Abstract
Simultaneous liver-kidney transplantation (SLKT) is increasingly common in the United States. However, little is known about the renal-related outcomes following SLKT, which are essential to maximize the health of these allografts. We examined the factors impacting renal function following SLKT. This is an observational multicenter cohort study from the US Multicenter SLKT Consortium consisting of recipients of SLKT aged ≥18 years of transplantations performed between February 2002 and June 2017 at 6 large US centers in 6 different United Network for Organ Sharing regions. The primary outcome was incident post-SLKT stage 4–5 chronic kidney disease (CKD) defined as <30 mL/minute/1.73 m2 or listing for kidney transplant. The median age of the recipients (n = 570) was 58 years (interquartile range, 51–64 years), and 37% were women, 76% were White, 33% had hepatitis C virus infection, 20% had nonalcoholic steatohepatitis (NASH), and 23% had alcohol-related liver disease; 68% developed ≥ stage 3 CKD at the end of follow-up. The 1-year, 3-year, and 5-year incidence rates of post-SLKT stage 4–5 CKD were 10%, 12%, and 16%, respectively. Pre-SLKT diabetes mellitus (hazard ratio [HR], 1.45; 95% CI, 1.00–2.15), NASH (HR, 1.58; 95% CI, 1.01–2.45), and delayed kidney graft function (HR, 1.72; 95% CI, 1.10–2.71) were the recipient factors independently associated with high risk, whereas the use of tacrolimus (HR, 0.44; 95% CI, 0.22–0.89) reduced the risk. Women (β = −6.22 ± 2.16 mL/minute/1.73 m2; P = 0.004), NASH (β = −7.27 ± 3.27 mL/minute/1.73 m2; P = 0.027), and delayed kidney graft function (β = −7.25 ± 2.26 mL/minute/1.73 m2; P = 0.007) were independently associated with low estimated glomerular filtration rate at last follow-up. Stage 4–5 CKD is common after SLKT. There remains an unmet need for personalized renal protective strategies, specifically stratified by sex, diabetes mellitus, and liver disease, to preserve renal function among SLKT recipients.
Simultaneous liver-kidney transplantation (SLKT) is an important option for liver transplantation (LT) candidates with stage 4 chronic kidney disease (CKD) and end-stage renal disease (ESRD), sustained acute kidney injury (AKI) deemed unlikely to recover after LT, and select inherited metabolic disorders such as primary hyperoxaluria.(1–4) The revised Organ Procurement and Transplantation Network (OPTN) SLKT policy implemented in 2017 included medical eligibility criteria that were lacking in the previous allocation guidance. In addition, a “safety-net” option was created to prioritize kidney transplantation for those LT-only recipients who were unlikely to recover their renal function within 60 to 365 days after LT.(3,4)
The Model for End-Stage Liver disease (MELD)–based policy, adopted in February 2002 for liver allocation, improved the access of deceased donor liver allografts to the sickest while maintaining optimal short-term and long-term posttransplant survival.(5–7) Since then, there have been several evidence-based modifications to the policy,(8–11) including the revised criteria for SLKT.(1–4,12) Increased SLKT use is one of the important unintended consequences of the MELD-based allocation policy(2,3,13) because each SLKT performed draws a renal allograft away from a kidney transplantation–only recipient. Although SLKT use has increased by 200% in the MELD era, data on CKD after SLKT is lacking, and the risk factors that impact the long-term estimated glomerular filtration rate (eGFR) after SLKT are not well studied.
Most large observational studies have used data from the OPTN to examine post-SLKT survival and not renal function because such data are lacking in the OPTN data.(3,14–16) Therefore, we formed a multicenter consortium called the US Multicenter SLKT Consortium study to improve our understanding of post-SLKT CKD and related outcomes using patient-level granular data, which makes this study unique and novel. In this study, we examined the long-term renal outcomes after SLKT including the incidence of stage 4–5 CKD after SLKT and predictors of estimated GFR at last follow-up.
Patients and Methods
PATIENTS AND DATA COLLECTION
The US Multicenter SLKT Consortium (Fig. 1) includes candidate, donor, and recipient data on all adult recipients (≥18 years) of SLKT performed at 6 large US centers (Columbia University Irving Medical Center; Duke University; Northwestern University; University of California, San Francisco; Michigan Medicine, University of Michigan; University of Washington) in 6 different United Network for Organ Sharing regions between February 2002 and June 2017. The study was approved by each participating center’s institutional review board, and the data use agreements were established. Deidentified coded data were uploaded in the Research Electronic Data Capture at the University of Michigan, the data coordinating center for this consortium.
FIG. 1.
The US Multicenter SLKT Consortium.
The data collection sheets included the recipients’ demographic information, listing, transplant, donor, and posttransplant characteristics as well as donor characteristics (see Supporting Information collection sheets S.1).
IMMUNOSUPPRESSION
The immunosuppression protocols among all 6 centers were similar. All of the centers use tacrolimus-based immunosuppression with mycophenolic acid and corticosteroids. Northwestern University revised their immunosuppression protocol in April 2015 and included induction with basiliximab on days 0 and 2 in addition to corticosteroids and a maintenance phase with tacrolimus, mycophenolic acid, and a corticosteroid taper to 5 mg indefinitely. In all other centers, immunosuppression protocols for SKLT were similar to the kidney transplantation immunosuppression protocol. Induction with thymoglobulin, basiliximab, and dacluzimab was based on the presence of panel reactive antibodies and sensitization. The therapeutic tacrolimus trough levels in all of the centers were similar and based on days after SLKT. The levels were maintained between 8 and 12 ng/mL in the first 90 days among all the centers.
