first 2 years following therapy and contribute to a significant loss of QOL. 48
Fatigue may arise from a variety of underlying medical conditions that may coexist with the diagnosis of HNC. This includes baseline frailty, anemia, anxiety, depression, poor cardiopulmonary reserve, malnutrition, psychological distress, sleep disturbances, thyroid dysfunction, pain related to cancer and/or its therapy, pro‐inflammatory or catabolic state, and substance abuse. 50 Patients with a history of HNC can be counseled and screened for fatigue upon initial evaluation and then periodically through their survivorship. 47 Patients identified with symptoms of fatigue should be assessed for underlying medical conditions through a comprehensive review of history, physical examination, appropriate hematology and biochemical testing (i.e., complete blood count, comprehensive metabolic panel, thyroid function testing, and nutritional markers), use of screening tools for psychologic distress and depression, and referral to specialists when appropriate.
In addition to counseling and treatment of comorbid medical conditions contributing to fatigue, patients should be encouraged to participate in a regular exercise regimen. 50 , 51 Physical therapists specializing in oncology care can provide treatment to address cancer‐related fatigue. Additionally, clinicians and patients may consider the use of cognitive‐behavioral therapy, yoga, massage therapy, and others to mitigate cancer‐related fatigue. 47 , 52
3.2.1. Recommendation
For patients who have undergone treatment for HNC:
Consider evaluating for underlying etiologies of fatigue by the multidisciplinary care team (Oxford Category 5).
3.3. Sexual dysfunction and intimacy
HNC and its associated treatment can have significant physical, psychosocial, and emotional consequences which may adversely affect sexual function and intimacy. Patients and partners report a significantly reduced frequency of vaginal and oral sex practices after treatment for head and neck carcinoma regardless of HPV status. 53 Up to 44% of patients treated for upper aerodigestive malignancies report at least some difficulty with sexual function and/or intimacy following treatment. 54 Furthermore, patients perceive that their questions and concerns regarding intimacy and sexuality are not being addressed by their oncology team. This is most significant among those treated for tumors that do not have a direct effect on fertility and urogenital function. 55 At the same time, no validated tool exists to address sexual function and intimacy among individuals specifically treated for HNC. 56 At this time, several oncologic and HNC‐related quality of life instruments do include items that pertain to sex and intimacy (e.g., FACT‐HN) 56 ; these may be utilized as screening tools until a validated head and neck‐specific intimacy questionnaire is created. Referral for psychosexual therapy may be beneficial in some circumstances. 57
Individuals treated with irradiation to the skull base are at risk of hypothalamic–pituitary axis disorders. 58 Specifically, up to 20% of patients treated with radiotherapy for nasopharyngeal carcinoma may experience hypogonadism. 59 As this can have profound effects on sexual function, it is encouraged that these patients undergo regular assessment of hypothalamic–pituitary function (e.g., screening for symptoms, laboratory testing) with referral to endocrinology as indicated.
3.3.1. Recommendation
Patients who have undergone treatment for HNC should be:
Screened for sexual and intimacy dysfunction, possibly using questionnaires (Oxford Category 2A).
Offered referrals for therapy for sexual function and intimacy issues (Oxford Category 2A).
Assessed for pituitary dysfunction if they have received skull base irradiation (Oxford Category 2A).
3.4. Chronic pain
Chronic pain may be present in up to 45% of patients treated for HNC. 60 , 61 This can have profound effects on function and QOL among HNC survivors. There is no clear universally accepted or standardized tool for assessing chronic pain in patients with HNC. Likert scales or validated QOL instruments may be utilized as adjuncts to better assess the degree of disability experienced by the patient as a result of pain. 62 Over 20% of patients with chronic pain meet criteria for a major depressive episode. 60 Patients should be evaluated for depressive symptoms and referred accordingly in this context.
While pain is most often associated with treatment sequelae, it can also be a harbinger of recurrent disease. Up to 70% of patients with recurrent carcinoma will present with pain as the first symptom. 63 A comprehensive examination of the upper aerodigestive tract is imperative in the context of chronic post‐treatment pain—particularly in the setting of lancinating focal pain that radiates to the unilateral auricle or mandible.
Though definitions vary in the literature, a generally accepted definition of chronic pain is pain that extends beyond 3 months beyond the acute phase of treatment—provided that the acute effects of therapy have resolved. 64 Thirteen to fifty percent of patients undergoing HNC‐directed therapy with curative intent will utilize opioids chronically. Chronic use and abuse following treatment are associated with pre‐treatment opioid use, alcohol disorders, and nicotine dependence, as well as the employment of induction chemotherapy or tri‐modal therapy. Furthermore, depression and anxiety are associated with chronic opioid use after cancer treatment. 60 , 65 , 66 , 67 Chronic opioid use has also been associated with decreased disease‐free survival among patients with oral cavity carcinoma. 68
Given the opioid crisis that currently exists, patients (particularly those in high‐risk groups) should be screened for chronic opioid use and abuse at 3 months after completion of treatment. Opioid alternatives with demonstrated success in managing chronic pain include nonsteroidal anti‐inflammatory drugs and acetaminophen. Neuromodulators, such as gabapentin, have also demonstrated efficacy in the management of acute treatment‐related pain during HNC therapy. 69 , 70 and in managing chronic cancer‐related pain. 71
For those patients whose chronic opioid use or pain refractory to non‐opioid therapy remains a concern, referral to a palliative care or multidisciplinary pain management team is recommended. These specialized teams typically take a more holistic approach toward chronic pain and can provide integration of pharmacotherapy, physiotherapy, psychotherapy, and non‐traditional resources. These wide‐ranging treatment modalities may be beneficial in reducing opioid dependence, cost, and pain‐related morbidity in certain contexts. 72 , 73
3.4.1. Recommendation
Patients who have undergone treatment for HNC should be:
Screened by the survivorship team for chronic pain at routine intervals (NCCN Category 2A).
Assessed for the quality and severity of their pain using pain assessment tools by the team (NCCN Category 2A).
Evaluated for depressive symptoms in the presence of chronic pain by the team (NCCN Category 2A).
Undergo comprehensive evaluation to rule out recurrent disease as a cause of their pain by the team (Category 3B).
Screened for opioid abuse by the team (NCCN Category 2A).
Offered non‐opioid analgesics including nonsteroidal anti‐inflammatory agents, acetaminophen (Oxford Category 2C), neuromodulators (NCCN Category 2A), and acupuncture (Oxford Category 1A) through palliative and/or pain management consultations by the team.
Referred to palliative and/or pain management specialists for refractory or opioid‐dependent pain (Oxford Category 5) by their team.
3.5. Physical therapy
Functional musculoskeletal limitations after HNC‐directed therapy are relatively common, though often under‐reported and under‐treated. The subsequent disability can result in significant reductions in QOL and livelihood of HNC survivors. Indeed, a minority of HNC multidisciplinary teams refer patients for physical therapy on a routine basis. 74 The most common musculoskeletal issues include shoulder dysfunction, seen in up to 70% of patients undergoing neck dissection, and trismus, which may be observed in up to one‐half of patients undergoing radiotherapy to the pterygoid musculature/temporomandibular joint region. 75 , 76 Diminished neck range of motion is also commonly encountered post‐treatment. Baseline assessment of shoulder, temporomandibular joint, and neck mobility should be completed before therapy and at routine post‐treatment visits as a component of a comprehensive physical examination by HNC providers.
The prevalence of shoulder dysfunction after neck dissection has been variably reported and is associated with the extent of surgery. One meta‐analysis demonstrated impaired abduction is observed in greater than 90% of patients undergoing radical neck dissection and 23% of patients undergoing modified radical neck dissection. 77 Objective assessment of shoulder strength and range of motion may be performed using the standard Medical Research Council “0–5” scale and a handheld goniometer, but may not be practical to execute in a surgical clinic. 78 , 79 Several patient‐directed questionnaires regarding shoulder function after neck dissection have been developed to quantify disability and assess rehabilitation needs. 80 One review noted that the Neck Dissection Impairment Index is the only patient‐reported outcome scale to specifically address shoulder function in patients who have undergone neck dissection. 80
When subjective or objective shoulder dysfunction is identified, the efficacy of physiotherapy is somewhat equivocal based upon the existing medical literature. McGarvey and colleagues noted in a 2011 review that while well‐tolerated by patients, there is little evidence to suggest that physical therapy is effective in ameliorating shoulder dysfunction after neck dissection. 81 This same group published a follow‐up study in which patients were prospectively randomized to either a 12‐week shoulder strength program or a control group after neck dissection. They found that the group enrolled in physical therapy had significant improvements in active abduction of the shoulder at 3 months postoperatively. 82
With advancements in radiation therapy, transverse myelitis following HNC treatment is very uncommon (<1% at 54 Gy with conventional fractionation). 83 However, mild spinal cord toxicity can occur in the form of L'Hermitte's sign, which is characterized as electric‐shock sensations down the spinal cord and into the extremities upon neck flexion. 84 With historical conformal, field‐based radiation therapy for HNC, rates between 3% and 13% are reported. 85 Interestingly, recent work focusing on intensity‐modulated radiation therapy for thoracic and head and neck malignancy describes an increased incidence of L'Hermitte's sign between 15% and 29%. Typically, L'Hermitte's sign develops in the first few months following radiation therapy and seldom lasts more than 6 months. 86 Treatment for patients suffering from this condition remains conservative in nature, including a soft neck collar, corticosteroids, and hyperbaric oxygen. 87
Trismus is variably defined but has been most compellingly validated at a 35 mm inter‐incisor distance. 88 Among patients with oral cavity or oropharyngeal primary tumors, studies estimate that 25%–40% of patients will develop trismus post‐therapy with risk factors including the initial tumor stage, need for reconstruction, receipt of chemotherapy, and the receipt of radiation. 89 , 90 Three recent systematic reviews note that trismus is improved by exercise therapy and that early initiation and compliance resulted in more favorable outcomes. In one review the TheraBite device increased mouth opening significantly with an effect size of 2.6 compared to tongue blades (1.5), forced opening (1.1.), or microcurrent therapy (0.3). However, a return to normal (i.e., >35 mm) levels was not feasible in most cases. 91 , 92 , 93
3.5.1. Recommendation
Patients who have undergone treatment for HNC may need to be:
Screened for physical rehabilitation needs early in the post‐treatment phase and at regular intervals by their survivorship team (Oxford Category 5).
Assessed for trismus, depending on the site of their cancer, before and at regular intervals following therapy by their survivorship team (NCCN Category 2A).
Referred to exercise and physiotherapy professionals for structured rehabilitation after HNC treatment by their survivorship team (Oxford Category 5).
Undergoing shoulder physiotherapy after completion of neck dissection as early as feasible by their survivorship team (Oxford Category 1B).
3.6. Lymphedema
Lymphedema results from an increase in lymphatic load and the need for drainage exceeding the capacity of the lymphatic system to transport. In HNC, lymphedema occurs secondarily after disruption of lymphatics due to either tumor burden or cancer treatment (surgery, radiation, and/or chemotherapy). 94 , 95 The lack of adequate drainage results in the accumulation of lymphatic fluid within soft tissues and interstitial spaces, which in turn can lead to inflammation and fibrosis. This will manifest as significant dense soft tissue edema with either external edema (face and neck) or internal edema (tongue, pharynx, and larynx). Symptoms can include cosmetic deformity, neck and shoulder dysfunction, visual disturbance, dysphonia, dysphagia, and difficulty breathing. 7 Rarely, lymphedema can be significant enough to require tracheotomy. The reported incidence of lymphedema varies and is likely related to cancer site, stage, and severity/combination of treatment, with published rates ranging between 12% and 90%. 95 , 96
Several studies have demonstrated the impact lymphedema has on QOL in HNC survivors. 97 , 98 , 99 , 100 Given its functional and psychosocial impact, early diagnosis and management are important. Identification of head and neck lymphedema can be objectively determined by physical exam with discrete measurements and flexible fiberoptic laryngoscopy to identify internal lymphedema. 94 The Foldi lymphedema rating scale allows for a qualitative rating system although its original design was intended for extremity lymphedema. 94 To address this limitation, the University of Texas M. D. Anderson Cancer Center Head and Neck Lymphedema Rating Scale was adapted from the Foldi lymphedema rating scale, which allows for the capture of subtle presentations of HNC lymphedema with a simple 5 point rating scale. 94
Treatment of lymphedema involves improving lymphatic drainage through manual massage and compressive bandages. Manual lymphatic drainage (MLD) employs gentle circular massage to improve lymphatic flow. Complete decongestive therapy (CDT) represents the “gold standard” for management; it combines MLD, exercise, skincare, and compressive bandages and is performed by certified lymphedema therapists. This therapy often involves intense, near‐daily outpatient therapy for the first 2–4 weeks followed by a transition to home therapy; however, a motivated patient or caregiver may be able to provide appropriate and effective home care if frequent clinic visits are not feasible. 101 Exercises include range of motion exercises of the face, neck, shoulders, and arms as well as swallowing exercises. The compressive bandages are often applied after MLD, though some institutions support applying the bandages before and after MLD. 94 These therapies are effective in managing lymphedema in HNC patients. 102
3.6.1. Recommendation
Patients who have undergone treatment for HNC should be:
Evaluated for lymphedema after treatment for HNC by their survivorship team (NCCN Category 2A).
