Abstract
Background
Inflammatory myofibroblastic tumors in the head and neck (HNIMTs) sometimes show aggressive clinical features and can be diagnosed as HNIMT with malignant transformation.
Methods
The clinicopathological features of 45 HNIMTs with or without malignant transformation were retrospectively investigated. Logistic regression and receiver operating characteristic analysis were used to establish the predictive model.
Results
HNIMT with malignant transformation was associated with worse prognosis. HNIMT with a tumor size of >4.4 cm, tumors located in the maxillary sinus, or a preoperative neutrophil‐to‐lymphocyte ratio (NLR) greater than 1.958 were associated with higher chance of malignant transformation, with an AUC value of 0.9189. Postoperative radiotherapy could benefit HNIMT patients with high risk of malignant transformation.
Conclusions
HNIMT patients with a tumor size of >4.4 cm, tumors located in the maxillary sinus, and a preoperative NLR over 1.958 were associated with a higher risk of malignant transformation. These patients can benefit from postoperative radiotherapy.
Keywords: head neck, inflammatory myofibroblastic tumor, malignant transformation, postoperative radiotherapy, predicative model
1. INTRODUCTION
According to the 2020 World Health Organization (WHO) classification, an inflammatory myofibroblastic tumor (IMT) is a mesenchymal neoplasm of intermediate malignancy (rarely metastasizing). Although rare, IMT in head and neck (HNIMT) accounts for nearly 18% of extrapulmonary IMT cases. 1 There are sporadic case reports about IMT originating from the mastoid, 2 neck, 3 maxillary sinus, 4 , 5 larynx, 6 oral cavity, 7 , 8 and skull base. 9 Unlike IMTs originating from other sites that are associated with better disease‐free and overall survival rates, 10 , 11 HNIMT patients have a worse prognosis, 1 , 12 which may partially be explained by anatomical complexity.
Moreover, IMT can be accompanied by malignant transformation during tumor progression, which has been reported for IMT located in the bladder, 13 kidney, 14 and hypopharynx. 15 Histologically, IMT is characterized by spindle cell proliferation, and malignant transformation is marked by the transition from uniform spindled cells to atypical polygonal cells and atypical mitoses. 16 , 17 More importantly, IMTs with malignant transformation pursue an aggressive course with rapid local recurrences and are frequently fatal. 17 Unveiling the clinicopathological features of HNIMT with malignant transformation may help surgeons to make predictions, evaluate the surgical resection range preoperatively, and provide adjuvant therapies.
In the current study, the clinicopathological features of HNIMT with or without malignant transformation were retrospectively investigated and compared in a single cohort. Logistic regression and receiver operating characteristic (ROC) analysis were used to establish the predictive model of HNIMT with malignant transformation.
2. MATERIALS AND METHODS
Ethical approval was granted by the Ethical Committee of the Shanghai Ninth People's Hospital affiliated with the Shanghai Jiaotong University School of Medicine. Data on all 45 histologically proven HNIMTs treated between 2005 and 2017 were retrieved from a database from the Department of Oral and Maxillofacial Head Neck Oncology in Shanghai Ninth People's Hospital affiliated with the Shanghai Jiaotong University School of Medicine. The data acquired include demographic details, medical history, clinical features, histopathologic characteristics, and follow‐up information.
According to the WHO soft tissue classification, all 41 specimens were divided into three histopathologic types: myxoid, compact, and hyalinized, as we earlier reported. 12 The inflammation degree of tumor tissues was determined according to the research of Bennett. 18 The immunohistochemistry staining of anaplastic lymphoma kinase (ALK) was reported in our earlier research. 12 The histopathological diagnosis of HNIMT with malignant transformation was described in our earlier report 12 and other studies. 16 , 17
The overall and disease‐free survival rates were determined by the Kaplan–Meier (KM) method. The constituent ratio of HNIMT patients with different clinicopathological features was compared using Pearson's χ 2 test and Fisher's exact test. Prognosis prediction performance was calculated by the area under the ROC curve (AUC). Youden's index was calculated for the prognostic models.
Statistical analysis was performed utilizing GraphPad Prism 6.0 software and SPSS 20.0 Statistics software. P < .05 was considered statistically significant.
