Abstract
Species of the genus Bartonella are currently recognized in growing numbers and are involved in an increasing variety of human diseases, mainly trench fever, Carrion's disease, bacillary angiomatosis, endocarditis, cat scratch disease, neuroretinitis, and asymptomatic bacteremia. Such a wide spectrum of infections makes it necessary to develop species and strain identification tools in order to perform phylogenetic and epidemiological studies. The 16S/23S rRNA intergenic spacer region (ITS) was sequenced for four previously untested species, B. vinsonii subsp. arupensis, B. tribocorum, B. alsatica, and B. koehlerae, as well as for 28 human isolates of B. quintana (most of them from French homeless people), six human or cat isolates of B. henselae, five cat isolates of B. clarridgeiae, and four human isolates of B. bacilliformis. Phylogenetic trees inferred from full ITS sequences of the 14 recognized Bartonella species using parsimony and distance methods revealed high statistical support, as bootstrap values were higher than those observed with other tested genes. Five well-supported lineages were identified within the genus and the proposed phylogenetic organization was consistent with that resulting from protein-encoding gene sequence comparisons. The ITS-derived phylogeny appears, therefore, to be a useful tool for investigating the evolutionary relationships of Bartonella species and to identify Bartonella species. Further, partial ITS amplification and sequencing offers a sensitive means of intraspecies differentiation of B. henselae, B. clarridgeiae, and B. bacilliformis isolates, as each strain had a specific sequence. The usefulness of this approach in epidemiological investigations should be highlighted. Among B. quintana strains, however, the genetic heterogenity was low, as only three ITS genotypes were identified. It was nevertheless sufficient to show that the B. quintana population infecting homeless people in France was not clonal.
Bacteria of the genus Bartonella are oxidase-negative, fastidious, gram-negative bacilli belonging to the α2 subclass of Proteobacteria (40). Common features of Bartonella include transmission by an ectoparasitic, arthropod vector and survival within mammalian reservoir hosts (52). During recent years, an increasing number of Bartonella species has been isolated and characterized, and the genus, extended by unification with the genera Rochalimaea and Grahamella, currently consists of 14 recognized species (7, 11). As seven of the 14 species have been reported to cause human disease, Bartonella is considered an emerging pathogen (1). B. bacilliformis is the causative agent of bartonellosis (Carrion's disease), a biphasic disease endemic from Andean valleys (13). B. quintana and B. henselae, etiologic agents of trench fever and cat scratch disease, respectively (18, 44, 45, 54), have also been involved in endocarditis (19, 27) and in bacillary angiomatosis in immunocompromised patients (34, 46, 55). B. elizabethae and B. vinsonii subsp. berkhoffii have also been shown to cause endocarditis (16, 49), while B. vinsonii subsp. arupensis was first isolated from a febrile patient with valvular disease in the United States (56). B. grahamii has been involved in cases of neuroretinitis (33), and B. clarridgeiae is suspected to be an additional agent of cat scratch disease (36). The other species, B. vinsonii subsp. vinsonii, B. grahamii, B. taylorii, B. doshiae, B. talpae, B. peromysci, B. alsatica, B. tribocorum, and B. koehlerae, were isolated from the blood of vertebrate animals but are not yet known to cause recognizable human disease (7, 21, 28, 29). Additionally, an increasing number of Bartonella strains has been recovered from a wide range of mammals, including rodents, cervids, and cattle, in Europe and America (5, 9, 12, 23). Although partial, the genetic characterization of these isolates suggests that some of them may represent new Bartonella species (37).
Because of the implication of Bartonella in a variety of animal hosts, arthropod vectors, and human diseases, it would be useful to develop species- and strain-specific molecular tools, for dignostic and epidemiologic purposes. DNA hybridization and pulsed-field gel electrophoresis are currently the most sensitive techniques for molecular characterization of Bartonella species (41, 48) but are not suitable for routine use in a clinical laboratory and require prior cultivation of the organism (31, 50). Conversely, amplification-based techniques enable detection and identification of the bacteria to be performed directly from clinical specimens. To date, protein-encoding genes such as those encoding citrate-synthase (gltA), riboflavin synthetase (ribC), cell division protein (ftsZ), and 17-kDa antigen have been used for that purpose, as well as the 16S rRNA gene and the 16S/23S rRNA intergenic spacer region (ITS). Determination of sequence variability of these genes among different Bartonella species has been assessed both indirectly, using restriction fragment length polymorphism (RFLP) analysis (6, 31, 39), and directly, using base sequence comparisons (3, 4, 10, 22, 32, 48, 53). While 16S ribosomal DNA (rDNA) was shown to be an insensitive tool for appreciating genetic variability among Bartonella species, the protein-encoding genes appeared to serve as good indicators of interspecies divergence, because different species possessed markedly different sequences whereas there was very little sequence variation among strains of the same species. This last feature, however, precluded the use of these genes as sensitive gauges of intraspecies divergence (8, 38).
The ITS which separates the 16S and 23S rRNA genes of many bacteria is widely recognized for its sequence hypervariation (26). Comparison of ITS sequence data from different Bartonella species has expectedly demonstrated a high degree of interspecies variability, and a single-step PCR assay based on ITS divergences has been recently developed for medically relevant species (10, 30, 42, 47, 48). Genotypic diversity among Bartonella strains was assessed using RFLP analysis of PCR-amplified ITS and allowed the differentiation of seven profiles among 11 strains of B. henselae (39). Based on ITS sequences, previous studies described four genotypic variants among four B. henselae strains, two genotypic variants among seven B. quintana strains, three genotypic variants among six B. taylorii strains, and three genotypic variants among five B. grahamii strains (10, 48). Thus, not only is sequence analysis strains more sensitive than PCR-RFLP analysis for studying genetic variability, but its results are unequivocal and transferable. Moreover, gene sequence comparisons enable phylogenetic analyses to be performed, and the use of new, independent, gene data sets has been proposed to overcome the limitations of currently published phylogenies, which still lack statistical support and show discrepancies (8, 38).
For these reasons, we determined the ITS nucleotide sequence for all recognized Bartonella species and subspecies that had remained undetermined, and we assessed the usefulness of ITS sequence-based schemes for the differentiation of these bacteria and for inferring interspecies phylogenetic relationships. Furthermore, we investigated the possibility of using ITS sequences as a means of subtyping B. henselae, B. quintana, B. clarridgeiae, and B. bacilliformis strains and the usefulness of this approach in the epidemiological investigation of infections.
MATERIALS AND METHODS
Bacterial strains and culture conditions.
All strains were grown on Columbia blood agar containing 5% whole sheep blood (Biomérieux). The strains were incubated at 37°C in a humidified CO2-enriched environment, except B. bacilliformis, which was incubated at 28°C. The Bartonella species and strains that have been studied for determination of the complete ITS sequence are shown in Table 1. The type strain was tested for B. vinsonii subsp. arupensis, B. tribocorum, B. alsatica, and B. koehlerae. In addition, ITS were sequenced for four B. bacilliformis strains, five B. clarridgeiae strains, six B. henselae strains, and seven B. quintana strains. The four strains of B. bacilliformis, recovered from Peruvian patients, were given by R. J. Birtles. The five B. clarridgeiae strains were isolated in our laboratory from the blood of cats. Isolates B. henselae URBHLLY 8 and URBHLIE 9 (20) and B. quintana URBQMTF95 were also obtained in our laboratory, from patients with cat scratch disease, endocarditis, and asymptomatic bacteremia, respectively. Moreover, 21 additional isolates of B. quintana, recovered in Marseilles from homeless, bacteremic patients or from body lice, were tested for amplification and sequencing of a 260-bp fragment of the ITS. All B. quintana isolates are listed in Table 2 with their clinical and geographic sources.
