Abstract
Background and aim:
The synchronous occurrence of renal cell carcinoma and urothelial carcinoma of the renal pelvis in the same kidney is extremely rare, although previously reported. With this study we aim to present our case and provide a literature review on this entity.
Methods:
An otherwise healthy 43-year-old military male with the chief complaint of left plank pain was seen in the office. Imaging revealed the presence of a 3.5 cm left renal mass. Left laparoscopic radical nephrectomy was performed for presumed renal malignancy. Pathology confirmed the presence of a clear cell RCC and revealed an area of low-grade UC arising from the ipsilateral renal pelvis, not visible in the preoperative imaging. Furthermore, a Pubmed database search in English language was conducted, up to June 2021, to identify the rate of simultaneous RCC and UC of the renal pelvis or ureter in RN specimen performed for presumed RCC or renal malignancy and subsequent management in these cases.
Results:
A total of 53 articles reporting on 56 patients with synchronous ipsilateral RCC and UC of the renal pelvis were identified, together with our case. Eight cases (14%) were initially managed with RN, thereby requiring further management of the ureteral stump. Of these, six (75%) were managed with distal ureterectomy, one (12.5%) with active surveillance of the ureteral stump, while one case (12.5%) did not report subsequent management. To our knowledge, we present the youngest patient recorded in the literature, with this histology combination presenting synchronously in the same kidney.
Conclusions:
Although uncommon, the final pathology report may reveal neoplasms of dissimilar histology that may involve the renal pelvis. It is crucial for urologists and pathologists to be vigilant of such cases during a solid renal mass workup. Additional therapeutic adjustments may be necessitated, derailing the initial treatment plan. (www.actabiomedica.it)
Keywords: Renal cell carcinoma, Transitional cell carcinoma, Urothelial carcinoma, Radical nephrectomy, Synchronous tumors
Introduction
Since the first reported case by Graves et al. in 1921 (1), synchronous renal cell carcinoma (RCC) and urothelial carcinoma (UC) in the same kidney remain infrequent, with approximately 56 cases to date.
We present a case of an incidentally discovered UC of the renal pelvis in the pathology specimen of a laparoscopic radical nephrectomy performed for presumed RCC in the same kidney. To our knowledge, this is the youngest patient with this type of histology combination, presenting concurrently in the same kidney, reported in English literature.
Case Description
A 43-year-old active-duty male was seen in the office with dull, left plank pain of five days’ duration. The patient also complained of generalized fatigue for the past month, which he attributed to increased workload. He denied any trauma, gross hematuria or pain during urination in the days leading up to the presentation. Past medical and surgical history was unremarkable. A 10-pack-year history of cigarette smoking, but no occupational chemical exposure was reported. Genitourinary or associated genetic disorders were also absent in any family member.
On initial assessment, the patient had a BMI of 28 kg/m2, a temperature of 36.50C, blood pressure 120/80 mm Hg, pulse 75/min, and respiration rate 16/min. No costovertebral angle tenderness or palpable masses were felt.
Routine blood work showed a marginally elevated aspartate transaminase (AST) and alanine transaminase (ALT), 35.6 IU/L (normal 5.0–34.0 g/dL) and 58.6 IU/L (normal 7.0–55.0 g/dL) respectively, LDH of 346.3 (normal 125.0-220.0 IU/L), creatinine in the upper limit of normal 1.19 mg/dL (normal 0.72–1.25 g/dL); full blood count, electrolytes, and coagulation studies were within normal limits. Urinalysis showed 8 red blood cells/hpf (high power field). Urine culture and a Meares-Stamey test came back negative for abnormal findings.
