Abstract
Objective
To demonstrate that oro‐pharyngo‐esophageal radionuclide scintigraphy (OPERS) not only detects tracheobronchial aspiration after swallowing, but also quantifies the amount of aspiration and subsequent clearance.
Methods
Data collected between 2014 and 2019 were reviewed for aspiration pneumonia at 12 and 24‐months after OPERS. The predictive value for aspiration pneumonia on flexible endoscopic evaluation of swallowing (FEES), videofluoroscopic swallowing study (VFSS), and OPERS, and the overall survival of patients with or without aspiration were determined.
Results
Thirty‐seven patients treated with radiotherapy for nasopharyngeal carcinoma (NPC) were reviewed. The incidence of aspiration detected on FEES, VFSS, and OPERS was 78.4%, 66.7%, and 44.4%, respectively. Using VFSS as a gold standard, the sensitivity and specificity of OPERS for aspiration was 73.7% and 100%. The positive and negative predictive values for aspiration were 100% and 66.7%, respectively, with an overall accuracy of 82.8%. A history of aspiration pneumonia was one factor associated with a higher chance of subsequent aspiration pneumonia within 12 months (odds ratio: 15.5, 95% CI 1.67–145.8, p < .05) and 24 months (odds ratio: 23.8, 95% CI 3.69–152.89, p < .01) of the swallowing assessment. Aspiration detected by OPERS was a significant risk factor for future aspiration pneumonia at 12 and 24 months respectively. Significantly, better survival was associated with an absence of aspiration on OPERS only, but not on FEES or VFSS.
Conclusion
OPERS predicts the safety of swallowing, the incidence of subsequent aspiration pneumonia, and the survival prognosis in post‐irradiated NPC dysphagia patients.
Level of Evidence
3.
Keywords: aspiration, dysphagia, nasopharyngeal carcinoma, pneumonia, radionuclide scintigraphy
1. INTRODUCTION
Aspiration pneumonia is a potentially fatal sequela of chronic aspiration. However, there is still uncertainty about the relationship between aspiration and pneumonia, as the association is not absolute and may vary between cancer and non‐cancer patients. Wang et al reported on a cohort of 113 patients with a history of pneumonia after radiotherapy for nasopharyngeal carcinoma (NPC), and showed that old age, smoking, weight loss, and lower cranial nerve palsies were predisposing factors for recurrent pneumonia. 1 On the other hand, Xiong et al. retrospectively reviewed 217 patients who received radiotherapy for NPC and showed that 26 patients (12%) developed swallowing related chest infections (SRCIs) with a median delay of 24.5 months after treatment and with a mortality of 4% (nine patients). 2 The same study also showed that advanced age, recurrent disease, and concurrent chemoradiotherapy were significant factors associated with the development of SRCIs.
Clearance of any aspirated food material from the chest depends on normal physiology, which, if abnormal, can increase the risk of aspiration pneumonia. This may depend on the nature of the food substance, amount of aspirated food material, sensation of the larynx and lower airway, coughing effort, and mucus production and its expulsion from the tracheobronchial tree by smooth muscle motility and muco‐ciliary clearance. 3 , 4 Rosenbeck's penetration‐aspiration scale (PAS) 5 has been widely used in flexible endoscopic evaluation of swallowing (FEES) studies and videofluoroscopic swallowing studies (VFSS) in an attempt to address the outcome of clearance of food residue from the airway during swallowing. 6 , 7 PAS Grade 7 indicates aspiration of food material below the vocal cords with incomplete clearance despite coughing, while PAS Grade 8 indicates aspiration without a cough reflex or attempt to clear aspirated food material. PAS Grade 8, also known as “silent aspiration,” is the most severe grade of aspiration. Although some studies have shown an association between the severity of grading by PAS and the incidence of aspiration pneumonia in certain neurological conditions, such an association has not been confirmed by other studies. 8 , 9 There is a paucity of reports in the literature that describe the relationship between aspiration, clearance of aspirated materials, and the incidence of pneumonia in treated NPC patients or other head and neck cancer patients, the major source of referrals (68%) to our dysphagia clinic.
Radionuclide scintigraphy has been widely used to evaluate various gastrointestinal conditions such as gastric emptying, gastrointestinal bleeding, gastrointestinal motility, and gastroesophageal and laryngopharyngeal reflux. 10 , 11 , 12 , 13 In head and neck oncology, Humphrey et al. was the first to report the application of radionuclide scintigraphy to detect and quantify food transit and stasis in the oro‐pharyngo‐esophageal region as well as pulmonary aspiration in patients which provided an important reference for future application and research. 14 Oro‐pharyngo‐esophageal radionuclide scintigraphy (OPERS) is an investigation that is currently done using a radionuclide salivagram to evaluate swallowing and pulmonary aspiration in children and adults. 15 , 16 , 17 , 18 Radionuclide scintigraphy not only detects penetration and aspiration, but also quantifies the amount of food material that enters the chest after swallowing. 19 , 20 However, there is a paucity of studies reporting on the application of OPERS in the evaluation of aspiration related to dysphagia. 14 , 21 , 22 To date, there is no study reporting on the application of OPERS for the diagnosis of aspiration and assessment of the clearance of aspirated food material which predicts the future risk of aspiration pneumonia and survival prognosis in patients treated for head and neck cancer. We hypothesize that OPERS can evaluate the safety of swallowing by predicting the risk of aspiration pneumonia following the treatment of head and neck cancer. This study aims to investigate the application of OPERS to evaluate swallowing safety and related survival prognosis in patients suffering from dysphagia after being treated with radiotherapy for NPC, and to compare its efficacy with FEES and VFSS.
2. MATERIAL AND METHODS
2.1. Subject recruitment and data retrieval
This retrospective study was approved by the Institutional Review Board of the New Territories East Hospital Cluster that oversees all research activities in the participating institution (CREC‐2019.667). Patients who developed dysphagia after being treated with radiotherapy for NPC and who were referred to the dysphagia clinic at the Prince of Wales Hospital between January 2014 and November 2017 were eligible for the study.