3. RESULTS
3.1. HNIMT patients with malignant transformation had worse prognosis
Among the 45 cases, 13 died at follow‐up (28.9%). The 1‐, 3‐, and 5‐year survival rates were 100%, 71.1%, and 71.1%, respectively. Fifteen patients were diagnosed with HNIMT with malignant transformation. Histologically, HNIMT with malignant transformation showed atypical polygonal cells and atypical mitoses (Figure 1A,B). Radiologically, HNIMT with malignant transformation showed obvious local aggression similar to malignant neoplasm. As shown in Figure 1C, HNIMT with malignant transformation could invade the orbits, masticator space, and skull base. The overall survival rate of HNIMT with or without malignant transformation was 40% and 86.7%, respectively. HNIMT patients with malignant transformation had worse overall survival (P = .004) and disease‐free survival rates (P = .0237) (Figure 2A,B).
FIGURE 1.
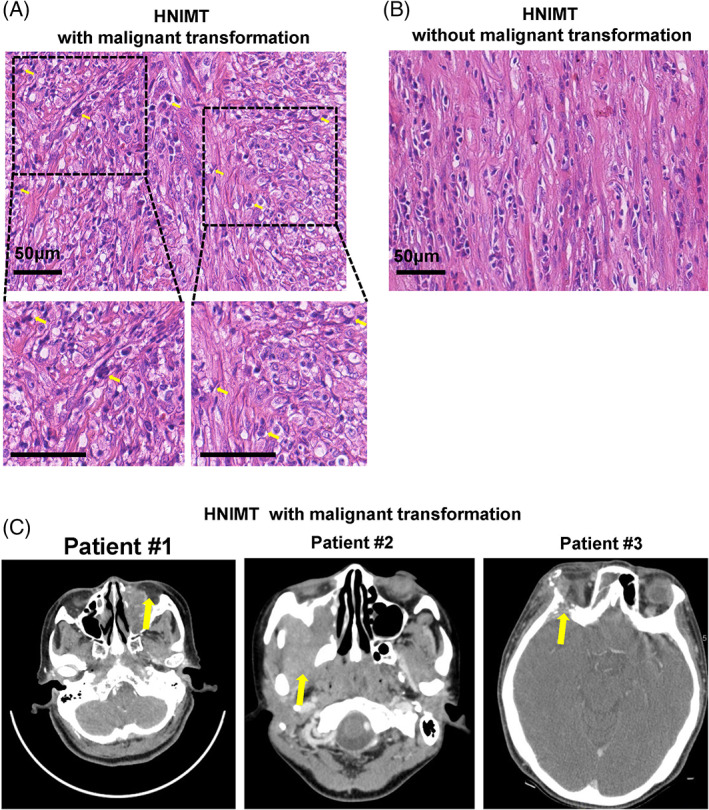
The histopathological and radiological features of inflammatory myofibroblastic tumor in the head and neck (HNIMT) with malignant transformation. (A) Hematoxylin–eosin staining of HNIMT with malignant transformation showed atypical polygonal cells and atypical mitoses. (B) Hematoxylin–eosin staining of HNIMT without malignant transformation. (C) CT images of HNIMT with malignant transformation showing great local invasion
FIGURE 2.