Referred for certified lymphedema therapists for management of lymphedema via CDT by their survivorship team (NCCN Category 2A).
3.7. Psycho‐oncology
The diagnosis and treatment of HNC can have a devastating impact on psychosocial function. As HNC arises in cosmetically and functionally critical areas, highly visible and socially significant changes are common following treatment. 103 , 104 The visible disfigurement, impaired smile, dysphagia, dysphonia, and fear of recurrence can result in substantial psychosocial morbidity, including body image concerns, depression, and anxiety. 104 , 105 , 106 , 107 , 108
3.7.1. Body image disturbance
Body image disturbance (BID) is a multidimensional phenomenon characterized by a displeasing self‐perceived change in appearance and/or function. 109 , 110 , 111 Body image concerns are expressed in up to 75% of patients with HNC; an increase in prevalence and severity early in the survivorship period is associated with social isolation, stigmatization, depression, and decreased QOL. 103 , 104 , 110 As a result, HNC survivors should be periodically assessed for BID. 7 Unfortunately, there is a lack of psychometrically sound, patient‐centered tools to measure BID in HNC survivors. Improved measurement strategies represent an area of clinical and scientific need. 112 Management of BID remains a critical component of HNC survivorship care. 7 Affected patients should be referred for psycho‐oncologic management, although further work is needed to develop effective interventions that specifically target BID in HNC survivors. 7 , 113 , 114 , 115
3.7.2. Distress, depression, and anxiety
Distress, depression, and anxiety are prevalent in HNC survivors, who are at higher risk for developing psychological sequelae than survivors with other types of cancer. 116 As a result, HNC survivors should be assessed periodically for distress, depression, and anxiety using validated screening tools (e.g., NCCN Distress Thermometer, Hospital Anxiety and Depression Scale, Beck Depression or Anxiety Inventories, Generalized Anxiety Disorder‐7, and Patient Health Questionnaire‐9). 7 , 117 , 118 , 119 For survivors with clinical findings of distress, depression, or anxiety, recommended treatments include psychotherapy, pharmacotherapy, and referral to psycho‐oncology. Unfortunately, mental health support is frequently underutilized. 7 , 120 There is evidence that preventative interventions, before initiation of HNC treatment, may decrease the risk of depression in HNC survivors. A landmark trial found that prophylactic escitalopram given to HNC patients at treatment initiation reduced the risk of depression by more than 50%, with beneficial effects seen primarily in those treated with definitive radiation‐based approaches. 121 Other multidisciplinary HNC programs have integrated health psychologists in the multidisciplinary treatment team to aid in pre‐treatment evaluation and identification of psychosocial risk factors, with close follow‐up with intervention as indicated. This integration has been well received by patients, caregivers, and medical professionals. 122
Although the body of literature remains small for HNC, there is evidence to suggest support group participation in patients with breast, lung, and colon cancer has been found to decrease anxiety and depression. 123 Furthermore, with the advent of internet technology, virtual support groups have been associated with reductions in physiological and psychological stress. 124 Online gatherings can promote social support by increasing functional status, personal empowerment, and self‐esteem as well as decrease feelings of depression, emotional distress, helplessness, and social isolation. 125 In a study of 199 patients with HNC, better health‐related QOL was associated with being a member of an online support group for a longer period (B = 0.07, p < .05). With these notable benefits, health care professionals should certainly encourage and refer patients to seek out regional and support groups and information related to their clinical condition.
Given the tremendous psychosocial burden of HNC for survivors, suicide is a critically important issue. 126 Up to 16% of HNC survivors report suicidal ideation within the first year, with the highest risk being in those with substance abuse and prior psychiatric disorders. 127 The adjusted suicide risk in HNC survivors ranges from 38 to 60/100,000 person‐years; HNC survivors are 2‐fold more likely to die from suicide than patients with other types of cancer and 3‐fold more likely than the general US population. 27 , 128 Even more concerning, the risk of suicide in HNC survivors is increasing over time. 27 There is thus an urgent need to develop novel strategies to prevent suicide in HNC survivors and integrate them into routine clinical care. 127
3.7.3. Recommendation
Patients who have undergone treatment for HNC should be:
Screened for BID concerns by their survivorship team (NCCN Category 2A).
Referred to psychology or psychiatry for the management of BID as indicated by their team (NCCN Category 2A).
Assessed for distress, depression, and/or anxiety at regular intervals with a validated questionnaire by their team (Oxford Category 1A).
Referred to psychology or psychiatry if distress, depression, and/or anxiety are present (Oxford Category 1B).
3.8. Hearing loss
HNC treatment can have a profound impact on auditory function. For cancers involving the ear, nasopharynx, skull base, and paranasal sinuses, both surgery and radiation can have a significant effect on the auditory pathway. Surgical resection of the external auditory canal (EAC), tympanic membrane, and/or middle ear space will result in conductive hearing loss (CHL). Similarly, surgery of the paranasal sinuses, nasopharynx, and/or skull base can lead to trauma, edema, or dysfunction of the Eustachian tube leading to CHL from serous otitis media.
Radiation therapy to the head and neck may also lead to both CHL and sensorineural hearing loss (SNHL). CHL may result via radiation changes to the Eustachian tube apparatus, additionally, external toxicity to the auricle or EAC may lead to otitis externa, EAC stenosis, or ossicular necrosis. 129 , 130 Radiation to the inner ear can also lead to SNHL anywhere along the auditory pathway from the brainstem to the cochlea. Studies have identified that the incidence of ototoxicity is associated with radiation dose and technique, patient age, and the combination of radiation with chemotherapy. Studies suggest a radiation dose greater than 40–45 Gy to the cochlea may be associated with a higher incidence of irreversible ototoxicity, though some have described SNHL with radiation doses as low as 20–25 Gy. 130 , 131 , 132 , 133 , 134 Reports of ototoxicity also range from 0% to 43% including a variety of radiation techniques. 131 , 132 , 135
Chemotherapy, particularly platinum‐based agents (primarily cisplatin but also carboplatin and oxaliplatin), can have strong effects on the inner ear and may result in high‐frequency SNHL and tinnitus. 129 These drugs have a cumulative dose‐dependent effect; a total dose of more than 100 mg/m2 of cisplatin places patients at risk for developing high‐frequency SNHL. 7 Ototoxic effects related to chemotherapy can be characterized by cochlear dysfunction (hearing loss, tinnitus, or hyperacusis), vestibular dysfunction (vertigo, dizziness, or imbalance), or a combination of phenomena. 136 Tinnitus, defined as a subjective perception of sound without an external source can lead to a significant negative impact on psychological status and QOL; it has been estimated that 40% of patients receiving chemotherapy develop tinnitus. 137 Vestibular symptoms can trigger a deterioration of QOL including interference with driving, riding a bicycle, and other activities as well as psychological symptoms. 138 Hearing loss related to cochleotoxicity may present immediately or in a delayed fashion and can be progressive, affecting as many as 18% of patients who underwent cisplatin therapy. 139 The presence of hearing loss in the middle of treatment may lead to consideration for an alternative chemotherapy regimen.
Chemoprotective agents have been studied, though none are standard of care. 129 Treating and managing ototoxicity are dependent on the cause and location. CHL secondary to edema will often resolve over time, while losses secondary to scarring/fibrosis or Eustachian tube dysfunction may require surgical approaches to relieve the focal obstruction. Serous otitis media may also resolve over time but those patients with persistent CHL for 3–6 months may benefit from either simple myringotomy or tympanostomy tube placement. 129 Unfortunately, there is no established treatment for SNHL with mixed results associated with high‐dose steroid administration or hyperbaric oxygen therapy. As outlined by the American Academy of Audiology, patients undergoing platinum‐based chemotherapy should undergo pure tone audiometry a few months following completion of chemotherapy followed by monitoring for at least 2 years in the setting of concomitant radiation therapy as hearing loss may progress over time (https://www.audiology.org/publications-resources/document-library/ototoxicity-monitoring). In addition, post‐treatment SNHL rehabilitation relies on the use of hearing amplification (hearing aids). 131 For patients with profound bilateral SNHL, cochlear implantation is effective with no difference in outcomes as compared to those who did not previously receive chemotherapy or radiation. 129 , 131
3.8.1. Recommendation
Patients who have undergone platinum‐based chemotherapy and/or radiation therapy for HNC should be:
Evaluated yearly for hearing loss via pure tone audiometry for at least 2 years (NCCN Category 2A).
3.9. Caregivers
Caregivers of patients with HNC face a unique burden in their role caring for HNC survivors. 140 , 141 , 142 Caregivers are tasked with coordinating complicated and demanding treatment regimens, survivorship care schedules, performing medical and nursing tasks to manage treatment toxicities in the setting of nutritional, speech, and addiction challenges, assisting patients with time‐consuming self‐management interventions to mitigate long‐term treatment effects, and managing ongoing psychosocial, emotional, and financial concerns. 6 , 140 , 143 , 144 , 145 , 146 , 147 , 148 , 149 , 150 , 151 , 152 Despite this impressive array of supportive tasks, caregivers typically receive little or no formal training. 151 It is thus not surprising that HNC caregiver distress is quite high, equaling or exceeding the distress levels of HNC patients for whom they care. 151 , 153 Caregiver psychological distress is related to task burden (frequency and complexity) of caring for the HNC survivors as well as caregiver unmet needs, which often differ from and are put aside for those of the survivor. 154 , 155 These unmet needs include balancing competing roles/responsibilities, making time for self‐care, finding effective strategies for encouraging patient self‐care, changes in intimacy and social/leisure activities, fears/anxiety regarding cancer recurrence, and the lack of coping strategies for others not acknowledging “the impact that having a partner experience cancer has had on my own life.” These findings suggest the need for information, counseling, and reassurance that partners and caregivers may require through education, interventions, and sharing of assessment and management plan by the clinicians. It is important to encourage caregivers to pause and take the time to take care of themselves, as this will allow them to continue taking care of HNC survivors. Caregivers should be encouraged to seek help from others, recognize the limits of their abilities and endurance, and take pride in the care they provide for HNC survivors. For physical health maintenance, they should similarly see their PCP regularly, adopt a healthy active lifestyle, and undergo routine age‐appropriate and gender‐appropriate cancer screening. For mental health concerns, caregivers should be counseled on a wide variety of emotions, such as guilt, anger, depression, and anxiety, particularly around the fear of cancer recurrence. Caregivers should also be encouraged to speak openly to their loved ones or talk with a counselor, the oncology team, PCP, close friends, or support groups. There remains an important clinical need for additional research to develop effective supportive interventions targeting the unique concerns of HNC caregiver–survivor dyads. 156 , 157 , 158 , 159
3.9.1. Recommendation
Care of patients who have undergone treatment for HNC should be:
Inclusive of partners and caregivers in all aspects of HNC care provided by the oncology team (Oxford Category 5).
3.10. Social support groups
The Commission on Cancer of the American College of Surgeons 2020 Standard 5.2 necessitates policies and procedures that are in place to ensure patient access to psychosocial services either on‐site or by referral. 160 Cancer support groups provide an environment for patients, families, and caregivers to share their experiences and be able to cope with all aspects of cancer care. Peer support can also help patients, families, and caregivers feel supported by a community or navigate the complex process of managing a life‐altering illness such as cancer. 161
There is a dearth of research examining support groups for HNC. However, as mentioned previously, online support groups often provide low‐threshold advice for acute problems to patients with high distress. 162 Patient support groups play an important role in the short‐term and long‐term management of HNC and should be offered in all programs offering HNC care.