Inclusion criteria were (i) history of primary NPC, (ii) completed radiotherapy or chemo‐radiotherapy for at least 12 months, (iii) diagnosed with dysphagia at a dysphagia clinic consultation, (iv) completed a FEES, OPERS, and a videofluoroscopic swallowing study (VFSS) within a 4‐week time frame, (v) and a regular follow up after the swallowing studies to monitor for the occurrence of aspiration pneumonia.
Exclusion criteria were (i) age under 18 years, (ii) known history of dysphagia before radiotherapy, (iii) history of surgery of the oral cavity, pharynx, larynx or neck, (vi) residual or recurrent nasopharyngeal or other head and neck cancer, (v) upper airway obstruction, (vi) history of cerebral vascular accident, (vii) other acute causes of dysphagia such as head and neck infection or abscesses, (viii) poor cognitive function or unable to follow the instructions of the study, (ix) pregnancy, and (x) severe nasal obstruction making an endoscopic swallowing evaluation difficult or impossible. Data collected between 2014 and 2019 were retrieved from the database of the combined dysphagia clinic and from hospital electronic medical records. These data included patient demographic information, symptoms, duration of dysphagia, pre‐morbid medical conditions, history of aspiration pneumonia, swallowing assessment results, and swallowing rehabilitation. The incidence or occurrence of aspiration pneumonia at the 12‐month and 24‐month follow‐up, at the last follow‐up, survival status, and the compliance with swallowing instructions after swallowing studies were also retrieved.
2.2. Flexible endoscopic evaluation of swallowing and videofluoroscopic study of swallowing to detect aspiration
The techniques of FEES and VFSS have been described in our previous publication on subjects with dysphagia after radiotherapy for NPC. 23 The video clips of the FEES and VFSS were rated by three observers who were three senior otolaryngologists with at least 10 years of experience in this field of practice. Rosenbeck's PAS 5 was used to rate the severity of aspiration during swallowing. Each otolaryngologist was required to rate the FEES and VFSS two times in random fashion on separate occasions without knowing the swallowing condition of the subjects. The intra and inter‐rater reliability were calculated. The final scoring of the PAS for each subject for the FEES and VFSS was obtained by taking an average of the scores from each of the observers.
2.3. Oro‐pharyngo‐esophageal radionuclide scintigraphy to detect aspiration
OPERS was performed by a dual‐headed gamma camera (Infinia Hawkeye; General Electric, Milwaukee [WI], USA) equipped with a parallel‐beam collimator. Technetium‐99m, which was used as a radiotracer mix with food during the swallowing study, has a half‐life of 6 hours and a decay factor of 0.841 during the longest scanning time of 90 minutes after the swallowing study (Table 1). We assume the level of radioactivity detected in the airway after swallowing corresponds to the amount of food residue in the chest.
TABLE 1.
Technetium‐99m decay factors calculated using a half‐life of 6.02 hours
| Elapsed time | 0 hour | 1 hour | 2 hour | 3 hour | 4 hour | 5 hour | 6 hour |
|---|---|---|---|---|---|---|---|
| 0 minute | 1.000 | 0.891 | 0.794 | 0.708 | 0.631 | 0.562 | 0.501 |
| 5 minute | 0.990 | 0.883 | 0.787 | 0.701 | 0.625 | 0.557 | 0.496 |
| 10 minute | 0.981 | 0.874 | 0.779 | 0.695 | 0.619 | 0.552 | 0.492 |
| 15 minute | 0.972 | 0.866 | 0.772 | 0.688 | 0.613 | 0.546 | 0.487 |
| 20 minute | 0.962 | 0.858 | 0.764 | 0.681 | 0.607 | 0.541 | 0.482 |
| 25 minute | 0.953 | 0.850 | 0.757 | 0.675 | 0.601 | 0.536 | 0.478 |
| 30 minute | 0.944 | 0.841 | 0.75 | 0.668 | 0.596 | 0.531 | 0.473 |
| 35 minute | 0.935 | 0.833 | 0.743 | 0.662 | 0.590 | 0.526 | 0.469 |
| 40 minute | 0.926 | 0.825 | 0.736 | 0.656 | 0.584 | 0.521 | 0.464 |
| 45 minute | 0.917 | 0.818 | 0.729 | 0.649 | 0.579 | 0.516 | 0.460 |
| 50 minute | 0.909 | 0.810 | 0.722 | 0.643 | 0.573 | 0.511 | 0.455 |
| 55 minute | 0.900 | 0.802 | 0.715 | 0.637 | 0.568 | 0.506 | 0.451 |
To prepare the food materials for OPERS, 5 mCi of Tc‐99 m sulfur colloid was mixed with 5 ml of water and added to three different consistencies of food: (i) water (sufficient for three mouthfuls of 5–10 ml + 1 ml of radioisotope), (ii) paste (35 ml water + 1 spoon of thickener + 2 ml of radioisotope), (iii) and solid (three small pieces of biscuit ~2 cm in size immersed in 2 ml of radioisotope or simply immersed in any residual paste). Each patient was allowed three swallow attempts for each consistency of food.
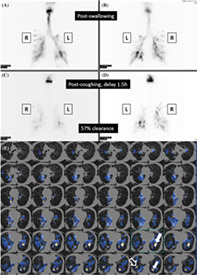
During the radionuclide scintigraphic study, patients were placed in an erect left lateral position, and the scanning started with the acquisition of 1 frame per second for 1 minute with a matrix of 128 × 128 for three swallow attempts of the radiolabeled food material for each phase, starting with liquid and followed by paste and then solid. Anterior and posterior planar images of the neck and chest with a matrix of 256 × 256 and an acquisition time of 300 seconds were performed immediately after the swallowing phases (early phase) were completed (Figure 1A,B). The anterior and posterior planar images of the neck and chest were repeated within 60–90 minutes (delay phase) of all food consistencies being tested, and with the same acquisition parameters as the early phase (Figure 1C,D), followed by a SPECT/CT of the neck and chest (Figure 1E). The SPECT/CT was performed with a low‐dose CT scan (140 kV and 2.5 mA) and image reconstruction with a matrix of 512 × 512 was performed followed by the SPECT images with a 3° angle view for a 360° angular range with a matrix of 128 × 128; zoom 1.33 by the step‐and‐shoot scan mode with an acquisition time of 15 seconds per projection, and a total scanning time of about 22 minutes for each SPECT/CT of the neck and thorax.
