Abstract
Background and aim:
Three-dimensional (3D) printing is prevailing in surgical planning of complex cases. The aim of this study is to describe the use of 3D printed models during the surgical planning for the treatment of four pediatric hip deformity cases. Moreover, pediatric pelvic deformities analyzed by 3D printed models have been object of a concise review.
Methods:
All treated patients were females, with an average age of 5 years old. Patients’ dysplastic pelvises were 3D-printed in real scale using processed files from Computed Tomography (CT) or Magnetic Resonance Imaging (MRI). Data about 3D printing, surgery time, blood loss and fluoroscopy have been recorded.
Results:
The Zanoli-Pemberton or Ganz-Paley osteotomies were performed on the four 3D printed models, then the real surgery was performed in the operating room. Time and costs to produce 3D printed models were respectively on average 17:26 h and 34.66 €. The surgical duration took about 87.5 min while the blood loss average was 1.9 ml/dl. Fluoroscopy time was 21 sec. MRI model resulted inaccurate and more difficult to produce. 10 papers have been selected for the concise literature review.
Conclusions:
3D printed models have proved themselves useful in the reduction of surgery time, blood loss and ionizing radiation, as well as they have improved surgical outcomes. 3D printed model is a valid tool to deepen the complex anatomy and orientate surgical choices by allowing surgeons to carefully plan the surgery. (www.actabiomedica.it)
Keywords: 3D printing, surgical planning, Zanoli-Pemberton osteotomy, Ganz-Paley osteotomy, mock surgery
Introduction
Developmental dysplasia of the hip (DDH) is a pathology which ranges from instable hip to real dislocation. (1) In case of DDH that does not respond to conservative treatment or in case of late presentation, surgery is mandatory to restore the normal femoral head acetabulum relationship. (2) Pelvic osteotomies (PO) were introduced to improve the stability in open reduction and to increase the coverage of the femoral head in case of well reduced hips. The PO are usually divided in: re-directional orientation, volume-reducing and salvage. (2) The choice among the different techniques depends on the type of acetabulum dysplasia, expected outcomes and surgeon confidence and predilection. (2-3) The Zanoli-Pemberton osteotomy, for example, was described in 1965 and suggested for treatments of acetabular dysplasia, hip subluxation, or hip dislocation in children. The technique consists in hinging the acetabular roof on the triradiate cartilage after an incomplete iliac osteotomy. The aim of this technique is to modify the acetabular index and to improve the anterior and lateral coverage of the femoral head by caudally and anteriorly reorientating the acetabular fragment. (4) On the other hand, hip deformities that concerns femoral heads must be treated by “femoral head reduction osteotomy” or “osteochondroplasty with relative neck lengthening”.
A typical reduction technique is the Ganz modified by Paley osteotomy, which consists in cutting the femoral head non-perpendicularly to femoral neck, and it requires a wedge resection after safe surgical hip dislocation. (5)
In patients younger than 6 months, the hip ultrasonography is usually the way to evaluate the DDH while radiographs are used for the older ones. Computed Tomography (CT) and/or Magnetic Resonance Imaging (MRI) are helpful for pre-operative surgical planning and recently, Three-Dimensional (3D) printing has been adopted in surgical planning of complex cases. (6) Indeed, 3D printed model can be a valid tool in the clinical practice to better understand the complex anatomy and orientate the surgical choice (7-8), and for the communication with guardians and patients too. (9) Moreover, patient-specific 3D printed models revealed themselves useful in the operating room (10) reducing surgery time. (11-13) Thanks to progress in manufacturing technologies, software development and the related cost reduction, it is easier to produce personalized and realistic 3D printing models from CT scan. 3D printed models can help in simulating the surgical procedures (10) and might be manipulated by using surgical instruments. (1)
The aim of this study is to describe the surgical planning and technique in the treatment of dysplastic hip associated with DDH, using 3D printing model and reporting four illustrative cases. Moreover, pediatric pelvic deformities studied thanks to 3D printed models have been object of a concise review.
Materials and Method
Four consecutive cases of developmental dysplasia of the hip that underwent preoperative planning with a 3D printed model before Zanoli-Pemberton osteotomy surgery from November 2018 to November 2019 were retrospectively reviewed. All patients or their guardians gave their informed consent at the ENROLMENT and were included into the study. Declaration of Helsinki and Guidelines for Good Clinical Practice were applied. All four patients treated were females, with an average age of 5 years old (from 1 to 9 years old). Surgery duration, hemoglobin loss and fluoroscopy time were the evaluated parameters throughout our study. A survey about 3D models’ utility and accuracy has been proposed to 5 main surgeons and 5 residents. They were asked to split a score from 1 to 5 for each of the different subjective fields (1= “low”; 5= “excellent”). (tab. 1)
Table 1.