ANALYTIC APPROACH
The continuous variables were expressed as median (interquartile range [IQR]), and the categorical variables were expressed as percentages. The eGFR was collected at 1 year, 2 years, 3 years, and 5 years after SLKT and at the end of follow-up. The Model for End-Stage Liver Disease–sodium (MELD-Na) score was calculated using the OPTN calculator. The renal risk index (RRI) score was calculated using the RRI calculator (https://rri.med.umich.edu). The RRI score combines 14 recipient factors at the time of transplant to summarize the post-LT ESRD risk into a single number. The RRI expresses the relative risk of incident ESRD for a given LT recipient compared with the reference LT recipient with an RRI of 1; values exceeding 1 have higher-than-expected ESRD risk than the reference LT recipient and vice versa. All of the components of the Kidney Donor Profile Index (KDPI) were not available on all the patients. Therefore, we used the kidney donor age as a covariate for donor quality in the models.
The primary outcome was post-SLKT stage 4–5 CKD defined as eGFR ≤ 30 mL/minute per 1.73 m2 at last follow-up or listed for kidney transplantation. The secondary outcomes were (1) eGFR at the last follow-up and (2) post-SLKT mortality.
Incidence and Risk Factors of New-Onset Stage 4–5 CKD
We used the Kaplan-Meier analysis to examine the cumulative incidence of post-SLKT stage 4–5 and post-SLKT survival. We used Cox regression to assess the risk factors of stage 4–5 CKD after SLKT. The covariates with P < 0.15 were used in the multivariable model to examine the independent association between donor and recipient factors and stage 4–5 CKD. We forced the center in the final adjusted model to examine the unmeasured center effect.
eGFR at Last Follow-Up and eGFR Slope
Using linear regression, we modeled eGFR at the last follow-up. For this model, we adjusted for covariates a priori: age, sex, etiology of liver disease, year of SLKT, pretransplant hypertension, diabetes mellitus, MELD-Na, body mass index (BMI), pretransplant renal dysfunction type, renal replacement therapy (RRT), time of follow-up, kidney delayed graft function (DGF), immunosuppression, induction therapy, donor age, cold ischemia time (CIT), warm ischemia time (WIT), and RRI.
Using linear regression, we modeled the slope of eGFR decline from the first available eGFR following SLKT or at the 1-year follow-up to estimate the eGFR decline per year. We tested the eGFR decline by sex, race, etiology of liver disease, and kidney DGF. This model was adjusted for age, sex, etiology of liver disease, year of SLKT, pretransplant hypertension, diabetes mellitus, MELD-Na, BMI, pretransplant renal dysfunction type, RRT, time of follow-up, kidney DGF, immunosuppression, induction therapy, donor age, CIT, WIT, and RRI.
Subset Analysis Limited to those Who Had Data on KDPI
We performed a subset analysis limiting to the patients who had information on KDPI. We fitted the Cox regression model to examine the effect of KDPI on kidney DGF, new-onset stage 4–5 CKD, and patient survival. This model was stratified by center and adjusted for age, sex, nonalcoholic steatohepatitis (NASH), RRT at SKLT, MELD score, donor age, and CIT.
All analyses were performed in SAS 9.4 (SAS Institute, Cary, NC).
Results
CLINICAL CHARACTERISTICS
Table 1 shows the clinical characteristics of the cohort. The median age of the cohort (n = 570) was 58 years, 63% were men, and 76% were White. The etiology of liver disease was hepatitis C virus infection in 33%, NASH or cryptogenic cirrhosis in 20%, alcohol-related liver disease in 23%, and 24% had other etiologies. The median MELD-Na at SLKT score was 28 (IQR, 23–34). Only 39% were on hemodialysis or RRT at the time of SLKT. The traditional risk factors of CKD, hypertension, and diabetes mellitus were seen in 45%, 54%, and 42% of the SLKT candidates, respectively. The median BMI was 27 kg/m2, and 25% had a BMI ≥32 kg/m2 at the time of SLKT (Table 1). The median RRI score was 7.57 and three-fourths of the cohort had an RRI score in the 10th decile (highest risk group; Table 1).
TABLE 1.
Baseline Characteristics at SLKT
| Variables at LT (n = 570) | Median (IQR) or n (%) |
|---|---|
| Recipient characteristics | |
| Age at LT, years | 58 (51–64) |
| Columbia | 47 (8.3) |
| Duke University | 43 (7.5) |
| Northwestern University | 281 (49.3) |
| University of California, San Francisco | 100 (17.5) |
| University of Michigan | 51 (9.0) |
| University of Washington | 48 (8.4) |
| Male | 361 (63) |
| Female | 209 (37) |
| White | 432 (75.8) |
| Black | 71 (12.5) |
| Other races | 67 (11.7) |
| Hepatitis C virus infection | 189 (33) |
| Alcohol-related cirrhosis | 131 (23) |
| NASH/cryptogenic cirrhosis | 112 (20) |
| Other etiologies | 138 (24) |
| AKI | 149 (26) |
| CKD | 257 (45) |
| Other | 164 (29) |
| Pre-LT dialysis or RRT | 220 (39) |
| Hypertension | 306 (54) |
| Diabetes mellitus | 237 (42) |
| BMI, kg/m2 | 27 (24–32) |
| RRI score | 7.57 (5.2–12.2) |
| MELD-NA score | 28 (23–34) |
| Donor and transplant characteristics | |
| Donor age, years | 36 (23–48) |
| DCD | 24 (4.2) |
| Donor, male | 318 (55.7) |
| Donor cause of death, cerebrovascular deaths | 382 (67) |
| KDPI score, n = 184 | 32 (15–66) |
| CIT, minutes | 360 (300–465) |
| WIT, minutes | 37.5 (25–60) |
| Tacrolimus | 541 (95) |
| Cyclosporine | 29 (5) |
| Induction, yes | 138 (24) |
| Kidney DGF | 133 (23.3) |
| Length of stay, transplant admission, days | 19 (9–33) |
| eGFR at last follow-up | 54 ml/minute per 1.73 m2 (33–65) |
| Time to last follow-up | 63.6 months (28.2–110.6) |
Donor characteristics are outlined in Table 1. The median donor age was 36 years (IQR, 23–48), 93% were donations after brain death, and cerebrovascular disease or head injury were the most common causes of death (68%) followed by anoxia or asphyxiation (20%). Donor biopsy data were not available across the center. KDPI information was available in 184 SLKT recipients. The features of the KDPI subset are described in the “Subset Analysis” section.