TABLE 1.
Bacterial strains studied for determination of the complete ITS nucleotide sequence
| Species | Strain | Source
|
ITS
|
||
|---|---|---|---|---|---|
| Collection no.a | Reference or source | GenBank no. | Reference | ||
| B. bacilliformis | KC584T | ATCC 35686 | 11 | L26364 | 42 |
| B. bacilliformis | Cuzco 8 | This study | AF312491 | This study | |
| B. bacilliformis | Cuzco 14 | This study | AF312492 | This study | |
| B. bacilliformis | Monzon 269 | This study | AF312493 | This study | |
| B. bacilliformis | Monzon 812 | This study | AF312494 | This study | |
| B. quintana | FullerT | ATCC VR-358 | 11 | L35100 | 48 |
| B. quintana | URBQTBAAH 1 | 41 | AF368396 | 48 | |
| B. quintana | Oklahoma | CDCb | AF368391 | 48 | |
| B. quintana | SH-PERM | CDC | AF368392 | 48 | |
| B. quintana | URBQMLY 4 | 43 | AF368393 | 48 | |
| B. quintana | URBQPIEH 2 | 19 | AF368394 | 48 | |
| B. quintana | URBQGBAA 3 | 19 | AF368395 | 48 | |
| B. quintana | URBQMTF95 | This study | AF369482 | This study | |
| B. henselae | Houston-1T | ATCC 49882 | 45 | L35101 | 48 |
| B. henselae | 90-615 | 55 | AF369528 | 48 | |
| B. henselae | CAL-1 | CDC | AF369527 | 48 | |
| B. henselae | Fizz | 20 | AF369526 | 48 | |
| B. henselae | San Ant 2 (SA2) | 18 | AF369529 | 48 | |
| B. henselae | URBHLLY 8 | 20 | AF312495 | This study | |
| B. henselae | URBHLIE 9 | 20 | AF312496 | This study | |
| B. clarridgeiae | Houston-2T | ATCC 51734 | 15 | AF167989 | 30 |
| B. clarridgeiae | C 23 | This study | AF312498 | This study | |
| B. clarridgeiae | C 44 | This study | AF312499 | This study | |
| B. clarridgeiae | C 48 | This study | AF312500 | This study | |
| B. clarridgeiae | C 49 | This study | AF312501 | This study | |
| B. clarridgeiae | C 78 | This study | AF312502 | This study | |
| B. elizabethae | F9251T | ATCC 49927 | 16 | L35103 | 48 |
| B. vinsonii subsp. vinsonii | BakerT | ATCC VR-152 | 2 | L35102 | 48 |
| B. vinsonii subsp. berkhoffii | 93-CO1T | ATCC 51672 | 35 | AF167988 | 30 |
| B. vinsonii subsp. arupensis | OK94-423T | ATCC 700727 | 56 | AF312504 | This study |
| B. doshiae | R18T | NCTC 12862 | 7 | AJ269786 | 10 |
| B. grahamii | V2T | NCTC 12860 | 7 | AJ269785 | 10 |
| B. taylorii | M6T | NCTC 12861 | 7 | AJ269784 | 10 |
| B. tribocorum | IBS506T | CIP 105476 | 28 | AF312505 | This study |
| B. alsatica | IBS382T | CIP 105477 | 29 | AF312506 | This study |
| B. koehlerae | C-29T | ATCC 700693 | 21 | AF312490 | This study |
Abbreviations: ATCC, American Type Culture Collection, Manassas, Va.; NCTC, National Collection of Type Cultures, Central Public Health Laboratory, London, United Kingdom; CIP, Collection de l'Institut Pasteur, Paris, France.
CDC, Centers for Disease Control and Prevention, Atlanta, Ga.
TABLE 2.
Clinical sources and place of isolation of B. quintana strains examined in this study
| B. quintana strain | Clinical sourcea | Geographic origin | ITS genotypeb | GenBank no.b |
|---|---|---|---|---|
| FullerT | Trench fever patient | Yugoslavia | I | L35100 |
| URBQTBAAH 1 | BA patient | France | I | AF368396 |
| Oklahoma | Bacteremia, HIV+ patient | United States | II | AF368391 |
| SH-PERM | NA | Russia | II | AF368392 |
| URBQMLY 4 | Adenitis patient (contact with a cat) | France | II | AF368393 |
| URBQPIEH 2 | Endocarditis, homeless patient | France | II | AF368394 |
| URBQGBAA 3 | Endocarditis, homeless patient | France | II | AF368395 |
| URBQMIE 48 | Endocarditis patient | France | II | AF368397 |
| URBQMTF 20 | Bacteremia, homeless patient | France | II | AF368398 |
| URBQMTF 44 | NA | France | II | AF368399 |
| URBQMTF 47 | Bacteremia, homeless patient | France | II | AF368400 |
| URBQMTF 85 | Bacteremia, homeless patient | France | II | AF368401 |
| URBQMTF 97 | Bacteremia, homeless patient | France | II | AF368402 |
| URBQMTF 98 | Bacteremia, homeless patient | France | II | AF368403 |
| URBQMTF 101 | Bacteremia, homeless patient | France | II | AF368404 |
| URBQMTF 107 | Bacteremia, homeless patient | France | II | AF368405 |
| URBQMTF 95 | Bacteremia, homeless patient | France | III | AF369482 |
| URBQMTF 96 | Bacteremia, homeless patient | France | III | AF368406 |
| URBQMTF 99 | Bacteremia, homeless patient | France | III | AF368407 |
| URBQMTF 100 | Bacteremia, homeless patient | France | III | AF368408 |
| URBQMTF 105 | Bacteremia, homeless patient | France | III | AF368409 |
| URBQMTF 110 | Body louse | France | II | AF368415 |
| URBQMTF 111 | Body louse | France | II | AF368416 |
| URBQMTF 112 | Body louse | France | II | AF368417 |
| URBQMTF 102 | Body louse | France | III | AF368410 |
| URBQMTF 103 | Body louse | France | III | AF368411 |
| URBQMTF 104 | Body louse | France | III | AF368412 |
| URBQMTF 108 | Body louse | France | III | AF368413 |
| URBQMTF 109 | Body louse | France | III | AF368414 |
Abbreviations: HIV+, human immunodeficiency virus positive; NA, not available; BA, bacillary angiomatosis.
Among B. quintana strains, three types of ITS have been identified and named: genotypes I, II, and III. Genotypes were determined on the basis of a 260-bp partial sequencing within the 3′ hypervariable region of ITS.
Nucleic acid preparation.