The patient was subsequently sent for computed tomography (CT), which revealed a 3.5 cm mass in the anterior aspect of the left mid-pole with heterogenous enhancement, following administration of intravenous contrast, and a normal-appearing contralateral kidney. The lesion was noted to slightly compress and displace without invading the left renal pelvis and renal vein. No enlargement of the retroperitoneal lymph nodes and adrenals was seen (Fig. 1). Additionally, the baseline chest CT scan was normal. Following the results of imaging our working diagnosis was presumed RCC warranting surgical removal. Left laparoscopic radical nephrectomy (LRN) using the transperitoneal approach was performed. The patient did well postoperatively and was discharged in 3 days.
Figure 1.
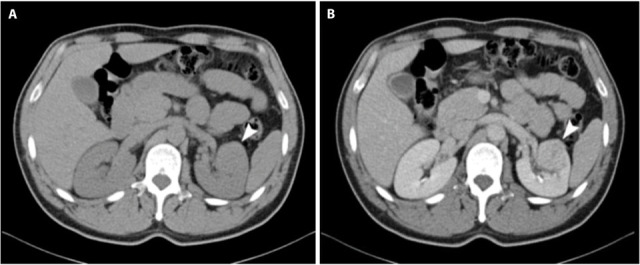
CT of the abdomen before (a) and after intravenous contrast administration (b) reveals the presence of a 3.5 cm mass in the anterior aspect of the mid-pole of the left kidney with heterogenous enhancement and a normal-appearing contralateral kidney. The lesion was noted to slightly compress and displace without invading the left renal pelvis and renal vein. No involvement of the retroperitoneal lymph nodes and adrenals was detected.
The left kidney weighing 320 gr., perinephric adipose tissue, and a 3.5 x 0.5 cm segment of the left ureter were resected. On cut section, a 3 cm well-circumscribed, centrally located mass, in close proximity to the renal hilum, was detected. The mass had a brown and orange-colored variegated cut surface. The perirenal fat tissue could be easily detached from the renal capsule, and no involvement of the Gerota’s fascia was appreciated. In addition, two whitish nodules of 0.4 to 0.6 cm were found in the periphery of the centrally located mass, arising from the collecting system. On light microscopy, the first tumor was a Fuhrman nuclear grade 3 clear cell RCC (Fig. 2). No capsular penetration and invasion of renal parenchyma and pelvis was noted. The grossly whitish nodules were interpreted as low-grade, papillary UC originating from the renal pelvis, pT1 (Fig. 2). No parenchymal invasion was seen. Tumor cells on this part were negative for cluster of differentiation 10 (CD10) and positive for cytokeratin 7 (CK7) on immunohistochemistry (Fig. 3). All surgical margins, including the ureteral margin, were free of tumor and no lymphovascular and neural invasion was seen.
Figure 2.
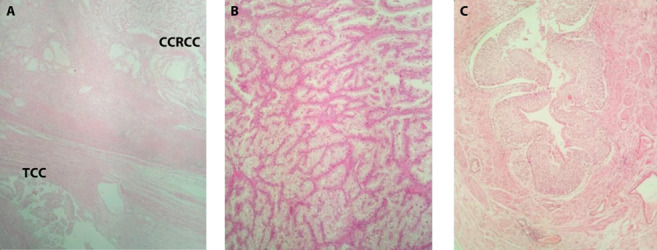
Section of the tumor at the middle part of the left kidney: (a) Renal cell carcinoma (upper right) and transitional cell carcinoma (lower left) captured together. (b) Clear cell renal cell carcinoma, Fuhrman’s nuclear grade 3. (c) Low-grade, papillary urothelial carcinoma arising from the renal pelvis (Hematoxylin and eosin, original magnifications x40, x100 and x100, respectively)
Figure 3.
