Abstract
Background
Monosodium glutamate (MSG) is the sodium salt of the non‐essential amino acid, glutamic acid, and is used as a flavour enhancer. It has been implicated in causing adverse reactions, which have been referred to as "Chinese restaurant syndrome". Over the last two decades there have been a number of studies investigating whether MSG ingestion induces an asthmatic response, and several reviews have been published (ILSI 1991; Stevenson 2000; Woods 2001), but no meta‐analysis or Cochrane systematic review has been performed.
Objectives
The objectives of this review are to: 1) identify randomised controlled trials (RCTs) of MSG ingestion and asthma response in adults and children older than two years of age with asthma; 2) assess the methodological quality of these trials; and 3) determine the effect of MSG ingestion on asthma outcomes.
Search methods
We searched the Cochrane Airways group's Specialised Register, the Cochrane Central Register of Controlled Trials (CENTRAL), and bibliographies of existing trials. Searches were current up to May 2012.
Selection criteria
We included RCTs that investigated the effect of MSG on chronic asthma in adults and children.
Data collection and analysis
Two authors independently extracted, entered and analysed data from included studies. We contacted study authors for additional information.
Main results
Only two cross‐over studies involving 24 adults met the eligibility criteria; the challenge dosages of MSG were 1 g, 5 g and 25 mg/kg. They reported the number of subjects who had a maximum fall in forced expiratory volume in the first second (FEV1) greater than 15% or 200 mL after MSG or the control challenge. The pooled data found no statistically significant difference between MSG and placebo. One trial reported the mean change at four hours and maximum fall in FEV1 over four hours after MSG or the placebo challenge, but found no statistically significant difference between interventions. There were no differences in symptom scores, non‐specific bronchial hyper‐responsiveness (BHR), eosinophil cationic protein (ECP) or tryptase levels in peripheral blood between MSG and control, although we were unable to perform meta‐analyses.
Authors' conclusions
The limited evidence available (n = 24) found no significant difference between MSG or the control challenge for the number of subjects who had a maximum fall in FEV1 greater than 15% or 200 mL. There is no evidence to support the avoidance of MSG in adults with chronic asthma, but as data were limited, this review cannot provide a reliable evidence base for determining whether MSG avoidance is a worthwhile strategy. We could not find any studies conducted on the effect of MSG in children with chronic asthma. There is therefore, a need for further RCTs to investigate any relationship between MSG and asthma, especially in children.
Keywords: Adult, Humans, Asthma, Asthma/chemically induced, Asthma/physiopathology, Cross‐Over Studies, Food Additives, Food Additives/adverse effects, Forced Expiratory Volume, Forced Expiratory Volume/drug effects, Sodium Glutamate, Sodium Glutamate/adverse effects
Plain language summary
Monosodium glutamate (MSG) avoidance for chronic asthma in adults and children
Monosodium glutamate (MSG) is used as a flavour enhancer and has been implicated in "Chinese Restaurant Syndrome", causing tightness, burning or numbness in the face, neck and upper chest (although there is no evidence to prove this syndrome). It has also been proposed that asthmatics may react badly to MSG. In two randomised controlled trials (RCTs), involving 24 adult asthmatics, there was no evidence that MSG worsened asthma when compared to control ingestion. Further RCTs are needed.
Summary of findings
for the main comparison.
| Monosoium glutamate (MSG) avoidance for chronic asthma | ||||||
|
Patient or population: adults and children with asthma Settings: community Intervention: monosodium glutamate (MSG) capsule Comparison: placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| placebo | MSG capsule | |||||
| Lung function | See comment | See comment | See comment | See comment | ⊕⊝⊝⊝ very low1,2,3 | We were unable to pool the two small trials on 24 people. The participants are different in each trial (Schwartzstein 1987: 12 people with chronic asthma and Woods 1998: 12 people who perceived themselves MSG‐intolerant) and we would not expect the same effect in these two trials |
| Hospital admissions | See comment | See comment | See comment | See comment | ⊕⊝⊝⊝ very low1,2,3 | See above |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk Ratio. | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1. (‐1 limitations) we were uncertain of methods for randomisation
2. (‐1 indirectness) participants are different in each trial (Schwartzstein 1987: chronic asthma and Woods 1998: people who perceived themselves MSG‐intolerant) and we would not expect the same treatment effect in these two trials (unless the treatment is ineffective)
3. (‐1 imprecision) small trials with few events
Background
Monosodium glutamate (MSG), a flavour enhancer, is the sodium salt of the non‐essential amino acid, glutamic acid. Glutamate is present in almost all proteins, and it plays an essential role in human metabolism as a key component of metabolic cycles (Filer 1994). Because there is no chemical difference between the MSG we find in foods and the MSG manufactured through fermentation by micro‐organisms, the effects of MSG on the body are the same.
Kwok 1968 originally noted that MSG may cause adverse reactions; he wrote a letter to the New England Journal of Medicine to explain how he felt after eating MSG in a Chinese restaurant. The symptoms were tightness, burning or numbness in the face, neck and upper chest. This syndrome was named "Chinese Restaurant Syndrome". Kwok hypothesised that this syndrome could be due to MSG, sodium or some other unidentified substance. In 1981, Allen and Baker reported two cases of asthma and proposed an association between asthma and MSG (Allen 1981). Over the last two decades there have been a number of studies investigating whether MSG ingestion can induce an asthmatic response, but results are conflicting.