A 200-μl sample of each bacterial suspension was mixed with 500 μl of a 20% Chelex suspension (Bio-Rad) in an Eppendorf tube, and the mixture was boiled for 30 min and centrifuged (14,000 × g for 10 min). The supernatant was transferred to a new Eppendorf tube and kept at 4°C until required.
Amplification and complete nucleotide sequence determination of ITS.
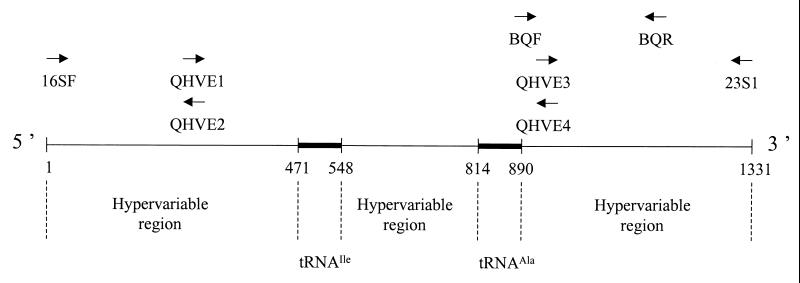
The primer pair BABF and BABR was designed by reference to regions flanking the ITS of B. bacilliformis (strain KC 584) as published by Minnick et al. (42), and these primers were used to amplify the ITS of all B. bacilliformis strains. For the other species, the ITS was amplified using the primers 16SF and 23S1 as described by Roux and Raoult (Table 3) (47). The PCR amplification was performed with 7 μl of extracted DNA in a 17.5-μl reaction mixture containing a 12.5 pM concentration of each primer, a 5 nM concentration of each deoxynucleoside triphosphate, 15 nM dUTP (Life Technologies), 0.75 U of EuroblueTaq DNA polymerase (Eurobio), 0.8 μl of a 25 mM solution of MgCl2 (Perkin-Elmer), and 2.5 μl of 10× reaction buffer. Amplification was carried out under the following conditions: an initial 3-min denaturation step at 95°C was followed by 39 cycles consisting of denaturation at 95°C for 30 s, annealing for 1 min at 52°C, and extension at 72°C for 90 s. Amplification was completed by incubation for 5 min at 72°C to allow complete extension of the PCR products. PCR products were visualized by ethidium bromide staining after electrophoresis through a 1% agarose gel, and their sizes were determined by comparison with the molecular weight standard marker VI (Boehringer). The samples were then purified using a QIAquick PCR purification kit (Qiagen). The purified products were incorporated into the d-rhodamine Terminator Cycle Sequencing Ready Reaction Buffer (DNA Sequencing kit; Perkin-Elmer). Nucleotide sequences were determined with an ABI PRISM 310 Genetic Analyzer (Perkin-Elmer) using the fluorescein-labeled primers QHVE1, QHVE2, QHVE3, and QHVE4, as described by Roux and Raoult (Table 3; Fig. 1) (47).
TABLE 3.
Oligonucleotide primers used for ITS amplification and sequencing
| Primer | Bartonella species | Nucleotide sequence (5′ to 3′) | Reference(s) |
|---|---|---|---|
| 16SFab | All (except B. bacilliformis) | AGAGGCAGGCAACCACGGTA | 47, 48 |
| 23S1abc | All (except B. bacilliformis) | GCCAAGGCATCCACC | 47, 48 |
| QHVE1b | All | TTCAGATGATGATCCCAA | 47, 48 |
| QHVE2bc | All | TTGGGATCATCATCTGAA | 47, 48 |
| QHVE3b | All | GATATATTCAGACATGTT | 47, 48 |
| QHVE4bc | All | AACATGTCTGAATATATC | 47, 48 |
| BABFab | B. bacilliformis | CTGGATCACCTCCTTTCTAA | This study |
| BABRabc | B. bacilliformis | ATGCCCTTAAGACACTTGAT | This study |
| BQFab | B. quintana | CTCCACCATTTTAGGTCATC | This study |
| BQRabc | B. quintana | GGTTTTGAGAATTCCCTTGC | This study |
Primer used for PCR. The 16SF primer was chosen from a conserved region of the 3′ end of the 16S rRNA-encoding gene of Bartonella species, and the 23S1 primer was chosen from the antisense strand of the 23S rRNA-encoding gene.
Primer used to sequence the PCR amplification product.
The primer was located on the complementary strand of DNA at the same position as the primer preceding it in the table.
FIG. 1.
Line diagram showing relative positions of oligonucleotide primers used for PCR amplification and sequencing of the ITS from Bartonella species. The positions of the oligonucleotides are with respect to the sequence of the ITS of B. quintana Fuller (accession number L35100). Nucleotide positions: 16SF, 1 to 20; 23S1, 1316 to 1331; QHVE1 and QHVE2, 274 to 292; QHVE3 and QHVE4, 898 to 915; BABF, 880 to 899; and BABR, 1121 to 1140.
Analysis of sequence data and construction of phylogenetic trees.
For each tested strain, the complete ITS primary sequences were assembled by alignment and by combining the sequences generated by each primer, using the Macintosh software ABI-Autoanalyzer Sequence Analysis package (Applied Biosystems). Complete ITS sequences for the 14 recognized type strains (including the 4 sequences determined in this study plus 10 sequences available in GenBank under the accession numbers listed in Table 1) were then aligned with each other by using version V of the CLUSTAL multisequence alignment program, which is part of the BISANCE software package (17). A total of 100 bootstraps were produced by using the program SEQBOOT from the 3.4 version of the PHYLIP software package (24), and a phylogenetic tree was inferred from each bootstrap sample by using parsimony (DNAPARS in the PHYLIP package). The resulting trees were combined to yield a consensus tree (CONSENSE in the PHYLIP package). A matrix of evolutionary distances was derived from each bootstrap alignment by the Jukes and Cantor method using DNADIST. Trees were inferred from the matrices by using the maximum-likelihood and the neighbor-joining methods as executed in the PHYLIP package (51). Levels of similarity between ITS sequences were determined by using the homology search functions of DNASIS (Hitachi Software Engineering America).
Amplification and partial sequencing of ITS for additional B. quintana strains.
DNA extracts from 21 additional isolates of B. quintana were incorporated into a PCR mix containing the primer pair BQF and BQR, which were designed to amplify a 260-bp DNA fragment located between positions 880 and 1140 of the B. quintana ITS sequence (Table 3; Fig. 1). The base sequences of these PCR products were obtained by following the protocol detailed above. Each product was included in two sequencing reactions that incorporated either BQF or BQR. Base differences located within this fragment were used as the basis for genotypic differentiation among B. quintana isolates.
Nucleotide sequence accession numbers.
The GenBank accession numbers for the ITS sequences which we determined are listed in Tables 1 and 2.
RESULTS
Comparison of ITS sequences for the recognized Bartonella species.