Questionnaire survey on children pelvis 3D models.
| Question | Subjective field | Score | Extreme values |
|---|---|---|---|
| 1 | Utility of 3D printed model in preoperative planning | 4.6 | [3-5] |
| 2 | Utility of 3D printed model in educational | 4.9 | [4-5] |
| 3 | Accuracy of TC 3D printed model | 5.0 | [5] |
| 4 | Accuracy of MRI 3D printed model | 3.5 | [2-4] |
| 5 | Maneuverability of 3D printed model | 3.2 | [2-4] |
Pre-operative planning
As usual, patients underwent second level imaging (CT or MRI) almost two weeks before surgery: three patients underwent preoperative CT, one underwent MRI. DICOM image stacks were imported, segmented, and processed into a 3D printable format by using a commercial software. Dysplastic hip models have been subsequently 3D printed. Mock surgeries were performed by a resident and a trained pediatric orthopedic senior surgeon a couple of days before the real operation on each specific 3D model per patient.
3D model production: CT 3D model
The patient underwent preoperative CT-scan of the pelvis (GE CT Lightspeed 16 rows; 120 kV, 200mA, rotation 1 s, 0.625 slice thickness, pitch 0.525, no iterative reconstruction), followed by 3D reconstruction and 3D printing of the dysplastic hip (fig.1a). A dedicated high-resolution reconstruction was implemented in the CT images sets (Detail Kernel filter). Digital Imaging and COmmunications in Medicine (DICOM) image stacks were processed using the commercial software prototype ”3D Printing” from Syngo.via Frontier (Siemens Healthineers, GE). CT images were segmented and processed into a 3D printable format “standard tessellation language” (.stl) (fig.1b). Bone tissues of the dysplastic hip were segmented semi-automatically by an expert and trained operator by using a threshold with a range from 200 HU to the maximum value. The segmentation was firstly manually inspected and cleaned from defects by “brush” function, and automatically by using “keep the largest” function. All the no-bone tissues were excluded, and bridges between isolated bone structures were created in order to obtain a one-piece model. Smoothing function has been applied at 2.0.
Figure 1.
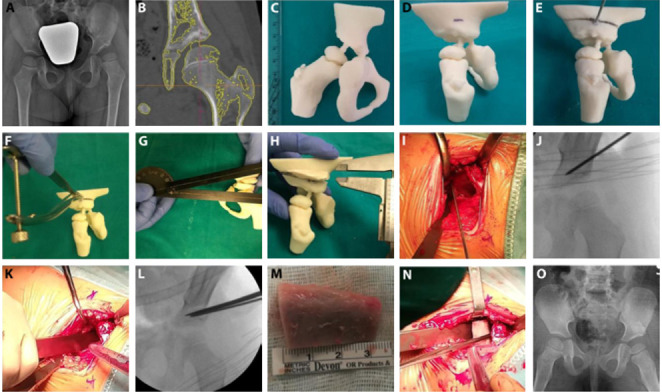
female patient with non-breech birth, no familiarity with DDH. No ultrasound screening for DHH had been performed. She did not respond to orthosis and cast treatment. Then a left hip close reduction surgery was performed with adductors muscle tenotomy. When she was 2 years old Pemberton-Zanoli osteotomy was proposed. a. preoperative X-ray. b. 3D model production from CT DICOM files on Syngo.via Frontier software. Bridges between isolated bone structures were created in order to obtain a one-piece model. c. left hip 3D printed model in real scale in ABS plus. d. mock surgery: landmark of the iliac cut, 1.5 cm above the superior hip joint line. e. mock surgery: using the Kirshner wire as a guide, a little osteotomy was realized to cut medially and anteriorly the iliac bone, following the direction to the sciatic notch. f. mock surgery: a spreader was used in order to enlarge the space created by the osteotomy and to push the distal fragment down. g., h. mock surgery: a protractor was used to define the dimension of the space created according to the bone graft’s size. A trapeze bone graft was prepared by wax and then inserted in the osteotomy space. i., j. real surgery: landmark of the iliac cut, 1.5 cm above the superior hip joint line. k., l. real surgery: iliac bone osteotomy. m., n. real surgery: bone graft and his positioning. o. final outcome, spica cast has been positioned.
MRI 3D model
T2 sequence has been chosen. The patient underwent preoperative MR-study of the pelvis (Philips, Achieva 1.5 T, 2D axial acquisition SPAIR T2 weighted, slice thickness 5 mm), followed by 3D reconstruction and 3D printing of the dysplastic hip. Images have been elaborated by the commercial software prototype “3D Printing” from Syngo.via Frontier (Siemens Healthineers, GE).