FIGURE 1.
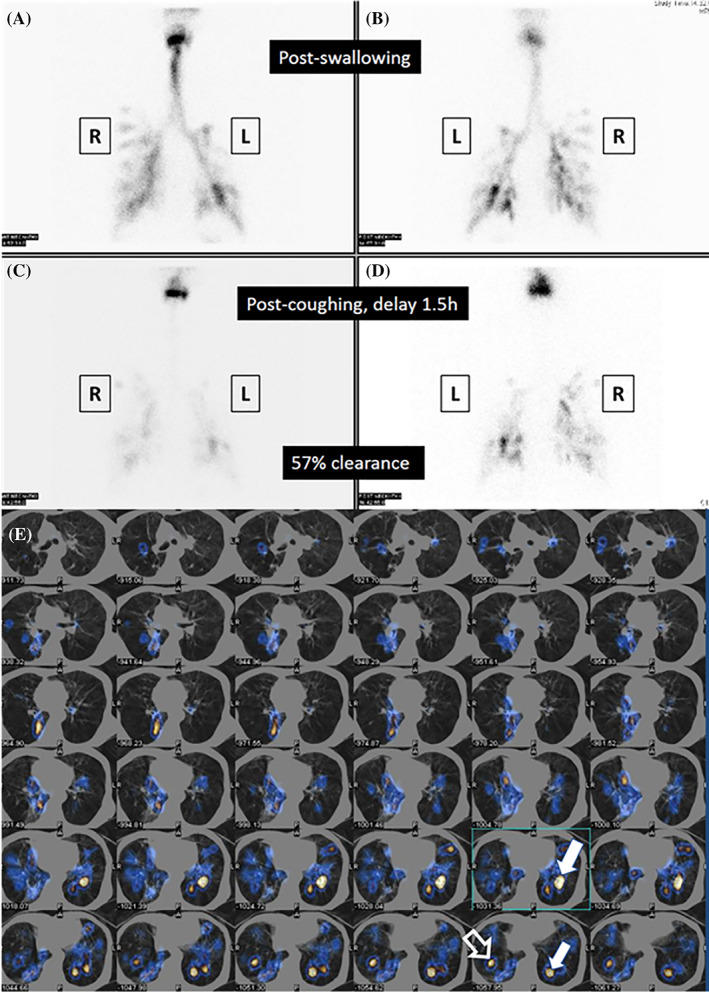
Aspiration as detected by planar cine view and SPECT/CT in oro‐pharyngo‐esophageal radionuclide scintigraphy (OPERS). (A) Aspiration of thin liquid in bilateral lower bronchial regions shown immediately after swallowing (anteroposterior planar view). (B) Aspiration of thin liquid in bilateral lower bronchial regions shown immediately after swallowing (posteroanterior planar view). (C) Clearance of radioactivity in the lower bronchial region 90 minutes after swallowing (anteroposterior planar view). (D) Clearance of radioactivity in the lower bronchial region 90 min after swallowing (posteroanterior planar view). (E) SPECT/CT scan showing radioactivity in the left and right lower bronchial trees suggesting aspiration which is more severe on the right side (solid white arrows) than on the left side (hollow white arrow). (L, left; R, right)
Swallowing was evaluated on the lateral view of the radionuclide scintigraphy to document the presence of any penetration and aspiration. The region of interest (ROI) included the radioactivity on the radionuclide scintigraphy of the airways and lungs that implied aspiration was detected. On both sets of early and delayed planar images, the radioactivity was calculated by a geometric mean method (square root of the product of the count of the ROI in anterior planar and posterior planar images). Clearance of any residual radioactivity in the radionuclide scintigraphy study from the airway and lungs, which was assumed to reflect clearance of residual aspirated food materials of all food consistencies, was calculated by the difference in the radioactivity between the early and late phases of the radionuclide scintigraphy study over the radioactivity in the early phase of the radionuclide scintigraphy study and multiplied by 100. The measurement of clearance of any residual radioactivity in the airways and lungs was performed twice to test the intra‐rater reliability.
2.4. Statistical analysis
All statistical analyses were performed using Statistical Product and Service Solutions version 23.0 (IBM, Armonk, NY, USA). Intraclass correlation coefficient (ICC) with a two‐way mixed effects model and absolute agreement was computed to measure the intra‐rater and inter‐rater reliability for the two sets of readings by three observers for the FEES and VFSS. ICC was also used to calculate intra‐rater reliability for the two sets of readings by a single observer for OPERS. Binary logistic regression was performed to identify if any variables were good predictors of aspiration pneumonia at 12 and 24 months after the swallowing studies. Gwet's agreement coefficients (Gwet's AC1) were computed to estimate the agreement for the diagnosis of penetration and aspiration on the FEES, VFSS, and OPERS. The Chi‐square test or Fisher's exact test was performed to identify if association existed between the incidence of aspiration pneumonia at 12 and 24 months after the swallowing studies and the diagnosis of aspiration on FEES, VFSS, and OPERS. Moreover, the same tests were used to identify if an association existed between the incidence of aspiration pneumonia at 12 months and 24 months after the swallowing studies and the percentage of clearance of radioactivity from the respiratory tracts after aspiration. Furthermore, chi‐square and Fisher's exact tests were also performed to identify factors that were associated with the incidence of aspiration as detected by FEES, VFSS, and OPERS. Receiver operating characteristic (ROC) curves were plotted to identify the optimal cut‐off values of percentage clearance and absolute value of radioactivity in the trachea and lungs after OPERS to predict aspiration pneumonia within 12 months and 24 months after OPERS. By using FEES and VFSS as the reference respectively, the sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPV) of FEES, VFSS, and OPERS to identify penetration and aspiration were calculated. Lastly, the Kaplan Meier analysis and Log Rank test were used to estimate and compare the survival of patients who were and who were not diagnosed with aspiration on the FEES, VFSS, and OPERS. For all statistical analyses, p < .05 was considered to be significant.