Almost all of the SLKT recipients were on tacrolimus (95%), 82% were on triple immunosuppression (calcineurin inhibitors, mycophenolate, and corticosteroids), and only 3% were on calcineurin inhibitor monotherapy. Of the patients, one-fourth (24%) received induction therapy after SLKT: 74% received basiliximab, 18% received thymoglobulin, and 7% received dacluzimab as induction therapy. Within the first 6 months after SLKT, there were 80 rejection episodes among 71 SLKT recipients; 36 were kidney rejection episodes and 44 were liver rejection episodes. Of note, 12 patients had both liver and kidney rejection episodes within the first 6 months after SLKT.
A total of 133 (23%) patients developed kidney DGF requiring RRT during transplant hospitalization. The median time spent on RRT was 13 days (IQR, 4–40). Post-SLKT stage 4–5 CKD was higher in patients with DGF versus those without (32% versus 17%; P < 0.001).
INCIDENCE OF STAGE 4–5 CKD
A total of 120 (21%) SLKT recipients developed stage 4–5 CKD at the end of the follow-up period. The median follow-up time was 63 months. The crude incidence rate was 3.8 per 100 patient-years. The cumulative incidence of posttransplant stage 4–5 CKD at 1 year, 3 years, 5 years, and 10 years was 10%, 12%, 16%, and 25%, respectively (Fig. 2).
FIG. 2.
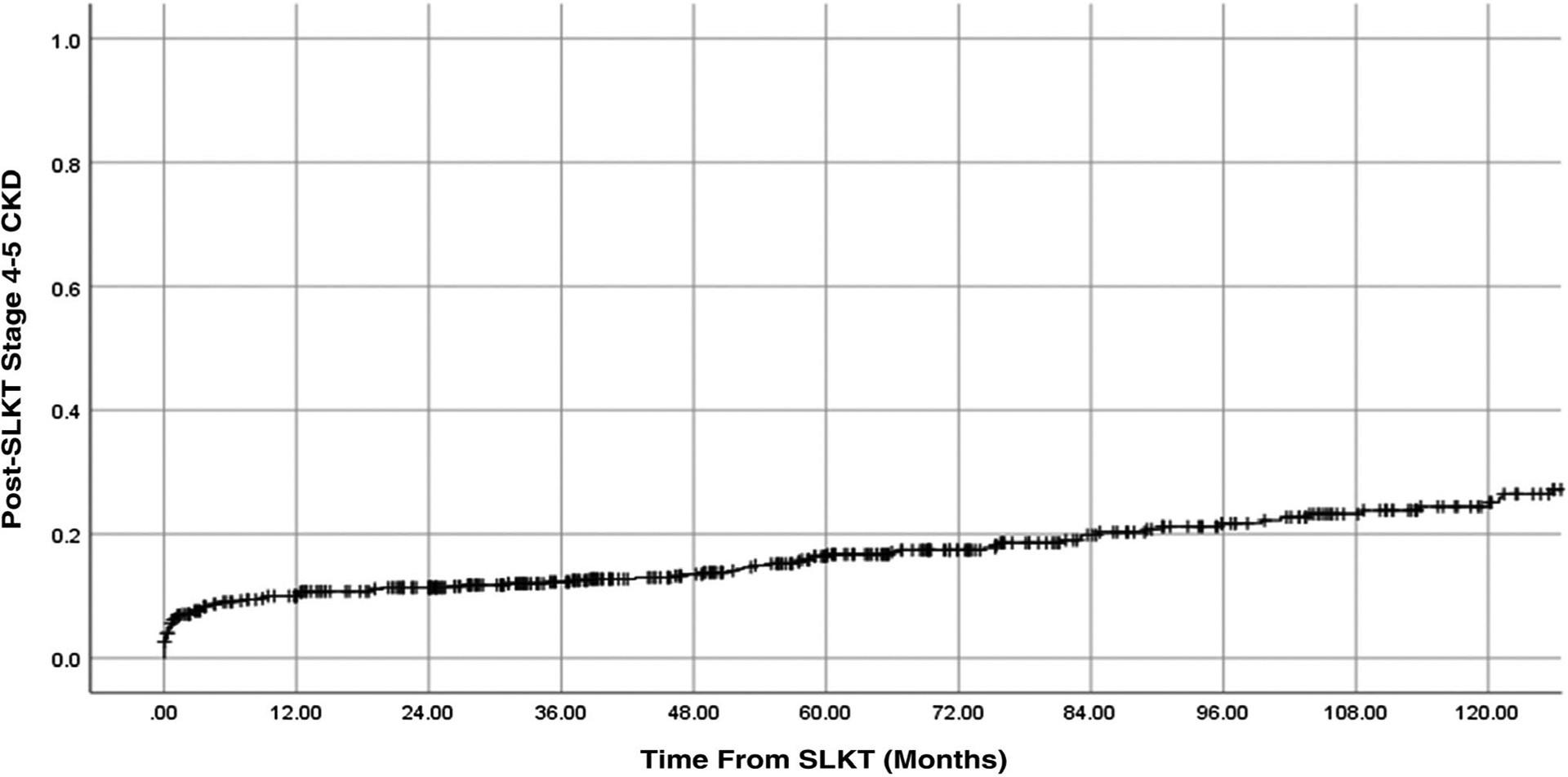
Cumulative incidence of stage 4–5 CKD after SLKT.