MRI images were segmented and processed into a 3D printable format (.stl) (fig.2a). Bone and cartilaginous tissues of the pelvis were segmented manually with the “brush” function by an expert and trained operator. Automated smoothing at 2.0 has been applied. All other tissues were excluded. Bridges between bony structures were created. Pelvis model were eventually exported in .stl printable format (fig.2b).
Figure 2.
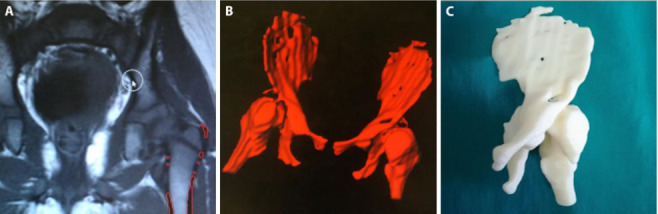
MRI 3D model: a. 3D model production from MRI DICOM files on Syngo.via Frontier software; b. Virtual MRI 3D model; c. MRI 3D printed model
3D model printing
Patients’ dysplastic hips were 3D-printed in Acrylonitrile Butadiene Styrene (ABS) plus in real scale (fig.1c and 2c). Printing was performed by using a fused deposition modeling 3D printer (Fortus 250 mc Stratasys). It was used SCA-1200HT to dissolve soluble support from 3D models.
Concise literature review
Pediatric pelvic deformities studied thanks to 3D printed models have been object of a concise review. The Pubmed database has been mined in order to find studies published in English from the 1st of January 2015 to the 31st of March 2020. The Mesh (Medical Subject Headings) terms chosen were: "Printing, Three-Dimensional" AND "Pelvic Bones” AND "Child”; "Femur" AND "Child" AND "Printing, Three-Dimensional". Research articles, reviews and cases reports have been selected relying on their titles and abstracts. Papers’ bibliography has also been analyzed to search for additional articles. We excluded the researches involving 16-year-old patients or older, as well as studies about 3D CT and Virtual Reality. We also excluded studies about lower limbs and other districts deformities except for pelvis. We looked for several parameters such as 3D model use, Software used, 3D printing information, surgery duration, blood loss, fluoroscopy time and accuracy, usefulness, and surgical outcomes evaluation.
Results
Zanoli-Pemberton osteotomy: surgical planning - mock surgery
In the three cases of dysplastic hip, the correct dimensions of bone graft have been evaluated in order to obtain a physiological acetabular index. The Zanoli-Pemberton osteotomy was performed on three 3D printed models, one for each patient. Specifically, as it is shown in the pictures 1d-1h, the Zanoli-Pemberton osteotomy started with a Kirshner wire as landmark in the exactly position of the iliac cut, 1.5 cm above the superior hip joint line (fig.1d). Using the Kirshner wire as a guide, a little osteotomy was realized to cut medially and anteriorly the iliac bone, following the direction to the sciatic notch (fig.1e). The osteotome did not go beyond the ilium medial cortical bone. A spreader was used to enlarge the space created by the osteotomy and to push the distal fragment down (fig.1f). The final objective was to restore a good acetabular index: a protractor was used to define the dimension of the space created according to the bone graft’s size (fig.1g-h). A trapeze bone graft was prepared by wax and then inserted in the osteotomy space. No Kirshner wires were used to fix the bone graft. Then the real surgery was performed in the operating room (fig.1i-o).
Ganz-Paley osteotomy: surgical planning - mock surgery
The correct dimensions and orientation of femoral head portion to remove have been evaluated in order to obtain a physiological epiphysis covered by acetabular roof in one case of dysplastic hip sequela. The Ganz-Paley osteotomy was performed on a 3D printed model. Specifically, as shown in the picture 3a, femoral head portion to remove has been assessed and drawn on the 3D printed model. The osteotomy was performed following the drawn line, and the femoral portion was removed (fig.3b). The two remaining parts were thus assembled (fig.3c). Then the real surgery was performed in the operating room (fig.3d-f).
Figure 3.