Amplification of the ITS for the type strains of B. vinsonii subsp. arupensis, B. tribocorum, B. alsatica, and B. koehlerae yielded a single product for each bacterium tested. Sizes of the PCR products were variable (B. vinsonii subsp. arupensis 1,405 bp, B. tribocorum 1,083 bp, B. alsatica 1,273 bp, and B. koehlerae 1,332 bp), and sequences could be clearly distinguished from one another and from those of other Bartonella species (10, 30, 42, 47, 48). All sequences were determined at least twice for each strand of DNA. All ITSs comprised five regions, namely, a 5′ hypervariable region, a 77-bp tRNAIle-encoding gene, an inter-tRNA hypervariable region, a 76-bp tRNAAla-encoding gene, and a 3′ hypervariable region (Fig. 1). The two tRNA encoding genes were completely conserved among all studied species, while hypervariable regions were characterized by substantial variation in sequence length and composition. A pairwise comparison of the ITS sequences obtained from the recognized Bartonella species revealed DNA similarity values ranging from 45.0 to 82.5% (Table 4).
TABLE 4.
Levels of ITS DNA sequence similarity and 16S rDNA sequence similarity for the main recognized Bartonella species
| Taxon | % Similaritya to:
|
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| B. bacilliformis | B. quintana | B. henselae | B. clarridgeiae | B. elizabethae | B. vinsonii subsp. vinsonii | B. vinsonii subsp. berkhoffii | B. vinsonii subsp. arupensis | B. doshiae | B. grahamii | B. taylorii | B. tribocorum | B. alsatica | B. koehlerae | |
| B. bacilliformis | 97.9 | 98.2 | 97.3 | 97.8 | 97.7 | 98.2 | 97.3 | 97.8 | 98.2 | 98.1 | 97.7 | 97.8 | 98.3 | |
| B. quintana | 55.4 | 98.9 | 97.8 | 98.2 | 98.9 | 98.9 | 98.4 | 98.9 | 98.8 | 98.6 | 98.5 | 98.2 | 98.9 | |
| B. henselae | 51.8 | 68.0 | 98.0 | 98.4 | 99.3 | 99.2 | 98.5 | 99.4 | 99.2 | 98.7 | 98.8 | 98.9 | 99.5 | |
| B. clarridgeiae | 50.2 | 61.8 | 45.2 | 97.3 | 98.0 | 98.2 | 97.9 | 98.1 | 98.2 | 98.0 | 97.8 | 98.0 | 98.1 | |
| B. elizabethae | 50.1 | 65.1 | 64.3 | 49.6 | 98.5 | 98.9 | 98.1 | 98.6 | 98.9 | 98.4 | 99.5 | 98.5 | 98.8 | |
| B. vinsonii subsp. vinsonii | 56.1 | 69.6 | 64.1 | 54.9 | 67.3 | 99.3 | 99.2 | 99.6 | 98.9 | 90.7 | 99.1 | 99.3 | 99.4 | |
| B. vinsonii subsp. berkhoffii | 46.1 | 58.2 | 46.7 | 51.6 | 66.1 | 74.7 | 98.2 | 99.4 | 99.6 | 97.5 | 98.5 | 98.3 | 99.2 | |
| B. vinsonii subsp. arupensis | 53.3 | 64.5 | 60.8 | 48.8 | 67.1 | 81.2 | 74.0 | 99.0 | 98.8 | 98.5 | 98.6 | 98.5 | 99.0 | |
| B. doshiae | 48.7 | 68.1 | 45.0 | 57.6 | 58.5 | 60.6 | 56.2 | 52.9 | 99.2 | 99.1 | 99.2 | 99.1 | 99.4 | |
| B. grahamii | 47.1 | 58.4 | 49.5 | 49.5 | 79.1 | 66.2 | 64.0 | 64.6 | 56.8 | 99.0 | 99.3 | 99.0 | 99.0 | |
| B. taylorii | 50.2 | 65.8 | 45.9 | 61.6 | 58.1 | 60.5 | 57.9 | 52.5 | 69.9 | 56.0 | 99.1 | 98.9 | 99.1 | |
| B. tribocorum | 52.1 | 65.1 | 62.7 | 49.3 | 82.5 | 68.8 | 64.8 | 67.3 | 57.6 | 80.3 | 56.1 | 98.9 | 98.8 | |
| B. alsatica | 55.1 | 70.8 | 67.6 | 53.5 | 65.6 | 70.7 | 63.7 | 65.9 | 62.5 | 63.9 | 62.4 | 67.0 | 99.0 | |
| B. koehlerae | 52.3 | 69.7 | 80.6 | 56.2 | 64.0 | 65.0 | 57.5 | 60.5 | 65.2 | 57.8 | 63.9 | 63.0 | 69.4 | |
The values on the upper right are levels of 16S rRNA gene sequence similarity, and the values on the lower left are levels of ITS sequence similarity.
ITS-based phylogeny of Bartonella species.
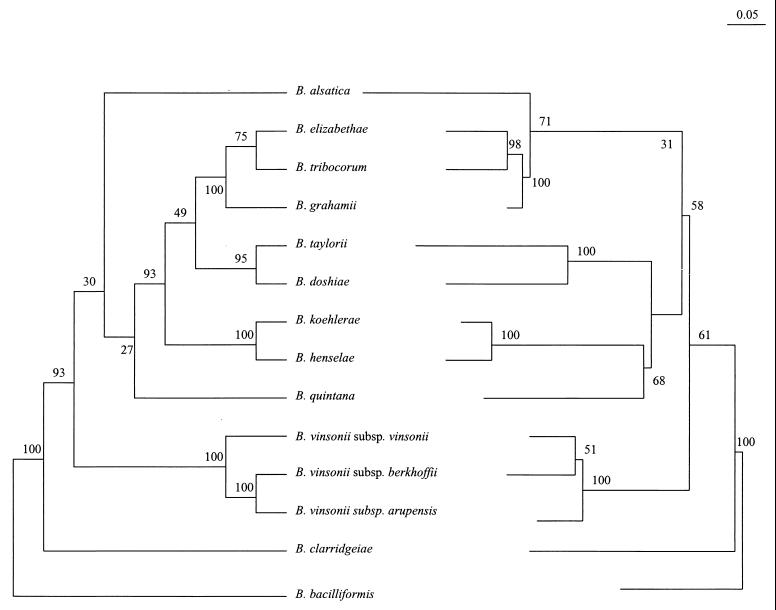
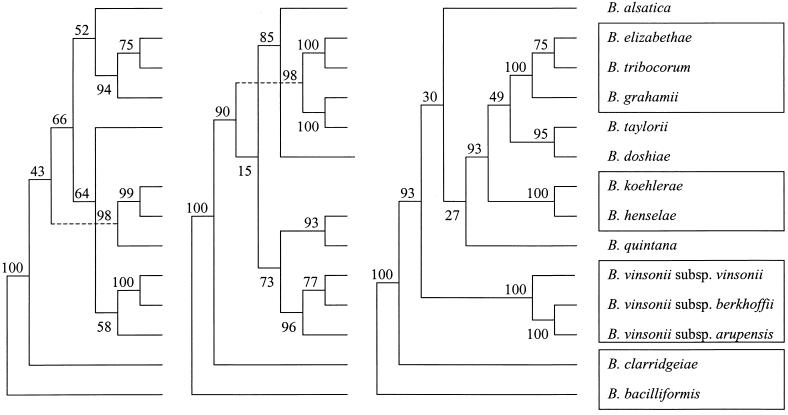
The sequence alignment of ITSs from Bartonella species was 1,193 characters long. The phylogenetic trees inferred from the ITS data set using parsimony and distance methods showed consistent topologies and statistical significance. Five well-supported (more than 90% of bootstrap samples with all analysis methods) clusters were identified within the genus (Fig. 2). One group consisted of B. bacilliformis only. Another cluster contained the cat-associated species B. henselae and B. koehlerae. The third group included the rodent-associated taxa B. grahamii, B. elizabethae, and B. tribocorum, while B. taylorii and B. doshiae clustered together in a separate clade and a fifth group contained the three B. vinsonii subspecies. The position of B. clarridgeiae appeared to be divergent according to all analysis methods, but this finding was supported by significant bootstrap values (>90%) only in the parsimony tree. The taxonomic position of B. quintana and B. alsatica could not be consistently determined using ITS-based phylogeny.