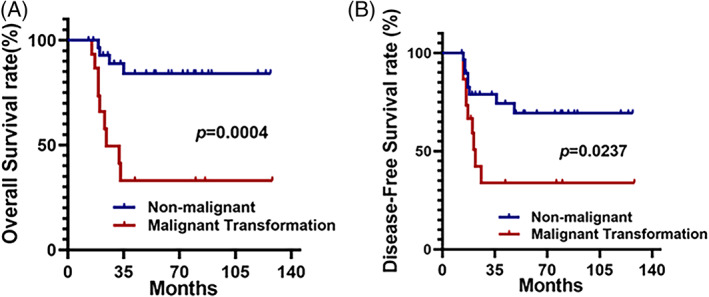
Inflammatory myofibroblastic tumor in the head and neck (HNIMT) patients with malignant transformation had worse prognosis. (A) HNIMT patients with malignant transformation were associated with worse overall survival compared to those without malignant transformation (P = .0004). (B) HNIMT patients with malignant transformation had a worse disease‐free survival rate compared to those without malignant transformation (P = .0237)
3.2. Different clinical features between HNIMT with or without malignant transformation
We next explored the potential clinicopathological features which are correlated with the malignant transformation of HNIMT. The average tumor size of HNIMT with and without malignant transformation was 4.980 cm versus 3.55 cm (P = .0017). The ROC analysis revealed that the best cutoff was 4.4 cm, which yielded a Youden index of 0.5667 with a sensitivity of 80% and a specificity of 76.67%. As expected, HNIMT patients with a tumor measuring >4.4 cm in diameter had a higher incidence of malignant transformation (63.2% vs. 11.5%, P = .0004) (Figure 3A). Tumor location was also closely correlated with the biological behavior of cancer cells. Among all 45 cases, there were 5 primary tumors located in the infratemporal fossa, 9 in the maxilla, 12 in the maxillary sinus, 5 in the mandible, 4 in the parotid gland, 4 in the pterygopalatine fossa, and 6 in the neck. Using our previously reported classification method based on the ala‐tragus line, there was no significant correlation between malignant transformation and tumor location (Figure 3B). To our surprise, HNIMT patients with the tumor located in the maxillary sinus had a much higher incidence of malignant transformation (83.3% vs. 15.2%, P < .0001) (Figure 3C).
FIGURE 3.
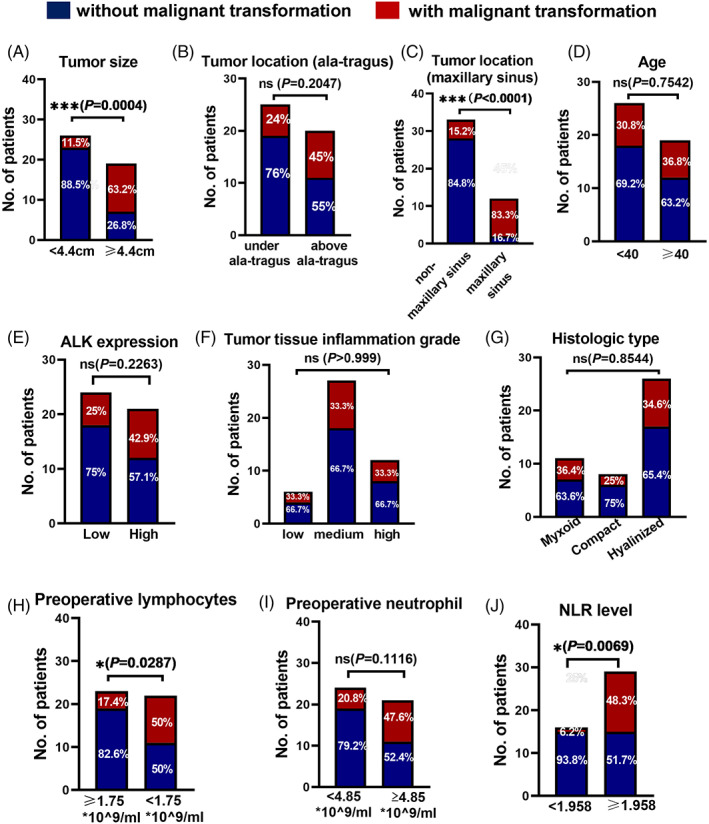
Different clinical features between inflammatory myofibroblastic tumor in the head and neck (HNIMT) patients with or without malignant transformation. (A) HNIMT patients with a tumor measuring >4.4 cm in diameter had a higher incidence of malignant transformation (63.2% vs. 11.5%, P = .0004). (B) There is no significant correlation between malignant transformation and tumor location based on the ala‐tragus line. (C) HNIMT patients with the tumor located in the maxillary sinus had a higher incidence of malignant transformation (83.3% vs. 15.2%, P < .0001). (D) There were no significant correlations between malignant transformation and tumor tissue ALK expression, histological type, and patient age. (E–G) There were no significant correlations between the malignant transformation and (E) tumor ALK expression, (F) tumor tissue inflammation grade, and (G) the histologic type. (H) HNIMT patients with preoperative lymphocyte counts below 1.75 × 109 cells/ml had a higher incidence of malignant transformation (50.0% vs. 17.4%, P = .0004). (I) There is no significant correlation between malignant transformation and the preoperative neutrophil count. (J) HNIMT patients with preoperative neutrophil‐to‐lymphocyte ratio (NLR) >1.958 had a higher incidence of malignant transformation (48.3% vs. 6.2%, P = .0004)
3.3. ALK expression and inflammation in tumor tissues was not correlated with malignant transformation of HNIMT
We have recently reported that the ALK level and inflammation in HNIMT tumor tissues are independent prognostic factors of the disease‐free survival rate. Here, we analyzed whether these two important pathological features are correlated with the malignant transformation of HNIMT. We found that the malignant transformation rate in the high ALK group was 42.9%, compared to 25% in the low ALK group; this difference was not statistically significant (P = .2263) (Figure 3E). Similarly, the inflammation grade (P > .999) and histological type (P = .8544) were not correlated with the malignant transformation of HNIMT (Figure 3F,G).