3.10.1. Recommendation
Patients who have undergone treatment for HNC should be:
Educated by their survivorship team on social support groups and their impact on understanding the wide‐ranging sequelae of oncologic treatment (Oxford Category 5).
3.11. Return to work
Returning to work after cancer treatment can be a daunting task for patients. For HNC patients, simple tasks such as eating in public, fatigue, radiation dermatitis, and oral ulcers can make functioning in a public work environment difficult and at times, embarrassing. Due to the impact on the patient's speech and voice, communication may be difficult with coworkers and performing daily work‐related duties. It is imperative for employers to support the employees' successful return to work and provide them engagement opportunities or accommodations to change the nature of their job. Cancelliere and colleagues demonstrated common factors associated with positive return to work outcomes include higher education and socioeconomic status, higher self‐efficacy and optimistic expectations for recovery and return to work, lower severity of injury/illness, return to work coordination, and multidisciplinary interventions that include the workplace and stakeholders. On the other hand, factors associated with negative return‐to‐work outcomes were advanced age, female gender, higher pain or disability, depression, higher physical work demands, previous sick leave and unemployment, and activity limitations. 163
It is important for physicians caring for HNC patients to understand medical disability and the protections afforded by the United States law. When employees are injured or disabled or become ill on the job, they may be entitled to medical and/or disability‐related leave under two federal laws: the Americans with Disabilities Act (ADA) and the Family and Medical Leave Act (FMLA). An individual with a disability is defined as a person who: (1) has physical or mental impairment that substantially limits one or more major life activities; (2) has a record of such an impairment; or (3) is regarded as having such an impairment. 164 The ADA does not specifically require employers to provide medical or disability‐related leave. However, it does require employers to make reasonable accommodations for qualified employees with disabilities if necessary to perform essential job functions or to benefit from the same opportunities and rights afforded employees without disabilities. 164 Accommodations can include modifications to work schedules (e.g., 8‐h shifts instead of 12‐h shifts or 5‐day work week, no overtime), restricted duties (i.e., limitations on lifting), or medical leave. 164
3.11.1. Recommendation
Patients who have undergone treatment for HNC should be:
Counseled by their survivorship team on medical disability rights and protections afforded by federal law (Oxford Category 5).
3.12. Financial burden
As a result of the convergence between improved oncologic outcomes, increased cost of cancer care, and continued shifting of cost burden to the patient, there has been a growing awareness of the devastating financial toxicity that can burden HNC survivors and their caregivers. 165 , 166 Financial toxicity is a multidimensional construct: (1) material hardship that results from increased out‐of‐pocket costs and lower‐income, (2) psychological distress resulting from material hardship, and (3) compensatory coping strategies that families develop in response to the financial cost of cancer and its treatment. 167 , 168
Patients with HNC are disproportionately burdened by the destructive consequences of financial toxicity relative to other types of cancer 165 HNC‐related financial toxicity is especially common, with cumulative incidence estimates ranging from 40% to 69%. 169 , 170 Patients with HNC who are particularly high‐risk include those with Medicaid, decreased wealth, higher perceived social isolation, and higher total out‐of‐pocket treatment costs. 165 , 170 Financial toxicity is thus yet another aspect of the cancer care continuum in which significant and disturbing disparities in outcomes exist. 166 Financial toxicity can be assessed using quantitative measures (e.g., financial burden, ratio of out‐of‐pocket health‐related spending to household income) or patient‐reported measures such as the Comprehensive Score for financial Toxicity questionnaire. 171 , 172 The downstream impact of financial toxicity on patients with cancer is significant, as increased levels of financial toxicity are associated with decreased adherence to cancer treatment, increased symptom burden, decreased QOL, higher unmet needs for HNC survivors, and decreased survival. 173 , 174 , 175 , 176 , 177 Financial toxicity is also a common problem for HNC caregivers and one that can have a devastating impact. 152 , 155
Although many sociodemographic determinants of financial toxicity are beyond the control of the multidisciplinary HNC team, providers can help by increasing awareness that financial toxicity is common following treatment for HNC, identifying at‐risk patients, alerting patients and caregiver to resources for its management, and making referrals to social workers and financial navigators who may further facilitate engagement with social welfare systems to help assess and manage HNC‐related financial toxicity. 152 , 166
3.12.1. Recommendation
Patients who have undergone treatment for HNC:
Can be referred to social workers and financial navigators by their survivorship team to navigate health care costs associated with HNC care (Oxford Category 5).
4. HEALTH MAINTENANCE
4.1. Primary care physician
PCPs have a critical and continuing role in the management of acute and chronic care of HNC survivors, supporting the diagnosis and management of HNC, and promoting good health behaviors. It is critical that the oncology team communicate regularly with the PCP. A treatment summary, risk of recurrence, short‐term and long‐term effects of treatment, and an individualized surveillance/survivorship plan should be available to PCP and HNC providers. Several documents exist to help communicate this information including those published by AHNS and ASCO. 6 , 7 Of note, the use of a standardized survivorship care plan has been integrated into the 2020 Commission on Cancer Standards for member organizations (Standard 4.8). 160
PCPs should be aware of the signs, symptoms, and physical findings of cancer recurrence or second malignancy and promptly refer patients back to the oncology team for further investigation of any suspicious findings. The PCP should engage in discussion and counseling regarding cardiovascular health, smoking cessation, alcohol consumption, diet/nutrition/weight, regular physical activity, dental hygiene, and mental/psychosocial health. General well‐being and QOL should be assessed including weight, energy/fatigue, mood (depression), sleep, activity (work, avocation). As HNC survivors are at an increased risk of developing second primary malignancies, both in head and neck and non‐head and neck sites, the PCP should also perform age‐appropriate and gender‐appropriate screening of HNC survivors for other neoplasms, as they would for patients in the general population. 178 , 179 , 180 , 181 The multidisciplinary cancer team and/or PCP should also routinely evaluate thyroid function by measuring TSH/T4 every 6–12 months in patients with a surgically compromised thyroid gland or history of neck irradiation. 182 , 183 , 184
4.1.1. Recommendation
Patients who have undergone treatment for HNC should be:
Followed by their PCP for age‐appropriate and gender‐appropriate screening of other neoplasms, general health, and well‐being (Oxford Category 2A).
4.2. Dental care
HNC survivors experience a wide range of changes that increase their risk of oral and dental diseases. 185 , 186 Dental providers have a role in oral and dental care from diagnosis to survivorship of HNC patients, establishing oral health and preventive programs, and manage HNC treatment complications. A comprehensive history and physical examination, including issues related to primary cancer treatment and potential survivorship symptoms, should be addressed at each dental visit. An understanding of prior cancer therapy, especially radiation dose and treatment fields, is required. The frequency of dental visits should be individualized, often requiring more frequent visits. At each visit, the history should address head and neck, oral/dental symptoms, tobacco/alcohol cessation, and surveillance for potential recurrence or second primary cancers. Symptoms include sensory changes (pain, taste), xerostomia, and dysphagia. Oral and dental evaluation should focus on mucosal and gingival integrity, oral hydration, dental demineralization/caries, periodontal status, oral hygiene, changes due to tissue fibrosis (tongue mobility, presence of trismus, oral aperture), local infection, and exposed bone. Diet and nutritional status should also be assessed. Dental prophylaxis should include brushing with remineralizing toothpaste, dental floss utilization, and fluoride supplementation. Consultation with the oncology team and review of the radiation fields are indicated prior to any invasive dental treatment is performed. Although there is limited evidence, consideration may be given to hyperbaric oxygen therapy in HNC patients with a prior history of radiotherapy who require dental extractions. 187 Following this, patient management and referral for oral and dental care can be performed as deemed appropriate. A recent study reported only 50% of HNC survivors received recommended post‐treatment oral/dental care, which further highlights the need for a more coordinated and enhanced multidisciplinary approach in educating and helping patients utilize oral/dental supportive care. 188
4.2.1. Recommendation
Patients who have undergone treatment for HNC:
Should be counseled by their survivorship team to maintain close follow‐up with a dental professional (Oxford Category 1B).
Can be encouraged by their survivorship team to avoid tobacco and alcohol to minimize the risk of dental disease (Oxford Category 5).
4.3. Substance abuse
According to pooled analyses of United States HNC cohorts (n = 7570, 1981–2006), 58% of HNC survivors were current smokers at diagnosis and 37% reported consuming >3 alcoholic beverages per day. 189 Over 50% of HNC survivors continue smoking after diagnosis and persistent tobacco or alcohol dependence has been associated with an increased risk of perioperative complications, recurrence, second primary cancers, and mortality in HNC survivors. 190 , 191 , 192 , 193 , 194 , 195 , 196 An estimated 10% of HNC survivors report ever using marijuana. 197 While studies have not identified a clear association between marijuana and HNC, some have reported an association with oropharyngeal squamous cell carcinoma (OPSCC). 196 , 197 Approximately 10% of HNC survivors exhibit new opioid dependence 6 months after treatment. In the general population, opioid use disorder, defined as a problematic pattern of utilizing opioids that leads to significant distress or impairment over 12 months, is associated with increased mortality from accidental overdose, trauma, suicide, or infectious disease. 65 , 67 , 198 Evidence demonstrating that directed tobacco or alcohol treatment in HNC patients yields improved abstinence rates compared to usual care is limited and underpowered. 199 , 200 , 201 Hence, guideline recommendations for HNC and other cancer patients are inferred from data confirming the efficacy of treatment in adults generally. 202 , 203 Timely management of tobacco or alcohol dependence before oncologic treatment is preferred and may associate most strongly with reduced complications, recurrence, and mortality. 191 , 192 , 193 , 194 , 195 , 196 In addition to managing tobacco dependence, clinicians should assess and treat or refer HNC survivors with alcohol or opioid abuse. Additional studies evaluating (1) interventions to improve abstinence among tobacco or alcohol‐dependent HNC survivors and (2) management of opioid abuse in HNC survivors are needed.
4.3.1. Recommendation
Patients who have undergone treatment for HNC should be:
Referred by their survivorship team for tobacco cessation counseling and alcohol abstinence resources (Oxford Category 1A).
Encouraged by their survivorship team to avoid tobacco and alcohol consumption (Oxford Category 5).
4.4. Physical activity/exercise
HNC survivor participation in physical activity, exercise, and strength training is poorly understood. Early studies describe variable findings: 19%–63% of patients reported any vigorous or moderate levels of physical activity and 9%–40% were compliant with national physical activity guidelines. 204 , 205 Several small cohort and randomized‐controlled studies have evaluated physical activity or resistance training interventions in HNC survivors during or after oncologic treatment. Most have reported significant improvements in at least some symptoms, health‐related QOL domains, or objective measures of weight, muscle mass, physical function (i.e., 6‐min walk test), and range of motion. 206 , 207 , 208 , 209 , 210 While physical activity may associate with improved overall, disease‐free, or disease‐specific survival among patients with other types of cancer (i.e., breast and colorectal cancer), these outcomes have not been evaluated in HNC patients. 211 , 212 , 213 Therefore, the authors support the ACS and ASCO survivorship guidelines on physical activity in HNC survivors: (a) avoid inactivity and return to normal daily activities as soon as possible following diagnosis, (b) aim for at least 150 min of moderate or 75 min of vigorous aerobic exercise per week, (c) include strength training exercises at least 2 days/week. 7 , 8 , 51 Further evaluation of the implementation of increased physical activity into routine clinical care and the optimal timing, intensity, and duration of exercise interventions in relation to oncologic treatment will be necessary. Assessment of associations between exercise and HNC recurrence or survival is also warranted.
4.4.1. Recommendation
Patients who have undergone treatment for HNC should be:
Encouraged by their survivorship team to engage in regular physical activity and exercise (Oxford Category 1B).
4.5. Respiratory therapy
HNC survivors may have significant needs for respiratory therapy secondary to their disease process. Following treatment, surgical changes to native anatomy as well as mucositis, edema, or fibrosis secondary to chemotherapy and radiation can impact a patient's ability to protect their airway. Comparing patients that aspirate post‐therapy to those that do not, there is evidence that expiratory pressure and cough is depressed in patients that aspirate. 214 Expiratory muscle strength training (EMST) has been shown to improve swallowing function and airway protection in patients with neurodegenerative disorders and several case series demonstrated improvement in swallowing parameters in HNC patients after treatment. 215 , 216 , 217 EMST involves expiration against a spring‐loaded relief valve that can provide a variable amount of resistance. Over time, the resistance can be increased as the patient's expiratory muscles strengthen, and an increased maximal expiratory pressure can be generated.