FIG. 2.
Comparison of parsimony tree (left side) and neighbor-joining tree (right side) derived from complete ITS sequences for recognized Bartonella species (type strains). The support of each branch, as determined from 100 bootstrap samples, is indicated by the value at the node. The lengths of vertical lines are not significant. For the parsimony tree, the lengths of horizontal lines are also not significant. For the neighbor-joining tree, the scale bar represents evolutionary distance as calculated by using the Kimura two-parameter distance calculation.
Comparison of ITS sequences for several isolates of the same species.
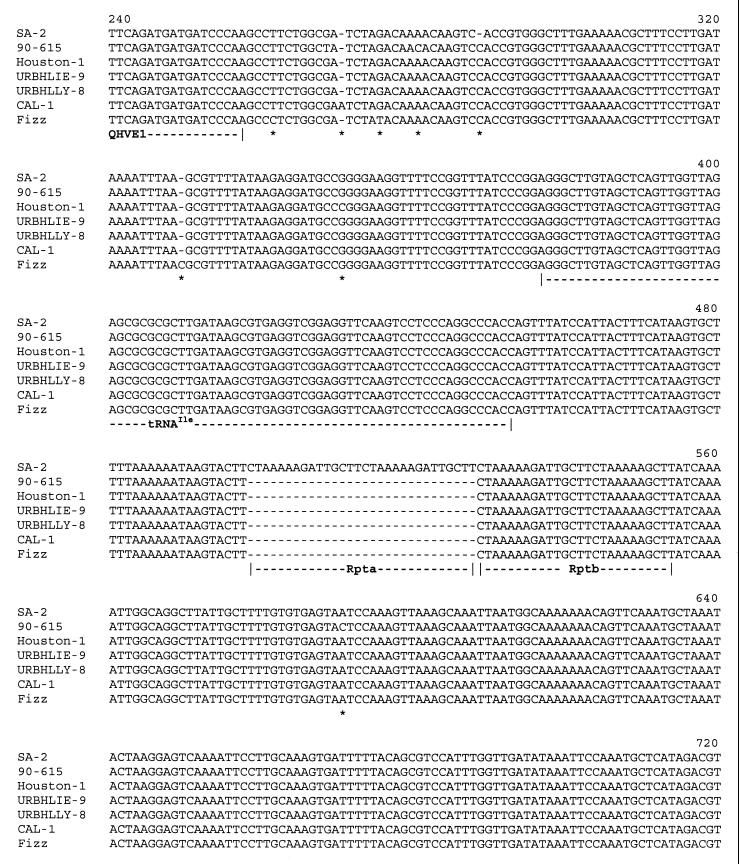
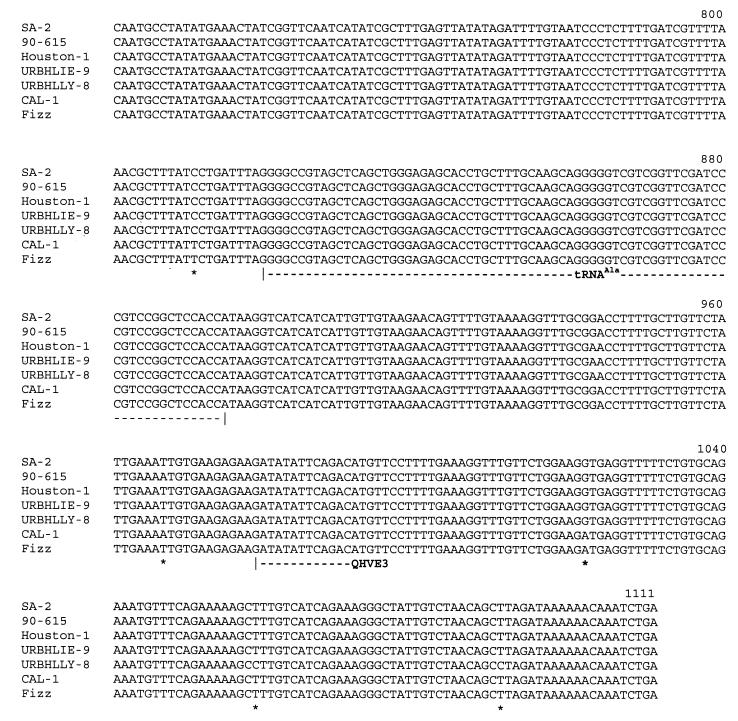
A complete ITS sequence was obtained for four B. bacilliformis strains, five B. clarridgeiae strains, six B. henselae strains, and seven B. quintana strains (Table 1). Interstrain comparison of DNA sequences revealed that levels of ITS sequence divergence among different species were not the same. B. quintana isolates exhibited ITS sequences that were 1,331 bp long. The only difference between these sequences consisted of base substitutions at positions 860, 940, 959, and 1004. Thus, three genotypic subgroups were identified among the eight B. quintana strains: one, including the Fuller and URBQTBAAH1 isolates, showed bases C, C, A and G at these respective positions; the second, including the Oklahoma, SH-PERM, URBQMLY4, URBQPIEH2, and URBQGBAA3 isolates, was characterized by G, G, G, and A, respectively; and the third group consisted of strain URBQMTF95 only and exhibited C, G, G, and G, respectively. Intraspecies heterogeneity was higher for B. henselae, B. bacilliformis, and B. clarridgeiae, with ITS sizes ranging from 1,345 to 1,382 bp, from 906 to 930 bp, and from 1,356 to 1,436 bp respectively. ITS sequences of B. henselae, B. bacilliformis, and B. clarridgeiae strains revealed DNA similarity values from 94.9 to 99.3%, from 91.3 to 99.7%, and from 91.2 to 99.8%, respectively, and each tested isolate had a different sequence. Among B. henselae strains, the 90–615, SA2, CAL-1, and Fizz isolates differed from the Houston-1 strain by four substitutions and, for the SA2 isolate only, by a 30-bp insertion between positions 498 and 499 (48). For the URBHLLY 8 isolate, we found T to C (T→C) mutations at positions 1029 and 1061 (Fig. 3). In comparison with the KC584 isolate, the four other B. bacilliformis strains had about 20 point mutations and a 25-bp long insertion fragment between positions 816 and 840; these four isolates varied from one to another by fewer than five point mutations. Genetic heterogeneity appeared to be higher among B. clarridgeiae isolates. In comparison with the Houston-2 isolate, the C 23 isolate had mutations at positions 314 and 327 (A→T and T→G, respectively) and deletion of an 80-bp fragment between positions 1207 and 1283; for the C 44 isolate, we found one mutation at position 5 (C→G) and a 46-bp deletion between positions 912 and 957; the C 48 isolate had the same deletion and also mutations at positions 5, 6, and 679 (C→G, A→G, and G→A, respectively); for the C 78 isolate, we found again the same deletion and a 7-bp insertion between positions 158 and 164. Phylogenetic analyses were performed at the intraspecies level using parsimony and distance methods in order to identify significant subgroups. However, no stable topology was obtained, as branching orders inferred from different methods revealed marked differences and had low statistical support (<55%) (data not shown).