In the univariate analysis, NASH (P = 0.004), pre-SLKT diabetes mellitus (P = 0.01), use of tacrolimus compared with cyclosporine (P = 0.015), kidney DGF (P < 0.001), donor age (P < 0.001), CIT (P = 0.002), and WIT (P = 0.044) were significant. In an adjusted model that included these factors and age at SLKT, alcohol-related liver disease (P < 0.15), and center, we found that pre-SLKT diabetes mellitus, NASH, and kidney DGF were independently associated with high risk, whereas the use of tacrolimus compared with cyclosporine was independently associated with the reduced risk of post-SLKT stage 4–5 CKD (Table 2). Donor age, CIT, and WIT were independent donor risk factors associated with a high risk of stage 4–5 CKD, and transplant center did not affect the risk of post-SLKT stage 4–5 CKD (Table 2).
TABLE 2.
Independent Predictors of Stage 4–5 CKD
| Covariates | HR (95% CI) | P Value |
|---|---|---|
| Recipient factors | ||
| NASH, reference: no NASH | 1.58 (1.01–2.45) | 0.044 |
| Pre-SLKT diabetes mellitus | 1.45 (1.00–2.15) | 0.049 |
| Tacrolimus, reference: cyclosporine | 0.44 (0.22–0.89) | 0.023 |
| Kidney DGF, reference: none | 1.72 (1.10–2.71) | 0.018 |
| Donor factors | ||
| Donor age, per year | 1.02 (1.01–1.03) | 0.001 |
| CIT, per 10 minutes | 1.01 (1.00–1.03) | 0.019 |
| WIT, per 10 minutes | 1.05 (1.00–1.10) | 0.05 |
| Center | ||
| 1 | 0.53 (0.24–1.18) | 0.12 |
| 2 | 0.51 (0.19–1.32) | 0.17 |
| 3 | 0.83 (0.45–1.53) | 0.56 |
| 4 | 1.09 (0.56–2.12) | 0.79 |
| 5 | 0.72 (0.31–1.66) | 0.44 |
| 6, reference | 1.00 |
FACTORS AFFECTING eGFR AT LAST FOLLOW-UP
The median eGFR at last follow-up was 54 mL/minute per 1.73 m2 (IQR, 33–65) with 68% having CKD stage 3 or higher (eGFR ≤ 60 mL/minute per 1.73 m2). The median time of last follow-up was 64 months (IQR, 28.2–110.7). Of those who had stage 3 CKD (eGFR 30–59 mL/minute per 1.73 m2) at the last follow-up, 43% had stage 3A (eGFR 45–59 mL/minute) and 56% had stage 3B CKD (eGFR 30–44 mL/minute). There were no significant differences between the baseline recipients and donor characteristics between these 2 groups. The mean decline in the slope of eGFR after SLKT was −0.76 mL/minute per 1.73 m2 per year. eGFR slope did not differ by sex, etiology, race, or presence of kidney DGF.
Female sex (β = −6.35 ± 2.11 mL/minute per 1.73 m2; P = 0.003), NASH (β = −7.58 ± 3.20 mL/minute per 1.73 m2; P = 0.018), kidney DGF (β = −7.74 ± 2.60 mL/minute per 1.73 m2; P = 0.003), and donor age per year (β = −0.36 ± 0.07 mL/minute per 1.73 m2; P < 0.0001) were independently associated with low eGFR at last follow-up, whereas each calendar year increase in SLKT procedure performed was associated with high eGFR (β = 1.46 ± 0.03 mL/minute per 1.73 m2; P < 0.001).
POST-SLKT MORTALITY
Figure 3 shows the unadjusted patient survival after SLKT. Posttransplant mortality was significantly higher in patients with post-SLKT stage 4–5 CKD compared with those without (55% versus 25%; P < 0.001). Although post-SLKT stage 4–5 CKD was higher in the kidney DGF group, patient mortality was similar (36% versus 32%; P = 0.3) in both groups.
FIG. 3.