Although there are hypotheses about the mechanisms of MSG‐induced asthma, for example, mediated by immunoglobulin E (IgE; a class of antibody found in mammals) or acetylcholine, findings are inconclusive. Yoneda 2011 investigated the effects of MSG on bronchial inflammation; they measured cytological, histological and functional changes in an ovalbumin‐induced asthma mouse model, but found no acute effects on lung inflammation or airway hyper‐responsiveness.
Although there are several narrative reviews on this topic (ILSI 1991; Stevenson 2000; Woods 2001), no meta‐analyses have been performed.
Objectives
The objectives of this review were to:
identify randomised controlled trials (RCTs) of monosodium glutamate (MSG) ingestion and asthma response in adults and children older than two years of age with asthma;
assess the methodological quality of these trials; and
determine the effect of MSG ingestion on asthma outcomes.
Methods
Criteria for considering studies for this review
Types of studies
We included RCTs only, with either parallel or cross‐over design. We preferred double‐blind trials, but we also reviewed single‐blind and open studies for possible inclusion. We did not limit inclusion of trials by their duration.
Types of participants
We included adults and children older than two years with a diagnosis of asthma. We accepted trialist‐defined asthma and recorded both the definition of asthma and the entry criteria used for each trial. We excluded studies on patients with acute asthma or exercise‐induced bronchospasm because it is difficult to identify whether the attack of asthma is caused by MSG. We considered studies which included patients with other conditions only if the results for subjects with asthma could be identified separately. We excluded studies in children less than two years of age.
Types of interventions
We included studies involving either a challenge of MSG to the diet, manipulation of dietary intake of MSG, or both. We considered only the oral route of administration (for example, capsule or liquid etc). We included placebo and untreated control groups.
Types of outcome measures
We anticipated that not all studies would have results pertaining to the entire outcome measures listed below, but included all outcomes that were reported or available through contact with the authors.
Primary outcomes
Lung function measurements, such as forced expiratory volume in one second (FEV1), peak expiratory flow (PEF) or forced vital capacity (FVC)
Hospital admissions
Secondary outcomes
Asthma symptom scores
Bronchial hyper‐responsiveness (BHR)
Soluble inflammatory markers, such as eosinophil cationic protein (ECP) and/or tryptase
Asthma medication usage
Search methods for identification of studies
Electronic searches
We searched the Cochrane Airways group "Asthma and Wheez* RCT" ProCite Specialised Register, with no language restrictions. We performed the latest search in May 2012.
We used the following search terms:
MSG or "monosodium glutamate*" or monoglutamate or monosodiumglutamate or *sodium or sodium* or glutam* or Glutavene or L‐glutamic or L‐glu or accent or vestin or (food* and (salt* or additive* or flavour* or flavor*)).
Searching other resources
We searched bibliographies of existing trials and approached primary authors of eligible trials and MSG manufacturing companies to ask if they were aware of any other published or unpublished trials. We also searched trial registries for current or recently completed trials.
Data collection and analysis
Selection of studies
Two review authors (YZ, MY) reviewed the title and abstract of references returned by the searches to identify potentially relevant trials for full review. Then we independently read the abstract and methods sections of the papers to select the trials for inclusion in this review. We resolved differences between reviewer authors by consensus.
Data extraction and management
Two review authors (YZ, MY) independently extracted data for inclusion in the Characteristics of included studies tables. We contacted the principal investigators of included studies, when necessary, to request additional data or confirm methodological aspects of their study. We combined trials using Review Manager 5.1 software (RevMan 2011).
Assessment of risk of bias in included studies
We assessed the risk of bias in the included studies as either 'high', 'low' or 'unclear' using the Cochrane Collaboration's 'Risk of bias' tool (Higgins 2011a) under the following headings: 1) sequence generation; 2) allocation concealment; 3) blinding; 4) incomplete outcome data; 5) selective outcome reporting; and 6) other potential bias.
Measures of treatment effect
Lung function measurements included the forced expiratory volume in one second (FEV1), the peak expiratory flow (PEF) and the forced vital capacity (FVC). Hospital admissions included the total number of admissions, the number of participants admitted to hospital and hospital admission rates. Asthma symptom scores included both validated and simple (for example, visual analogue scales) scores. Bronchial hyper‐responsiveness (BHR) to a range of non‐specific provocants were included, either measured as the provocation concentration or provocation dose of the provocant. Soluble inflammatory markers included eosinophil cationic protein (ECP), tryptase, or both. Asthma medication usage was recorded as number of puffs of inhaled therapy over specific time periods.
Unit of analysis issues
We planned to combine data from cross‐over studies using generic inverse variance in Review Manager 5.1 software (RevMan 2011).
Dealing with missing data
We requested information from the trial authors when sufficient details were not available in the published reports to conduct analyses.