3. RESULTS
Thirty‐seven eligible patients who had previously been treated with radiotherapy for NPC and who attended the dysphagia clinic between 2014 and 2017 were entered into this study. All had undergone a FEES and OPERS for the evaluation of their swallowing, while only 30 had undergone a VFSS examination. Nine females and 28 males had a mean age of 58.4 years (range 31–73 years). The mean duration of dysphagia was 41 months (range 1–120 months). The main complaints of patients who attended the dysphagia clinic were dysphagia (70.3%) and choking (18.9%). Sixteen patients had a history of aspiration pneumonia prior to attending the dysphagia clinic for swallowing evaluation. The mean follow‐up after the swallowing studies was 53.6 months (range 2–92 months). Fifty‐one percent of the patients had had Stage I or II disease. Eighteen patients received radiotherapy alone and 19 patients received chemoradiotherapy. Prior to the swallowing studies, 14 patients (37.8%) were on non‐oral feeding. Overall, 12 patients (34.3%) developed aspiration pneumonia within 24 months of the swallowing studies. Eight patients (21.6%) developed aspiration pneumonia during the first year and four (11.4%) developed aspiration pneumonia during the second year after their swallowing study.
The intraclass correlation coefficient (ICC) for the two sets of measurements of the OPERS by the radiologist was 0.775 with a 95% CI that ranged from 0.358 to 0.928, which reflected an excellent agreement between the two sets of readings for the radioactivity on the scan. The ICC for the three observers in rating the FEES and VFSS is summarized in Table 2 and demonstrates moderate to excellent reliability.
TABLE 2.
Intra and inter class correlation coefficients computed to measure the intra‐rater (A) and inter‐rater (B) reliability
| (A) Intra‐rater reliability of the raters | ||
|---|---|---|
| Rater | FEES | VFSS |
| Rater A | 0.995 (95% CI 0.991–0.998) | 0.995 (95% CI 0.990–0.998) |
| Rater B | 0.955 (95% CI 0.910–0.977) | 0.959 (95% CI 0.915–0.981) |
| Rater C | 0.886 (95% CI 0.738–0.946) | 0.902 (95% CI 0.796–0.953) |
| (B) Inter‐rater reliability of the raters: | |
|---|---|
| Swallowing study | ICC |
| FEES | 0.676 (95% CI 0.174–0.859) |
| VFSS | 0.700 (95% CI 0.351–0.860) |
Abbreviations: FEES, flexible endoscopic evaluation of swallowing; ICC, intraclass correlation coefficient; VFSS, videofluoroscopic swallowing study.
With different food consistencies given during the swallowing studies, aspiration of fluid was detected in 29 patients (78.4%) by FEES (PAS score: 6–8). Twenty‐five patients (67.6%) had PAS graded as 7 or 8 on thin fluid and thick fluid respectively, which suggested partial or no clearance of aspirated food material from the subglottis or trachea. On the other hand, VFSS detected aspiration of fluid in 20 of 30 patients (66.7%) and OPERS aspiration in 16 patients (44.4%). The agreement among FEES, VFSS, and OPERS for detecting aspiration of fluid was variable with a good agreement between VFSS and OPERS (82.8%, Gwet's AC1: 0.66) and a fair agreement between FEES and OPERS (66.7%, Gwet's AC1: 0.37; Table 3).
TABLE 3.
Agreement for detection of aspiration by FEES, VFSS, and OPERS
| FEES | Total cases | |||
|---|---|---|---|---|
| No aspiration | Aspiration | |||
| OPERS | No aspiration | 8 | 12 | 20 |
| Aspiration | 0 | 16 | 16 | |
| Total cases | 8 | 28 | 36 | |
|
Gwet's AC1 = 0.37 (95% CI: 0.04–0.69) Percentage agreement = 66.7% | ||||
| VFSS | Total cases | |||
|---|---|---|---|---|
| No aspiration | Aspiration | |||
| OPERS | No aspiration | 10 | 5 | 15 |
| Aspiration | 0 | 14 | 14 | |
| Total cases | 10 | 19 | 29 | |
|
Gwet's AC1 = 0.66 (95% CI: 0.38–0.95) Percentage agreement = 82.8% | ||||
| FEES | Total cases | |||
|---|---|---|---|---|
| No aspiration | Aspiration | |||
| VFSS | No aspiration | 4 | 6 | 10 |
| Aspiration | 1 | 19 | 20 | |
| Total cases | 5 | 25 | 30 | |
|
Gwet's AC1 = 0.63 (95% CI: 0.33–0.92) Percentage agreement = 76.7% | ||||
Abbreviations: FEES, flexible endoscopic evaluation of swallowing; OPERS, oro‐pharyngo‐esophageal radionuclide scintigraphy; VFSS, videofluoroscopic swallowing study.
Based on the evaluation by FEES, 17 patients were recommended for non‐oral feeding, while nine patients had a percutaneous endoscopic gastrostomy and eight patients had a nasogastric tube. One patient was advised to partially oral feed while using gastrostomy feeding. Eighteen patients required a modified diet to continue safe oral feeding. Thirty‐three patients were offered oro‐motor and neck exercise as swallowing training by speech therapists. In the 12 months follow up after the swallowing study, 4 of 20 patients (20%) who were advised for oral feeding eventually required tube feeding, and 2 of 17 patients (11.8%) who were advised for tube feeding were found to be noncompliant with their swallowing recommendations.