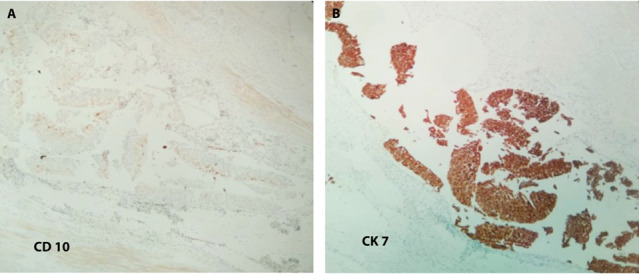
Transitional Cell Carcinoma of the renal pelvis (Original magnifications x100): (a) Cells stained negative for CD10 immunohistochemical stain. (b) Cells stained positive for CK7 immunohistochemical stain
In light of the UC and following extensive discussion, regarding the recurrence rates and the stringent follow-up that a remaining ureteral stump requires, the patient elected to undergo further surgical treatment. Cystoscopy followed by open left ureterectomy with bladder cuff excision was performed three weeks after the first operation. Cystoscopy, urinary cytology, and distal ureter stump pathology showed no evidence of disease. The patient was discharged to be followed by a modified surveillance protocol of cystoscopy, urine cytology, and CT for the next five years.
Discussion
RCC and TCC represent the majority of renal malignancies in adults comprising together 90-95% (2). The coexistence, however, of the two most common histological subtypes of renal tumors in the same kidney is extremely rare, approximating 0.14% as per a previous study (3).
Concurrent RCC and TCC of the renal pelvis present with a mean age of 64.5 years, more commonly in the left side, such as in our case, and are twice as common in males, resembling the epidemiology of independent RCC or TCC of the kidney, as demonstrated by Hart et al. in a review of 23 cases (4). Strikingly enough, our patient was 43 years old, making him the youngest case on record to the best of our knowledge. Moreover, it represents the first report in active military personnel. Previously, Kline et al. had reported on a 47-year-old patient in an era when CT urography was practically nonexistent (5).
In the same retrospective study by Hart et al., cigarette smoking was implicated in 24% of the cases (4). TCC of the renal pelvis may be associated with the abuse of phenacetin-based compounds. Anseline et al. in 1977 described this histological combination in a woman with analgesic nephropathy (6), in accordance with Bengtsson et al. initial findings in 1968 (7). Our patient denied the use of analgesics and exposure to other known renal carcinogens apart from tobacco. Moreover, Park et al. proposed that c-MET and p53 may be associated with the development of papillary TCC of the renal pelvis, based on their immunohistochemistry findings (8).
Interestingly, we are witnessing a paradigm shift in the preoperative diagnosis of such cases. Traditionally, the renal pelvic tumor was diagnosed first, using retrograde pyelograms, during the workup of hematuria, and the RCC was an incidental pathology finding (3). Nowadays, most renal tumors and more than 50% of RCCs are detected accidentally due to the US and CT’s widespread use for other medical indications (9). Consequently, similarly to our case, detection of an asymptomatic RCC usually precedes that of TCC.
There are several issues that need to be addressed concerning renal pain in renal tumors. First, renal tumors cause pain by renal capsule or pelvis distention or compression, obstruction, or via neighboring structures invasion (10). Second, pain and/or hematuria at presentation indicate poor prognosis because both are associated with advanced disease. It is uncommon for stage 1 RCC to present with flank pain (10), contrary to our case. Pain is expected in stage 3 or stage 4 tumor that has spread locally or in distant sites (10). Conversely, malignancies of the renal collecting system and ureter may cause pain due to urinary flow obstruction. One-third of patients with renal pelvis cancer experience pain (10). Third, experimental evidence suggests that the peripelvic renal capsule, pelvis, renal artery, or vein are the anatomical locations sensitive to stimuli causing the visceral renal pain, over the costovertebral angle, contrary to regions away from the pelvis (11). Consequently, we can only speculate that two were the culprits in our case. First, the peripelvic and close to hilum location and, second, minor bleeding from the UC in the pelvis, which might have irritated or obstructed the outflow tract acting like a kidney stone. Lastly, DeWolf and Fraley in their review about renal pain noted that severe pain in the absence of radiographically apparent obstruction is possible, clearing up the misconception that the degree of dilation in imaging correlates with the subjective pain felt by the patient (11).