For binary outcomes, we used data from intention‐to‐treat analyses. If intention‐to‐treat data were not available in the publications, we used 'on‐treatment' data (i.e. the data of those who complete the trial) and indicated it as such.
Assessment of heterogeneity
We had planned to measure statistical variation between studies using the I2 statistic (Higgins 2011b). If we had found significant heterogeneity we had planned to investigate this further using sensitivity analyses as indicated below.
Assessment of reporting biases
We had planned to visually inspected funnel plots to test for publication bias if we had found sufficient trials on a single meta‐analysis.
Data synthesis
We had planned to combine data in Review Manager 5.1 (RevMan 2011) using a fixed‐effect mean difference (MD) and the 95% confidence intervals (CIs) for continuous variables measured on the same scale. We would have calculated the standardised mean difference (SMD) and 95% CIs for variables measured on different scales. We had planned to use a fixed‐effect odds ratio (OR) for dichotomous variables. We had planned to compare random‐effects and fixed‐effect models; if we found a major difference in the pooled effect sizes and their 95% CIs, we would have opted for the random‐effect model.
Subgroup analysis and investigation of heterogeneity
We had planned to perform the following subgroup analyses if we had found sufficient data:
MSG versus placebo:
MSG versus no treatment;
low dose versus high dose; and
adults versus children.
Sensitivity analysis
We had planned to perform sensitivity analyses if we had found sufficient included trials to explore the influence of the:
risk of bias for allocation concealment; and
risk of bias for outcome evaluation.
Results
Description of studies
Results of the search
From the preliminary searches (latest search May 2012) we found a total of 86 references. By searching bibliographies of existing trials, we identified five additional studies. After removing 15 duplicate references from the searches, we identified 76 studies as potentially relevant; only two of these studies met the entry criteria and we included them in this review.
Included studies
Both studies that met the inclusion criteria were double‐blind, randomised, cross‐over trials that recruited adult participants (Schwartzstein 1987; Woods 1998). Full details of these included studies can be found in the Characteristics of included studies section.
The purpose of the Schwartzstein 1987 trial was to study the effect of oral MSG on airway function in 12 subjects with a history of chronic asthma (diagnosed using criteria from the American Thoracic Society). Participants had a history of food sensitivity, or not, and they were clinically stable. Clincal stability was defined as: 1) no increase in symptoms requiring medical attention within the past month; 2) no participants receiving corticosteroid therapy for at least one month; and 3) being able to discontinue medications for at least 12 hours without adverse effects. The article did not report how the participants were recruited. The participants (eight men and four women) were 22 to 44 years old (mean 28 years), and the mean duration of asthma was 16 years (3 to 30 years). None of the participants used bronchodilators within 12 hours of the challenges. Two participants had a history of milk sensitivity, and only one subject believed that she was MSG‐sensitive. Six subjects were receiving bronchodilator therapy on a daily basis. The interventions were MSG (25 mg/kg) versus sodium chloride (equimolar to MSG), given in identical capsules. Subjects fasted for at least six hours prior to study visits. The forced expiratory volume in the first second (FEV1) and forced vital capacity (FVC) were measured before and after the challenge for two hours, with a 15‐minute interval, and then they were measured every 30 minutes for a further two hours. If at the end of four hours observation, the FEV1 had declined by 10% or more from baseline, measurement of pulmonary function continued at 30‐minute intervals for an additional two hours. At the end, subjects were asked whether they had experienced any side effects, 24 to 48 hours after each study day.
The main purpose of the Woods 1998 trial was to determine if MSG would induce bronchoconstriction in adults with asthma who perceived they were MSG‐sensitive. Twelve participants were recruited through The Alfred Hospital Asthma and Allergy Clinic, from patients registered on a computer database, between March 1995 and September 1996. The participants were aged between 19 and 57 years (mean 35.3 years), and seven (58%) of them were female. All of the participants had a history of reversible airway obstruction, however, they had been clinically stable without any change in their regular asthma medications in the past four weeks before the study. The study exclusion criteria were: 1) a previous life‐threatening attack of asthma; 2) life‐threatening anaphylaxis; 3) females who were pregnant or lactating or 4) a baseline FEV1 greater than 60% predicted or less than 1.5 L. An elimination diet was commenced after the baseline visit. Subjects continued with their regular inhaled bronchodilators and anti‐inflammatory asthma medications during the study period, but they were instructed to withhold their short‐acting beta2‐ agonist bronchodilator medication for at least four hours before attending the laboratory on each of the control days. The amount of relief medication and the time it was taken, if required on the control day, was duplicated on the subsequent control and challenge days. The participants received MSG (1 g), MSG (5 g), and placebo (5 g lactose) challenges as single doses on an empty stomach. Subjects received the lower dose (1 g) of MSG before the higher dose (5 g), with the placebo randomly interspersed on separate study days. Spirometry was conducted every 15 minutes, commencing 45 minutes before the challenge. The participants continued to monitor their peak expiratory flow (PEF) and FEV1 at 15, 30, and 45 minutes and regular intervals after each challenge until 12 hours. Symptoms, symptom scores, non‐specific bronchial hyper‐responsiveness (BHR) and soluble inflammatory marker activity (tryptase and eosinophil cationic protein (ECP)) were also measured.