4.5.1. Tracheostomy care
Patients who are tracheostomy‐dependent in the post‐surgical or post‐treatment period require specific care. Humidification can reduce the thickness and quantity of secretions. Heat and moisture exchange (HME) filters can also provide humidification while allowing the patient to be active and mobile. Additionally, suction equipment may be necessary to clear secretions. The inner cannula should be replaced or cleaned on a routine basis to clear any crusting or retained mucus along the tube's path. The degree and frequency of suctioning or inner cannula maintenance will be dependent on several patient factors including mucus quality, amount produced, and aspiration status. 218 As HNC patients recover function after treatment, consideration can be given to decannulation or removal of the tracheostomy tube if the patient is able to breathe comfortably with the tracheostomy tube occluded and demonstrate a lack of overt aspiration. One‐way speaking valves such as the Passy Muir Valve may provide an interim stage between tracheostomy dependence and decannulation as well as facilitation of verbal communication and cough.
4.5.2. Laryngectomy care
Patients who are status post‐laryngectomy permanently bypass oral and nasal humidification as well as filtration mechanisms of inspired air with a permanent stoma between the tracheal wall and anterior cervical skin. As such, patients with a laryngectomy stoma will require a suction apparatus or evidence of strong cough reflex as well as a method of humidification. In the setting of patient self‐care, a handheld mirror can be helpful with cleaning and suctioning. Additionally, humidification can be provided either via an external humidified air (delivered via a mist collar) or by wearing an HME device which can fit over the stoma and can reduce the reliance on pulmonary toilet and suctioning. 219 , 220 Patients with a tracheoesophageal puncture for phonation will also need to keep the prosthesis clean of crusting and debris with a bristle brush. Should the tracheoesophageal prosthesis become incompetent, it will start leaking esophageal contents into the airway, resulting in frank aspiration and mandating replacement. Some patients may require routine use of a “laryngectomy tube” to maintain stoma patency and minimize stomal stenosis; these stents should also be removed and cleaned of crusting and debris regularly. 219
4.5.3. Recommendation
Patients who have undergone treatment for HNC should be:
Educated in the tracheostomy and laryngectomy stoma care if either is present by their survivorship team (Oxford Category 5).
4.6. HPV counseling
The incidence of HPV‐associated OPSCC is rising; the disease now comprises 70% of OPSCC in the United States. Despite this, there is a significant gap in knowledge and awareness about HPV and HPV‐associated OPSCC among both general adults and HPV OPSCC survivors. 221 , 222 , 223 , 224 , 225 HPV OPSCC survivors report reduced sexual activity, 53 informational, emotional, and psychosocial needs related to their diagnosis including self‐blame, guilt, anxiety, distress, and concern about transmitting HPV to others. 224 , 225 , 226 Broad patient information needs may include knowledge regarding: the prevalence, nature, and transmission of HPV infection, risk to current and future partners, and utility of preventative vaccination. Recent publications have provided helpful resources for both clinicians and patients. 227 , 228 To better understand general knowledge and advocacy intent, a cross‐sectional study (n = 200) of HPV‐related cancer survivors (i.e., cervical, vaginal, vulvar, penile, anal, or oropharyngeal) revealed only 33% of respondents were aware that their cancer was HPV‐associated and 57% reported that the HPV vaccine is safe. Of those participants who understood that their cancer was HPV‐related, they were significantly more likely to have their children vaccinated, recommend the HPV vaccine, serve as a peer mentor for HPV‐related cancer patients, and advocate for increasing vaccination rates. Raising awareness for HPV‐related cancer allows for the empowerment of survivors, which results in the development of key opinion leaders in the realm of HPV vaccine advocacy. 229 Thus, clinicians should routinely attend to the informational needs of HPV‐associated OPSCC patients and survivors as well as offer treatment or referral for the emotional, psychosocial, or sexual needs of HPV OPSCC survivors. 230 , 231 , 232 Further evaluation of HPV counseling implementation into routine care and corollary outcomes will be needed.
4.6.1. Recommendation
Patients who have undergone treatment for HPV OPSCC should be:
Counseled by their survivorship team on the sequelae of HPV‐related oncologic disease as well as the clinical evidence relating to the benefits of HPV vaccine (Oxford Category 5).
5. CONCLUSION
Cancer survivorship necessarily incorporates a multidisciplinary approach centered around the patient and the caregiver, led by oncologists (surgical, medical, and radiation) and primary care providers and managed by a wide variety of allied health providers including but not limited to therapists (speech, physical, occupational, and respiratory), dieticians, social workers, dentists, and psycho‐oncologists. With increasing survival related to HNC treatment, addressing post‐treatment concerns will have a profound impact on patient quality of life and survival. There continues to be a need to define effective and efficient programs that can coordinate this multidisciplinary effort toward survivorship.
CONFLICT OF INTEREST
None of the authors have any conflicts of interest.
ACKNOWLEDGMENTS
The authors would like to acknowledge Jenna Loewus and Isabella Yeung for administrative support.
Goyal N, Day A, Epstein J, et al. Head and neck cancer survivorship consensus statement from the American Head and Neck Society. Laryngoscope Investigative Otolaryngology. 2022;7(1):70‐92. doi: 10.1002/lio2.702
This manuscript represents a consensus statement from the Survivorship, Supportive Care & Rehabilitation Service of the American Head and Neck Society.
Contributor Information
Neerav Goyal, Email: ngoyal1@pennstatehealth.psu.edu.
Nishant Agrawal, Email: na@uchicago.edu.
REFERENCES
- 1. Siegel RL, Miller KD, Goding Sauer A, et al. Colorectal cancer statistics, 2020. CA Cancer J Clin. 2020;70(3):145‐164. [DOI] [PubMed] [Google Scholar]
- 2. National Comprehensive Cancer Network . Head and neck cancer. 2020. https://www.nccn.org/professionals/physician_gls/pdf/head-and-neck.pdf
- 3. Chaturvedi AK, Engels EA, Pfeiffer RM, et al. Human papillomavirus and rising oropharyngeal cancer incidence in the United States. J Clin Oncol. 2011;29(32):4294‐4301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Patel MA, Blackford AL, Rettig EM, Richmon JD, Eisele DW, Fakhry C. Rising population of survivors of oral squamous cell cancer in the United States. Cancer. 2016;122(9):1380‐1387. [DOI] [PubMed] [Google Scholar]
- 5. Mullan F. Seasons of survival: reflections of a physician with cancer. N Engl J Med. 1985;313(4):270‐273. [DOI] [PubMed] [Google Scholar]
- 6. Miller MC, Shuman AG, American Head and Neck Society's Committee on Survivorship . Survivorship in head and neck cancer: a primer. JAMA Otolaryngol Head Neck Surg. 2016;142(10):1002‐1008. [DOI] [PubMed] [Google Scholar]
- 7. Cohen EE, LaMonte SJ, Erb NL, et al. American Cancer Society head and neck cancer survivorship care guideline. CA Cancer J Clin. 2016;66(3):203‐239. [DOI] [PubMed] [Google Scholar]
- 8. Nekhlyudov L, Lacchetti C, Davis NB, et al. Head and neck cancer survivorship care guideline: American Society of Clinical Oncology clinical practice guideline endorsement of the American Cancer Society guideline. J Clin Oncol. 2017;35(14):1606‐1621. [DOI] [PubMed] [Google Scholar]
- 9. Ball C, Sackett D, Phillips B, Haynes B, Straus S. Oxford Centre for evidence‐based medicine – levels of evidence. 2009. Accessed February 4, 2020. https://www.cebm.net/2009/06/oxford-centre-evidence-based-medicine-levels-evidence-march-2009/
- 10. Brennan KE, Hall SF, Owen TE, Griffiths RJ, Peng Y. Variation in routine follow‐up care after curative treatment for head‐and‐neck cancer: a population‐based study in Ontario. Curr Oncol. 2018;25(2):e120‐e131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Watanabe A, Hosokawa M, Taniguchi M, Sasaki S. Periodic pharyngolaryngoscopy detects early head and neck cancer and improves survival in esophageal cancer. Ann Thorac Surg. 2003;76(5):1699‐1705. [DOI] [PubMed] [Google Scholar]
- 12. Zhou H, Zhang J, Guo L, Nie J, Zhu C, Ma X. The value of narrow band imaging in diagnosis of head and neck cancer: a meta‐analysis. Sci Rep. 2018;8(1):515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Siddiqui F, Movsas B. Management of radiation toxicity in head and neck cancers. Semin Radiat Oncol. 2017;27(4):340‐349. [DOI] [PubMed] [Google Scholar]
- 14. Galloway TA, Robert J. Management and prevention of complications during initial treatment of head and neck cancer. 2020. https://www.uptodate.com/contents/management-and-prevention-of-complications-during-initial-treatment-of-head-and-neck-cancer
- 15. Seretny M, Currie GL, Sena ES, et al. Incidence, prevalence, and predictors of chemotherapy‐induced peripheral neuropathy: a systematic review and meta‐analysis. Pain. 2014;155(12):2461‐2470. [DOI] [PubMed] [Google Scholar]
- 16. Staff NP, Grisold A, Grisold W, Windebank AJ. Chemotherapy‐induced peripheral neuropathy: a current review. Ann Neurol. 2017;81(6):772‐781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Smith EM, Pang H, Cirrincione C, et al. Effect of duloxetine on pain, function, and quality of life among patients with chemotherapy‐induced painful peripheral neuropathy: a randomized clinical trial. JAMA. 2013;309(13):1359‐1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Fallon MT, Storey DJ, Krishan A, Weir CJ, Mitchell R, Fleetwood‐Walker SM, Scott AC, Colvin LA. Cancer treatment‐related neuropathic pain: proof of concept study with menthol—a TRPM8 agonist. Supportive Care in Cancer. 2015;23:(9):2769‐2777. 10.1007/s00520-015-2642-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Anand P, Elsafa E, Privitera R, et al. Rational treatment of chemotherapy‐induced peripheral neuropathy with capsaicin 8% patch: from pain relief towards disease modification. J Pain Res. 2019;12:2039‐2052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Duregon F, Vendramin B, Bullo V, et al. Effects of exercise on cancer patients suffering chemotherapy‐induced peripheral neuropathy undergoing treatment: a systematic review. Crit Rev Oncol Hematol. 2018;121:90‐100. [DOI] [PubMed] [Google Scholar]
- 21. Sacco AGCC, Sanghvi P, et al. Development of care pathways to standardize and optimally integrate multidisciplinary care for head and neck cancer. Oncol Issues. 2018;33(6):28‐44. [Google Scholar]
- 22. Taplin SH, Clauser S, Rodgers AB, Breslau E, Rayson D. Interfaces across the cancer continuum offer opportunities to improve the process of care. J Natl Cancer Inst Monogr. 2010;2010(40):104‐110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ng SP, Pollard C 3rd, Berends J, et al. Usefulness of surveillance imaging in patients with head and neck cancer who are treated with definitive radiotherapy. Cancer. 2019;125(11):1823‐1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ward MC, Lee NY, Caudell JJ, et al. A competing risk nomogram to predict severe late toxicity after modern re‐irradiation for squamous carcinoma of the head and neck. Oral Oncol. 2019;90:80‐86. [DOI] [PubMed] [Google Scholar]