Regarding management, surgical treatment of RCC and upper tract UC are fundamentally different. RCC is managed by radical or partial nephrectomy, with the latter suggested mainly for localized T1 RCC (9). Upper tract UC management, on the other hand, relies on risk stratification to select those patients who will benefit more from a kidney-sparing treatment. Histological and cytological grades of cancer cells are crucial factors, although not the only ones, to consider for risk stratification. Accordingly, kidney-sparing surgery (KSS) for low-risk disease and RNU with bladder cuff excision for high-risk disease are recommended (12).
Due to the unfavorable location of close proximity to the renal hilum, we found it more technically feasible to proceed with a laparoscopic RN, instead of partial nephrectomy, even though we had to deal with a T1 tumor.
In view of the incidental UC in the renal pelvis after RN, management of the remaining ureteric stump remained a therapeutic dilemma. Previous series have shown recurrence rates ranging from 20 to 58 % in the ureteral stump after incomplete nephroureterectomy for TCC of the upper tract and seem to increase proportionately to the length of the remaining ureter (13). On top of that, a study from Mayo Clinic suggested that grade 2 tumors carry a 30 % rate of ipsilateral tumor recurrence, meriting a radical nephroureterectomy. Conversely, the less frequent ipsilateral recurrence in grade 1 tumors may allow a less radical approach (14).
In our case of a low-grade TCC of the renal pelvis found after RN, one could argue in favor of surveillance instead of a more extensive surgical treatment. An interesting case of active surveillance with cystoscopy, ureterograms, ureteral washings, and ureteroscopy of the ureteral stump is reported by Michel and Belldegrun (15). Of note, the patient must be aware of the stringent follow-up that active surveillance entails to make an educated choice. Our patient elected to complete the surgical treatment to avoid this strict surveillance and eliminate anxiety around a potential recurrence.
We performed a Pubmed database search up to June 2021, to identify studies of synchronous ipsilateral RCC and renal pelvis UC in English language. The following search terms were included: “renal cell carcinoma”, “urothelial carcinoma”, and “transitional cell carcinoma”. References of the included papers were hand-searched. Including our case, 53 articles reporting on 56 patients were retrieved. That is in contrast to the 40 cases as previously reported in the literature.
A total of eight cases (14%), together with our case, were initially managed with RN for presumed RCC or renal malignancy, thus requiring further management after RN (Table 1). Open RN was performed in three cases (3/8, 37.5%), while all five cases published after 2009 (5/8, 62.5%) used the laparoscopic approach, either as conventional laparoscopy (4/5, 80%) or robot-assisted (1/5, 20%). Distal ureterectomy was performed in six of eight cases (75%), active surveillance of the ureteral stump in one (12.5%), while one case (12.5%) did not report subsequent management. Among patients managed with distal ureterectomy, most were performed in a second operation at two weeks or later from the first surgery (4/6, 66.6%) and two at the time of the first surgery due to intraoperative frozen section biopsy (2/6, 33.3%). Notably, intraoperative frozen section biopsy was used in three cases, but failed to reveal the UC in one case, in which case a second operation was needed. Lastly, the patient on active surveillance had two recurrences of bladder UC at 8 and 10 months with grade progression from 1 to 2. One patient managed with LRNU experienced bladder recurrence of UC at 5 months and ipsilateral adrenal metastasis of RCC at 15 months.
Table 1.
