To the Editor: Using an active nationwide surveillance system administered by the Israeli Ministry of Health, we previously found a higher incidence of myocarditis among persons 16 years of age or older who had received the BNT162b2 vaccine (Pfizer–BioNTech) than among historical controls and unvaccinated persons; the incidence was highest among young male recipients.1 The Food and Drug Administration recently granted emergency use authorization for the two-dose regimen of the BNT162b2 vaccine in adolescents 12 to 15 years of age. Here, we report the incidence of hospitalization for myocarditis between June 2 and October 20, 2021, among adolescents in this age group within 21 days after receipt of the first vaccine dose and within 30 days after receipt of the second dose.
Clinical data that involved International Classification of Diseases, 10th Revision, 422.0-9x and 429.0x codes were reviewed by a cardiologist and a rheumatologist, and the severity of disease was classified according to the Brighton Collaboration Case Definition for myocarditis (see the Supplementary Appendix, available with the full text of this letter at NEJM.org).2 These data were collected by the Israeli Ministry of Health. Pfizer–BioNTech had no role in the collection or analysis of the data or in the reporting of data in this letter.
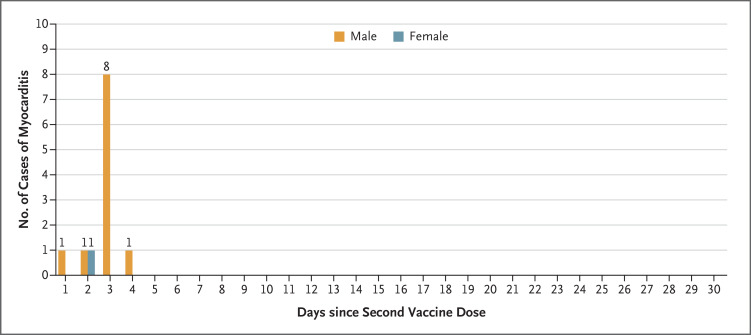
During the period under study, 404,407 adolescents (195,579 of whom were male) received the first dose of vaccine, 326,463 adolescents (157,153 of whom were male) received the second dose, and 18 cases of myocarditis leading to hospitalization were reported. Two cases of myocarditis were excluded from the study owing to reasonable alternative diagnoses. Of the remaining 16 cases, 1 occurred in an unvaccinated adolescent and 15 occurred in vaccinated adolescents — 1 case within 21 days after receipt of the first vaccine dose, 12 cases within 1 week after receipt of the second dose (Figure 1), and 2 later cases (1 each at 46 days and 70 days after receipt of the second dose); the 2 later cases were considered by the investigators as unlikely to be related to the vaccine.
Figure 1. Myocarditis after BNT162b2 Vaccination in Adolescents 12 to 15 Years of Age, According to Sex and Timing of Diagnosis.
Shown is the timing of diagnosis of myocarditis, according to sex, among adolescents within 30 days after receipt of the second dose of the BNT162b2 vaccine. Myocarditis developed in one male adolescent 5 days after receipt of the first vaccine dose (data not shown).
The demographic characteristics of the 13 adolescents with myocarditis occurring within 21 days after receipt of the first vaccine dose or within 30 days after receipt of the second dose are shown in Table S1 in the Supplementary Appendix. These 13 cases were classified as probable or definitive myocarditis according to the case definition. All the cases were clinically mild, involving a mean duration of hospitalization of 3.1 days (range, 1 to 6) and no readmissions during 30 days of follow-up. Symptoms at presentation, laboratory features, and echocardiographic findings are shown in Table S2.
The risk estimates of myocarditis among male recipients in the 21 days after the first and second doses were 0.56 cases per 100,000 after the first dose and 8.09 cases per 100,000 after the second dose; the risk estimates among female recipients were 0 cases per 100,000 after the first dose and 0.69 cases per 100,000 after the second dose. The risk of myocarditis after receipt of the second vaccine dose among male adolescents 12 to 15 years of age was estimated to be 1 case per 12,361; the corresponding risk among female adolescents was estimated to be 1 case per 144,439.
The risk estimates per person in this study were lower than the previously reported risks among male recipients 16 to 24 years of age,1 but they were slightly higher than the Centers for Disease Control and Prevention estimate of approximately 1 case per 16,129 male recipients 12 to 17 years of age after receipt of the second dose.3 These differences may be explained by the active surveillance in our population. In a phase 3 trial of the BNT162b2 vaccine, the relatively small number of vaccinated adolescents 12 to 15 years of age (1131), of whom 567 were male, is a possible explanation for the absence of reported cases of myocarditis during the trial.4
Limitations of the current study are that myocarditis was not validated on myocardial biopsy, that misclassification and reporting bias may have taken place, and that we acquired only reports of cases of myocarditis that led to hospitalization. In conclusion, the incidence of myocarditis leading to hospitalization among adolescents who received the second dose of the BNT162b2 vaccine was low but was higher than among recipients of the first vaccine dose and proportionately numerically higher than in recent estimates of incidence among unvaccinated persons.
Supplementary Appendix
Disclosure Forms
This letter was published on January 26, 2022, at NEJM.org.
Footnotes
Disclosure forms provided by the authors are available with the full text of this letter at NEJM.org.
References
- 1.Mevorach D, Anis E, Cedar N, et al. Myocarditis after BNT162b2 mRNA vaccine against Covid-19 in Israel. N Engl J Med 2021;385:2140-2149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brighton Collaboration of the Task Force for Global Health. Myocarditis/pericarditis case definition. 2021. (https://brightoncollaboration.us/myocarditis-case-definition-update/).
- 3.Wallace M, Oliver S. COVID-19 mRNA vaccines in adolescents and young adults: benefit-risk discussion. Meeting of the Advisory Committee on Immunization Practices (ACIP). Atlanta: Centers for Disease Control and Prevention, June 23, 2021. (https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2021-06/05-COVID-Wallace-508.pdf). [Google Scholar]
- 4.Frenck RW Jr, Klein NP, Kitchin N, et al. Safety, immunogenicity, and efficacy of the BNT162b2 Covid-19 vaccine in adolescents. N Engl J Med 2021;385:239-250. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.