Excluded studies
We excluded 74 references for the following reasons.
The studies did not examine the relationship between MSG and asthma (N = 45).
They were review articles (N = 10), rather than trials.
The studies were not randomised (N = 9).
The studies involved subjects with diseases other than asthma (N = 10).
Full details can be found in the Characteristics of excluded studies table.
Risk of bias in included studies
We judged risk of bias in included studies as unclear using the Cochrane Collaboration's 'Risk of bias' tool (see Figure 1, Figure 2). Our judgement of each risk of bias can be found in Characteristics of included studies.
1.
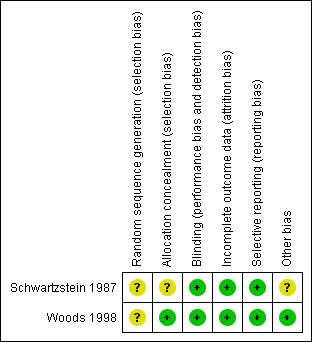
Methodological quality summary: review authors' judgements about each methodological quality item for each included study.
2.
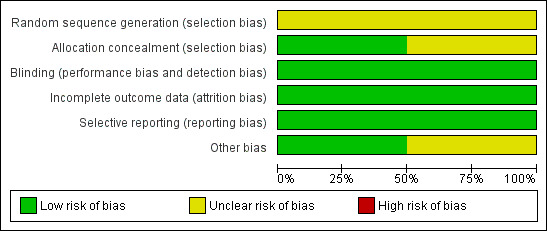
Methodological quality graph: review authors' judgements about each methodological quality item presented as percentages across all included studies.
Both studies (Schwartzstein 1987; Woods 1998) were reported as randomised, but gave no information of the methods used, and we therefore judged them to be at unclear risk of bias. Schwartzstein 1987 did not describe the allocation concealment, but there was reference to personnel carrying‐out the randomisation in Woods 1998, and so we judged the latter as low risk of bias. Subjects and investigators were blinded as to the identity of the medications. Because of the unique flavour of MSG, capsules were used in both studies so that participants were unable to tell the difference between a capsule containing MSG and one containing placebo. All of the outcomes were reported according to the protocol. Schwartzstein 1987 was supported in part by the International Glutamate Technical Committee, and we are unsure if such sponsorship would influence the result.
Effects of interventions
See: Table 1
Two cross‐over trials involving a total of 24 subjects were included, with all subjects being exposed to MSG and control interventions. Both reported changes in FEV1 after MSG and placebo challenges (Schwartzstein 1987; Woods 1998). Woods 1998 reported other outcomes, including non‐specific BHR, ECP levels in peripheral blood and symptom scores.
We were unable to perform meta‐analyses because of the heterogeneity of the studies. The participants in included studies were different; Schwartzstein 1987 included people with chronic asthma, with or without a history of food sensitivity, and Woods 1998 included people with clinically documented asthma and who perceived themselves MSG‐intolerant). Furthermore, to be able to combine dichotomous data from cross‐over studies, we would need to know the number of participants who form discordant pairs, but this information was not reported in Schwartzstein 1987. Hence, we presented the results in narrative form.
Primary outcome: lung function
In Woods 1998, eight out of 12 people experienced a fall in FEV1, PEF, or both of greater than 15% of their pre‐challenge spirometry after receiving an MSG challenge (each received two separate challenges of 1 g and 5 g). Two participants experienced a fall in lung function measurement after placebo only, three after both active and placebo treatment, and two to the 1 g MSG challenge only, but not the 5 g MSG challenge. One subject had a greater than 15% fall in spirometry to both high and low doses of MSG, but the change was not statistically significant and the trialists interpreted this as a negative effect. All participants except one experienced a drop of 20% or more of their pre‐challenge spirometry measurement, so the authors stated that they "did not find any conclusive evidence of true MSG‐induced asthma by using this rigorous method of analysis" (Woods 1998).
Schwartzstein 1987 gave only one MSG challenge of 25 mg/kg. Schwartzstein 1987 reported the mean FEV1 change at four hours post‐challenge and the difference between these values was not statistically significant.
In summary, there was no statistically significant difference between MSG and placebo for a positive FEV1 response at any of the doses studied.
Secondary outcomes
Symptom scores
Woods 1998 reported that there were no statistically significant differences in daytime or night time symptom scores between placebo and 1 g MSG challenges (P = 0.5 and P = 1.0, respectively), placebo and 5 g MSG challenges (P = 1.0 and P = 1.0, respectively), or 1 g and 5 g MSG challenges (P = 0.25 and P = 1.0, respectively). Schwartzstein 1987 reported the number of participants who had unusual sensations (two in the MSG group and two in the placebo group), but did not score the symptoms.
Non‐specific bronchial hyper‐responsiveness
Woods 1998 found that there was no evidence of MSG affecting non‐specific BHR.
Eosinophil cationic protein levels in peripheral blood
Woods 1998reported that five participants had elevated ECP levels following the MSG challenge, however, baseline ECP levels were elevated in four participants. In the fifth subject, the ECP level was raised after both the 1 g and 5 g MSG challenge (the level was higher after the 1 g than the 5 g MSG challenge), but was normal after the placebo challenge.