Studies of synchvronous ipsilateral RCC and UC of renal pelvis that required distal ureterectomy or active surveillance of the ureteral stump after a RN
| First authort | Year | Country | Sex | Age, years | Smoking Status | Presenting Symptom | Imaging modalities at initial diagnosis | Imaging finding or Working diagnosis | Tumor size (cm) and location | Surgery | Management after UC diagnosis | Side | RCC type | RCC | UC | Follow-up, months, mean (range) / Outcome | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| RCC | UC | Stage | Grade | Stage | Grade | ||||||||||||||
| Michel(15) | 1999 | USA | M | 74 | NR | Gross hematuria | IVP, abdominal+ pelvic CT with contrast | 4 x 3 x 4 cm heterogenous enhancing mass in the anterior left lower pole | 4.0 - anterior lower pole | NR - renal pelvis | RN | Active surveillance | L | Clear cell | T1 | Grade 3 | NR | Grade 2 | 8/ 3 small papillary bladder tumors grade 1, Ta TCC; 10/ 5 papillary bladder tumors grade 2,Ta TCC |
| Bernie(16) | 2000 | USA | M | 72 | CS | Incidental renal mass on US for splenomegaly | US, gadolinium enhanced MRI | 4 cm lower pole mass | 5.0 - lower pole | NR - renal pelvis | RN | Distal ureterectomy after 6 weeks | R | Clear cell | T1 | NR | Ta | NR | NR/ uneventful recovery |
| Demir(17) | 2004 | Turkey | M | 61 | NR | Flank pain, hematuria | US, CT abdomen and chest/ MRI | 57 x 50 mm irregular and infiltrative upper right kidney mass with necrotic components | 3.0 - upper | 7.0 – upper renal pelvis to adrenal gland | RN | Intraoperative frozen section biopsy showed high grade malignant tumor. Distal ureterectomy after 8 weeks following final pathology. | R | Clear cell | T1a | Grade 2 | T4 | High | NR/ uneventful recovery |
| Leveridge (18) | 2009 | Canada | F | 85 | NS | Abdominal pain, hematuria | US, CT-U | RCC with invasion into the collecting system | 5.5 - anterior upper pole | 2.5 - upper pelvis | LRNU | Ureterectomy after intraoperative frozen section biopsy revealed the UC | R | Clear cell | T3 | NR | T3 | High | NR/ uneventful in-hospital course, and well at her first post-op follow-up |
| Fang(19) | 2015 | Taiwan | M | 76 | NR | Flank pain | MRI | Mass lesion about 6.4 x 5.4 cm in lower pole of left kidney, in favor of RCC | 6.4 - posterior lower pole | NR - renal pelvis | Robot-assisted LRN | NR | L | Clear cell | pT3a | NR | T1 | NR | NR/ NR |
| Qun Lu(20) | 2017 | China | F | 76 | NR | Flank pain | US, CXR, CT | 1) a 7.5 cm solid mass on the posterior lower pole 2) a solid mass protruding into the upper collecting system suspicious for RCC with invasion into the collecting system or for UC of the renal pelvis | 7.5 - posterior lower | 4.0 - upper renal pelvis | LRNU | Ureterectomy after intraoperative frozen section biopsy revealed the UC | L | Clear cell | T2a | Grade 3 | T3 | High | 5/ bladder recurrence of UC, 15/ left adrenal metastasis of RCC |
| Chhajed (21) | 2021 | India | F | 75 | NS | Hematuria, abdominal pain | US, CT | 4 × 4 cm lower pole strongly enhancing mass, highly suspicious for RCC | 4 - lower pole | 0.8 - renal pelvis close to UPJ | LRN | Distal ureteric stump with bladder cuff excision after 2 weeks | R | Clear cell | T1 | Grade 1-2 | T1 | Low (Grade 1-2) | NR / post-op course uneventful |
| Current case | 2021 | Greece | M | 43 | CS | Flank pain, fatigue | Abdominal CT with contrast, Chest CT | 3.5 cm mass in the anterior aspect of left mid-pole with heterogenous enhancement, suspicious for RCC | 3.5 - anterior mid-pole | 0.4-0.6 - renal pelvis and parenchyma | LRN | Distal ureterectomy with bladder cuff excision after 3 weeks | L | Clear cell | T1 | Grade 3 | T1 | Low | NR/ post-op course uneventful |
1. Abbreviations: NR, Not reported; M, male; F, female; L, left; R, right; UPJ, ureteropelvic junction; CS, current smoker; FS, former smoker; NS, never smoked; UC, urothelial
2. carcinoma; TCC, transitional cell carcinoma; RCC, renal cell carcinoma; RN, radical nephrectomy, LRNU, laparoscopic radical nephroureterectomy; US, ultrasound; CT-U,
3. computed tomography urography; MRI, magnetic resonance imaging; IVP, intravenous pyelography
Two lessons can be drawn from the above. First, intraoperative frozen section biopsy may be of use during nephrectomy of masses with suspicion of invasion into the renal pelvis. Frozen section biopsy could spare the patients a second surgery allowing for ureterectomy at first surgery. Second, a partial nephrectomy for T1 RCC in 5/8 of cases might have missed the UC in the renal pelvis, thereby increasing future morbidity and mortality. Meticulous study of preoperative imaging is needed to identify involvement of the collecting system.