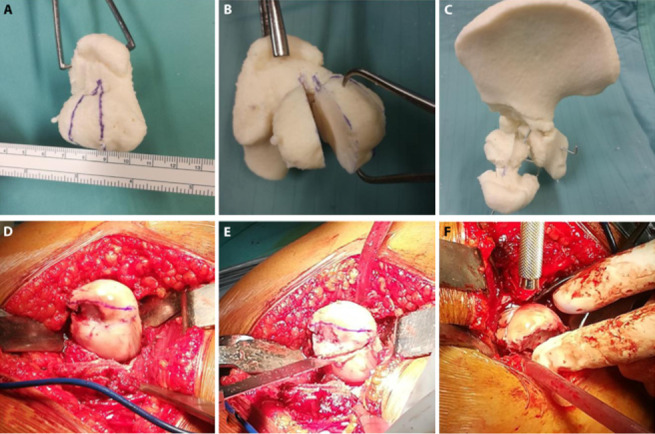
Ganz-Paley osteotomy: mock and real surgeries. a., d.: femoral head portion to remove has been assessed and drawn on the 3D printed model and during the real surgery. b., e.: the osteotomy was performed following the drawn line, and the femoral portion was removed. c., f.: the two remaining parts were put closer and assembled.
Parameters evaluated
Less than one hour was spent to produce each CT model .stl file. The production of MRI model .stl file required about two hours. Time and costs to produce 3D printed models were respectively on average 17:26 h and 34.66 €. The surgical duration took about 87.5 min (from 80 to 100 min) while the blood loss average was 1.9 ml/dl (from 1.0 to 2.4 mg/dl). Fluoroscopy time was 21 sec (from 30 to 10 sec). Data of our study have been inserted in the table 2.
Table 2.
Parameters evaluated in this study. For 0-3 years-old patients, months have been specified
| Patient | Age (years) | Duration (h) software | Duration (h) printing | Model material (cm³) | Support material (cm³) | Costs (€) | Surgery duration (min) | Hb loss (mg/dl) | Fluoroscopy (sec) |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 (35 months) | 0:45 | 8:01 | 31.69 | 25.50 | 19.10 | 85 | 2.4 | 30 |
| 2 | 8 | 1:00 | 22:38 | 78.66 | 60.52 | 46.47 | 85 | 1.0 | 10 |
| 3 | 1 (22 months) | 2:15 | 10:31 | 40.67 | 39.13 | 26.65 | 100 | 2.2 | 27 |
| 4 | 9 | 0:50 | 28:36 | 98.77 | 65.45 | 46.41 | 80 | 2.0 | 17 |
| Total | / | 4:50 | 69:46 | 249.79 | 190.60 | 138.63 | 350 | 7.6 | 84 |
| Media | 5 | 1:12 | 17:26 | 62.45 | 47.65 | 34.66 | 87.5 | 1.9 | 21 |
According to questionnaire score results, the utility of 3D models for preoperative planning and for educational was respectively 4.6 and 4.9 points. Accuracy of 3D printed model was quantified as 5 points for CT and 3.5 points for MRI. The assigned score for the maneuverability of 3D printed model has been 3.2 points (tab. 1).
Concise literature review
Concerning the concise literature review, 10 papers have been selected relying on their titles and abstracts and looking for paper’ bibliography. Concise review results are shown in the table 3.
Table 3.
Concise review of literature. LCP-PHP: Locking Compression Pediatric Hip Plate; VDFO: Varus de-rotation femoral osteotomy PAO: periacetabular osteotomy
| Paper | Patients included | Imaging for 3D model | 3D model use | Software to produce and to act on 3D model | Model material | Costs (€) | Surgery duration | Blood loss | Fluoroscopy | Survey (useful and accurate) | Surgical outcomes evaluation |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Zheng P. (2016) (27) | 9 6M-3F |
CT | 3D navigation template for cannulated screws and LCP-PHP position in femoral neck fracture treatment. | Mimics 17.0 Magic 17.0 |
PLA | / | LCP-PHP 24.5 ± 1.91 Screws 13.6±1.14 |
/ | LCP-PHP 5.5 ± 1,29 times Screws 4.2±3.53 |
/ | Ratliff criteria |
| Zheng P. (2017) (11) | 11 7M-4F |
CT | individualized navigation template of LCP-PHP in DDH or femoral neck fracture. | Mimics 17.0 | PLA | / | 26.50 min ± 4.07 | / | 6.00 times ± 0.73 | / | / |
| Zheng P. (2017) (21) | 12 2M-10F |
CT | 3D-printed navigation template in VDFO with LCP-PHP for DDH. | Mimics 17.0, Geomagic Design Direct software | PLA | / | 21.08 min ± 4.64 min |
/ | 3.92 times ± 0.90 times | / | McKay clinical classification system, Severin radiographic scale |
| Holt AM (2017) (18) | 1F | CT | 3D model for communication and PAO and VDFO surgical planning in late DDH. Fluoroscopic comparison between model and patient. | Slicer 4.1.1. | ABS | / | / | / | / | / | Clinical examination and radiological parameters |
| Cherkasskiy L (2017) (13) | 5 | CT | 3D printing in triplane proximal femoral osteotomy surgical planning for slipped capital femoral epiphysis | Mimics 17.0 MeshLab 1.3.3 NetFabb Basic 5.2.1 MeshMixer 10.9.297 |
ABS | 10$ | 125.8 min ± 25.4 min | 979.8 ml ± 316.2 ml | 0.3 min ± 0.3 min | / | Clinical examination and radiological parameters |
| Cai Z (2018) (15) | 186 46M-140F | CT | Evaluation of accuracy of acetabular anteversion CT measure in DDH. 3D printed model as gold standard. | Mimics 10.01 | PLA | / | / | / | / | / | / |