Using FEES as the gold standard for the detection of aspiration, the sensitivity of OPERS was 57.1% and the specificity 100%. The PPV was 100% and the NPV was 40%, with an overall accuracy of 66.7%. However, if VFSS was used as the gold standard for the detection of aspiration, the sensitivity of OPERS was 73.7% and the specificity 100%, and the PPV was 100% and the NPV was 66.7%, with an overall accuracy of 82.8%.
The chi‐square test and Fisher's exact tests showed that several factors were statistically significantly associated with the incidence of aspiration detected by FEES, VFSS, and OPERS. These factors were a history of aspiration pneumonia, gender, trismus, voluntary cough, pharyngeal contraction, supraglottic sensation, pharyngeal coating of food, food residue in a vallecula and/or pyriform fossa, and the feeding method. However, binary logistic regression showed patients with a history of aspiration pneumonia before swallowing evaluation was one factor that was associated with a higher chance of developing aspiration pneumonia again within 12 months (odds ratio: 15.5, 95% CI 1.67–145.8, p < .05) and 24 months (odds ratio: 23.8, 95% CI 3.69–152.89, p < .01) after the swallowing assessment.
An analysis was performed to evaluate whether aspiration detected by either FEES, VFSS or OPERS could predict future aspiration pneumonia within the 12 and 24 months of follow up after the swallowing study. We found that only aspiration detected by OPERS was a statistically significant risk factor for future aspiration pneumonia at 12 months (odds ratio: 5.4, 95% CI 1.02–31.933, p < .05) and 24 months (odds ratio: 10.2, 95% CI 1.97–52.78, p < .05), respectively, after the swallowing study, while aspiration detected on FEES was not. Aspiration detected by VFSS was also a significant factor for future aspiration pneumonia but only at 24 months after the study (odds ratio: 10, 95% CI 1.05–95.24, p < .05; Table 4).
TABLE 4.
Incidence of aspiration pneumonia at 12 and 24 months post‐swallowing study based on the findings on FEES, VFSS, and OPERS
| Swallowing test | Post‐study 12 months | Post‐study 24 months | ||||
|---|---|---|---|---|---|---|
| Pneumonia | Pneumonia | |||||
| FEES | Yes | No | p value | Yes | No | p value |
| Aspiration | 7 | 22 | p = .655 | 11 | 16 | p = .216 |
| No aspiration | 1 | 7 | 1 | 7 | ||
| VFSS | Pneumonia | Pneumonia | ||||
|---|---|---|---|---|---|---|
| Yes | No | p value | Yes | No | p value | |
| Aspiration | 6 | 14 | p = .053 | 10 | 9 | * p = .025 |
| No aspiration | 0 | 10 | 1 | 9 | ||
| OPERS | Pneumonia | Pneumonia | ||||
|---|---|---|---|---|---|---|
| Yes | No | p value | Yes | No | p value | |
| Aspiration | 6 | 10 | * p = .049 | 9 | 5 | * p < .01 |
| No aspiration | 2 | 18 | 3 | 17 | ||
Abbreviations: FEES, flexible endoscopic evaluation of swallowing; OPERS, oro‐pharyngo‐esophageal radionuclide scintigraphy; VFSS, videofluoroscopic swallowing study.
p < .05 = statistical significance.
Regarding the clearance of radioactivity which was assumed to correspond to the clearance of aspirated food from the lung, Fisher's Exact test showed a statistically significant association between aspiration pneumonia at 12 and 24 months after the swallowing study, and less than 60% clearance of radioactivity from the tracheobronchial tree on the 60–90 minutes follow‐up scans on OPERS (Table 5).
TABLE 5.
Incidence of aspiration pneumonia at 12 and 24 months post oro‐pharyngo‐esophageal radionuclide scintigraphy (OPERS) based on different clearances of radiotracer from the chest
| Chest condition | Post‐study 12 months | Post‐study 24 months | ||||
|---|---|---|---|---|---|---|
| 50% clearance of radioactivity | 50% clearance of radioactivity | |||||
| Above | Below | p value | Above | Below | p value | |
| Pneumonia | 4 | 2 | p = .051 | 7 | 2 | p = .255 |
| No pneumonia | 10 | 0 | 5 | 0 | ||
| 55% clearance of radioactivity | 55% clearance of radioactivity | |||||
|---|---|---|---|---|---|---|
| Above | Below | p value | Above | Below | p value | |
| Pneumonia | 2 | 4 | * p < .01 | 6 | 3 | p = .145 |
| No pneumonia | 10 | 0 | 5 | 0 | ||
| 60% clearance of radioactivity | 60% clearance of radioactivity | |||||
|---|---|---|---|---|---|---|
| Above | Below | p value | Above | Below | p value | |
| Pneumonia | 0 | 6 | * p < .01 | 4 | 5 | * p = .038 |
| No pneumonia | 9 | 1 | 5 | 0 | ||
p < .05 = statistical significance.
ROC curve analysis was performed to obtain the optimal cut‐off values in radioactivity that may determine the chance of aspiration pneumonia at 12 and 24‐months with the highest sensitivity and specificity after OPERS. These results are shown in Table 6. However, no significant difference in mean survival difference was noted with these cut‐off values.
TABLE 6.
Receiver operating characteristic (ROC) curve analysis to determine the optimal cut‐off values in radioactivity that can predict aspiration pneumonia at 12 and 24 months after oro‐pharyngo‐esophageal radionuclide scintigraphy (OPERS) with the optimal sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) given
| OPERS and post study duration | Optimal cut‐off radioactivity | Sensitivity | Specificity | PPV | NPV |
|---|---|---|---|---|---|
| a Delayed 60–90 minutes, 12 months | a 10837.50 | 100% | 100% | 100% | 100% |
| Immediate, 12 months | 41,523.50 | 83.3% | 100% | 100% | 90.91% |
| Delayed 60–90 minutes, 24 months | 6588.50 | 55.6% | 100% | 100% | 55.56% |
| Immediate, 24 months | 17,311.00 | 66.7% | 100% | 100% | 62.50% |
Optimal cut‐off value for residual radioactivity in the chest by delayed OPERS with 100% sensitivity, specificity, PPV, and NPV.