3.4. Elevated neutrophil‐to‐lymphocyte ratio represented a phenotype of inflammation in HNIMT with malignant transformation
We then evaluated the inflammation grade based on preoperative peripheral immune cells. The mean number of preoperative lymphocytes in HNIMT with or without malignant transformation was 1.547 versus 2.137 × 109 cells/ml (P = .0127). The best cutoff value is 1.75 × 109 cells/ml. The mean number of preoperative neutrophils in HNIMT with or without malignant transformation was 5.553 versus 4.400 × 109 cells/ml (P = .0653). The best cutoff value is 4.85 × 109 cells/ml. To our surprise, HNIMT patients with preoperative lymphocyte counts below 1.75 × 109 cells/ml had a significantly higher incidence of malignant transformation (50.0% vs. 17.4%, P < .0001) (Figure 3H). There was no significant correlation between malignant transformation and the preoperative neutrophil count (Figure 3I). Furthermore, we used the preoperative neutrophil‐to‐lymphocyte ratio (NLR) to evaluate the system immune status of HNIMT patients. The best cutoff value is 1.958, and the NLR was significantly higher in HNIMT patients with malignant transformation (Figure 3J).
3.5. Predictive model for HNIMT with malignant transformation
We next established a predictive model for HNIMT with malignant transformation. First, univariate logistic regression was used. The tumor size (≥4.4 cm) (OR = 13.143, P = .001), tumor site (maxillary sinus) (OR = 28, P < .0001), preoperative lymphocyte count (≤1.75 × 109 cells/ml) (OR = 4.750, P = .025), and preoperative NLR (≥1.958) (P = .016) were significant risk factors of the malignant transformation of HNIMT. We next used ROC analysis to explore the predictive power of these clinicopathological features. Tumor size alone yielded an AUC value of 0.7833 (P = .0021), but the positive predictive power (PPP) was only 0.6361. The preoperative NLR alone yielded an AUC value of 0.7167 (P = .0189). The preoperative lymphocyte level alone yielded an AUC value of 0.6833 (P = .0470). The clinicopathological feature that showed the strongest predictive value is tumor location. Tumors located in the maxillary sinus yield an AUC value of 0.8000, with a negative predictive power (NPP) of 0.8485 and a PPP of 0.8333 (Figure 4A). We next combined the three clinicopathological features to establish predictive models. The combination of tumor size, tumor location, and NLR yielded an AUC value of 0.9189 (P < .0001), with an NPP of 85.29% and a PPP of 90.91%, which revealed that HNIMTs located in the maxillary sinus with a tumor size of >4.4 cm and a preoperative NLR greater than 1.985 had a high chance of malignant transformation (Figure 4B). We thus combined the three indices as one signature. The logistic value was calculated, and cases with a value above the median were classified as the high‐risk group and the others as the low‐risk group. The KM analysis revealed that HNIMT patients in the high‐risk group had a significantly worse overall survival (P = .0025) (Figure 4C).
FIGURE 4.