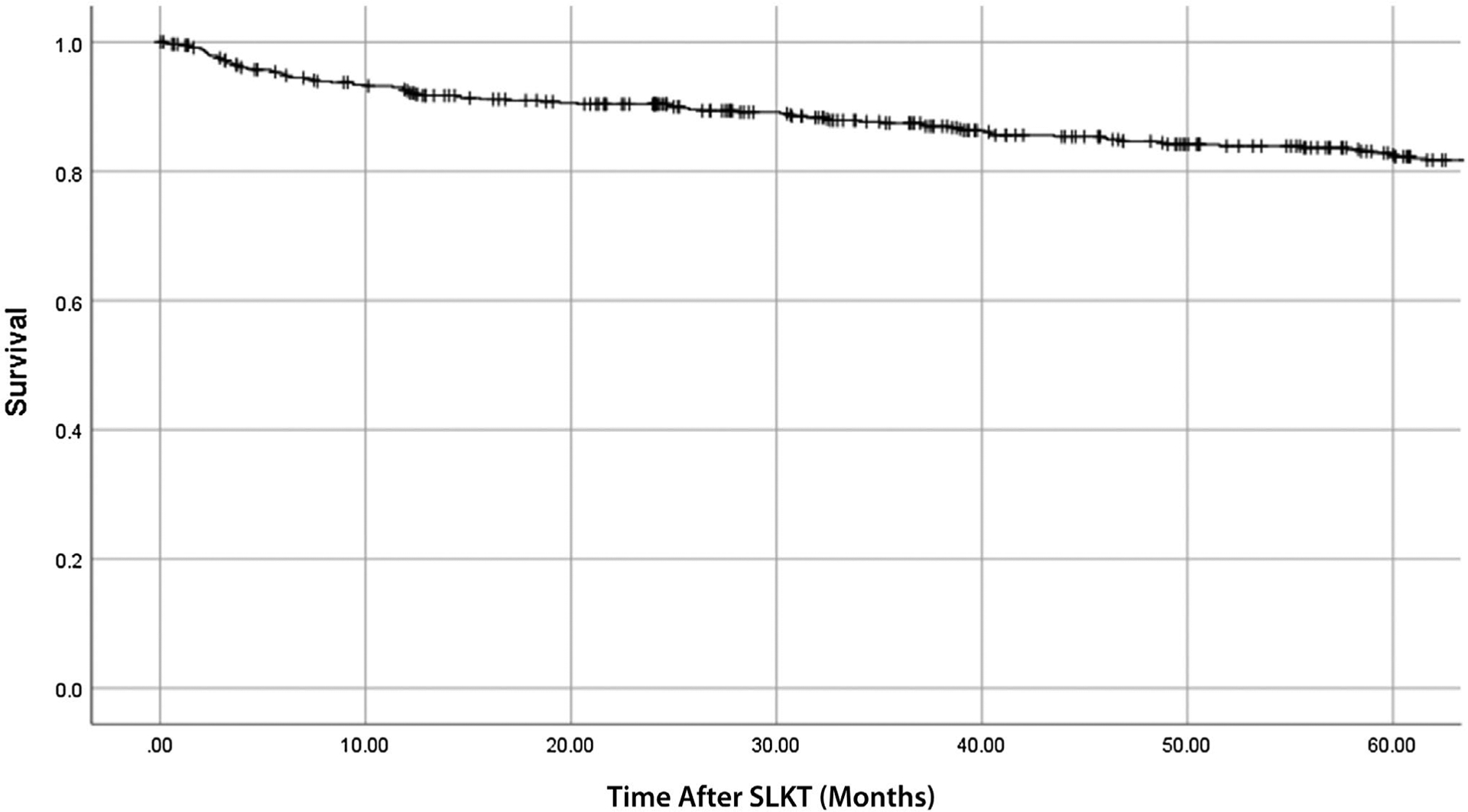
Patient survival after SLKT.
Subset Analysis
We performed the subset analysis on SLKT recipients with data on KDPI (n = 184). The median age of this group was 59 years (IQR, 51–64); 63% were men; 64% White, 13% were Black, and 23% were other races; 34% had hepatitis C virus infection; 22% had alcohol-related liver disease; 20% had NASH; 24% had other etiologies of liver disease; 49% were hypertensive; 45% had diabetes mellitus; and 57% were on RRT at SLKT. The median MELD score at SLKT was 25 (IQR, 21–32). The median donor age was 34 years (IQR, 23–47), and the median KDPI was 32% (IQR, 16%−55%).
In this subset, 80 had kidney DGF, 33 developed stage 4–5 CKD and 32 died. The median follow-up time was 49.2 (IQR, 29.3–74.6) months. In an adjusted model, KDPI was neither associated with stage 4–5 CKD nor with post-SLKT mortality.
Discussion
In this largest (to date) study of long-term renal outcomes following SLKT, we have shown that recurrent CKD impacts most patients after SLKT. Two-thirds had at least stage 3 CKD and one-fifth had advanced CKD (stage 4–5) after a median follow-up of 5 years. There are several recipient and donor factors that affect the risk of incident stage 4–5 CKD after SLKT; female sex, NASH, kidney DGF, and advanced donor age were all associated with significant reduction in eGFR over time. Although kidney DGF was 1 of the independent predictors of posttransplant stage 4–5 CKD, it did not affect overall survival. Donor age, CIT, and WIT were also associated with incident stage 4–5 CKD. Posttransplant stage 4–5 CKD, similar to other studies,(17,18) was associated with increased mortality among SLKT recipients.
The true incidence of stage 4–5 CKD after primary kidney transplantation is not known. In 2018, 12% of kidney allograft listing occurred in patients with primary kidney transplantation.(19) In a previous large cohort study of 43,514 LT recipients that excluded SLKT, the 5-year cumulative incidence of post-LT ESRD for those who had RRI ≥5.22 (10th decile) was 18%.(20) We believe that the 5-year cumulative incidence in their study would be even higher if they included stage 4 CKD in addition to ESRD. RRI score stratifies LT recipients at the varying risk of posttransplant ESRD. The risk of posttransplant ESRD and mortality increases with the increase in RRI score. Our study found the 5-year cumulative incidence of stage 4–5 CKD following SLKT to be 16%. The median RRI of SLKT patients in our data was 7.57, which falls into the 10th decile (highest risk group).
In our study, eGFR at the last follow-up was significantly lower in women compared with men. Studies comparing post–kidney transplantation graft outcomes between men and women demonstrated conflicting results with many showing either no difference or survival advantage in favor of women attributed to the smaller body size and therefore lower metabolic demand on the transplanted organ.(21–26) The pattern of sex differences in eGFR we observed may likely be attributed to the interplay of several factors, including the high incidence of autoimmune diseases in women, the effect of sex hormones on immune activation, and sex differences in adherence to immunosuppressive medications in women.(27) We, similar to others, had previously reported the sex-based differences in the relative risk of incident stage 4–5 CKD after LT alone.(28,29)
There is an emerging association between NASH and CKD because of the traditional risk factors such as hypertension, diabetes mellitus, and obesity, which are common to both. The important implication of this association may lead to an increase in SLKT listing among candidates with NASH cirrhosis. Our study showed a higher risk of stage 4–5 CKD and lower eGFR among NASH SLKT recipients compared with all other etiologies of liver diseases. NASH is also a risk factor for stage 4–5 CKD after LT.(28) Because NASH is the leading indication for LT in females,(30) we examined the impact of this relationship on eGFR. This interaction was not significant.