ROC curves were also constructed to find the optimal cut‐off levels. The areas under the curve (AUC) were all above 0.75 which indicates that the percentage clearance of radioactivity from the tracheobronchial tree on the 60–90 minutes follow‐up scan was a good to excellent tool to predict subsequent aspiration pneumonia in the 12 and 24 months after the swallowing assessment. ROC estimation showed that the clearance of radioactivity from the tracheobronchial tree by less than 59% on the 60–90 minutes follow‐up scan during OPERS predicts a 97% chance of developing aspiration pneumonia in the following 12 months with a sensitivity of 100% and specificity of 90% (AUC: 0.97, p < .05). The same ROC estimation showed the optimal cut‐off value for clearance of radioactivity from the tracheobronchial tree. Clearance of less than 73.5% of radioactivity from the chest on the delayed 60–90 minutes scan was associated with an 83% chance of an aspiration pneumonia within the following 24 months after OPERS, with a sensitivity of 77.8% and a specificity of 100% (AUC: 0.83, p < .05).
Eighteen patients (48.6%) had died at the time of data analysis of this study. The mean survival for patients with pre‐study aspiration pneumonia was 45.9 months, for the post‐study aspiration pneumonia at 12 months the mean survival was 31.5 months, and at 24 months, it was 43.3 months. These were all shorter times than for patients who never developed aspiration pneumonia, who survived for a mean of 69.9–75.4 months (Table 7). On the other hand, the mean survival of patients who had no aspiration detected by FEES, VFSS, and OPERS was generally longer than for patients in whom aspiration was detected by these swallowing studies (Table 8). However, the only statistical difference in survival shown was for patients with (mean survival 49 months) and without (mean survival 73 months) aspiration detected by OPERS (p < .05; Figure 2).
TABLE 7.
Mean survival for patients with or without aspiration pneumonia before and after their swallowing study
| Chest condition | Pre‐study | Post‐study 12 months | Post‐study 24 months |
|---|---|---|---|
| Mean survival (months) | |||
| Aspiration pneumonia | 45.9 | 31.5 | 43.2 |
| No aspiration pneumonia | 72.6 | 69.9 | 75.4 |
| p value | *.027 | *<.01 | *<.01 |
Log Rank test, p < .05: statistical significance.
TABLE 8.
Mean survival for patients with or without aspiration detected on FEES, VFSS, and OPERS
| Chest condition | FEES | VFSS | OPERS |
|---|---|---|---|
| Mean survival (months) | |||
| Aspiration | 57.4 | 51.9 | 47 |
| No aspiration | 70.9 | 69.6 | 72.8 |
| p value | .47 | .173 | * .024 |
Abbreviations: FEES, flexible endoscopic evaluation of swallowing; OPERS, oro‐pharyngo‐esophageal radionuclide scintigraphy; VFSS, videofluoroscopic swallowing study.
Log Rank test, p < .05: statistical significance.
FIGURE 2.
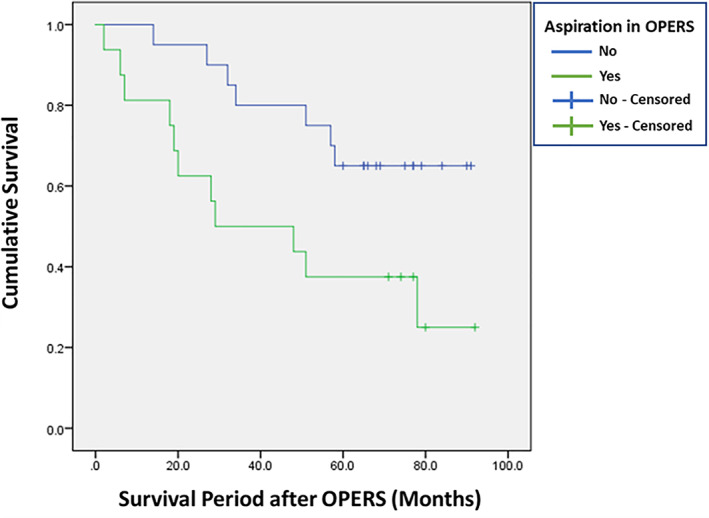
Survival curves for patients with and without aspiration detected by oro‐pharyngo‐esophageal radionuclide scintigraphy (OPERS)
4. DISCUSSION
In this study, the PAS rating for aspiration by FEES and VFSS was not found to be significantly associated with the incidence of aspiration pneumonia at 12 months nor at 24 months after the swallowing studies. However, aspiration detected by OPERS did have a significant prognostic value for the subsequent incidence of aspiration pneumonia after the swallowing evaluation. This can be explained by the fact that OPERS allows for the demonstration of deeper and more distal infiltration of aspirated food material into the tracheobronchial tree, which may not be possible to detect on a standard FEES nor on the lateral views of a VFSS, as both studies only examine aspiration down to the subglottis and upper trachea respectively. 24 Further, this phenomenon cannot be adequately addressed by the PAS score during FEES and VFSS, as the grading scale only addresses the level of penetration of food material with reference to above or below the true vocal folds, the integrity of the cough reflex and the strength of coughing to expel any aspirated food material effectively from the airway.
The antero‐posterior view of the chest during VFSS can detect tracheobronchial aspiration which may predict the risk of aspiration pneumonia, but it does not allow quantification of the amount of aspiration nor of the subsequent clearance of aspirated food material from the chest, which provides extra parameters with which to estimate the subsequent risk of chest infections in the 12 and 24 months after the swallowing study. We are very interested to identify other factors that can be used to further stratify “silent aspiration” and which may have a prognostic value in the management of dysphagia. In this respect, OPERS not only allows quantification of the food entering the chest, but also of the extent of infiltration into the tracheobronchial tree, and of the clearance of residual food material from the chest. The use of SPECT/CT views can improve identification and characterization of the anatomic structures affected by aspiration. 25 OPERS can fill in missing data of either FEES or VFSS and add information other than penetration or aspiration, which can further predict safety and prognosis on deglutition.