- 25. Chen MC, Kuan FC, Huang SF, et al. Accelerated risk of premature ischemic stroke in 5‐year survivors of nasopharyngeal carcinoma. Oncologist. 2019;24(9):e891‐e897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Eytan DF, Blackford AL, Eisele DW, Fakhry C. Prevalence of comorbidities and effect on survival in survivors of human papillomavirus‐related and human papillomavirus‐unrelated head and neck cancer in the United States. Cancer. 2019;125(2):249‐260. [DOI] [PubMed] [Google Scholar]
- 27. Osazuwa‐Peters N, Simpson MC, Zhao L, et al. Suicide risk among cancer survivors: head and neck versus other cancers. Cancer. 2018;124(20):4072‐4079. [DOI] [PubMed] [Google Scholar]
- 28. Massa ST, Rohde RL, McKinstry C, et al. An assessment of patient burdens from head and neck cancer survivorship care. Oral Oncol. 2018;82:115‐121. [DOI] [PubMed] [Google Scholar]
- 29. Monterosso L, Platt V, Bulsara M, Berg M. Systematic review and meta‐analysis of patient reported outcomes for nurse‐led models of survivorship care for adult cancer patients. Cancer Treat Rev. 2019;73:62‐72. [DOI] [PubMed] [Google Scholar]
- 30. Thom B, Boekhout AH, Corcoran S, Adsuar R, Oeffinger KC, McCabe MS. Advanced practice providers and survivorship care: they can deliver. J Oncol Pract. 2019;15(3):e230‐e237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Absolom K, Eiser C, Michel G, et al. Follow‐up care for cancer survivors: views of the younger adult. Br J Cancer. 2009;101(4):561‐567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Brennan ME, Butow P, Marven M, Spillane AJ, Boyle FM. Survivorship care after breast cancer treatment – experiences and preferences of Australian women. Breast. 2011;20(3):271‐277. [DOI] [PubMed] [Google Scholar]
- 33. Cancer Survivorship Algorithms . MD Anderson Cancer Center. Accessed December 1, 2020. https://www.mdanderson.org/for-physicians/clinical-tools-resources/clinical-practice-algorithms/survivorship-algorithms.html
- 34. Wall LR, Ward EC, Cartmill B, Hill AJ. Physiological changes to the swallowing mechanism following (chemo)radiotherapy for head and neck cancer: a systematic review. Dysphagia. 2013;28(4):481‐493. [DOI] [PubMed] [Google Scholar]
- 35. Lin YS, Jen YM, Lin JC. Radiation‐related cranial nerve palsy in patients with nasopharyngeal carcinoma. Cancer. 2002;95(2):404‐409. [DOI] [PubMed] [Google Scholar]
- 36. Gillespie MB, Brodsky MB, Day TA, Lee FS, Martin‐Harris B. Swallowing‐related quality of life after head and neck cancer treatment. Laryngoscope. 2004;114(8):1362‐1367. [DOI] [PubMed] [Google Scholar]
- 37. Hutcheson KA, Nurgalieva Z, Zhao H, et al. Two‐year prevalence of dysphagia and related outcomes in head and neck cancer survivors: an updated SEER‐Medicare analysis. Head Neck. 2019;41(2):479‐487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Garcia‐Peris P, Paron L, Velasco C, et al. Long‐term prevalence of oropharyngeal dysphagia in head and neck cancer patients: impact on quality of life. Clin Nutr. 2007;26(6):710‐717. [DOI] [PubMed] [Google Scholar]
- 39. Hutcheson KA, Barringer DA, Rosenthal DI, May AH, Roberts DB, Lewin JS. Swallowing outcomes after radiotherapy for laryngeal carcinoma. Arch Otolaryngol Head Neck Surg. 2008;134(2):178‐183. [DOI] [PubMed] [Google Scholar]
- 40. Maurer J, Hipp M, Schafer C, Dysphagia KO. Impact on quality of life after radio(chemo)therapy of head and neck cancer. Strahlenther Onkol. 2011;187(11):744‐749. [DOI] [PubMed] [Google Scholar]
- 41. Clarke P, Radford K, Coffey M, Stewart M. Speech and swallow rehabilitation in head and neck cancer: United Kingdom National Multidisciplinary Guidelines. J Laryngol Otol. 2016;130(suppl 2):S176‐S180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Pauloski BR, Rademaker AW, Logemann JA, et al. Pretreatment swallowing function in patients with head and neck cancer. Head Neck. 2000;22(5):474‐482. [DOI] [PubMed] [Google Scholar]
- 43. Kotz T, Federman AD, Kao J, et al. Prophylactic swallowing exercises in patients with head and neck cancer undergoing chemoradiation: a randomized trial. Arch Otolaryngol Head Neck Surg. 2012;138(4):376‐382. [DOI] [PubMed] [Google Scholar]
- 44. Carnaby‐Mann G, Crary MA, Schmalfuss I, Amdur R. “Pharyngocise”: randomized controlled trial of preventative exercises to maintain muscle structure and swallowing function during head‐and‐neck chemoradiotherapy. Int J Radiat Oncol Biol Phys. 2012;83(1):210‐219. [DOI] [PubMed] [Google Scholar]
- 45. Hutcheson KA, Bhayani MK, Beadle BM, et al. Eat and exercise during radiotherapy or chemoradiotherapy for pharyngeal cancers: use it or lose it. JAMA Otolaryngol Head Neck Surg. 2013;139(11):1127‐1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Langmore SE. Normal swallowing: the endoscopic perspective. Endoscopic Evaluation and Treatment of Swallowing Disorders, pp. 37–37; Thieme Medical Publishers, Incorporated; 2000. [Google Scholar]
- 47. National Comprehensive Cancer Network . NCCN guidelines version 1.2019. Cancer‐related fatigue. Accessed May 30, 2019. https://www.nccn.org/professionals/physician_gls/pdf/fatigue.pdf
- 48. Spratt DE, Sakae M, Riaz N, et al. Time course and predictors for cancer‐related fatigue in a series of oropharyngeal cancer patients treated with chemoradiation therapy. Oncologist. 2012;17(4):569‐576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Jereczek‐Fossa BA, Santoro L, Alterio D, et al. Fatigue during head‐and‐neck radiotherapy: prospective study on 117 consecutive patients. Int J Radiat Oncol Biol Phys. 2007;68(2):403‐415. [DOI] [PubMed] [Google Scholar]
- 50. Bower JE, Bak K, Berger A, et al. Screening, assessment, and management of fatigue in adult survivors of cancer: an American Society of Clinical oncology clinical practice guideline adaptation. J Clin Oncol. 2014;32(17):1840‐1850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Rock CL, Doyle C, Demark‐Wahnefried W, et al. Nutrition and physical activity guidelines for cancer survivors. CA Cancer J Clin. 2012;62(4):243‐274. [DOI] [PubMed] [Google Scholar]
- 52. Gielissen MF, Verhagen CA, Bleijenberg G. Cognitive behaviour therapy for fatigued cancer survivors: long‐term follow‐up. Br J Cancer. 2007;97(5):612‐618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Taberna M, Inglehart RC, Pickard RK, et al. Significant changes in sexual behavior after a diagnosis of human papillomavirus‐positive and human papillomavirus‐negative oral cancer. Cancer. 2017;123(7):1156‐1165. [DOI] [PubMed] [Google Scholar]
- 54. Rogers SN, Hazeldine P, O'Brien K, Lowe D, Roe B. How often do head and neck cancer patients raise concerns related to intimacy and sexuality in routine follow‐up clinics? Eur Arch Otorhinolaryngol. 2015;272(1):207‐217. [DOI] [PubMed] [Google Scholar]
- 55. Hordern AJ, Street AF. Communicating about patient sexuality and intimacy after cancer: mismatched expectations and unmet needs. Med J Aust. 2007;186(5):224‐227. [DOI] [PubMed] [Google Scholar]
- 56. Hoole J, Kanatas A, Calvert A, Rogers SN, Smith AB, Mitchell DA. Validated questionnaires on intimacy in patients who have had cancer. Br J Oral Maxillofac Surg. 2015;53(7):584‐593. [DOI] [PubMed] [Google Scholar]
- 57. Hoole J, Kanatas AN, Mitchell DA. Psychosexual therapy and education in patients treated for cancer of the head and neck. Br J Oral Maxillofac Surg. 2015;53(7):601‐606. [DOI] [PubMed] [Google Scholar]
- 58. Ratnasingam J, Karim N, Paramasivam SS, et al. Hypothalamic pituitary dysfunction amongst nasopharyngeal cancer survivors. Pituitary. 2015;18(4):448‐455. [DOI] [PubMed] [Google Scholar]
- 59. Huang S, Wang X, Hu C, Ying H. Hypothalamic‐pituitary‐thyroid dysfunction induced by intensity‐modulated radiotherapy (IMRT) for adult patients with nasopharyngeal carcinoma. Med Oncol. 2013;30(4):710. [DOI] [PubMed] [Google Scholar]
- 60. Cramer JD, Johnson JT, Nilsen ML. Pain in head and neck cancer survivors: prevalence, predictors, and quality‐of‐life impact. Otolaryngol Head Neck Surg. 2018;159(5):853‐858. [DOI] [PubMed] [Google Scholar]
- 61. Terkawi AS, Tsang S, Alshehri AS, Mulafikh DS, Alghulikah AA, AlDhahri SF. The burden of chronic pain after major head and neck tumor therapy. Saudi J Anaesth. 2017;11(suppl 1):S71‐S79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Ojo B, Genden EM, Teng MS, Milbury K, Misiukiewicz KJ, Badr H. A systematic review of head and neck cancer quality of life assessment instruments. Oral Oncol. 2012;48(10):923‐937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Smit M, Balm AJ, Hilgers FJ, Tan IB. Pain as sign of recurrent disease in head and neck squamous cell carcinoma. Head Neck. 2001;23(5):372‐375. [DOI] [PubMed] [Google Scholar]
- 64. Dowell D, Haegerich TM, Chou R. CDC guideline for prescribing opioids for chronic pain – United States. JAMA. 2016;315(15):1624‐1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Smith WH, Luskin I, Resende Salgado L, et al. Risk of prolonged opioid use among cancer patients undergoing curative intent radiation therapy for head and neck malignancies. Oral Oncol. 2019;92:1‐5. [DOI] [PubMed] [Google Scholar]
- 66. Silver N, Dourado J, Hitchcock K, et al. Chronic opioid use in patients undergoing treatment for oropharyngeal cancer. Laryngoscope. 2019;129(9):2087‐2093. [DOI] [PubMed] [Google Scholar]
- 67. McDermott JD, Eguchi M, Stokes WA, et al. Short‐ and long‐term opioid use in patients with oral and oropharynx cancer. Otolaryngol Head Neck Surg. 2019;160(3):409‐419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Pang J, Tringale KR, Tapia VJ, et al. Chronic opioid use following surgery for oral cavity cancer. JAMA Otolaryngol Head Neck Surg. 2017;143(12):1187‐1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Sanders JG, Dawes PJ. Gabapentin for perioperative analgesia in otorhinolaryngology‐head and neck surgery: systematic review. Otolaryngol Head Neck Surg. 2016;155(6):893‐903. [DOI] [PubMed] [Google Scholar]
- 70. Bar AV, Weinstein G, Dutta PR, Chalian A, Both S, Quon H. Gabapentin for the treatment of pain related to radiation‐induced mucositis in patients with head and neck tumors treated with intensity‐modulated radiation therapy. Head Neck. 2010;32(2):173‐177. [DOI] [PubMed] [Google Scholar]
- 71. Bar AV. Gabapentin for the treatment of cancer‐related pain syndromes. Rev Recent Clin Trials. 2010;5(3):174‐178. [DOI] [PubMed] [Google Scholar]
- 72. Binczak M, Navez M, Perrichon C, et al. Management of somatic pain induced by head‐and‐neck cancer treatment: definition and assessment. Guidelines of the French Oto‐Rhino‐Laryngology‐Head and Neck Surgery Society (SFORL). Eur Ann Otorhinolaryngol Head Neck Dis. 2014;131(4):243‐247. [DOI] [PubMed] [Google Scholar]
- 73. Katz J, Weinrib A, Fashler SR, et al. The Toronto General Hospital Transitional Pain Service: development and implementation of a multidisciplinary program to prevent chronic postsurgical pain. J Pain Res. 2015;8:695‐702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Robinson M, Ward L, Mehanna H, Paleri V, Winter SC. Provision of physiotherapy rehabilitation following neck dissection in the UK. J Laryngol Otol. 2018;132(7):624‐627. [DOI] [PubMed] [Google Scholar]
- 75. Dijkstra PU, van Wilgen PC, Buijs RP, et al. Incidence of shoulder pain after neck dissection: a clinical explorative study for risk factors. Head Neck. 2001;23(11):947‐953. [DOI] [PubMed] [Google Scholar]
- 76. Weber C, Dommerich S, Pau HW, Kramp B. Limited mouth opening after primary therapy of head and neck cancer. Oral Maxillofac Surg. 2010;14(3):169‐173. [DOI] [PubMed] [Google Scholar]