On average, the rate of kidney DGF is about 30.8% in US deceased donor kidney transplant recipients, and donor factors significantly affect the likelihood of DGF.(19,31–33) In our study, 23% of SLKT recipients developed DGF, which is somewhat lower than kidney transplant recipients. However, we cannot draw this inference without directly comparing kidney transplantation–only recipients with SLKT recipients. Although kidney donor age has been associated with increased incidence of DGF, the median donor age in our cohort was lower than the donor age reported for kidney transplantation–only recipients.(34) This could be the plausible explanation for lower DGF in SLKT recipients than kidney transplant recipients.
Our study showed modest rate of rejection in the kidney grafts. This may be attributed to the protective effect of liver allograft against antibody-mediated kidney rejection as shown by previous studies.(35–37) Some studies suggest the role of preformed cytotoxic and neutralizing antibodies through the release of soluble class I antigens in reducing kidney allograft rejection in patients with SLKT.(38,39) A previous study demonstrated a 26% higher risk of stage 4–5 CKD with cyclosporine compared with tacrolimus among nonrenal organ transplantation.(17) Our study validated these results in SLKT recipients.
CKD progression adversely impacts patient and graft survival and adds to health care costs.(17,18,20,40,41) We hypothesize that CKD progression is accelerated in LT and SLKT recipients given the prevalent traditional risk factors such as diabetes mellitus, hypertension, obesity, and calcineurin inhibitors. Therefore, recognizing the phenotypes that are at the highest risk of CKD progression is an important first step to focus on intervention(s) that may reduce the CKD progression. Hence, there is an unmet need to develop personalized risk-based immunosuppression regimens to further prevent the renal insult because 1 size does not fit all.
A majority of SLKT patients remain on triple immunosuppression including prednisone that can worsen preexisting diabetes mellitus and may cause new-onset diabetes mellitus in these patients. Metabolic disorder, hypertension, diabetes mellitus, and obesity are also prevalent among SLKT recipients. Although we cannot modify many of the risk factors associated with stage 4–5 CKD and low eGFR, attempts should be made to minimize any additional insult to the allograft kidneys to prevent further reduction in eGFR. Immunosuppression (for example, discontinuation of prednisone in those with stable renal function), risk factor modification with life style modification and weight management, stricter control of diabetes and hypertension, especially in high-risk group identified in our study (females, NASH and kidney DGF). Implementation of these measures may attenuate CKD progression and improve renal as well as overall health outcomes among SLKT recipients.
In our study, more than half of the patients were not on dialysis or RRT at the time of SLKT. This is likely related to the changing practices and SLKT guidance during the 15-year study period.(1,2,42) The first policy was put forth in 2009 by the OPTN based on the recommendations from the first SLKT consensus conference.(1) The SLKT guidance changed substantially between 2002 and 2012, especially for sustained AKI deemed irreversible with respect to dialysis or renal dysfunction duration before transplant. Recently, the OPTN implemented an SLKT policy that has the following 2 important components: medical eligibility criteria and the option of a “safety net.”(4) This change in SLKT allocation has streamlined the SLKT usage to some extent. The earliest signs of this policy change resulted in a slight reduction in SLKT rates in 2018.
Our study has limitations that include the retrospective design, heterogeneity, and variability in practices during the long study period across the 6 centers, resulting in potential bias as a result of unmeasured characteristics and patient selection. To overcome some of these limitations, we adjusted for the year of SLKT and forced the center in the final model for incident stage 4–5 CKD. Although the KDPI data were not available on all of the patients, we performed the subset analysis to examine its effect on long-term renal function and survival. However, the KDPI data did not affect long-term renal function or survival. Finally, one may argue that the lack of a comparison arm makes it difficult to put these results in perspective. The valid comparison group would be either a lung-kidney group or heart-kidney group. There are no studies to date that examined the renal outcomes after simultaneous lung-kidney or heart-kidney transplant.(43,44) Despite these shortcomings, this is the first and the largest study to examine the renal outcomes after SLKT.
In conclusion, incident stage 4–5 CKD impacts SLKT recipients. Several recipient and donor factors affect the renal function after SKLT. Further prospective studies are warranted to identify the role of personalized immunosuppression based on sex and etiology of liver disease in preserving renal function among SLKT recipients.
Supplementary Material
Acknowledgments
This work was supported by the AST-LICOP educational committee and intramural MCUBE 3.0 grant from Michigan Medicine. Lisa VanWagner is supported by the National Heart, Lung, and Blood Institute Grant K23 HL136891.
Giuseppe Cullaro has stock in Fate and has received grants from Mallinckrodt. Yuval Patel consults for Intercept Pharmaceuticals. Elizabeth C. Verna has received grants from Salix.
Abbreviations:
- AKI
acute kidney injury
- BMI
body mass index
- CKD
chronic kidney disease
- CIT
cold ischemia time
- DCD
donation after circulatory death
- DGF
delayed graft function
- ESRD
end-stage renal disease
- eGFR
estimated glomerular filtration rate
- HR
hazard ratio
- IQR
interquartile range
- KDPI
Kidney Donor Profile Index
- LT
liver transplantation
- MELD
Model for End-Stage Liver Disease
- MELD-Na
Model for End-Stage Liver Disease–sodium
- NASH
nonalcoholic steatohepatitis
- OPTN
Organ Procurement and Transplantation Network
- PI
principal investigator
- RRI
renal risk index
- RRT
renal replacement therapy
- SLKT
simultaneous liver-kidney transplantation
- WIT
warm ischemia time
Footnotes
Additional supporting information may be found in the online version of this article.