FIG. 3.
Alignment of QHVE1 and QHVE3 ITS amplicons derived from seven B. henselae strains. ∗, substitution or point insertion or deletion; Rpta and Rptb, repeat regions.
Strain differentiation of B. quintana by partial sequencing of the ITS.
A 260-bp fragment including the four base substitutions that characterized the three ITS genotypes of B. quintana was amplified and sequenced for a total of 28 strains from various geographic and clinical sources (Table 2). B. quintana Fuller and one isolate from a French patient with bacillary angiomatosis were the only strains to exhibit ITS genotype I. Seventeen strains (58.6%) possessed ITS genotype II, and 10 strains (34.5%) possessed ITS genotype III. Of the 26 strains isolated in France, 1 was genotype I, and 15 (57.7%) and 10 (38.5%) were genotypes II and III, respectively. Twelve strains were recovered from the blood of asymptomatic, bacteremic homeless men. Of these, seven (58.3%) and five (41.6%) exhibited ITS genotype II and III, respectively. Among the eight strains recovered from body lice, three were genotype II and five were genotype III.
DISCUSSION
As Bartonella species are involved in an increasing variety of human diseases, species-specific tools for diagnosis are necessary (1). Molecular, sequence-based methods appear, therefore, to be useful techniques for detection and identification of Bartonella species. Moreover, as Bartonella is being isolated in large numbers from a wide range of animals, sensitive means for strain differentiation are required to perform epidemiologic studies. Sequencing of the entire ITS of the type strains of four further Bartonella species led to confirmation that this genetic region is a discriminant tool for species differentiation. Although many bacteria possess multiple ITSs of different sizes and base sequences (25), PCR amplification using the primer pairs described above resulted in a single product for all Bartonella species. Our results confirmed that Bartonella possesses an exceptionally long ITS of 900 to 1,500 bp, with three hypervariable, species-specific regions surrounding two tRNA-encoding genes which are conserved throughout the genus (42, 47, 48).
16S rDNA gene sequence comparison has been shown to provide reliable data about the phylogenetic position of the genus Bartonella among Proteobacteria. However, within the Bartonella cluster, use of this gene lacks sensitivity because of high DNA similarity among Bartonella species. Conversely, several protein-encoding genes have been found to be useful for inferring evolutionary relationships between Bartonella strains because they possess high interspecies variability and low intraspecies variability (3, 8, 32, 38, 53). As ITS sequence variation appears to be high at the interspecies level but also among different strains of a same species, its usefulness for phylogenetic purposes has been disputed (10, 39, 47, 50). The first attempt at this type of dispute was that of Jensen et al. (30), although they tested only seven Bartonella species and did not indicate which method was used to infer the tree; the resulting branching order had only low support but seemed to be consistent with gltA-based organization. We constructed phylogenetic trees using entire ITS sequences for all Bartonella species and found, with three different methods, consistent topologies which were supported by the highest bootstrap values observed up to now among tested genes (Fig. 2) (8, 38, 57). Of the five clusters that were significantly supported by ITS-based analyses, four were congruent to those provided by groEL and gltA gene sequence comparisons, suggesting that they can be considered to be reliable (Fig. 4). The unique, deep-rooted divergence of B. bacilliformis is reflected by its particular growth requirements and colony morphology, and by fatty acid, protein, and antigenic profiles (15). Although groEL- and ITS-based parsimony analyses suggest that B. clarridgeiae may be, as B. bacilliformis, an early divergent species, such an organization lacks statistical support when distance methods are used (30, 38). Comparison of ITS sequences provided for the first time statistically significant data about the taxonomic position of the three B. vinsonii subspecies, and parsimony analysis suggested that B. vinsonii subsp. berkhoffii and B. vinsonii subsp. arupensis (two species involved in human infections) were more closely related to each other than to B. vinsonii subsp. vinsonii (49, 56). Three species associated with Old World rodent species (B. grahamii, B. elizabethae, and B. tribocorum) were found to cluster together with 100% bootstrap values (5, 7, 23). Within this cluster, the subgroup including B. elizabethae and B. tribocorum, two rat (Rattus sp.)-associated species, was stable (8, 9, 28). These findings, together with the close evolutionary relationships observed between B. henselae and B. koehlerae (to cat-associated species) support the hypothesis that coevolution occurred between Bartonella and its mammalian hosts (12, 23, 37).
FIG. 4.
Comparison of parsimony trees derived from the gltA gene (left side), from the groEL gene (middle), and from the ITS sequences (right side) for recognized Bartonella species. The support of each branch, as determined from 100 bootstrap samples, is indicated by the value at the node. This analysis provided tree topology only, and the lengths of both vertical lines and horizontal lines are not significant. Boxes contain the clusters which are found consistently in all analyses performed.
In common with previous studies, our results confirm that the level of ITS intraspecies divergence is variable from one species to another (10, 48). A high variability was observed among B. henselae, B. bacilliformis, or B. clarridgeiae strains; as each strain tested to date exhibited a different sequence, it is necessary to test further strains to assess the genetic heterogeneity of ITS for these species. In contrast, only three ITS genotypes were identified among 29 B. quintana strains. Two events, base substitutions and insertion or deletion of 3- to 80-bp DNA fragments, were responsible for the ITS sequence variation and were found in variable proportion according to the species considered. Thus, the diversity among B. quintana strains was related to only a few base substitutions (fewer than five) as observed for B. grahamii strains. The higher diversity among B. bacilliformis strains, related to 20- to 25-base substitutions and a 24-bp insertion, was similar to that observed for B. taylorii (12). The variability of B. clarridgeiae and B. henselae was due mainly to long fragment insertions (up to 30 and 80 bp, respectively), while base substitutions remained rare (fewer than five). In these two species, the presence in variable number of 20- to 30-bp repeating elements of DNA was responsible for a significant proportion of variations among different strains as described by Birtles et al. (12) in B. taylorii. However, the relative significance of these different events as indicators of molecular evolution remains unclear (10, 48). Our attempts to infer evolutionary relationships among strains of a same species from ITS sequences compared using parsimony and distance methods did not provide consistent dendrograms (data not shown).