Tryptase levels in peripheral blood
Woods 1998 found that the only elevated tryptase level occurred on a baseline sample.
Discussion
This review summarises evidence from two small clinical trials assessing the effects of the MSG challenge on short‐term physiological, symptomatic and biomarker outcomes. Long‐term dietary avoidance has not been addressed in any clinical trials involving asthma patients to date. The evidence from this review, which found no difference in outcomes following MSG or control exposure, does not provide a reliable basis for recommendations on avoidance of MSG.
There are two reasons for this. First of all, only two small trials met the eligibility criteria of the review. The sample sizes of both studies were small and identical in number (n = 12), and the quality of one study was poor. As data from the included studies were limited, no firm conclusions can be drawn from this review regarding the effects of MSG in asthma. Secondly, the participants recruited had been clinically stable; those with a baseline FEV1 of less than 60% predicted were excluded. Therefore, these results cannot be applied to people with severe or unstable asthma, nor children, since both trials recruited only adult patients.
The dose of MSG in studies is reasonable. The average daily MSG intake in Western countries is 0.3 g to 1 g, but it may be as high as 4 g to 6 g in a highly seasoned restaurant meal (Allen 1987). The doses in the studies were 0.3 g to 7.5 g (0.3, 1, 2.5, 5, 6, 7.5) (Raiten 1995), and the dosage used most frequently was 2.5 g. The doses used in challenges in the included studies are similar to quantities in meals, which means that it is unlikely that the absence of effect is due to under dosing.
Another limitation of this review is the lack of numerical outcome data on clinical scores such as symptoms, for which we were unable to extract raw data from trials assessing these outcomes.
We did not find any RCTs assessing the effects of MSG in children, so were unable to explore the relationship between childhood asthma and MSG.
Our result is consistent with most clinical (Freeman 2006; Stevenson 2000; Williams 2009) and mechanical (Yoneda 2011) studies, and it is difficult to draw a firm conclusion.
Authors' conclusions
Implications for practice.
Limited evidence from people with stable chronic asthma did not provide any evidence that MSG could pose a risk. However, this review has not been able to establish the effect of MSG exposure on people with stable chronic asthma, as data were limited by small sample sizes. Because of the lack of data, these results cannot be applied to people with severe or unstable asthma, nor children since both trials recruited only adult patients.
Implications for research.
There is a need for large, placebo‐controlled randomised trials that would address as many MSG‐related symptoms as possible, and researchers should pay attention to other aspects such as psychological factors. There is a need for further studies in children.
What's new
| Date | Event | Description |
|---|---|---|
| 4 June 2014 | Amended | PLS title amended |
Acknowledgements
We are grateful to Jo Picot and Toby Lasserson for their help. We'd like to thank Elizabeth Stovold for searching studies for us. We would like to thank the referees for their helpful comments and Ryan Westhoff for his suggestions in writing the review.
Sponsored by the Chinese Cochrane Center.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Schwartzstein 1987.
| Methods | Double‐blind, randomised, cross‐over trial | |
| Participants | 12 patients (male = 8, female = 4) with chronic stable asthma Mean age 28 years (22 to 44) Mean duration of asthma 16 years (3 to 30) |
|
| Interventions |
Treatments administered in identical capsules |
|
| Outcomes | FEV1 and FVC collected at: 0, 15, 30, 45, 60, 75, 90, 105, 120, 150, 180, 210, 240 minutes
If FEV1 declined ≥ 10%, FEV1 and FVC were also measured at 270, 300, 330, 360 minutes Symptoms: 24, 48 hours |
|
| Notes | Diagnostic criteria: American Thoracic Society This study was supported in part by the International Glutamate Technical Committee |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "The order in which the test substances were given was randomly assigned" Comment: Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "both subjects and investigators were blinded as to the identity of the medications being given." The drugs were administered in identical capsules |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Results of all the subjects were reported |
| Selective reporting (reporting bias) | Low risk | All the results described in method chapter were reported |
| Other bias | Unclear risk | "This study was supported in part by the International Glutamate Technical Committee" Comment: we are not certain whether it would influence the result |
Woods 1998.