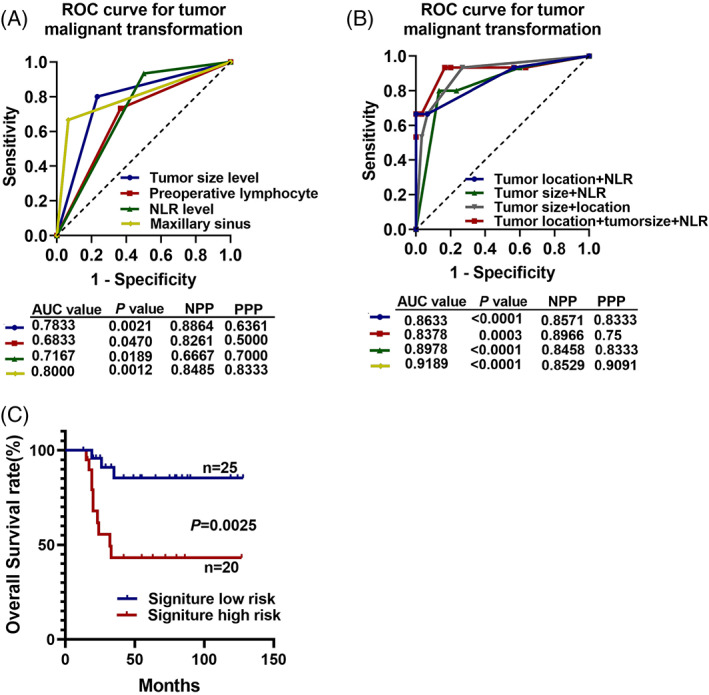
Receiver operating characteristic (ROC) analysis for inflammatory myofibroblastic tumor in the head and neck (HNIMT) with malignant transformation. (A) The tumor size, tumor location, preoperative lymphocyte count, and preoperative neutrophil‐to‐lymphocyte ratio (NLR) yielded AUC values of 0.7878, 0.8000, 0.6833, and 0.7167, respectively, to predict malignant transformation. (B) The combination of tumor size, tumor location, and preoperative NLR yielded an AUC value of 0.9189 with a negative predictive value of 0.8529 and a positive predictive value of 0.9091. (C) HNIMTs with the high‐risk signature of malignant transformation had a worse overall survival rate (P = .0025)
3.6. Postoperative radiotherapy benefits HNIMT patients with high risk of malignant transformation
After describing the clinicopathological features and establishing the predictive model of HNIMT with malignant transformation, we explored the treatment strategy which might benefit HNIMT with malignant transformation. All 45 patients underwent radical surgical resection, and 20 patients also received postoperative radiotherapy. According to the KM analysis, postoperative radiotherapy did not benefit the HNIMT patients (P = .3526), but in patients with malignant transformation, postoperative radiotherapy significantly improved overall survival (P = .045) (Figure 5A–C). Consistently, postoperative radiotherapy improved the overall survival of HNIMT patients with a high risk of malignant transformation (P = .016) (Figure 5D).
FIGURE 5.
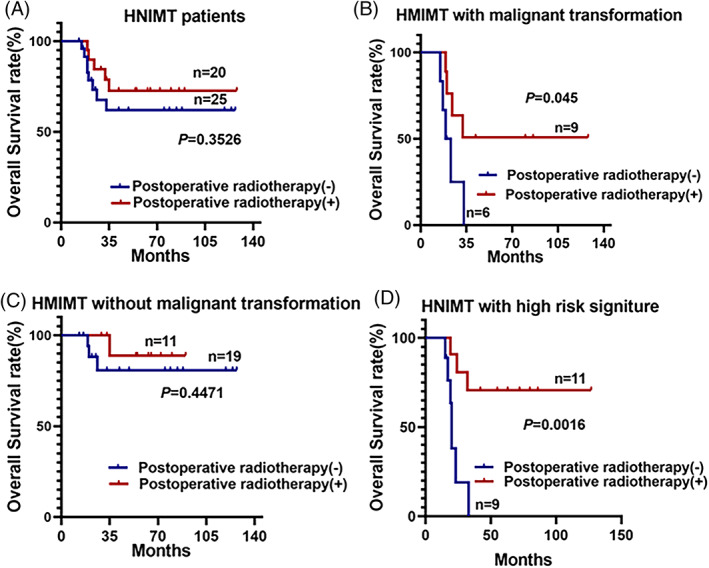
Postoperative radiotherapy benefits inflammatory myofibroblastic tumor in the head and neck (HNIMT) patients with a high risk of malignant transformation. (A) Postoperative radiotherapy did not improve the overall survival rate of HNIMT patients. (B) Postoperative radiotherapy benefited HNIMT patients with malignant transformation. (C) Postoperative radiotherapy did not improve the overall survival rate of HNIMT patients without malignant transformation. (D). Postoperative radiotherapy benefited HNIMT patients with a high risk of malignant transformation
4. DISCUSSION
HNIMT has features of both tumor and inflammation. 12 Recent advances in image‐guided fine needle aspiration biopsy have made the preoperative diagnosis more accurate, giving the clinician the chance to perform radical excision immediately. However, the resection extents of HNIMT are usually not as radical as in other sites due to the esthetic needs of patients and adjacent vital anatomical structures such ad eyes, skull bases, and carotid arteries. Normally, these compromises are acceptable due to the relatively good prognosis of IMT. However, there are sporadic case reports about IMT with malignant transformation at different sites, including HNIMT, which does not receive much attention although it is associated with a high rate of recurrence. 13 , 14 , 15 Here, we retrospectively explored the clinicopathological features of HNIMT and found that HNIMT patients with a tumor size of >4.4 cm, with a tumor located in the maxillary sinus, and with a preoperative NLR above 1.958 had a higher risk of malignant transformation. These patients can benefit from postoperative radiotherapy.