With seven identified variants, ITS sequencing appears to be a more discriminative tool for subtyping B. henselae than any other technique proposed to date, including pulsed-field gel electrophoresis (3, 22, 39, 48). No correlation was found between the ITS type and either the two 16S rDNA genotypes that were described by Bergmans et al. (4) in cat scratch disease patients or the two serotypes (Houston and Marseille) that we reported (20). Genetic heterogeneity appears to be extremely high in B. henselae populations, but no specific correlation has been made between genotypes and clinical manifestations. For B. quintana, ITS genotypes II and III were found predominantly among homeless bacteremic patients living in France, but lack of isolates from other geographic and clinical origins prevented significant epidemiologic correlations from being made. It is interesting to note that the B. quintana population infecting French homeless people does not stem from a unique clone, although it is likely to spread in an epidemic way. It would be interesting to correlate the genotypes of isolates from body lice and from patients to understand the spread of the current outbreak.
Conclusion.
Comparison of ITS nucleotide sequence for all currently recognized Bartonella species confirmed that each Bartonella possessed a single, species-specific ITS. Comparison of ITS sequences appears to be a useful tool for phylogenetic analyses at the interspecies level and in this study provided results that were consistent with those from other gene-based phylogenies. We confirmed the usefulness of ITS sequencing for subtyping of Bartonella species and demonstrated a high genetic heterogeneity among B. henselae, B. bacilliformis, and B. clarridgeiae isolates, while B. quintana strains exhibited only three genotypes. Application of these subtyping methods to a high number of Bartonella isolates, from various human, animal, and geographic origins, will be useful in understanding the epidemiology of these bacteria.
REFERENCES
- 1.Anderson B E, Neuman M A. Bartonella spp. as emerging human pathogens. Clin Microbiol Rev. 1997;10:203–219. doi: 10.1128/cmr.10.2.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baker J A. A rickettsial infection in Canadian voles. J Exp Med. 1946;84:37–51. [PubMed] [Google Scholar]
- 3.Bereswill S, Hinkelmann S, Kist M, Sander A. Molecular analysis of riboflavin synthesis genes in Bartonella henselae and use of the ribC gene for differentiation of Bartonella species by PCR. J Clin Microbiol. 1999;37:3159–3166. doi: 10.1128/jcm.37.10.3159-3166.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bergmans A M C, Schellekens J F P, Van Embden J D A, Schouls L M. Predominance of two Bartonella variants among cat scratch disease patients in The Netherlands. J Clin Microbiol. 1996;34:254–260. doi: 10.1128/jcm.34.2.254-260.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Birtles R J, Harrison T G, Molyneux D H. Grahamella in small woodland mammals in the U.K.: isolation, prevalence and host specificity. Ann Trop Med Parasitol. 1994;88:317–327. doi: 10.1080/00034983.1994.11812872. [DOI] [PubMed] [Google Scholar]
- 6.Birtles R J. Differentiation of Bartonella species using restriction endonuclease analysis of PCR-amplified 16S rRNA genes. FEMS Microbiol Lett. 1995;129:261–266. doi: 10.1111/j.1574-6968.1995.tb07590.x. [DOI] [PubMed] [Google Scholar]
- 7.Birtles R J, Harrison T G, Saunders N S, Molyneux D H. Proposals to unify the genera Grahamella and Bartonella, with descriptions of Bartonella talpae comb. nov., Bartonella peromysci comb. nov., and three new species, Bartonella grahamii sp. nov., Bartonella taylorii sp. nov., and Bartonella doshiae sp. nov. Int J Syst Bacteriol. 1995;45:1–8. doi: 10.1099/00207713-45-1-1. [DOI] [PubMed] [Google Scholar]
- 8.Birtles R J, Raoult D. Comparison of partial citrate-synthase gene (gltA) sequences for phylogenetic analysis of Bartonella species. Int J Syst Bacteriol. 1996;46:891–897. doi: 10.1099/00207713-46-4-891. [DOI] [PubMed] [Google Scholar]
- 9.Birtles R J, Canales J, Ventosilla P, Alvarez E, Guerra H, Llanos-Cuentas A, Raoult D, Doshi N, Harrison T G. Survey of Bartonella species infecting intradomicillary animals in the Huayllacallan valley, Ancash, Peru, a region endemic for human bartonellosis. Am J Trop Med Hyg. 1999;60:799–805. doi: 10.4269/ajtmh.1999.60.799. [DOI] [PubMed] [Google Scholar]
- 10.Birtles R J, Hazel S, Bown K, Raoult D, Begon M, Bennett M. Subtyping of uncultured bartonellae using sequence comparison of 16S/23S rRNA intergenic spacer regions amplified directly from infected blood. Mol Cell Probes. 2000;14:79–87. doi: 10.1006/mcpr.2000.0289. [DOI] [PubMed] [Google Scholar]
- 11.Brenner D J, O'Connor S P, Winkler H H, Steigerwalt A G. Proposals to unify the genera Bartonella and Rochalimaea, with descriptions of Bartonella quintana comb. nov., and Bartonella elizabethae comb. nov., and to remove the family Bartonellaceae from the order Rickettsiales. Int J Syst Bacteriol. 1993;43:777–786. doi: 10.1099/00207713-43-4-777. [DOI] [PubMed] [Google Scholar]
- 12.Breitschwerdt E B, Kordick D L. Bartonella infection in animals: carriership, reservoir potential, pathogenicity, and zoonotic potential for human infection. Clin Microbiol Rev. 2000;13:428–438. doi: 10.1128/cmr.13.3.428-438.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brouqui P, La Scola B, Roux V, Raoult D. Chronic Bartonella quintana bacteremia in homeless patients. N Engl J Med. 1999;340:184–189. doi: 10.1056/NEJM199901213400303. [DOI] [PubMed] [Google Scholar]
- 14.Chang C C, Chomel B B, Kasten R W, Heller R, Kocan K M, Ueno H, Yamamoto K, Bleich V C, Pierce B M, Gonzales B J, Swift P K, Boyce W M, Jang S S, Boulouis H J, Piémont Y. Bartonella spp. isolated from wild and domestic ruminants in North America. Emerg Infect Dis. 2000;6:306–311. doi: 10.3201/eid0603.000313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Clarridge J E, III, Raich T J, Pirwani D, Simon B, Tsai L, Rodriguez-Barradas M C, Regnery R L, Zollo A, Jones D C, Rambo C. Strategy to detect and identify Bartonella species in routine laboratory yields Bartonella henselae from human immunodeficiency virus-positive patient and unique Bartonella strain from his cat. J Clin Microbiol. 1995;33:2107–2113. doi: 10.1128/jcm.33.8.2107-2113.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Daly J S, Worthington M G, Brenner D J, Moss C W, Hollis D G, Weyant R S, et al. Rochalimaea elizabethae sp. nov. isolated from a patient with endocarditis. J Clin Microbiol. 1993;31:872–881. doi: 10.1128/jcm.31.4.872-881.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dessen P, Fondrat C, Valencien C, Munier G. BISANCE: a French service for access to biomolecular sequences databases. CABIOS. 1990;6:355–356. doi: 10.1093/bioinformatics/6.4.355. [DOI] [PubMed] [Google Scholar]