| Methods | Double‐blind, randomised, cross‐over, placebo‐controlled trial | |
| Participants | 12 patients (male = 5, female = 7) who believed they had previously had reactions to MSG Mean age 35.3 years (19 to 57) |
|
| Interventions |
|
|
| Outcomes | FEV1, PEF at ‐15, ‐30, ‐45, 0, 15, 30, 45 minutes; 1, 1.25, 1.5, 2, 2.5, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12 hours on 15 days , 18 days, 20 days (challenge visit 6, 7, 8 days) Soluble inflammatory marker activity (ECP and tryptase)(2 days, 6 days, 7 days, 8 days) Non‐specific BHR to methacholine (1 day, 5 days, 9 days) |
|
| Notes | Diagnostic criteria: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "This study was conducted as a randomised, cross‐over, double‐blind, placebo‐controlled trial" "We thank Ms. Anne James, Pharmacist, for the preparation of the challenge capsules and Ms. Pam Liakakis, Respiratory Scientist, for the randomisation and administration of the capsules" Comment: Not described |
| Allocation concealment (selection bias) | Low risk | "We thank Ms. Anne James, Pharmacist, for the preparation of the challenge capsules and Ms. Pam Liakakis, Respiratory Scientist, for the randomisation and administration of the capsules" |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "Each challenge dose comprised 10 size 00 opaque capsules, which were manufactured with a capsule machine filler (Sandell, Switzerland) by a pharmacist not otherwise involved in the study. All capsules were wiped clean after filling and rolled in lactose powder to prevent any MSG being detected on the outside of the capsules" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Results of all the subjects were reported |
| Selective reporting (reporting bias) | Low risk | All the results described in the method chapter were reported |
| Other bias | Low risk | "The participants had a diet that was low in other chemicals perceived to cause asthma symptoms" Comment: it minimised the influence of other materials |
BHR: bronchial hyper‐responsiveness FEV1:forced expiratory volume in the first second FVC: forced vital capacity MSG: monosodium glutamate PEF: peak expiratory flow
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Allen 1987 | Not randomised |
| Bernstein 1978 | Not focused on the relationship between MSG and asthma |
| Briese 1987 | Not focused on the relationship between MSG and asthma |
| Businco 1990 | Not focused on the relationship between MSG and asthma |
| D'Elia 1972 | Not randomised |
| Dahl 1978 | Not focused on the relationship between MSG and asthma |
| Daniliak 1995 | Not focused on the relationship between MSG and asthma |
| Demissie 1996 | Not focused on the relationship between MSG and asthma |
| Doeglas 1975 | Involved subjects with other diseases, such as urticaria, rather than asthma |
| Fiocchi 1995 | Involved subjects with other diseases, such as urticaria, rather than asthma |
| FSA 1998 | Involved subjects with other diseases, such as urticaria, rather than asthma |
| Fuchs 1998 | Not focused on the relationship between MSG and asthma |
| Fuglsang 1994 | Not focused on the relationship between MSG and asthma |
| Germano 1991 | Not randomised |
| Harries 1978 | Not focused on the relationship between MSG and asthma |
| Hodge 1996 | Not randomised |
| James 1999 | Review |
| Novembre 1988 | Review |
| Onorato 1986 | Not randomised |
| Pacor 1992 | Not randomised |
| Patil 1997 | Not focused on the relationship between MSG and asthma |
| Prieto 1988 | Not focused on the relationship between MSG and asthma |
| Raiten 1995 | Review |
| Reinert 1991 | Involved subjects with other diseases, such as urticaria, rather than asthma |
| Sabbah 1990 | Review |
| Stenius 1976 | Not focused on the relationship between MSG and asthma |
| Stevenson 1997 | Not randomised |
| Stevenson 2000 | Review |
| Tarlo 1982 | Not focused on the relationship between MSG and asthma |
| Walker 1999 | Review |
| William 1997 | Not focused on the relationship between MSG and asthma |
| Wilson N 1989 | Not focused on the relationship between MSG and asthma |
| Woessner 1999 | Not randomised |
| Yang 1997 | Involved subjects with other diseases, such as urticaria, rather than asthma |
MSG: monosodium glutamate
Contributions of authors
Yan Zhou selected the trials, extracted data, performed analyses, and wrote the review.
Ming Yang selected the trials.
Birong Dong gave advice on methodology.
Richard Wood‐Baker edited the review and revised text.
Sources of support
Internal sources
No sources of support supplied
External sources
Chinese Cochrane Centre, China.
Declarations of interest
There are no known conflicts of interest.
Edited (no change to conclusions)
References
References to studies included in this review
Schwartzstein 1987 {published data only}
- Schwartzstein RM, Kelleher M, Weinberger SE, Weiss JW, Drazen JM. Airway effects of monosodium glutamate in subjects with chronic stable asthma. Journal of Asthma 1987;24(3):167‐72. [DOI] [PubMed] [Google Scholar]