Conclusions
This case report highlights a synchronous occurrence of RCC and UC of the renal pelvis in a 43-year-old patient . The rarity of two simultaneous primary renal malignancies of dissimilar histology and the patient’s age merits reporting. Oftentimes, physicians rest assured of a diagnosis once detection of a renal parenchymal tumor on imaging is being made. Meticulous pathologic examination and clinical suspicion are necessary to uncover the co-existence of a second malignancy providing the optimal treatment, even when that requires a change in the initial therapeutic plan.
Consent for publication:
Written informed consent was obtained from the patient for publication of this case report and any accompanying images. A copy is available for review by the editor of this journal.
Conflict of Interest:
Each author declares that he or she has no commercial associations (e.g. consultancies, stock ownership, equity interest, patent/licensing arrangement etc.) that might pose a conflict of interest in connection with the submitted article
References
- Graves RC, Templeton ER. Combined Tumors of the Kidney. J Urol. 1921 Jun;5(6):517–38. [Google Scholar]
- Kidney Cancer - Introduction [Internet] Cancer.Net. 2012 [cited 2021 Apr 19] Available from: https://www.cancer.net/cancer-types/kidney-cancer/introduction . [Google Scholar]
- Voneschenbach AV, Johnson DE, Ayala AG. Simultaneous occurrence of renal adenocarcinoma and transitional cell carcinoma of the renal pelvis. J Urol. 1977 Jul;118(1 Pt 1):105–6. doi: 10.1016/s0022-5347(17)57907-0. [DOI] [PubMed] [Google Scholar]
- Hart AP, Brown R, Lechago J, Truong LD. Collision of transitional cell carcinoma and renal cell carcinoma. An immunohistochemical study and review of the literature. Cancer. 1994 Jan 1;73(1):154–9. doi: 10.1002/1097-0142(19940101)73:1<154::aid-cncr2820730126>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- Kline DW, Marshall M, Johnson SH, Reed G. Concurrent dissimilar malignancies of the urinary tract. J Urol. 1955 Jun;73(6):964–9. doi: 10.1016/S0022-5347(17)67507-4. [DOI] [PubMed] [Google Scholar]
- Anseline P, Howarth VS. A case of transitional cell carcinoma of the renal pelvis, clear cell renal carcinoma, and analgesic nephropathy. Aust N Z J Surg. 1977 Aug;47(4):521–3. doi: 10.1111/j.1445-2197.1977.tb04340.x. [DOI] [PubMed] [Google Scholar]
- Bengtsson U, Angervall L, Ekman H, Lehmann L. Transitional cell tumors of the renal pelvis in analgesic abusers. Scand J Urol Nephrol. 1968;2(3):145–50. doi: 10.3109/00365596809135358. [DOI] [PubMed] [Google Scholar]
- Park JY, Kwak EK, Park TI. Ipsilateral Synchronous Renal Cell Carcinoma and Transitional Cell Carcinoma: A Case Report. Korean J Pathol. 36(6):429–32. [Google Scholar]
- Ljungberg B, Albiges L, Abu-Ghanem Y, Bensalah K, Dabestani S, Fernández-Pello S, et al. European Association of Urology Guidelines on Renal Cell Carcinoma: The 2019 Update. Eur Urol. 2019 May;75(5):799–810. doi: 10.1016/j.eururo.2019.02.011. [DOI] [PubMed] [Google Scholar]