- 77. Gane EM, Michaleff ZA, Cottrell MA, et al. Prevalence, incidence, and risk factors for shoulder and neck dysfunction after neck dissection: a systematic review. Eur J Surg Oncol. 2017;43(7):1199‐1218. [DOI] [PubMed] [Google Scholar]
- 78. Jepsen J, Laursen L, Larsen A, Hagert CG. Manual strength testing in 14 upper limb muscles: a study of inter‐rater reliability. Acta Orthop Scand. 2004;75(4):442‐448. [DOI] [PubMed] [Google Scholar]
- 79. Fan E, Ciesla ND, Truong AD, Bhoopathi V, Zeger SL, Needham DM. Inter‐rater reliability of manual muscle strength testing in ICU survivors and simulated patients. Intensive Care Med. 2010;36(6):1038‐1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Goldstein DP, Ringash J, Bissada E, et al. Evaluation of shoulder disability questionnaires used for the assessment of shoulder disability after neck dissection for head and neck cancer. Head Neck. 2014;36(10):1453‐1458. [DOI] [PubMed] [Google Scholar]
- 81. McGarvey AC, Chiarelli PE, Osmotherly PG, Hoffman GR. Physiotherapy for accessory nerve shoulder dysfunction following neck dissection surgery: a literature review. Head Neck. 2011;33(2):274‐280. [DOI] [PubMed] [Google Scholar]
- 82. McGarvey AC, Hoffman GR, Osmotherly PG, Chiarelli PE. Maximizing shoulder function after accessory nerve injury and neck dissection surgery: a multicenter randomized controlled trial. Head Neck. 2015;37(7):1022‐1031. [DOI] [PubMed] [Google Scholar]
- 83. Kirkpatrick JP, van der Kogel AJ, Schultheiss TE. Radiation dose–volume effects in the spinal cord. Int J Radiat Oncol Biol Phys. 2010;76(3 suppl):S42‐S49. [DOI] [PubMed] [Google Scholar]
- 84. Laidley HM, Noble DJ, Barnett GC, et al. Identifying risk factors for L'Hermitte's sign after IMRT for head and neck cancer. Radiat Oncol. 2018;13(1):84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Esik O, Csere T, Stefanits K, et al. A review on radiogenic Lhermitte's sign. Pathol Oncol Res. 2003;9(2):115‐120. [DOI] [PubMed] [Google Scholar]
- 86. Pak D, Vineberg K, Feng F, Ten Haken RK, Eisbruch A. Lhermitte sign after chemo‐IMRT of head‐and‐neck cancer: incidence, doses, and potential mechanisms. Int J Radiat Oncol Biol Phys. 2012;83(5):1528‐1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Gemici C. Lhermitte's sign: review with special emphasis in oncology practice. Crit Rev Oncol Hematol. 2010;74(2):79‐86. [DOI] [PubMed] [Google Scholar]
- 88. van der Geer SJ, van Rijn PV, Kamstra JI, Roodenburg JLN, Dijkstra PU. Criterion for trismus in head and neck cancer patients: a verification study. Support Care Cancer. 2019;27(3):1129‐1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Pauli N, Johnson J, Finizia C, Andrell P. The incidence of trismus and long‐term impact on health‐related quality of life in patients with head and neck cancer. Acta Oncol. 2013;52(6):1137‐1145. [DOI] [PubMed] [Google Scholar]
- 90. van der Geer SJ, van Rijn PV, Kamstra JI, et al. Prevalence and prediction of trismus in patients with head and neck cancer: a cross‐sectional study. Head Neck. 2019;41(1):64‐71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Kamstra JI, van Leeuwen M, Roodenburg JL, Dijkstra PU. Exercise therapy for trismus secondary to head and neck cancer: a systematic review. Head Neck. 2017;39(1):160‐169. [DOI] [PubMed] [Google Scholar]
- 92. Scherpenhuizen A, van Waes AM, Janssen LM, Van Cann EM, Stegeman I. The effect of exercise therapy in head and neck cancer patients in the treatment of radiotherapy‐induced trismus: a systematic review. Oral Oncol. 2015;51(8):745‐750. [DOI] [PubMed] [Google Scholar]
- 93. Dijkstra PU, Kalk WW, Roodenburg JL. Trismus in head and neck oncology: a systematic review. Oral Oncol. 2004;40(9):879‐889. [DOI] [PubMed] [Google Scholar]
- 94. Smith BG, Lewin JS. Lymphedema management in head and neck cancer. Curr Opin Otolaryngol Head Neck Surg. 2010;18(3):153‐158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Deng J, Ridner SH, Murphy BA. Lymphedema in patients with head and neck cancer. Oncol Nurs Forum. 2011;38(1):E1‐E10. [DOI] [PubMed] [Google Scholar]
- 96. Ridner SH, Dietrich MS, Niermann K, Cmelak A, Mannion K, Murphy B. A prospective study of the lymphedema and fibrosis continuum in patients with head and neck cancer. Lymphat Res Biol. 2016;14(4):198‐205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Klernas P, Johnsson A, Horstmann V, Johansson K. Health‐related quality of life in patients with lymphoedema – a cross‐sectional study. Scand J Caring Sci. 2018;32(2):634‐644. [DOI] [PubMed] [Google Scholar]
- 98. Murphy BA, Gilbert J. Dysphagia in head and neck cancer patients treated with radiation: assessment, sequelae, and rehabilitation. Semin Radiat Oncol. 2009;19(1):35‐42. [DOI] [PubMed] [Google Scholar]
- 99. Poulsen MG, Riddle B, Keller J, Porceddu SV, Tripcony L. Predictors of acute grade 4 swallowing toxicity in patients with stages III and IV squamous carcinoma of the head and neck treated with radiotherapy alone. Radiother Oncol. 2008;87(2):253‐259. [DOI] [PubMed] [Google Scholar]
- 100. Penner JL. Psychosocial care of patients with head and neck cancer. Semin Oncol Nurs. 2009;25(3):231‐241. [DOI] [PubMed] [Google Scholar]
- 101. Yao T, Beadle B, Holsinger CF, Starmer HM. Effectiveness of a home‐based head and neck lymphedema management program: a pilot study. Laryngoscope. 2020;130:E858‐E862. [DOI] [PubMed] [Google Scholar]
- 102. Smith BG, Hutcheson KA, Little LG, et al. Lymphedema outcomes in patients with head and neck cancer. Otolaryngol Head Neck Surg. 2015;152(2):284‐291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Fingeret MC, Yuan Y, Urbauer D, Weston J, Nipomnick S, Weber R. The nature and extent of body image concerns among surgically treated patients with head and neck cancer. Psychooncology. 2012;21(8):836‐844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Graboyes EM, Hill EG, Marsh CH, Maurer S, Day TA, Sterba KR. Body image disturbance in surgically treated head and neck cancer patients: a prospective cohort pilot study. Otolaryngol Head Neck Surg. 2019;161(1):105‐110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Fingeret MC, Hutcheson KA, Jensen K, Yuan Y, Urbauer D, Lewin JS. Associations among speech, eating, and body image concerns for surgical patients with head and neck cancer. Head Neck. 2013;35(3):354‐360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Rieke K, Schmid KK, Lydiatt W, Houfek J, Boilesen E, Watanabe‐Galloway S. Depression and survival in head and neck cancer patients. Oral Oncol. 2017;65:76‐82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Henry M, Fuehrmann F, Hier M, et al. Contextual and historical factors for increased levels of anxiety and depression in patients with head and neck cancer: a prospective longitudinal study. Head Neck. 2019;41(8):2538‐2548. [DOI] [PubMed] [Google Scholar]
- 108. Wu YS, Lin PY, Chien CY, et al. Anxiety and depression in patients with head and neck cancer: 6‐month follow‐up study. Neuropsychiatr Dis Treat. 2016;12:1029‐1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Fingeret MC, Vidrine DJ, Reece GP, Gillenwater AM, Gritz ER. Multidimensional analysis of body image concerns among newly diagnosed patients with oral cavity cancer. Head Neck. 2010;32(3):301‐309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Rhoten BA, Murphy B, Ridner SH. Body image in patients with head and neck cancer: a review of the literature. Oral Oncol. 2013;49(8):753‐760. [DOI] [PubMed] [Google Scholar]
- 111. Ellis MA, Sterba KR, Day TA, et al. Body image disturbance in surgically treated head and neck cancer patients: a patient‐centered approach. Otolaryngol Head Neck Surg. 2019;161(2):278‐287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Ellis MA, Sterba KR, Brennan EA, et al. A systematic review of patient‐reported outcome measures assessing body image disturbance in patients with head and neck cancer. Otolaryngol Head Neck Surg. 2019;160(6):941‐954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Fingeret MC, Teo I, Epner DE. Managing body image difficulties of adult cancer patients: lessons from available research. Cancer. 2014;120(5):633‐641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Chen SC, Huang BS, Lin CY, et al. Psychosocial effects of a skin camouflage program in female survivors with head and neck cancer: a randomized controlled trial. Psychooncology. 2017;26(9):1376‐1383. [DOI] [PubMed] [Google Scholar]
- 115. Huang S, Liu HE. Effectiveness of cosmetic rehabilitation on the body image of oral cancer patients in Taiwan. Support Care Cancer. 2008;16(9):981‐986. [DOI] [PubMed] [Google Scholar]
- 116. Lydiatt WM, Moran J, Burke WJ. A review of depression in the head and neck cancer patient. Clin Adv Hematol Oncol. 2009;7(6):397‐403. [PubMed] [Google Scholar]
- 117. Denlinger CS, Sanft T, Baker KS, et al. Survivorship, version 2.2017, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2017;15(9):1140‐1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Beck AT, Steer RA. Beck Anxiety Inventory Manual. Psychological Corporation; 1993. [Google Scholar]
- 119. Kroenke K, Spitzer RL, Williams JB. The PHQ‐9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16(9):606‐613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Chen AM, Daly ME, Vazquez E, et al. Depression among long‐term survivors of head and neck cancer treated with radiation therapy. JAMA Otolaryngol Head Neck Surg. 2013;139(9):885‐889. [DOI] [PubMed] [Google Scholar]
- 121. Lydiatt WM, Bessette D, Schmid KK, Sayles H, Burke WJ. Prevention of depression with escitalopram in patients undergoing treatment for head and neck cancer: randomized, double‐blind, placebo‐controlled clinical trial. JAMA Otolaryngol Head Neck Surg. 2013;139(7):678‐686. [DOI] [PubMed] [Google Scholar]
- 122. Jesse MT, Ryan ME, Eshelman A, et al. Integrated psychological care in head and neck cancer: views from health care providers, patients, and supports. Laryngoscope. 2015;125(6):1345‐1351. [DOI] [PubMed] [Google Scholar]
- 123. Schou I, Ekeberg O, Karesen R, Sorensen E. Psychosocial intervention as a component of routine breast cancer care – who participates and does it help? Psychooncology. 2008;17(7):716‐720. [DOI] [PubMed] [Google Scholar]
- 124. Golant M, Lieberman M, Giese‐Davis J, Winzelberg A, Ellis W. Emotional expression and facilitation of online vs. face‐to‐face breast cancer support groups. Psychooncology. 2004;13(1):S18‐S19. [Google Scholar]
- 125. Algtewi EE, Owens J, Baker SR. Analysing people with head and neck cancers’ use of online support groups. Cyberpsychology: Journal of Psychosocial Research on Cyberspace. 2015;9:(4). 10.5817/cp2015-4-6 [DOI] [Google Scholar]
- 126. Zeller JL. High suicide risk found for patients with head and neck cancer. JAMA. 2006;296(14):1716‐1717. [DOI] [PubMed] [Google Scholar]
- 127. Henry M, Rosberger Z, Bertrand L, et al. Prevalence and risk factors of suicidal ideation among patients with head and neck cancer: longitudinal study. Otolaryngol Head Neck Surg. 2018;159(5):843‐852. [DOI] [PubMed] [Google Scholar]
- 128. Kam D, Salib A, Gorgy G, et al. Incidence of suicide in patients with head and neck cancer. JAMA Otolaryngol Head Neck Surg. 2015;141(12):1075‐1081. [DOI] [PubMed] [Google Scholar]
- 129. Nader ME, Gidley PW. Challenges of hearing rehabilitation after radiation and chemotherapy. J Neurol Surg B Skull Base. 2019;80(2):214‐224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Bhandare N, Antonelli PJ, Morris CG, Malayapa RS, Mendenhall WM. Ototoxicity after radiotherapy for head and neck tumors. Int J Radiat Oncol Biol Phys. 2007;67(2):469‐479. [DOI] [PubMed] [Google Scholar]