REFERENCES
- 1).Eason JD, Gonwa TA, Davis CL, Sung RS, Gerber D, Bloom RD. Proceedings of consensus conference on simultaneous liver kidney transplantation (SLK). Am J Transplant 2008;8:2243–2251. [DOI] [PubMed] [Google Scholar]
- 2).Nadim MK, Sung RS, Davis CL, Andreoni KA, Biggins SW, Danovitch GM, et al. Simultaneous liver-kidney transplantation summit: current state and future directions. Am J Transplant 2012;12:2901–2908. [DOI] [PubMed] [Google Scholar]
- 3).Formica RN, Aeder M, Boyle G, Kucheryavaya A, Stewart D, Hirose R, Mulligan D. Simultaneous liver-kidney allocation policy: a proposal to optimize appropriate utilization of scarce resources. Am J Transplant 2016;16:758–766. [DOI] [PubMed] [Google Scholar]
- 4).OPTN. Organ Procurement and Transplantation Network Policies. https://optntransplanthrsagov/media/1200/optn_policiespdf. Published 2017. Accessed December 12, 2020. [Google Scholar]
- 5).Wiesner R, Edwards E, Freeman R, Harper A, Kim R, Kamath P, et al. Model for end-stage liver disease (MELD) and allocation of donor livers. Gastroenterology 2003;124:91–96. [DOI] [PubMed] [Google Scholar]
- 6).Wiesner RH, McDiarmid SV, Kamath PS, Edwards EB, Malinchoc M, Kremers WK, et al. MELD and PELD: application of survival models to liver allocation. Liver Transpl 2001;7:567–580. [DOI] [PubMed] [Google Scholar]
- 7).Institute of Medicine. Organ Procurement and Transplantation: Assessing Current Policies and the Potential Impact of the DHHS Final Rule. Washington, DC: National Academies Press; 1999. [PubMed] [Google Scholar]
- 8).Kim WR, Biggins SW, Kremers WK, Wiesner RH, Kamath PS, Benson JT, et al. Hyponatremia and mortality among patients on the liver-transplant waiting list. N Engl J Med 2008;359:1018–1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9).Merion RM, Sharma P, Mathur AK, Schaubel DE. Evidence-based development of liver allocation: a review. Transpl Int 2011;24:965–972. [DOI] [PubMed] [Google Scholar]
- 10).Merion RM, Schaubel DE, Dykstra DM, Freeman RB, Port FK, Wolfe RA. The survival benefit of liver transplantation. Am J Transplant 2005;5:307–313. [DOI] [PubMed] [Google Scholar]
- 11).Sharma P, Schaubel DE, Gong Q, Guidinger M, Merion RM. End-stage liver disease candidates at the highest model for end-stage liver disease scores have higher wait-list mortality than status-1A candidates. Hepatology 2012;55:192–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12).Sharma P Liver-kidney: indications, patient selection, and allocation policy. Clin Liver Dis 2019;13:165–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13).Formica RN Jr. Simultaneous liver-kidney allocation: let’s not make perfect the enemy of good. Am J Transplant 2016;16:2765. [DOI] [PubMed] [Google Scholar]
- 14).Locke JE, Warren DS, Singer AL, Segev DL, Simpkins CE, Maley WR, et al. Declining outcomes in simultaneous liver-kidney transplantation in the MELD era: ineffective usage of renal allografts. Transplantation 2008;85:935–942. [DOI] [PubMed] [Google Scholar]
- 15).Sharma P, Shu X, Schaubel DE, Sung RS, Magee JC. Propensity score-based survival benefit of simultaneous liver-kidney transplant over liver transplant alone for recipients with pretransplant renal dysfunction. Liver Transpl 2016;22:71–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16).Goldberg DS, Vianna RM, Martin EF, Martin P, Arosemena Benitez LR, O’Brien CB, Bhamidimarri KR. Simultaneous liver kidney transplant in elderly patients with chronic kidney disease. Is there an appropriate upper age cutoff? Transplantation 2020;104:2538–2546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17).Ojo AO, Held PJ, Port FK, Wolfe RA, Leichtman AB, Young EW, et al. Chronic renal failure after transplantation of a nonrenal organ. N Eng J Med 2003;349:931–940. [DOI] [PubMed] [Google Scholar]
- 18).Sharma P, Schaubel DE, Guidinger MK, Goodrich NP, Ojo AO, Merion RM. Impact of MELD-based allocation on end-stage renal disease after liver transplantation. Am J Transplant 2011;11:2372–2378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19).Hart A, Smith JM, Skeans MA, Gustafson SK, Wilk AR, Castro S, et al. OPTN/SRTR 2018 annual data report: kidney. Am J Transplant 2020;20(suppl 1):20–130. [DOI] [PubMed] [Google Scholar]
- 20).Sharma P, Goodrich NP, Schaubel DE, Guidinger MK, Merion RM. Patient-specific prediction of ESRD after liver transplantation. J Am Soc Nephrol 2013;24:2045–2052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21).Neugarten J, Acharya A, Silbiger SR. Effect of gender on the progression of nondiabetic renal disease: a meta-analysis. J Am Soc Nephrol 2000;11:319–329. [DOI] [PubMed] [Google Scholar]
- 22).Chen PD, Tsai MK, Lee CY, Yang CY, Hu RH, Lee PH, Lai HS. Gender differences in renal transplant graft survival. J Formos Med Assoc 2013;112:783–788. [DOI] [PubMed] [Google Scholar]