| Wei YP (2019) (10) | 1M | CT | 3D model for surgical planning in post-osteomyelitis deformity: Pemberton osteotomy and VDFO | / | / | 582.37 USD | / | / | / | / | Clinical examination and radiological parameters |
| Caffrey JP (2019) (1) | 14 13F-1M |
CT | 3D printed model for comparison of 3 pelvic osteotomies (Pemberton, Dega, San Diego) in DDH | Mimics 19.0 Simplify 3D |
ABS for bone, NinjaFlex for cartilage | / | / | / | / | / | Post mock-surgery CT and radiographic evaluation |
| Hedelin H (2019) (12) | 1M | CT | 3D printed model for pelvic triple osteotomy surgical planning in Legg-Calvé-Perthes disease. 3D model accuracy evaluation | Volume Viewer 12.3 ext. 8 Meshmixer |
ABS | 150€ | / | / | / | / | Clinical examination and radiological parameters |
| Kalenderer Ö (2019) (14) | 2 | CT | 3D model in femoral head reduction osteotomy surgical planning in Legg Calve Perthes and DDH | Mimics 17.0 | / | / | 150 min and 200 min | 230 cc and 300 cc | / | / | Clinical examination and radiological parameters |
Discussion
In the past, orthopedic surgeons only used pelvis radiographs for DDH preoperative planning. The most used angles and parameters to assess acetabular dysplasia include acetabular index, acetabular index of sharp, acetabular depth, sourcil and teardrop. (6) Complex surgical procedures may not fit with a X-ray planning: drawing, cutting and pasting the bone fragments on X-ray could become inaccurate and insufficient. (14) CT scan could help to overcome this challenge. CT scans permit a better understanding of the deformity in three planes (coronal, sagittal and axial) and quantifies the area or volume of the femoral head covered by the acetabulum. CT scans may help in preoperative planning to identify the acetabulum osteotomy location and suitable techniques. (6,14,15) A significant boost for surgical planning procedures is constituted by 3D Printing. It consists in the production of a physical object obtained by a virtual model and composed by a fusion or deposition of different materials. (16) Starting with Charles Hull in 1986, the 3D Printing idea was implemented in clinical practice for custom prosthetics and dental implants at the beginning of 2000s. (16) In 2000 Curodeau in his article presented the new 3D printing fabrication method to produce orthopedic prostheses. Unfortunately, this new procedure would still have needed several improvements. (17) Nowadays 3D Printing applications have raised, thanks to costs reduction and to technologies simplification, even if improvement and development are still needed. (7) As a matter of fact, 3D printing still entails high costs, (18) which mainly depend on the manufacturing facility. It takes a long time to learn the producing procedure of 3D models. High quality standard requires quality approvals and controls, which might be granted by affordable 3D models produced by cheap desktop 3D-printers. (19) On the other hand, 3D printing gives a lot of new opportunities to orthopedics surgery procedures (e.g. 3D anatomic model for surgical planning, education and communication with patients, surgical guides, custom made prosthetics, cell-printing). (16) About surgical planning, for example, Caffrey in his study demonstrated that specific 3D-printed models can be an effective tool to quantify volume changes of each pelvic osteotomy. Therefore, a 3D-printed model permits a personalized surgical planning and helps in choosing the best osteotomy to cover the regional deficiency. (1) 3D printed models have also proved themselves useful in the reduction of surgery time, (10,13) blood loss and ionizing radiation (13), as well as they have improved surgical outcomes. (14,18,19) As regards surgery time and blood loss reduction, studies by Chen analyzed the utility of a 3D printed model in adults distal radial fracture. (20) He also reported that 3D model group surgical time was shorter of 9 minutes than routine treatment group (66.5 ± 5.3 vs. 75.4 ± 6.0 minutes P<0.001). Concerning blood loss volume, he evidenced an improvement of 10 mL in 3D model group (41.1 ± 7.5 vs. 54.2 ± 7.9 mL, P < 0.05). He also highlighted a reduction in frequency of fluoroscopy in 3D model group (5.6 ± 1.6 vs. 4.4 ± 1.4 times, P = 0.011). (20)