- 131. Strojan P, Hutcheson KA, Eisbruch A, et al. Treatment of late sequelae after radiotherapy for head and neck cancer. Cancer Treat Rev. 2017;59:79‐92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Zuur CL, Simis YJ, Lamers EA, et al. Risk factors for hearing loss in patients treated with intensity‐modulated radiotherapy for head‐and‐neck tumors. Int J Radiat Oncol Biol Phys. 2009;74(2):490‐496. [DOI] [PubMed] [Google Scholar]
- 133. Hitchcock YJ, Tward JD, Szabo A, Bentz BG, Shrieve DC. Relative contributions of radiation and cisplatin‐based chemotherapy to sensorineural hearing loss in head‐and‐neck cancer patients. Int J Radiat Oncol Biol Phys. 2009;73(3):779‐788. [DOI] [PubMed] [Google Scholar]
- 134. McDowell LJ, Rock K, Xu W, et al. Long‐term late toxicity, quality of life, and emotional distress in patients with nasopharyngeal carcinoma treated with intensity modulated radiation therapy. Int J Radiat Oncol Biol Phys. 2018;102(2):340‐352. [DOI] [PubMed] [Google Scholar]
- 135. Wakisaka H, Yamada H, Motoyoshi K, Ugumori T, Takahashi H, Hyodo M. Incidence of long‐term ipsilateral and contralateral ototoxicity following radiotherapy for nasopharyngeal carcinoma. Auris Nasus Larynx. 2011;38(1):95‐100. [DOI] [PubMed] [Google Scholar]
- 136. Cianfrone G, Pentangelo D, Cianfrone F, et al. Pharmacological drugs inducing ototoxicity, vestibular symptoms and tinnitus: a reasoned and updated guide. Eur Rev Med Pharmacol Sci. 2011;15(6):601‐636. [PubMed] [Google Scholar]
- 137. Dille MF, Ellingson RM, McMillan GP, Konrad‐Martin D. ABR obtained from time‐efficient train stimuli for cisplatin ototoxicity monitoring. J Am Acad Audiol. 2013;24(9):769‐781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Sun DQ, Ward BK, Semenov YR, Carey JP, Della Santina CC. Bilateral vestibular deficiency: quality of life and economic implications. JAMA Otolaryngol Head Neck Surg. 2014;140(6):527‐534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Frisina RD, Wheeler HE, Fossa SD, et al. Comprehensive audiometric analysis of hearing impairment and tinnitus after cisplatin‐based chemotherapy in survivors of adult‐onset cancer. J Clin Oncol. 2016;34(23):2712‐2720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Longacre ML, Ridge JA, Burtness BA, Galloway TJ, Fang CY. Psychological functioning of caregivers for head and neck cancer patients. Oral Oncol. 2012;48(1):18‐25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Sterba KR, Zapka J, Cranos C, Laursen A, Day TA. Quality of life in head and neck cancer patient–caregiver dyads: a systematic review. Cancer Nurs. 2016;39(3):238‐250. [DOI] [PubMed] [Google Scholar]
- 142. Bond SM, Schumacher K, Sherrod A, et al. Development of the head and neck cancer caregiving task inventory. Eur J Oncol Nurs. 2016;24:29‐38. [DOI] [PubMed] [Google Scholar]
- 143. Murphy BA, Deng J. Advances in supportive care for late effects of head and neck cancer. J Clin Oncol. 2015;33(29):3314‐3321. [DOI] [PubMed] [Google Scholar]
- 144. Murphy BA, Ridner S, Wells N, Dietrich M. Quality of life research in head and neck cancer: a review of the current state of the science. Crit Rev Oncol Hematol. 2007;62(3):251‐267. [DOI] [PubMed] [Google Scholar]
- 145. Ringash J, Bernstein LJ, Devins G, et al. Head and neck cancer survivorship: learning the needs, meeting the needs. Semin Radiat Oncol. 2018;28(1):64‐74. [DOI] [PubMed] [Google Scholar]
- 146. Balfe M, O'Brien KM, Timmons A, et al. Informal caregiving in head and neck cancer: caregiving activities and psychological well‐being. Eur J Cancer Care (Engl). 2018;27(2):e12520. [DOI] [PubMed] [Google Scholar]
- 147. Chen SC, Lai YH, Liao CT, et al. Unmet supportive care needs and characteristics of family caregivers of patients with oral cancer after surgery. Psychooncology. 2014;23(5):569‐577. [DOI] [PubMed] [Google Scholar]
- 148. Longacre ML, Galloway TJ, Parvanta CF, Fang CY. Medical communication‐related informational need and resource preferences among family caregivers for head and neck cancer patients. J Cancer Educ. 2015;30(4):786‐791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Penner JL, McClement S, Lobchuk M, Daeninck P. Family members' experiences caring for patients with advanced head and neck cancer receiving tube feeding: a descriptive phenomenological study. J Pain Symptom Manage. 2012;44(4):563‐571. [DOI] [PubMed] [Google Scholar]
- 150. Sterba KR, Zapka J, Armeson KE, et al. Physical and emotional well‐being and support in newly diagnosed head and neck cancer patient–caregiver dyads. J Psychosoc Oncol. 2017;35(6):646‐665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Badr H, Herbert K, Reckson B, Rainey H, Sallam A, Gupta V. Unmet needs and relationship challenges of head and neck cancer patients and their spouses. J Psychosoc Oncol. 2016;34(4):336‐346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Balfe M, Butow P, O'Sullivan E, Gooberman‐Hill R, Timmons A, Sharp L. The financial impact of head and neck cancer caregiving: a qualitative study. Psychooncology. 2016;25(12):1441‐1447. [DOI] [PubMed] [Google Scholar]
- 153. Verdonck‐de Leeuw IM, Eerenstein SE, Van der Linden MH, Kuik DJ, de Bree R, Leemans CR. Distress in spouses and patients after treatment for head and neck cancer. Laryngoscope. 2007;117(2):238‐241. [DOI] [PubMed] [Google Scholar]
- 154. Castellanos EH, Dietrich MS, Bond SM, et al. Impact of patient symptoms and caregiving tasks on psychological distress in caregivers for head and neck cancer (HNC). Psychooncology. 2019;28(3):511‐517. [DOI] [PubMed] [Google Scholar]
- 155. Giuliani M, Milne R, McQuestion M, et al. Partner's survivorship care needs: an analysis in head and neck cancer patients. Oral Oncol. 2017;71:113‐121. [DOI] [PubMed] [Google Scholar]
- 156. Sterba KR, Zapka J, LaPelle N, et al. Development of a survivorship needs assessment planning tool for head and neck cancer survivors and their caregivers: a preliminary study. J Cancer Surviv. 2017;11(6):822‐832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Sterba KR, Armeson K, Zapka J, et al. Evaluation of a survivorship needs assessment planning tool for head and neck cancer survivor–caregiver dyads. J Cancer Surviv. 2019;13(1):117‐129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Badr H, Lipnick D, Diefenbach MA, et al. Development and usability testing of a web‐based self‐management intervention for oral cancer survivors and their family caregivers. Eur J Cancer Care (Engl). 2016;25(5):806‐821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Badr H, Krebs P. A systematic review and meta‐analysis of psychosocial interventions for couples coping with cancer. Psychooncology. 2013;22(8):1688‐1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. American College of Surgeons . Commission on Cancer's (CoC) Cancer Program Standards: Ensuring Patient‐Centered Care (2020 Edition). https://www.facs.org/quality-programs/cancer/coc/standards/2020
- 161. Schapira L. Why Peer Support is Important for People Coping with Cancer. Vol 2020. Cancer.Net; 2017. [Google Scholar]
- 162. Huber J, Muck T, Maatz P, et al. Face‐to‐face vs. online peer support groups for prostate cancer: a cross‐sectional comparison study. J Cancer Surviv. 2018;12(1):1‐9. [DOI] [PubMed] [Google Scholar]
- 163. Cancelliere C, Donovan J, Stochkendahl MJ, et al. Factors affecting return to work after injury or illness: best evidence synthesis of systematic reviews. Chiropr Man Ther. 2016;24(1):32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Office of Disability Employment Policy–United States Department of Labor. Department of Labor.
- 165. Massa ST, Osazuwa‐Peters N, Adjei Boakye E, Walker RJ, Ward GM. Comparison of the financial burden of survivors of head and neck cancer with other cancer survivors. JAMA Otolaryngol Head Neck Surg. 2019;145(3):239‐249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Deschler DG. Head and neck cancer compared with other cancers – does the great equalizer equalize equally? JAMA Otolaryngol Head Neck Surg. 2019;145(3):249‐250. [DOI] [PubMed] [Google Scholar]
- 167. Zafar SY, Abernethy AP. Financial toxicity, part I: a new name for a growing problem. Oncology (Williston Park). 2013;27(2):80‐149. [PMC free article] [PubMed] [Google Scholar]
- 168. Altice CK, Banegas MP, Tucker‐Seeley RD, Yabroff KR. Financial hardships experienced by cancer survivors: a systematic review. J Natl Cancer Inst. 2017;109(2):djw205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Krouse JH, Krouse HJ, Fabian RL. Adaptation to surgery for head and neck cancer. Laryngoscope. 1989;99(8 Pt 1):789‐794. [DOI] [PubMed] [Google Scholar]
- 170. de Souza JA, Kung S, O'Connor J, Yap BJ. Determinants of patient‐centered financial stress in patients with locally advanced head and neck cancer. J Oncol Pract. 2017;13(4):e310‐e318. [DOI] [PubMed] [Google Scholar]
- 171. Banthin JS, Cunningham P, Bernard DM. Financial burden of health care, 2001–2004. Health Aff (Millwood). 2008;27(1):188‐195. [DOI] [PubMed] [Google Scholar]
- 172. de Souza JA, Yap BJ, Hlubocky FJ, et al. The development of a financial toxicity patient‐reported outcome in cancer: the COST measure. Cancer. 2014;120(20):3245‐3253. [DOI] [PubMed] [Google Scholar]
- 173. Neugut AI, Subar M, Wilde ET, et al. Association between prescription co‐payment amount and compliance with adjuvant hormonal therapy in women with early‐stage breast cancer. J Clin Oncol. 2011;29(18):2534‐2542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Lathan CS, Cronin A, Tucker‐Seeley R, Zafar SY, Ayanian JZ, Schrag D. Association of financial strain with symptom burden and quality of life for patients with lung or colorectal cancer. J Clin Oncol. 2016;34(15):1732‐1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Zafar SY, McNeil RB, Thomas CM, Lathan CS, Ayanian JZ, Provenzale D. Population‐based assessment of cancer survivors' financial burden and quality of life: a prospective cohort study. J Oncol Pract. 2015;11(2):145‐150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176. O'Brien KM, Timmons A, Butow P, et al. Associations between neighbourhood support and financial burden with unmet needs of head and neck cancer survivors. Oral Oncol. 2017;65:57‐64. [DOI] [PubMed] [Google Scholar]
- 177. Ramsey SD, Bansal A, Fedorenko CR, et al. Financial insolvency as a risk factor for early mortality among patients with cancer. J Clin Oncol. 2016;34(9):980‐986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. Lee DH, Roh JL, Baek S, et al. Second cancer incidence, risk factor, and specific mortality in head and neck squamous cell carcinoma. Otolaryngol Head Neck Surg. 2013;149(4):579‐586. [DOI] [PubMed] [Google Scholar]
- 179. Atienza JA, Dasanu CA. Incidence of second primary malignancies in patients with treated head and neck cancer: a comprehensive review of literature. Curr Med Res Opin. 2012;28(12):1899‐1909. [DOI] [PubMed] [Google Scholar]
- 180. Dikshit RP, Boffetta P, Bouchardy C, et al. Risk factors for the development of second primary tumors among men after laryngeal and hypopharyngeal carcinoma. Cancer. 2005;103(11):2326‐2333. [DOI] [PubMed] [Google Scholar]
- 181. Baxi SS, Pinheiro LC, Patil SM, Pfister DG, Oeffinger KC, Elkin EB. Causes of death in long‐term survivors of head and neck cancer. Cancer. 2014;120(10):1507‐1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182. Bernat L, Hrusak D. Hypothyroidism after radiotherapy of head and neck cancer. J Craniomaxillofac Surg. 2014;42(4):356‐361. [DOI] [PubMed] [Google Scholar]
- 183. Diaz R, Jaboin JJ, Morales‐Paliza M, et al. Hypothyroidism as a consequence of intensity‐modulated radiotherapy with concurrent taxane‐based chemotherapy for locally advanced head‐and‐neck cancer. Int J Radiat Oncol Biol Phys. 2010;77(2):468‐476. [DOI] [PubMed] [Google Scholar]