Woods 1998 {published data only}
- Woods RK, Weiner JM, Thien F, Abramson M, Walters EH. The effects of monosodium glutamate in adults with asthma who perceive themselves to be monosodium glutamate‐intolerant. Journal of Allergy and Clinical Immunology 1998;101(6 Pt 1):762‐71. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Allen 1987 {published data only}
- Allen DH, Delohery J, Baker G. Monosodium L‐glutamate‐induced asthma. Journal of Allergy and Clinical Immunology 1987;80(4):530‐7. [DOI] [PubMed] [Google Scholar]
Bernstein 1978 {published data only}
- Bernstein IL, Johnson CL. Therapy with cromolyn sodium. Annals of Internal Medicine 1978;89(2):228‐33. [DOI] [PubMed] [Google Scholar]
Briese 1987 {published data only}
- Briese D, Dorn B, Krauns P, Koch HF, Schnitker J. Absorption of theophylline from Bronchoparat depending on the preceding food intake. Praxis und Klinik der Pneumologie 1987;41(2):37‐42. [PubMed] [Google Scholar]
Businco 1990 {published data only}
- Businco L, Cantani A. Food allergy in children: diagnosis and treatment with sodium cromoglycate. Allergologia Et Immunopathologia 1990;18(6):339‐48. [PubMed] [Google Scholar]
D'Elia 1972 {published data only}
- D'Elia‐A, Colella‐C, Contursi‐R. Controlled clinical trial a gastroprotective steroid (prednisone + xylamide). Minerva Medica 1972;63(65):3554‐8. [PubMed] [Google Scholar]
Dahl 1978 {published data only}
- Dahl R, Zetterström. The effect of orally administered sodium cromoglycate on allergic reactions caused by food allergens. Clinical Allergy 1978;8(5):419‐22. [DOI] [PubMed] [Google Scholar]
Daniliak 1995 {published data only}
- Daniliak IG, Kogan AK, Bolevich S. Aevit and glutamic acid in the treatment of patients with bronchial asthma. Klinicheskaia Meditsina 1995;73(5):50‐3. [PubMed] [Google Scholar]
Demissie 1996 {published data only}
- Demissie K, Ernst P, Gray DK, Joseph L. Usual dietary salt intake and asthma in children: A case‐control study. Thorax 1996;51(1):59‐63. [DOI] [PMC free article] [PubMed] [Google Scholar]
Doeglas 1975 {published data only}
- Doeglas HM. Reactions to aspirin and food additives in patients with chronic urticaria, including the physical urticarias. British Journal of Dermatology 1975;93(2):135‐44. [DOI] [PubMed] [Google Scholar]
Fiocchi 1995 {published data only}
- Fiocchi A, Restani P, Riva E, Qualizza R, Bruni P, Restelli AR, et al. Meat allergy: I‐Specific IgE to BSA and OSA in atopic, beef sensitive children. Journal of the American College of Nutrition 1995;14(3):239‐44. [DOI] [PubMed] [Google Scholar]
FSA 1998 {published data only}
- Food Standards Agency. Do food additives cause hyperactivity and behaviour problems in a geographically defined population of 3 year olds?. www.foodbase.org.uk/results.php?f_report_id=536.
Fuchs 1998 {published data only}
- Fuchs WS, Nieciecki A, Molz KH, Popescu G, Weil A, Barkworth MF, et al. The effect of bile secretion on the pharmacokinetics of a theophylline sustained‐release preparation. Arzneimittelforschung 1998;48(5A):597‐604. [PubMed] [Google Scholar]
Fuglsang 1994 {published data only}
- Fuglsang G, Madsen G, Halken S, Jorgensen S, Ostergaard PA, Osterballe O. Adverse reactions to food additives in children with atopic symptoms. Allergy 1994;49(1):31‐7. [DOI] [PubMed] [Google Scholar]
Germano 1991 {published data only}
- Germano P, Cohen SG, Hahn B, Metcalfe D. An evaluation of clinical reactions to monosodium glutamate (MSG) in asthmatics using a blinded, placebo‐controlled challenge. Journal of Allergy and Clinical Immunology 1991;87:177. [Google Scholar]
Harries 1978 {published data only}
- Harries MG, O'Brien IM, Burge PS, Pepys J. Effects of orally administered sodium cromoglycate in asthma and urticaria due to foods. Clinical Allergy 1978;8(5):423‐7. [DOI] [PubMed] [Google Scholar]
Hodge 1996 {published data only}
- Hodge L, Yan KY, Loblay RL. Assessment of food chemical intolerance in adult asthmatic subjects. Thorax 1996;51(8):805‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
James 1999 {published data only}
- James JM. Food‐induced allergic reactions of the respiratory tract. Canadian Journal of Allergy and Clinical Immunology 1999;4(8):374‐80. [Google Scholar]
Novembre 1988 {published data only}
- Novembre E, Martino M, Vierucci A. Foods and respiratory allergy. Journal of Allergy and Clinical Immunology 1988;81(5 Pt 2):1059‐65. [DOI] [PubMed] [Google Scholar]
Onorato 1986 {published data only}
- Onorato J, Merland N, Terral C, Michel FB, Bousquet J. Placebo‐controlled double‐blind food challenge in asthma. Journal of Allergy and Clinical Immunology 1986;78:1139‐46. [DOI] [PubMed] [Google Scholar]
Pacor 1992 {published data only}
- Pacor ML, Marchi G, Cortina P, Nicolis F, Lunardi C, Corrocher R. Food allergy and asthma. Recenti Progressi in Medicina 1992;83(2):64‐6. [PubMed] [Google Scholar]
Patil 1997 {published data only}