In addition of Chen’s work, Zheng in his controlled study analyzed operation time and X-rays exposure parameters on 3D printed navigation templates (to guide internal fixation) for surgical planning in DDH. Better results were achieved in template-guided patient group with a reduction of surgical time (21.08 min vs. 46.92 min) and X-ray exposure (3.92 vs. 6.69). It was found a correlation between 3D printed navigation templates and improvement in surgical outcomes, even if there was no significant difference. (21) As reported by Tack, the reduction of fluoroscopy and surgical time constitutes an additional, desirable economical outcome, considering the difficulties involving healthcare funds and resources. (19)
In our experience, 3D Printing allowed surgeons to carefully plan and simulate the surgery. It ensured a better sizing of the bone graft for its positioning, as well as the correct dimensions and orientation of femoral head portion which had to be removed. Furthermore, it did not require additional studies or ionizing radiations since the Authors routinely require a CT scan in the pre-operative evaluation. The surgical duration took about 87.5 min (from 80 to 100 min) while the blood loss average was 1.9 ml/dl (from 1.0 to 2.4 mg/dl). Fluoroscopy time was 21 sec (from 30 to 10 sec). Patients treated using 3D printed model for surgical planning did not show any complications at follow-up (fig. 4).
Figure 4.
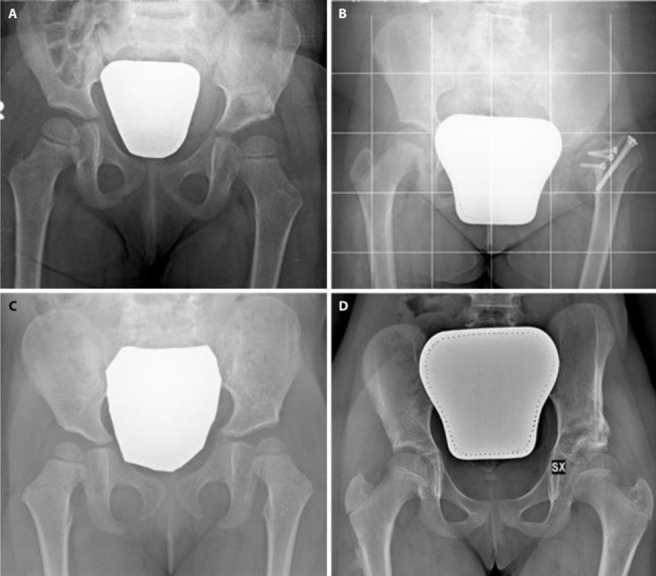
Pelvic X-rays: a. patient 1 six months follow-up; b. patient 2 six months follow-up; c. patient 3 six months follow-up; d. patient 4 one-year follow-up
We cannot declare if the 3D printing model improved these parameters and a possible enhance of this study could consist in creating a control group to reach a better data analysis.
Answering the survey questionnaire, main surgeons claimed that the 3D printed model was useful and accurate for the pre-operative planning of pediatric hip deformities surgical correction, because it permitted a clearer understanding of the hip morphology and a good training on it. Residents also said that 3D printed model was useful for the same reasons, and specifically because it allowed to figure out all procedural steps by training on them. Main surgeons and residents pointed out a limit of the type of thermoplastic used to build the model, which did not reproduce the elastic response of triradiate cartilage and bones.
3D Printed models can be obtained both from CT and MRI images. The outcomes difference between MRI and CT application has often been under the spotlight of the scientific debate. MRI is normally used to visualize soft tissue structures (22) avoiding any radiation for patients. (23,24) Additional 3D osseous reconstructions from MRI data thus constitute an advantage both for surgeons and patients. (24) Along with these advantages, MRI bears also some limits and problems, as reported in Arezoomand’s works, namely manual analysis and inhomogeneity of tissues and boundaries. The former could be excessively time-consuming, (23,25) while the latter affects several types of artifacts, because of the adjacency of bones with soft tissues such as cartilage and fat, that appear with similar intensity and texture. (23)
Despite these flaws, a clear MRI advantage is the fact that patients will not be exposed to radiations, which constitutes a part and parcel of CT examination. (24) An optimization of the workflow and the use of new CT scan technologies may obtain a high-quality image (high resolution on all the planes and low noise) at an acceptable dose. In his work on hip dysplasia, Wells has recently shown that pelvic CT radiation expose the patient to a low dose of radiation, especially if compared to 3-5 pelvic radiographs (0.75-1.25 mSv). (26)
This study presents the following restrictions: (1) the study retrospectivity and non-randomness; (2) the tiny number of involved patients; (3) the CT blindness in determining triradiate cartilage; (4) the MRI scarce accuracy for model design; (5) the low malleability of plastic materials involved. Further randomized prospective studies with higher number of patients enrolled would be useful to better analysis. Moreover, MRI for cartilage tissue and CT for bone tissue used together can improve 3D model production technique. In addition, mock surgery on 3D printed model could be strongly enhanced by using more malleable materials.