To our knowledge, we are the first to report that IMT in the maxillary sinus is associated with a worse prognosis. The maxillary sinus is adjacent to many vital anatomical structures in the head and neck region. Tumors derived from the maxillary sinus can break through its superior wall into orbital contents, through its interior wall into the nasal cavity, or through its back wall into the pterygopalatine space. As shown by our present results, IMT located in the maxillary sinus also showed obvious local invasion. It remains unknown whether this worse prognosis was due to the special biological behavior of the tumor cells or the complexity of the maxillary sinus. Further studies should use single‐cell sequencing and protein mass spectrometry to analyze the heterogeneity of IMT originating from different anatomical sites.
We also found that the preoperative NLR was closely correlated with the malignant transformation of HNIMT. The NLR has been proved to be associated with the survival rates and responses to chemotherapy in many cancers, such as prostate cancer, 19 colorectal cancer, 20 , 21 and non‐small cell lung cancer. 22 The NLR, to some extent, reflects the inflammation degree in the tumor microenvironment, represented by enhanced nonspecific inflammation and impaired antitumor immunity. 23 Because the NLR often has a significant meaning in malignant tumors, we argue that the inflammation status of HNIMT may also significantly affect the biological behavior of HNIMT, thus making immune therapy feasible for HNIMT. More importantly, we argue that differences in the levels of different types of immune cells in preoperative blood and postoperative tumor tissue may help surgeons to stratify risk before and after surgery.
Furthermore, based on our results, postoperative radiotherapy can benefit HNIMT with malignant transformation as well as patients with a high risk of malignant transformation. Although surgery remains the main option for IMT treatment, 1 there are sporadic case reports about successful treatment by radiotherapy in IMT located in the maxillary sinus 24 and trachea. 25 However, the effectiveness of radiotherapy for IMT treatment has not been verified through randomized controlled trials. Our retrospective study strongly recommends that treatment of HNIMT with a high risk of malignant transformation should include postoperative radiotherapy.
5. CONCLUSIONS
Overall, we found that HNIMT patients with a tumor size of >4.4 cm, a tumor located in the maxillary sinus, and a preoperative NLR over 1.958 had a higher risk of malignant transformation. These patients can benefit from postoperative radiotherapy.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
ACKNOWLEDGMENTS
This work was supported by National Natural Science Foundation of China (No. 81872189), Biobank Program of Shanghai Ninth People's Hospital (YBKB201908), and Shenkang Development Center (SHDC22017101) to Tong Ji. The authors declare that there is no financial disclosure that could be perceived as prejudicing the impartiality of the research reported.
Peng C, Chen MT, Liu Z, Guo Y, Zhang Y, Ji T. A clinical signature predicting the malignant transformation of inflammatory myofibroblastic tumor in the head and neck. Laryngoscope Investigative Otolaryngology. 2022;7(1):145‐152. doi: 10.1002/lio2.731
Cangbang Peng, MingTao Chen, and Yu Zhang were contributed equally to this work.