- Barone JG. Evaluation of Flank Pain. In: Lowry SF, Ciocca RG, Rettie CS, Vodarsik M, editors. Learning Surgery: The Surgery Clerkship Manual [Internet] New York, NY: Springer; 2005 [cited 2021 Jun 2]. pp. 670–92. Available from: https://doi.org/10.1007/0-387-28310-2_38 . [Google Scholar]
- DeWolf WC, Fraley EE. Renal pain. Urology. 1975 Oct;6(4):403–8. doi: 10.1016/0090-4295(75)90618-4. [DOI] [PubMed] [Google Scholar]
- Rouprêt M, Babjuk M, Burger M, Capoun O, Cohen D, Compérat EM, et al. European Association of Urology Guidelines on Upper Urinary Tract Urothelial Carcinoma: 2020 Update. Eur Urol. 2021 Jan;79(1):62–79. doi: 10.1016/j.eururo.2020.05.042. [DOI] [PubMed] [Google Scholar]
- Strong DW, Pearse HD, Tank ES, Hodges CV. The ureteral stump after nephroureterectomy. J Urol. 1976 Jun;115(6):654–5. doi: 10.1016/s0022-5347(17)59324-6. [DOI] [PubMed] [Google Scholar]
- Murphy DM, Zincke H, Furlow WL. Management of high grade transitional cell cancer of the upper urinary tract. J Urol. 1981 Jan;125(1):25–9. doi: 10.1016/s0022-5347(17)54881-8. [DOI] [PubMed] [Google Scholar]
- Michel K, Belldegrun A. Synchronous RCC and TCC of the Kidney in a Patient With Multiple Recurrent Bladder Tumors. Rev Urol. 1999;1(2):99–103. [PMC free article] [PubMed] [Google Scholar]
- Bernie JE, Albers L, Baird S, Parsons CL. Synchronous ipsilateral renal adenocarcinoma, transitional cell carcinoma of the renal pelvis and metastatic renal lymphoma. J Urol. 2000 Sep;164(3 Pt 1):773–4. doi: 10.1097/00005392-200009010-00037. [DOI] [PubMed] [Google Scholar]
- Demir A, Onol FF, Bozkurt S, Türkeri L. Synchronous ipsilateral conventional renal cell and transitional cell carcinoma. Int Urol Nephrol. 2004;36(4):499–502. doi: 10.1007/s11255-004-0855-8. [DOI] [PubMed] [Google Scholar]
- Leveridge M, Isotalo PA, Boag AH, Kawakami J. Synchronous ipsilateral renal cell carcinoma and urothelial carcinoma of the renal pelvis. Can Urol Assoc J. 2009 Feb;3(1):64–6. doi: 10.5489/cuaj.1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang C-W, Wang B-F, Chen J, Chen P-H, Chien Y-C, Kuo C-W. Synchronous ipsilateral renal cell carcinoma and urothelial carcinoma of kidney in a patient with prostate adenocarcinoma: A case report and review of literature. Urol Sci. 2015 Jun 1;26:S66. [Google Scholar]
- Lu Q, Zhuang J, Guo H. Renal cell carcinoma with synchronous ipsilateral urothelial carcinoma of the renal pelvis. Oncol Lett. 2017 Jun;13(6):4521–5. doi: 10.3892/ol.2017.5981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chhajed A, Baraniya J, Chhajed S, Choudhary A, Jain N. Pathologically diagnosed incidentaloma transition cell carcinoma (TCC) of renal pelvis in a laproscopic radical nephrectomy specimen done for a lower pole renal mass. Urol Case Rep. 2021 Jul 1;37:101607. doi: 10.1016/j.eucr.2021.101607. [DOI] [PMC free article] [PubMed] [Google Scholar]