In conclusion, for preoperative planning 3D printed models can be obtained by both MRI and CT scans, thus allowing a better understanding of the individually deformities (14,19) which will be extremely useful. Luckily, a fully experienced surgeon can easily operate without appealing to mock surgery and 3D Printing. Despite of that, these tools are suitable both for young and less-experienced surgeons learning processes, and for future Artificial Intellingence improvements to develope more and more accurate surgical techniques. This study is the first of its kind which employs a MRI 3D printed model for surgical planning in Zanoli-Pemberton osteotomy surgical planning with children. Moreover, it is the first study where a questionnaire survey on children pelvis 3D models has been proposed.
Conflict of Interest:
Each author declares that he or she has no commercial associations (e.g. consultancies, stock ownership, equity interest, patent/licensing arrangement etc.) that might pose a conflict of interest in connection with the submitted article.
References
- Caffrey JP, Jeffords ME, Farnsworth CL, Bomar JD, Upasani VV. Comparison of 3 Pediatric Pelvic Osteotomies for Acetabular Dysplasia Using Patient-specific 3D-printed Models. J Pediatr Orthop. 2019;39(3):e159–e164. doi: 10.1097/BPO.0000000000001271. [DOI] [PubMed] [Google Scholar]
- Murphy RF, Kim YJ. Surgical Management of Pediatric Developmental Dysplasia of the Hip. J Am Acad Orthop Surg. 2016;24(9):615–624. doi: 10.5435/JAAOS-D-15-00154. [DOI] [PubMed] [Google Scholar]
- Coppa V, Marinelli M, Specchia N. Unilateral uniplanar modular external fixator for percutaneous proximal femoral osteotomy in children: surgical technique. Eur J Orthop Surg Traumatol. 2019;29(1):205–211. doi: 10.1007/s00590-018-2295-7. [DOI] [PubMed] [Google Scholar]
- Baki ME, Baki C, Aydin H, Ari B, Özcan M. Single-stage medial open reduction and Pemberton acetabuloplasty in developmental dysplasia of the hip. J Pediatr Orthop B. 2016;25(6):504–508. doi: 10.1097/BPB.0000000000000360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paley D, Feldman DS. Femoral Head Reduction Osteotomy. In: Saran N, Hamdy R.C, editors. Pediatric Pelvic and Proximal Femoral Osteotomies: a case-based approach. Springer; 2018. pp. 379–420. [Google Scholar]
- Hamdy RC, Epstein DS. Preoperative Planning for Pelvic and/or Proximal Femoral Osteotomies. In: Saran N, Hamdy R.C, editors. Pediatric Pelvic and Proximal Femoral Osteotomies: A Case-Based Approach. Springer; 2018. pp. 1–11. [Google Scholar]
- Iobst CA. New Technologies in Pediatric Deformity Correction. Orthop Clin North Am. 2019;50(1):77–85. doi: 10.1016/j.ocl.2018.08.003. [DOI] [PubMed] [Google Scholar]
- Liu X, Dong K, Zheng S, et al. Separation of pygopagus, omphalopagus, and ischiopagus with the aid of three-dimensional models. J Pediatr Surg. 2018;53(4):682–687. doi: 10.1016/j.jpedsurg.2017.06.016. [DOI] [PubMed] [Google Scholar]
- Cho J, Park CS, Kim YJ, Kim KG. Clinical Application of Solid Model Based on Trabecular Tibia Bone CT Images Created by 3D Printer. Healthc Inform Res. 2015;21(3):201–5. doi: 10.4258/hir.2015.21.3.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei YP, Lai YC, Chang WN. Anatomic three-dimensional model-assisted surgical planning for treatment of pediatric hip dislocation due to osteomyelitis. J Int Med Res. 2019:300060519854288. doi: 10.1177/0300060519854288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng P, Yao Q, Xu P, Wang L. Application of computer-aided design and 3D-printed navigation template in Locking Compression Pediatric Hip PlateTM placement for pediatric hip disease. Int J Comput Assist Radiol Surg. 2017;12(5):865–871. doi: 10.1007/s11548-017-1535-3. [DOI] [PubMed] [Google Scholar]