Funding information Biobank Program of Shanghai Ninth People's Hospital, Grant/Award Number: YBKB201908; National Natural Science Foundation of China, Grant/Award Number: 81872189; Shenkang Development Center, Grant/Award Number: SHDC22017101
Contributor Information
Yu Zhang, Email: zhyzhy1818@sjtu.edu.cn.
Tong Ji, Email: jitong70@sjtu.edu.cn.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Ong HS, Ji T, Zhang CP, et al. Head and neck inflammatory myofibroblastic tumor (IMT): evaluation of clinicopathologic and prognostic features. Oral Oncol. 2012;48(2):141‐148. doi: 10.1016/j.oraloncology.2011.09.004 [DOI] [PubMed] [Google Scholar]
- 2. Wang J, Sun Z, Zhuo S, Wang K. Sigmoid sinus occlusion infiltrated by inflammatory myofibroblastic tumor from mastoid. Head & Neck. 2015;37(1):E4‐E7. doi: 10.1002/hed.23704 [DOI] [PubMed] [Google Scholar]
- 3. Zhao J, Han D, Gao M, et al. Inflammatory myofibroblastic tumor of the neck with thyroid invasion: a case report and literature review. Gland Surg. 2020;9(4):1042‐1047. doi: 10.21037/gs-20-355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Salehinejad J, Pazouki M, Gerayeli M. Malignant inflammatory myofibroblastic tumor of the maxillary sinus. J Oral Maxillofac Pathol. 2013;17(2):306‐310. doi: 10.4103/0973-029x.119754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kim J, Hong K, Kim J, Song J. Medical therapy of maxillary sinus inflammatory myofibroblastic tumors. Am J Otolaryngol. 2016;37(4):376‐378. doi: 10.1016/j.amjoto.2016.01.019 [DOI] [PubMed] [Google Scholar]
- 6. Völker H, Scheich M, Zettl A, Hagen R, Müller‐Hermelink H, Gattenlöhner S. Laryngeal inflammatory myofibroblastic tumors: different clinical appearance and histomorphologic presentation of one entity. Head & Neck. 2010;32(11):1573‐1578. doi: 10.1002/hed.21232 [DOI] [PubMed] [Google Scholar]
- 7. Dhivakar C, Govindasamy B, Pandian D, Vishnu R. Inflammatory myofibroblastic tumor of gingiva in a patient with constitutional growth delay: a rare case report. J Indian Soc Periodontol. 2021;25(3):250‐253. doi: 10.4103/jisp.jisp_279_20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Tateishi Y, Okudela K, Kawai S, et al. Intraosseous inflammatory myofibroblastic tumor of the mandible with a novel ATIC‐ALK fusion mutation: a case report. Diagn Pathol. 2016;11(1):132. doi: 10.1186/s13000-016-0586-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Spinazzi E, Desai S, Fang C, et al. Lateral skull base inflammatory pseudotumor: a systematic review. Laryngoscope. 2015;125(11):2593‐2600. doi: 10.1002/lary.25308 [DOI] [PubMed] [Google Scholar]
- 10. Casanova M, Brennan B, Alaggio R, et al. Inflammatory myofibroblastic tumor: the experience of the European pediatric Soft Tissue Sarcoma Study Group (EpSSG). Eur J Cancer. 2020;127:123‐129. doi: 10.1016/j.ejca.2019.12.021 [DOI] [PubMed] [Google Scholar]
- 11. Pandya N, Bhatt U. Inflammatory myofibroblastic tumor of hard palate: a lesion of extreme rarity. Pan Afr Med J. 2021;38:267. doi: 10.11604/pamj.2021.38.267.28236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zhang Y, Peng C, Tian Z, Cao W, Yang X, Ji T. Inflammatory myofibroblastic tumor in the head and neck‐a neoplasm with both tumor features and inflammation. Oral Surg Oral Med Oral Pathol Oral Radiol. 2020;130(5):e316‐e323. doi: 10.1016/j.oooo.2020.02.008 [DOI] [PubMed] [Google Scholar]
