To the Editor: On November 26, 2021, the World Health Organization (WHO) named the B.1.1.529 (omicron) variant of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), first detected in South Africa, as a variant of concern.1 By November 29, 2021, three days after the announcement by the WHO, cases of infection with the omicron variant had already been detected in many other countries.
Whether the BNT162b2 vaccine (Pfizer–BioNTech), which was previously shown to have 95% efficacy against coronavirus disease 2019 (Covid-19),2,3 will effectively neutralize infection with the omicron variant is unclear. We compared neutralization of omicron-infected cells in serum samples obtained from participants who had received two doses of vaccine with neutralization in samples obtained from participants who had received three doses.
Microneutralization assays with wild-type virus and B.1.351 (beta), B.1.617.2 (delta), and omicron variant isolates were performed with the use of serum samples obtained from two groups of 20 health care workers. One group comprised participants who had received two doses of the BNT162b2 vaccine (mean, 165.6 days since receipt of the second dose), and the second group comprised those who had received three vaccine doses (mean, 25 days since receipt of the third dose) (Table S1 in the Supplementary Appendix, available with the full text of this letter at NEJM.org). Significance was assessed with the use of a Wilcoxon matched-pairs signed-rank test.
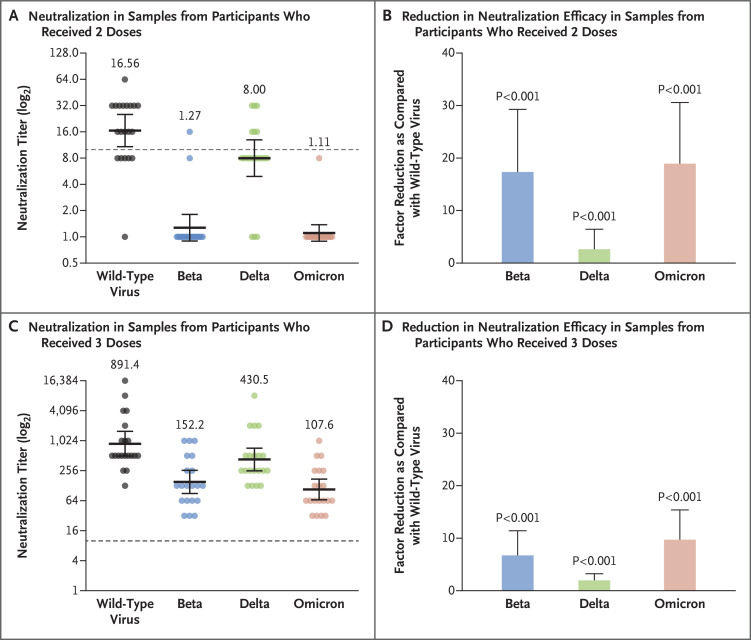
Receipt of three vaccine doses led to better neutralization of the wild-type virus and the three variants than receipt of two vaccine doses (Figure 1). The geometric mean titers of the wild-type virus and the beta, delta, and omicron variants were 16.56, 1.27, 8.00, and 1.11, respectively, after receipt of the second vaccine dose and 891.4, 152.2, 430.5, and 107.6, respectively, after receipt of the third dose. A significantly lower neutralization efficiency of the BNT162b2 vaccine against all the tested variants of concern (beta, delta, and omicron) than against the wild-type virus was observed in samples obtained from participants who had received two doses than in those obtained from participants who had received three doses (Figure 1B and 1D). The lower neutralization efficiency against the beta and omicron variants than against the wild-type virus was similar in samples obtained from participants who had received two doses and in those obtained from participants who had received three doses. The third dose of the BNT162b2 vaccine efficiently neutralized infection with the omicron variant (geometric mean titer, 1.11 after the second dose vs. 107.6 after the third dose) (Figure 1A and 1C).
Figure 1. Neutralization Efficiency against Wild-Type Virus and the Beta, Delta, and Omicron Variants of Concern.
Serum samples were obtained from 20 health care workers who had received two doses of the BNT162b2 vaccine (Panels A and B) and from 20 who had received three doses (Panels C and D). Samples were tested by microneutralization against wild-type severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and the B.1.351 (beta), B.1.617.2 (delta), and B.1.1.529 (omicron) variants of concern. Dashed lines in Panels A and C indicate the cutoff titer. Geometric mean titers (horizontal lines) with 95% confidence intervals (𝙸 bars) are presented, as well as the geometric mean titer value. Dots indicate individual serum samples. The factor reduction as compared with wild-type virus is shown for samples obtained from participants who had received two doses of vaccine (Panel B) and those obtained from participants who had received three doses (Panel D). For these analyses, the mean factor differences between wild-type SARS-CoV-2 and the variants of concern were calculated for each participant; the means of the individual values are shown here. Error bars in Panels B and D indicate the standard error.
We analyzed the neutralization efficiency of the BNT162b2 vaccine against wild-type SARS-CoV-2 and the beta, delta, and omicron variants of concern. Limitations of the study include the small cohort tested and the fact that the test was only an in vitro assay. Nevertheless, we found low neutralization efficiency with two doses of the BNT162b2 vaccine against the wild-type virus and the delta variant, assessed more than 5 months after receipt of the second dose, and no neutralization efficiency against the omicron variant. The importance of a third vaccine dose is clear, owing to the higher neutralization efficiency (by a factor of 100) against the omicron variant after the third dose than after the second dose; however, even with three vaccine doses, neutralization against the omicron variant was lower (by a factor of 4) than that against the delta variant. The durability of the effect of the third dose of vaccine against Covid-19 is yet to be determined.
Supplementary Appendix
Disclosure Forms
This letter was published on December 29, 2021, at NEJM.org.
Footnotes
Disclosure forms provided by the authors are available with the full text of this letter at NEJM.org.
References
- 1.World Health Organization. Classification of omicron (B.1.1.529): SARS-CoV-2 variant of concern. November 26, 2021. (https://www.who.int/news/item/26-11-2021-classification-of-omicron-(b.1.1.529)-sars-cov-2-variant-of-concern).
- 2.Bar-On YM, Goldberg Y, Mandel M, et al. Protection of BNT162b2 vaccine booster against Covid-19 in Israel. N Engl J Med 2021;385:1393-1400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Haas EJ, McLaughlin JM, Khan F, et al. Infections, hospitalisations, and deaths averted via a nationwide vaccination campaign using the Pfizer-BioNTech BNT162b2 mRNA COVID-19 vaccine in Israel: a retrospective surveillance study. Lancet Infect Dis 2021. September 22 (Epub ahead of print). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.