- Hedelin H, Swinkels CS, Laine T, Mack K, Lagerstrand K. Using a 3D Printed Model as a Preoperative Tool for Pelvic Triple Osteotomy in Children: Proof of Concept and Evaluation of Geometric Accuracy. J Am Acad Orthop Surg Glob Res Rev. 2019;3(3):e074. doi: 10.5435/JAAOSGlobal-D-18-00074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherkasskiy L, Caffrey JP, Szewczyk AF, et al. Patient-specific 3D models aid planning for triplane proximal femoral osteotomy in slipped capital femoral epiphysis. J Child Orthop. 2017;11(2):147–153. doi: 10.1302/1863-2548-11-170277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalenderer Ö, Erkuş S, Turgut A, İnan İH. Preoperative planning of femoral head reduction osteotomy using 3D printing model: A report of two cases. Acta Orthop Traumatol Turc. 2019;53(3):226–229. doi: 10.1016/j.aott.2019.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai Z, Zhao Q, Li L, Zhang L, Ji S. Can. Computed Tomography Accurately Measure Acetabular Anterversion in Developmental Dysplasia of the Hip? Verification and Characterization Using 3D Printing Technology. J Pediatr Orthop. 2018;38(4):e180–e185. doi: 10.1097/BPO.0000000000001141. [DOI] [PubMed] [Google Scholar]
- Ventola C L. Medical Applications for 3D Printing: Current and Projected Uses. P.T. 2014;39(10):704–711. [PMC free article] [PubMed] [Google Scholar]
- Curodeau A, Sachs E, Caldarise S. Design and fabrication of cast orthopedic implants with freeform surface textures from 3-D printed ceramic shell. J Biomed Mater Res. 2000;53(5):525–535. doi: 10.1002/1097-4636(200009)53:5<525::aid-jbm12>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Holt AM, Starosolski Z, Kan JH, Rosenfeld SB. Rapid Prototyping 3D Model in Treatment of Pediatric Hip Dysplasia: A Case Report. Iowa Orthop J. 2017;37:157–162. [PMC free article] [PubMed] [Google Scholar]
- Tack P, Victor J, Gemmel P, Annemans L. 3D printing techniques in a medical setting: a systematic literature review. Biomed Eng Online. 2016;15:115. doi: 10.1186/s12938-016-0236-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C, Cai L, Zheng W, Wang J, Guo X, Chen H. The efficacy of using 3D printing models in the treatment of fractures: a randomised clinical trial. BMC Musculoskelet Disord. 2019;20(1):65. doi: 10.1186/s12891-019-2448-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng P, Xu P, Yao Q, Tang K, Lou Y. 3D-printed navigation template in proximal femoral osteotomy for older children with developmental dysplasia of the hip. Sci Rep. 2017;7:44993. doi: 10.1038/srep44993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukagoshi Y, Kamada H, Takeuchi R, et al. Three-dimensional MRI analyses of prereduced femoral head sphericity in patients with developmental dysplasia of the hip after Pavlik harness failure. J Pediatr Orthop B. 2018;27(5):394–398. doi: 10.1097/BPB.0000000000000494. [DOI] [PubMed] [Google Scholar]
- Arezoomand S, Lee WS, Rakhra KS, Beaulé PE. A 3D active model framework for segmentation of proximal femur in MR images. Int J Comput Assist Radiol Surg. 2015;10(1):55–66. doi: 10.1007/s11548-014-1125-6. [DOI] [PubMed] [Google Scholar]
- Wadhwa V, Malhotra V, Xi Y, Nordeck S, Coyner K, Chhabra A. Bone and joint modeling from 3D knee MRI: feasibility and comparison with radiographs and 2D MRI. Clin Imaging. 2016;40(4):765–768. doi: 10.1016/j.clinimag.2016.02.017. [DOI] [PubMed] [Google Scholar]
- Brochard S, Mozingo JD, Alter KE, Sheehan FT. Three dimensionality of gleno-humeral deformities in obstetrical brachial plexus palsy. J Orthop Res. 2016;34(4):675–682. doi: 10.1002/jor.23049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells J, Nepple JJ, Crook K, et al. Femoral Morphology in the Dysplastic Hip: Three-dimensional Characterizations With CT. Clin Orthop Relat Res. 2017;475(4):1045–1054. doi: 10.1007/s11999-016-5119-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng P, Yao Q, Xu P, Tang K, Chen J, Li Y, et al. Application of three dimensional printed navigation template in pediatric femoral neck fracture. Digit Med. 2016;2:113–9. [Google Scholar]