Our study showed that OPERS was not only useful in predicting aspiration pneumonia at 12 and 24 months after the swallowing evaluation respectively, but also had significant implications for the survival prognosis of the patient, as the survival period for patients who had aspiration detected on FEES, VFSS, and OPERS was generally shorter than for those patients without aspiration. However, only the difference in survival between aspiration and non‐aspiration detected on OPERS was statistically significant. This can be explained by the fact OPERS is able to detect those patients with significant aspiration into their tracheobronchial tree rather than subtle to mild aspiration at the level of the subglottis or upper trachea with a standard FEES and VFSS, which in itself carries an unfavorable survival prognosis. 26 , 27
We assume that the clearance of the radiotracer from the chest is correlated with the clearance of aspirated food material. By using ROC analysis, this study showed that when the clearance of radioactivity from the chest was less than 59% on the 60–90 minutes post‐swallow follow‐up scan of the OPERS study, it was associated with subsequent aspiration pneumonia at 12 and 24 months with a sensitivity of 100% and specificity of 90%. On the other hand, the absolute level of radioactivity in delayed scanning of the chest at 60–90 minutes could also achieve a 100% sensitivity and 100% specificity in predicting aspiration pneumonia at 12‐months after the swallowing study. Although OPERS is less sensitive than FEES and VFSS in detecting aspiration in our study, the information obtained from the OPERS is clinically more meaningful and practical in guiding a speech therapist or clinician's decision regarding the mode of feeding and swallowing rehabilitation. OPERS exposes patients to lower doses of radiation than does VFSS, and so is a good supplementary swallowing study to support FEES, which is more accessible for swallowing evaluation and is more sensitive in detecting aspiration, defining the anatomy of the pharynx, and pharyngeal stasis of food. 28 OPERS is thus an invaluable swallowing test to combine with a FEES to assess swallowing safety in high‐risk head and neck cancer patients. FEES can conveniently be performed in a clinic or at the bedside using a standard flexible endoscope and basic audiovisual equipment available in an ambulatory otolaryngology practice. The cost of operating and maintain FEES hardware is lower than for VFSS and OPERS which require more expensive and sophisticated equipment in fixed locations, as well as having a higher maintenance cost. However, the equipment setup for VFSS and OPERS may also be shared by other specialties for other imaging purposes, so that the cost of the VFSS and OPERS can be reduced by the economy of scale.
The limitation of our study is that it is retrospective in nature and in data retrieval. Some patients did default on their VFSS examination. For OPERS, the protocol did not include the measurement of the ratio of aspirated food into the tracheobronchial tree per mouthful of food. Moreover, there was a small time lag between the performance of the FEES, VFSS, and OPERS, as the three swallowing evaluations were not done on the same day. However, the time lag did not result in a significant effect on the outcome of the assessments of our subjects as their dysphagia was chronic with a mean duration of symptoms of 41 months, and in our experience, it takes months or years for any significant deterioration to occur. There was no disconnect between the swallowing evaluation outcome and the clinical condition. Moreover, this was not a one‐off study but a continuation of care to patients regularly followed up in our clinics for review of their swallowing condition, and any deterioration would have been noted and recorded.
Although this study is limited by its retrospective nature and availability of resources for swallowing studies, it does provide preliminary evidence, based on available data from our center, on the suitability of OPERS to supplement our existing swallowing studies of FEES and VFSS, and hopefully provide extra information to clinicians regarding the safety of swallowing in patients with dysphagia and their relative prognosis. A future larger scale prospective study is warranted to further investigate the application of OPERS for dysphagia in other head and neck cancer patients.
5. CONCLUSION
OPERS can predict the safety of swallowing in post‐irradiated NPC dysphagia patients, and the incidence of future aspiration pneumonias, and the prognosis for their survival. OPERS can also supplement a FEES in high‐risk dysphagia patients being assessed for mode of feeding determination.
CONFLICT OF INTERESTS
The authors declare that they have no competing interests or financial relationships to disclose.
Ku PKM, Wang K, Vlantis AC, et al. Oro‐pharyngo‐esophageal radionuclide scintigraphy predicts aspiration pneumonia risk and associated survival in post‐irradiated nasopharyngeal carcinoma patients. Laryngoscope Investigative Otolaryngology. 2022;7(1):170‐179. doi: 10.1002/lio2.704
Michael C. F. Tong and Andrew van Hasselt are co‐senior authors for this article.