- 184. Garcia‐Serra A, Amdur RJ, Morris CG, Mazzaferri E, Mendenhall WM. Thyroid function should be monitored following radiotherapy to the low neck. Am J Clin Oncol. 2005;28(3):255‐258. [DOI] [PubMed] [Google Scholar]
- 185. Sroussi HY, Epstein JB, Bensadoun RJ, et al. Common oral complications of head and neck cancer radiation therapy: mucositis, infections, saliva change, fibrosis, sensory dysfunctions, dental caries, periodontal disease, and osteoradionecrosis. Cancer Med. 2017;6(12):2918‐2931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186. Epstein JB, Barasch A. Oral and dental health in head and neck cancer patients. Cancer Treat Res. 2018;174:43‐57. [DOI] [PubMed] [Google Scholar]
- 187. Chuang SK. Limited evidence to demonstrate that the use of hyperbaric oxygen (HBO) therapy reduces the incidence of osteoradionecrosis in irradiated patients requiring tooth extraction. J Evid Based Dent Pract. 2012;12(3 suppl):248‐250. [DOI] [PubMed] [Google Scholar]
- 188. Epstein JB, Smith DK, Villines D, et al. Patterns of oral and dental care education and utilization in head and neck cancer patients. Support Care Cancer. 2018;26(8):2591‐2603. [DOI] [PubMed] [Google Scholar]
- 189. Voltzke KJ, Lee YA, Zhang ZF, et al. Racial differences in the relationship between tobacco, alcohol, and the risk of head and neck cancer: pooled analysis of US studies in the INHANCE Consortium. Cancer Causes Control. 2018;29(7):619‐630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190. Burris JL, Studts JL, DeRosa AP, Ostroff JS. Systematic review of tobacco use after lung or head/neck cancer diagnosis: results and recommendations for future research. Cancer Epidemiol Biomarkers Prev. 2015;24(10):1450‐1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191. Shields PG, Herbst RS, Arenberg D, et al. Smoking cessation, version 1.2016, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2016;14(11):1430‐1468. [DOI] [PubMed] [Google Scholar]
- 192. Densky J, Eskander A, Kang S, et al. Risk factors associated with postoperative delirium in patients undergoing head and neck free flap reconstruction. JAMA Otolaryngol Head Neck Surg. 2019;145(3):216‐221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193. Browman GP, Wong G, Hodson I, et al. Influence of cigarette smoking on the efficacy of radiation therapy in head and neck cancer. N Engl J Med. 1993;328(3):159‐163. [DOI] [PubMed] [Google Scholar]
- 194. Day GL, Blot WJ, Shore RE, et al. Second cancers following oral and pharyngeal cancers: role of tobacco and alcohol. J Natl Cancer Inst. 1994;86(2):131‐137. [DOI] [PubMed] [Google Scholar]
- 195. Deleyiannis FW, Thomas DB, Vaughan TL, Davis S. Alcoholism: independent predictor of survival in patients with head and neck cancer. J Natl Cancer Inst. 1996;88(8):542‐549. [DOI] [PubMed] [Google Scholar]
- 196. Gillison ML, Zhang Q, Jordan R, et al. Tobacco smoking and increased risk of death and progression for patients with p16‐positive and p16‐negative oropharyngeal cancer. J Clin Oncol. 2012;30(17):2102‐2111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197. Berthiller J, Lee YC, Boffetta P, et al. Marijuana smoking and the risk of head and neck cancer: pooled analysis in the INHANCE consortium. Cancer Epidemiol Biomarkers Prev. 2009;18(5):1544‐1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198. Schuckit MA. Treatment of opioid‐use disorders. N Engl J Med. 2016;375(4):357‐368. [DOI] [PubMed] [Google Scholar]
- 199. Day AT, Tang L, Karam‐Hage M, Fakhry C. Tobacco treatment programs at National Cancer Institute‐designated cancer centers: a systematic review and online audit. Am J Clin Oncol. 2019;42(4):407‐410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200. Nayan S, Gupta MK, Sommer DD. Evaluating smoking cessation interventions and cessation rates in cancer patients: a systematic review and meta‐analysis. ISRN Oncol. 2011;2011:849023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201. Shingler E, Robles LA, Perry R, et al. Systematic review evaluating randomized controlled trials of smoking and alcohol cessation interventions in people with head and neck cancer and oral dysplasia. Head Neck. 2018;40(8):1845‐1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202. Force USPST, Curry SJ, Krist AH, et al. Screening and behavioral counseling interventions to reduce unhealthy alcohol use in adolescents and adults: US preventive services task Force recommendation statement. JAMA. 2018;320(18):1899‐1909. [DOI] [PubMed] [Google Scholar]
- 203. Moyer VA. Preventive services task F. screening and behavioral counseling interventions in primary care to reduce alcohol misuse: U.S. preventive services task force recommendation statement. Ann Intern Med. 2013;159(3):210‐218. [DOI] [PubMed] [Google Scholar]
- 204. Rogers LQ, Courneya KS, Robbins KT, et al. Physical activity and quality of life in head and neck cancer survivors. Support Care Cancer. 2006;14(10):1012‐1019. [DOI] [PubMed] [Google Scholar]
- 205. Sammut L, Fraser LR, Ward MJ, Singh T, Patel NN. Participation in sport and physical activity in head and neck cancer survivors: associations with quality of life. Clin Otolaryngol. 2016;41(3):241‐248. [DOI] [PubMed] [Google Scholar]
- 206. Capozzi LC, Boldt KR, Lau H, Shirt L, Bultz B, Culos‐Reed SN. A clinic‐supported group exercise program for head and neck cancer survivors: managing cancer and treatment side effects to improve quality of life. Support Care Cancer. 2015;23(4):1001‐1007. [DOI] [PubMed] [Google Scholar]
- 207. Eades M, Murphy J, Carney S, et al. Effect of an interdisciplinary rehabilitation program on quality of life in patients with head and neck cancer: review of clinical experience. Head Neck. 2013;35(3):343‐349. [DOI] [PubMed] [Google Scholar]
- 208. Lonbro S, Dalgas U, Primdahl H, et al. Lean body mass and muscle function in head and neck cancer patients and healthy individuals – results from the DAHANCA 25 study. Acta Oncol. 2013;52(7):1543‐1551. [DOI] [PubMed] [Google Scholar]
- 209. Zhao SG, Alexander NB, Djuric Z, et al. Maintaining physical activity during head and neck cancer treatment: results of a pilot controlled trial. Head Neck. 2016;38(suppl 1):E1086‐E1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210. Samuel SR, Maiya GA, Babu AS, Vidyasagar MS. Effect of exercise training on functional capacity & quality of life in head & neck cancer patients receiving chemoradiotherapy. Indian J Med Res. 2013;137(3):515‐520. [PMC free article] [PubMed] [Google Scholar]
- 211. Ibrahim EM, Al‐Homaidh A. Physical activity and survival after breast cancer diagnosis: meta‐analysis of published studies. Med Oncol. 2011;28(3):753‐765. [DOI] [PubMed] [Google Scholar]
- 212. Qiu S, Jiang C, Zhou L. Physical activity and mortality in patients with colorectal cancer: a meta‐analysis of prospective cohort studies. Eur J Cancer Prev. 2020;29(1):15‐26. [DOI] [PubMed] [Google Scholar]
- 213. Schmid D, Leitzmann MF. Association between physical activity and mortality among breast cancer and colorectal cancer survivors: a systematic review and meta‐analysis. Ann Oncol. 2014;25(7):1293‐1311. [DOI] [PubMed] [Google Scholar]
- 214. Hutcheson KA, Barrow MP, Warneke CL, et al. Cough strength and expiratory force in aspirating and nonaspirating postradiation head and neck cancer survivors. Laryngoscope. 2018;128(7):1615‐1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215. Laciuga H, Rosenbek JC, Davenport PW, Sapienza CM. Functional outcomes associated with expiratory muscle strength training: narrative review. J Rehabil Res Dev. 2014;51(4):535‐546. [DOI] [PubMed] [Google Scholar]
- 216. Hutcheson KA, Barrow MP, Plowman EK, et al. Expiratory muscle strength training for radiation‐associated aspiration after head and neck cancer: a case series. Laryngoscope. 2018;128(5):1044‐1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217. Palmer AD, Bolognone RK, Thomsen S, Britton D, Schindler J, Graville DJ. The safety and efficacy of expiratory muscle strength training for rehabilitation after supracricoid partial laryngectomy: a pilot investigation. Ann Otol Rhinol Laryngol. 2018;128:169‐176. [DOI] [PubMed] [Google Scholar]
- 218. Mitchell RB, Hussey HM, Setzen G, et al. Clinical consensus statement: tracheostomy care. Otolaryngol Head Neck Surg. 2013;148(1):6‐20. [DOI] [PubMed] [Google Scholar]
- 219. Garg S, Kozin E, Deschler D. Care of the post‐laryngectomy stoma #281. J Palliat Med. 2014;17(7):857‐858. [DOI] [PubMed] [Google Scholar]
- 220. Brook I, Bogaardt H, van As‐Brooks C. Long‐term use of heat and moisture exchangers among laryngectomees: medical, social, and psychological patterns. Ann Otol Rhinol Laryngol. 2013;122(6):358‐363. [DOI] [PubMed] [Google Scholar]
- 221. Blake KD, Ottenbacher AJ, Finney Rutten LJ, et al. Predictors of human papillomavirus awareness and knowledge in 2013: gaps and opportunities for targeted communication strategies. Am J Prev Med. 2015;48(4):402‐410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222. Luryi AL, Yarbrough WG, Niccolai LM, et al. Public awareness of head and neck cancers: a cross‐sectional survey. JAMA Otolaryngol Head Neck Surg. 2014;140(7):639‐646. [DOI] [PubMed] [Google Scholar]
- 223. Williams MU, Carr MM, Goldenberg D. Public awareness of human papillomavirus as a causative factor for oropharyngeal cancer. Otolaryngol Head Neck Surg. 2015;152(6):1029‐1034. [DOI] [PubMed] [Google Scholar]
- 224. Milbury K, Rosenthal DI, El‐Naggar A, Badr H. An exploratory study of the informational and psychosocial needs of patients with human papillomavirus‐associated oropharyngeal cancer. Oral Oncol. 2013;49(11):1067‐1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225. D'Souza G, Zhang Y, Merritt S, et al. Patient experience and anxiety during and after treatment for an HPV‐related oropharyngeal cancer. Oral Oncol. 2016;60:90‐95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226. Schorr M, Carlson LE, Lau HY, et al. Distress levels in patients with oropharyngeal vs. non‐oropharyngeal squamous cell carcinomas of the head and neck over 1 year after diagnosis: a retrospective cohort study. Support Care Cancer. 2017;25(10):3225‐3233. [DOI] [PubMed] [Google Scholar]
- 227. Fakhry C, D'Souza G. Discussing the diagnosis of HPV‐OSCC: common questions and answers. Oral Oncol. 2013;49(9):863‐871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228. Reich M, Licitra L, Vermorken JB, et al. Best practice guidelines in the psychosocial management of HPV‐related head and neck cancer: recommendations from the European Head and Neck Cancer Society's Make Sense Campaign. Ann Oncol. 2016;27(10):1848‐1854. [DOI] [PubMed] [Google Scholar]
- 229. Shelal Z, Cho D, Urbauer DL, et al. Knowledge matters and empowers: HPV vaccine advocacy among HPV‐related cancer survivors. Support Care Cancer. 2020;28(5):2407‐2413. [DOI] [PubMed] [Google Scholar]
- 230. Finnigan JP Jr, Sikora AG. Counseling the patient with potentially HPV‐related newly diagnosed head and neck cancer. Curr Oncol Rep. 2014;16(3):375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231. Khariwala SS, Moore MG, Malloy KM, Gosselin B, Smith RV. The “HPV discussion”: effective use of data to deliver recommendations to patients impacted by HPV. Otolaryngol Head Neck Surg. 2015;153(4):518‐525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232. D'Souza G, Robbins HA. Sexual and relationship health among survivors of oropharyngeal or oral cavity squamous cell carcinoma. Cancer. 2017;123(7):1092‐1094. [DOI] [PMC free article] [PubMed] [Google Scholar]