- 23).Meier-Kriesche HU, Ojo AO, Leavey SF, Hanson JA, Leichtman AB, Magee JC, et al. Gender differences in the risk for chronic renal allograft failure. Transplantation 2001;71:429–432. [DOI] [PubMed] [Google Scholar]
- 24).Kim SJ, Gill JS. H-Y incompatibility predicts short-term outcomes for kidney transplant recipients. J Am Soc Nephrol 2009;20:2025–2033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25).Oh CK, Lee BM, Jeon KO, Kim HJ, Pelletier SJ, Kim SI, Kim YS. Gender-related differences of renal mass supply and metabolic demand after living donor kidney transplantation. Clin Transplant 2006;20:163–170. [DOI] [PubMed] [Google Scholar]
- 26).Gratwohl A, Dohler B, Stern M, Opelz G. H-Y as a minor histocompatibility antigen in kidney transplantation: a retrospective cohort study. Lancet 2008;372:49–53. [DOI] [PubMed] [Google Scholar]
- 27).Muller V, Szabo A, Viklicky O, Gaul I, Pörtl S, Philipp T, Heemann UW. Sex hormones and gender-related differences: their influence on chronic renal allograft rejection. Kidney Int 1999;55:2011–2020. [DOI] [PubMed] [Google Scholar]
- 28).Sharma P, Sun Y, Neal J, Erley J, Shen J, Tischer S, et al. Renal outcomes of liver transplantation recipients receiving standard immunosuppression and early renal sparing immunosuppression: a retrospective single center study. Transplant Direct 2019;5:e480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29).Fussner LA, Charlton MR, Heimbach JK, Fan C, Dierkhising R, Coss E, Watt KD. The impact of gender and NASH on chronic kidney disease before and after liver transplantation. Liver Int 2014;34:1259–1266. [DOI] [PubMed] [Google Scholar]
- 30).Noureddin M, Vipani A, Bresee C, Todo T, Kim IK, Alkhouri N, et al. NASH leading cause of liver transplant in women: updated analysis of indications for liver transplant and ethnic and gender variances. Am J Gastroenterol 2018;113:1649–1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31).Perico N, Cattaneo D, Sayegh MH, Remuzzi G. Delayed graft function in kidney transplantation. Lancet 2004;364:1814–1827. [DOI] [PubMed] [Google Scholar]
- 32).Mogulla MR, Bhattacharjya S, Clayton PA. Risk factors for and outcomes of delayed graft function in live donor kidney transplantation—a retrospective study. Transpl Int 2019;32:1151–1160. [DOI] [PubMed] [Google Scholar]
- 33).Siedlecki A, Irish W, Brennan DC. Delayed graft function in the kidney transplant. Am J Transplant 2011;11:2279–2296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34).Reese PP, Veatch RM, Abt PL, Amaral S. Revisiting multi-organ transplantation in the setting of scarcity. Am J Transplant 2014;14:21–26. [DOI] [PubMed] [Google Scholar]
- 35).Taner T, Heimbach JK, Rosen CB, Nyberg SL, Park WD, Stegall MD. Decreased chronic cellular and antibody-mediated injury in the kidney following simultaneous liver-kidney transplantation. Kidney Int 2016;89:909–917. [DOI] [PubMed] [Google Scholar]
- 36).Taner T, Park WD, Stegall MD. Unique molecular changes in kidney allografts after simultaneous liver-kidney compared with solitary kidney transplantation. Kidney Int 2017;91:1193–1202. [DOI] [PubMed] [Google Scholar]
- 37).Askar M, Schold JD, Eghtesad B, Flechner SM, Kaplan B, Klingman L, et al. Combined liver-kidney transplants: allosensitization and recipient outcomes. Transplantation 2011;91:1286–1292. [DOI] [PubMed] [Google Scholar]
- 38).Gugenheim J, Amorosa L, Gigou M, Fabiani B, Rouger P, Gane P, et al. Specific absorption of lymphocytotoxic alloantibodies by the liver in inbred rats. Transplantation 1990;50:309–313. [DOI] [PubMed] [Google Scholar]
- 39).Sumimoto R, Kamada N. Specific suppression of allograft rejection by soluble class I antigen and complexes with monoclonal antibody. Transplantation 1990;50:678–682. [DOI] [PubMed] [Google Scholar]
- 40).USRDS. Cost of ESRD. 2012 USRDS Annual Data Report. 2020. https://adr.usrds.org/2020/chronic-kidney-disease/6-healthcare-expenditures-for-persons-with-ckd. Accessed November 14, 2020. [Google Scholar]
- 41).Ruebner RL, Reese PP, Denburg MR, Rand EB, Abt PL, Furth SL. Risk factors for end-stage kidney disease after pediatric liver transplantation. Am J Transplant 2012;12:3398–3405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42).Nadim MK, Davis CL, Sung R, Kellum JA, Genyk YS. Simultaneous liver-kidney transplantation: a survey of US transplant centers. Am J Transplant 2012;12:3119–3127. [DOI] [PubMed] [Google Scholar]
- 43).Awad M, Czer LS, Esmailian F, Jordan S, De Robertis Ma, Mirocha J et al. Combined heart and kidney transplantation: a 23-year experience. Transplant Proc 2017;49:348–353. [DOI] [PubMed] [Google Scholar]
- 44).Reich HJ, Chan JL, Czer LS, Mirocha J, Annamalai AA, Cheng W, et al. Combined lung-kidney transplantation: an analysis of the UNOS/OPTN database. Am Surg 2015;81:1047–1052. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