- 13. Inamdar A, Pulinthanathu R. Malignant transformation of inflammatory myofibroblastic tumor of urinary bladder: a rare case scenario. Bladder (San Franc). 2019;6(2):e39. doi: 10.14440/bladder.2019.805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zhang G, Guo X, Liang G, Wang Q. Kidney inflammatory myofibroblastic tumor masquerading as metastatic malignancy: a case report and literature review. World J Clin Cases. 2019;7(24):4366‐4376. doi: 10.12998/wjcc.v7.i24.4366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Muscarella L, Rossi G, Trombetta D, et al. EML4‐ALKA malignant inflammatory myofibroblastic tumor of the hypopharynx harboring the 3a/b variants of the fusion gene. Oncol Lett. 2017;13(2):593‐598. doi: 10.3892/ol.2016.5504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Guillou L, Coindre J, Bonichon F, et al. Comparative study of the National Cancer Institute and French Federation of Cancer Centers Sarcoma Group grading systems in a population of 410 adult patients with soft tissue sarcoma. J Clin Oncol. 1997;15(1):350‐362. doi: 10.1200/jco.1997.15.1.350 [DOI] [PubMed] [Google Scholar]
- 17. Mariño‐Enríquez A, Wang W, Roy A, et al. Epithelioid inflammatory myofibroblastic sarcoma: an aggressive intra‐abdominal variant of inflammatory myofibroblastic tumor with nuclear membrane or perinuclear ALK. Am J Surg Pathol. 2011;35(1):135‐144. doi: 10.1097/PAS.0b013e318200cfd5 [DOI] [PubMed] [Google Scholar]
- 18. Bennett J, Nardi V, Rouzbahman M, Morales‐Oyarvide V, Nielsen G, Oliva E. Inflammatory myofibroblastic tumor of the uterus: a clinicopathological, immunohistochemical, and molecular analysis of 13 cases highlighting their broad morphologic spectrum. Modern Pathol. 2017;30(10):1489‐1503. doi: 10.1038/modpathol.2017.69 [DOI] [PubMed] [Google Scholar]
- 19. Lorente D, Mateo J, Templeton A, et al. Baseline neutrophil‐lymphocyte ratio (NLR) is associated with survival and response to treatment with second‐line chemotherapy for advanced prostate cancer independent of baseline steroid use. Ann Oncol. 2015;26(4):750‐755. doi: 10.1093/annonc/mdu587 [DOI] [PubMed] [Google Scholar]
- 20. Feliciano E, Kroenke C, Meyerhardt J, et al. Association of Systemic Inflammation and Sarcopenia With Survival in Nonmetastatic Colorectal Cancer: results from the C SCANS study. JAMA Oncol. 2017;3(12):e172319. doi: 10.1001/jamaoncol.2017.2319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Dell'Aquila E, Cremolini C, Zeppola T, et al. Prognostic and predictive role of neutrophil/lymphocytes ratio in metastatic colorectal cancer: a retrospective analysis of the TRIBE study by GONO. Ann Oncol. 2018;29(4):924‐930. doi: 10.1093/annonc/mdy004 [DOI] [PubMed] [Google Scholar]
- 22. Simonaggio A, Elaidi R, Fournier L, et al. Variation in neutrophil to lymphocyte ratio (NLR) as predictor of outcomes in metastatic renal cell carcinoma (mRCC) and non‐small cell lung cancer (mNSCLC) patients treated with nivolumab. Cancer Immunol Immunother. 2020;69(12):2513‐2522. doi: 10.1007/s00262-020-02637-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Mei Z, Shi L, Wang B, et al. Prognostic role of pretreatment blood neutrophil‐to‐lymphocyte ratio in advanced cancer survivors: a systematic review and meta‐analysis of 66 cohort studies. Cancer Treat Rev. 2017;58:1‐13. doi: 10.1016/j.ctrv.2017.05.005 [DOI] [PubMed] [Google Scholar]
- 24. Biswas R, Halder A, Gangopadhyay M, Biswas D. Inflammatory myofibroblastic tumor of maxillary sinus successfully treated with radiotherapy and corticosteroid: report of a rare case. J Egypt Natl Canc Inst. 2020;32(1):26. doi: 10.1186/s43046-020-00038-0 [DOI] [PubMed] [Google Scholar]
- 25. Lisi R, Abate G, D'Urso P, et al. Successful role of adjuvant radiotherapy in a rare case of tracheal inflammatory myofibroblastic tumor: a case report. Tumori. 2019;105(6):NP1‐NP3. doi: 10.1177/0300891619838333 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