- Patil SP, Niphadkar PV, Bapat MM. Allergy to fenugreek (Trigonella foenum graecum). Annals of Allergy. Asthma and Immunology 1997;78(3):297‐300. [DOI] [PubMed] [Google Scholar]
Prieto 1988 {published data only}
- Prieto L, Juyol M, Paricio A, Martinez MA, Palop J, Castro J. Oral challenge test with sodium metabisulfite in steroid‐dependent asthmatic patients. Allergologia Et Immunopathologia 1988;16(6):393‐6. [PubMed] [Google Scholar]
Raiten 1995 {published data only}
- Daniel J. Raiten, John M. Talbot, Kenneth D. Fisher. Executive Summary from the Report: Analysis of adverse reactions to monosodium glutamate (MSG). In: Raiten DJ, Talbot JM, Fisher KD editor(s). American Institute of Nutrition. American Institute of Nutrition, 1995. [DOI] [PubMed] [Google Scholar]
Reinert 1991 {published data only}
- Reinert P, Narcy P, Paliwoda A, Rouffiac E. Evaluation of tixocortol pivalate‐neomycin combination versus ++a placebo excipient in acute rhinopharyngitis in children. Annales de Pédiatrie 1991;38(7):503‐8. [PubMed] [Google Scholar]
Sabbah 1990 {published data only}
- Sabbah A. Food allergy in childhood asthma. Allergie Et Immunologie 1990;22(8):325‐31. [PubMed] [Google Scholar]
Stenius 1976 {published data only}
- Stenius BS, Lemola M. Hypersensitivity to acetylsalicylic acid ASA and tartrazine in patients with asthma. Clinical Allergy 1976;6(2):119‐29. [DOI] [PubMed] [Google Scholar]
Stevenson 1997 {published data only}
- Stevenson DD, Simon RA, Woessner KM. The role of Monosodium L‐Glutamate (MSG) in asthma: Does it exit?. Journal of Allergy and Clinical Immunology 1997;99:S411. [DOI] [PubMed] [Google Scholar]
Stevenson 2000 {published data only}
- Stevenson DD. Monosodium glutamate and asthma. Journal of Nutrition 2000;130:1067S‐73S. [DOI] [PubMed] [Google Scholar]
Tarlo 1982 {published data only}
- Tarlo SM, Broder I. Tartrazine and benzoate challenge and dietary avoidance in chronic asthma. Clinical Allergy 1982;12(3):303‐12. [DOI] [PubMed] [Google Scholar]
Walker 1999 {published data only}
- Walker R. The significance of excursions above the ADI. Case study: monosodium glutamate. Regulatory Toxicology and Pharmacology 1999;30(2 Pt 2):S119‐21. [DOI] [PubMed] [Google Scholar]
William 1997 {published data only}
- Yang WH, Drouin MA, Herbert M, Mao Y, Karsh J. The monosodium glutamate symptom complex: Assessment in a double‐blind, placebo‐controlled, randomized study. Journal of Allergy and Clinical Immunology 1997;99(6):757‐62. [DOI] [PubMed] [Google Scholar]
Wilson N 1989 {published data only}
- Wilson N, Scott A. A double‐blind assessment of additive intolerance in children using a 12 day challenge period at home. Clinical and Experimental Allergy 1989;19(3):267‐72. [DOI] [PubMed] [Google Scholar]
Woessner 1999 {published data only}
- Woessner, KM, Simon RA, Stevenson DD. Monosodium glutamate sensitivity in asthma. Journal of Allergy and Clinical Immunology 1999;104(2 I):305‐10. [DOI] [PubMed] [Google Scholar]
Yang 1997 {published data only}
- Yang WH, Drouin MA, Herbert M, Mao Y, Karsh J. The monosodium glutamate symptom complex: Assessment in a double‐blind, placebo‐controlled, randomized study. Journal of Allergy and Clinical Immunology 1997;99(6):757‐62. [DOI] [PubMed] [Google Scholar]
Additional references
Allen 1981
- Allen DH, Baker GJ. Chinese restaurant asthma (letter). New England Journal of Medicine 1981;305(19):1154‐5. [DOI] [PubMed] [Google Scholar]
Filer 1994
- Filer LJ Jr, Stegink LD. A report of the proceedings of an MSG workshop held August 1991. Critical Reviews in Food Science and Nutrition 1994;34(2):159‐74. [DOI] [PubMed] [Google Scholar]
Freeman 2006
- Freeman M. Reconsidering the effects of monosodium glutamate: a literature review. Journal of the American Academy of Nurse Practitioners 2006;18(10):482‐6. [DOI] [PubMed] [Google Scholar]
Higgins 2011a
- Higgins JPT, Green S, Altman DG, Sterne JAC (editors). Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Higgins 2011b
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
ILSI 1991
- International Life Sciences Institute (ILSI). The scientific facts about MSG. International Life Sciences Institute Australia Symposium. Food Australia 1991;43:S1‐S20. [Google Scholar]
Kwok 1968
- Kwok RHM. Chinese Restaurant Syndrome (letter). New England Journal of Medicine 1968;278:796. [DOI] [PubMed] [Google Scholar]
RevMan 2011 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.1. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2011.
Williams 2009
- Williams AN, Woessner KM. Monosodium glutamate 'allergy': menace or myth?. Clinical and Experimental Allergy 2009;39(5):640‐6. [DOI] [PubMed] [Google Scholar]
Woods 2001
- Woods RK. MSG and asthma ‐ what is the evidence?. Food Australia 2001;53:555‐9. [Google Scholar]
Yoneda 2011
- Yoneda J, Chin K, Torii K, Sakai R. Effects of oral monosodium glutamate in mouse models of asthma. Food and Chemical Toxicology 2011;49(1):299‐304. [DOI] [PubMed] [Google Scholar]