REFERENCES
- 1. Wang JJ, Jiang RS, Yen TT, Liang KL. Risk factors for recurrent pneumonia in post‐irradiated patients with nasopharyngeal carcinoma. J Chin Med Assoc. 2017;80:558‐562. [DOI] [PubMed] [Google Scholar]
- 2. Xiong J, Krishnaswamy G, Raynor S, Loh KS, Kwa ALH, Lim CM. Risk of swallowing‐related chest infections in patients with nasopharyngeal carcinoma treated with definitive intensity‐modulated radiotherapy. Head Neck. 2016;38(suppl 1):E1660‐E1665. [DOI] [PubMed] [Google Scholar]
- 3. Niimi A, Matsumoto H, Ueda T, et al. Impaired cough reflex in patients with recurrent pneumonia. Thorax. 2003;58:152‐153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Woisard V, Victor G. Importance of swallowing scintigraphy in the evaluation of the risk of pulmonary complications in patients with a swallowing disorder. Rev Laryngol Otol Rhinol (Bord). 2008;129:91‐95. [PubMed] [Google Scholar]
- 5. Rosenbeck JC, Robbins JA, Roecker EB, Coyle JL, Wood JL. A penetration‐aspiration scale. Dysphagia. 1996;11:93‐98. [DOI] [PubMed] [Google Scholar]
- 6. Yoon JA, Kim SH, Jang MH, Kim SD, Shin YB. Correlations between aspiration and pharyngeal residue scale scores for fiberoptic endoscopic evaluation and videofluoroscopy. Yonsei Med J. 2019;60:1181‐1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Butler SG, Markley L, Sanders B, Stuart A. Reliability of the penetration aspiration scale with flexible endoscopic evaluation of swallowing. Ann Otol Rhinol Laryngol. 2015;124:480‐483. [DOI] [PubMed] [Google Scholar]
- 8. Lee JH, Lee KW, Kim SB, Lee SJ, Chun SM, Jung SM. The functional dysphagia scale is a useful tool for predicting aspiration pneumonia in patients with Parkinson disease. Ann Rehabil Med. 2016;40:440‐446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Yu KJ, Moon H, Park D. Different clinical predictors of aspiration pneumonia in dysphagic stroke patients related to stroke lesion: a STROBE‐complaint retrospective study. Medicine (Baltimore). 2018;97:e13968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Orthey P, Dadparvar S, Kamat B, Parkman HP, Maurer AH. Using gastric emptying scintigraphy to evaluate antral contractions and duodenal bolus propagation. Am J Physiol Gastrointest Liver Physiol. 2020;318:G203‐G209. [DOI] [PubMed] [Google Scholar]
- 11. Speir EJ, Newsome JM, Bercu ZL, Miller MJ Jr, Martin JG. Correlation of CT angiography and 99m technetium‐labeled red blood cell scintigraphy to catheter angiography for lower gastrointestinal bleeding: a single‐institution experience. J Vasc Interv Radiol. 2019;30:1725‐1732.e7. [DOI] [PubMed] [Google Scholar]
- 12. Solnes LB, Sheikhbahaei S, Ziessman HA. Nuclear scintigraphy in practice: gastrointestinal motility. AJR Am J Roentgenol. 2018;211:260‐266. [DOI] [PubMed] [Google Scholar]
- 13. Falk GL, Beattie J, Ing A, et al. Scintigraphy in laryngopharyngeal and gastroesophageal reflux disease: a definitive diagnostic test? World J Gastroenterol. 2015;21:3619‐3627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Humphreys B, Mathog R, Rosen R, Miller P, Muz J, Nelson R. Videofluoroscopic and scintigraphic analysis of dysphagia in the head and neck cancer patient. Laryngoscope. 1987;97:25‐32. [DOI] [PubMed] [Google Scholar]
- 15. Park D, Woo SB, Lee DH, et al. The correlation between clinical characteristics and radionuclide salivagram findings in patients with brain lesions: a preliminary study. Ann Rehabil Med. 2017;41:915‐923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hou P, Deng H, Wu Z, et al. Detection of salivary aspiration using radionuclide salivagram SPECT/CT in patients with COPD exacerbation: a preliminary study. J Thorac Dis. 2016;8:2730‐2737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Yang J, Codreanu I, Servaes S, Zhuang H. Radionuclide salivagram and gastroesophageal reflux scintigraphy in pediatric patients: targeting different types of pulmonary aspiration. Clin Nucl Med. 2015;40:559‐563. [DOI] [PubMed] [Google Scholar]
- 18. Kim GE, Sung IY, Ko EJ, Choi KH, Kim JS. Comparison of videofluoroscopic swallowing study and radionuclide salivagram for aspiration pneumonia in children with swallowing difficulty. Ann Rehabil Med. 2018;42:52‐58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Jang DH, Choi KH, Kim DH, Lim CM, Kim JS. Comparison between the radionuclide salivagram and videofluoroscopic swallowing study methods for evaluating patients with aspiration pneumonia. Ann Nucl Med. 2013;27:247‐252. [DOI] [PubMed] [Google Scholar]
- 20. Muz J, Mathog RH, Miller PR, Rosen R, Borrero G. Detection and quantification of laryngotracheopulmonary aspiration with scintigraphy. Laryngoscope. 1987;97:1180‐1185. [DOI] [PubMed] [Google Scholar]
- 21. Muz J, Mathog RH, Hamlet SL, Davis LP, Kling GA. Objective assessment of swallowing function in head and neck cancer patients. Head Neck. 1991;13:33‐39. [DOI] [PubMed] [Google Scholar]
- 22. Muz J, Hamlet S, Mathog R, Farris R. Scintigraphic assessment of aspiration in head and neck cancer patients with tracheostomy. Head Neck. 1994;16:17‐20. [DOI] [PubMed] [Google Scholar]
- 23. Ku PK, Vlantis AC, Hui TS, et al. Assessment of pharyngeal motor function using a novel velopharyngeal squeeze maneuver and a novel endoscopic pharyngeal contraction grade scale in patients with dysphagia after radiotherapy for nasopharyngeal carcinoma. Head Neck. 2021;43:3586‐3597. doi: 10.1002/hed.26871. Online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Silver KH, Van Nostrand D, Kuhlemeier KV, Siebens AA. Scintigraphy for the detection and quantification of subglottic aspiration: preliminary observations. Arch Phys Med Rehabil. 1991;72:902‐910. [DOI] [PubMed] [Google Scholar]
- 25. Grosso M, Duce V, Fattori B, et al. The value of oro‐pharyngo‐esophageal scintigraphy in the management of patients with aspiration into the tracheo‐bronchial tree and consequent dysphagia. N Am J Med Sci. 2015;7:533‐536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Fattori B, Giusti P, Mancini V, et al. Comparison between videofluoroscopy, fiberoptic endoscopy and scintigraphy for diagnosis of oro‐pharyngeal dysphagia. Acta Otorhinolaryngol Ital. 2016;36:395‐402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Silver KH, Van Nostrand D. The use of scintigraphy in the management of patients with pulmonary aspiration. Dysphagia. 1994;9:107‐115. [DOI] [PubMed] [Google Scholar]
- 28. Takahashi N, Kikutani T, Tamura F, Groher M, Kuboki T. Videoendoscopic assessment of swallowing function to predict the future incidence of pneumonia of the elderly. J Oral Rehabil. 2012;39:429‐437. [DOI] [PubMed] [Google Scholar]