- 18.Dolan M J, Wong M T, Regnery R L, Jorgensen J H, Garcia M, Peters J, Drehner D. Syndrome of Rochalimaea henselae adenitis suggesting cat-scratch disease. Ann Intern Med. 1993;118:331–336. doi: 10.7326/0003-4819-118-5-199303010-00002. [DOI] [PubMed] [Google Scholar]
- 19.Drancourt M, Mainardi J L, Brouqui P, Vandenesch F, Carta A, Lehnert F, Etienne J, Goldstein F, Acar J, Raoult D. Bartonella (Rochalimaea) quintana endocarditis in homeless men. N Engl J Med. 1995;332:419–423. doi: 10.1056/NEJM199502163320702. [DOI] [PubMed] [Google Scholar]
- 20.Drancourt M, Birtles R, Chaumentin G, Vandenesch F, Etienne J, Raoult D. New serotype of Bartonella henselae in endocarditis and cat-scratch disease. Lancet. 1996;347:441–443. doi: 10.1016/s0140-6736(96)90012-4. [DOI] [PubMed] [Google Scholar]
- 21.Droz S, Chi B, Horn E, Steigerwalt A G, Whitney A M, Brenner D J. Bartonella koelherae sp. nov., isolated from cats. J Clin Microbiol. 1999;37:1117–1122. doi: 10.1128/jcm.37.4.1117-1122.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ehrenborg C, Wesslen L, Jakobson A, Friman G, Holmberg M. Sequence variation in the ftsZ gene of Bartonella henselae isolates and clinical samples. J Clin Microbiol. 2000;38:682–687. doi: 10.1128/jcm.38.2.682-687.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ellis B A, Regnery R L, Beati L, Bacellar F, Rood M, Glass G G, Marston E, Ksiazek T G, Jones D, Childs J E. Rats of the genus Rattus are reservoir hosts for pathogenic Bartonella species: an old world origin for a new world disease? J Infect Dis. 1999;180:220–224. doi: 10.1086/314824. [DOI] [PubMed] [Google Scholar]
- 24.Felsenstein J. Phylip-phylogeny inference package (version 3.2) Cladistics. 1989;5:164–166. [Google Scholar]
- 25.Frothingham R, Wilson K H. Sequence-based differentiation of strains in the Mycobacterium avium complex. J Bacteriol. 1993;175:2818–2825. doi: 10.1128/jb.175.10.2818-2825.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gurtler V, Mayall B C. rDNA rearrangements and concerted evolution. Microbiology. 1999;145:2–3. doi: 10.1099/13500872-145-1-2. [DOI] [PubMed] [Google Scholar]
- 27.Hadfield T L, Warren R, Kass M, Brun E, Levy C. Endocarditis caused by Rochalimaea henselae. Hum Pathol. 1993;24:1140–1141. doi: 10.1016/0046-8177(93)90196-n. [DOI] [PubMed] [Google Scholar]
- 28.Heller R, Riegel P, Hansmann Y, Delacour G, Bermond D, Dehio C, Lamarque F, Monteil H, Chomel B, Piemont Y. Bartonella tribocorum sp. nov., a new Bartonella species isolated from the blood of wild rats. Int J Syst Bacteriol. 1998;48:1333–1339. doi: 10.1099/00207713-48-4-1333. [DOI] [PubMed] [Google Scholar]
- 29.Heller R, Kubina M, Mariet P, Riegel P, Delacour G, Dehio C, Lamarque F, Kasten R, Boulouis H J, Monteil H, Chomel B, Piémont Y. Bartonella alsatica sp. nov., a new Bartonella species isolated from the blood of wild rabbits. Int J Syst Bacteriol. 1999;49:283–288. doi: 10.1099/00207713-49-1-283. [DOI] [PubMed] [Google Scholar]
- 30.Jensen W A, Fall M Z, Rooney J, Kordick D L, Breitschwerdt E B. Rapid identification and differentiation of Bartonella species using a single-step PCR assay. J Clin Microbiol. 2000;38:1717–1722. doi: 10.1128/jcm.38.5.1717-1722.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Joblet C, Roux V, Drancourt M, Gouvernet J, Raoult D. Identification of Bartonella (Rochalimaea) species among fastidious gram-negative bacteria on the basis of the partial sequence of the citrate-synthase gene. J Clin Microbiol. 1995;33:1879–1883. doi: 10.1128/jcm.33.7.1879-1883.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kelly T M, Padmalayam I, Baumstark B R. Use of the cell division protein ftsZ as a means of differentiating among Bartonella species. Clin Diagn Lab Immunol. 1998;5:766–772. doi: 10.1128/cdli.5.6.766-772.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kerkhoff F T, Bergmans A M, Van der Zee A, Rothova A. Demonstration of Bartonella grahamii DNA in ocular fluids of a patient with neuroretinitis. J Clin Microbiol. 1999;37:4034–4038. doi: 10.1128/jcm.37.12.4034-4038.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Koehler J E, Quinn F D, Berger T G, LeBoit P E, Tappero J W. Isolation of Rochalimaea species from cutaneous and osseous lesions of bacillary angiomatosis. N Engl J Med. 1992;327:1625–1631. doi: 10.1056/NEJM199212033272303. [DOI] [PubMed] [Google Scholar]
- 35.Kordick L K, Swaminathan B, Greene C E, Wilson K H, Whitney A M, O'Connor S, Hollis D G, Matar G M, Steigerwalt A G, Malcolm G B, Hayes P S, Hadfield T L, Breitschwerdt E B, Brenner D J. Bartonella vinsonii subsp. berkhoffii subsp. nov., isolated from dogs; Bartonella vinsonii subsp. vinsonii; and emended description of Bartonella vinsonii. Int J Syst Bacteriol. 1996;46:704–709. doi: 10.1099/00207713-46-3-704. [DOI] [PubMed] [Google Scholar]
- 36.Kordick D L, Hilyard E J, Hadfield T L, Wilson K H, Steigerwalt A G, Brenner D J, Breitschwerdt E B. Bartonella clarridgeiae, a newly recognized zoonotic pathogen causing inoculation papules, fever, and lymphadenopathy (cat scratch disease) J Clin Microbiol. 1997;35:1813–1818. doi: 10.1128/jcm.35.7.1813-1818.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kosoy M Y, Regnery R L, Tzianabos T, Marston E L, Jones D C, Green D, Maupin G O, Olson J G, Childs J E. Distribution, diversity, and host specificity of Bartonella in rodents from the southeastern United States. Am J Trop Med Hyg. 1997;57:578–588. doi: 10.4269/ajtmh.1997.57.578. [DOI] [PubMed] [Google Scholar]
- 38.Marston E L, Sumner J W, Regnery R L. Evaluation of intraspecies genetic variation within the 60 kDa heat-shock protein gene (groEL) of Bartonella species. Int J Syst Bacteriol. 1999;49:1015–1023. doi: 10.1099/00207713-49-3-1015. [DOI] [PubMed] [Google Scholar]
- 39.Matar G M, Swaminathan B, Hunter S B, Slater L, Welch D. Polymerase chain reaction-based restriction fragment length polymorphism analysis of a fragment of the ribosomal operon from Rochalimaea species for subtyping. J Clin Microbiol. 1993;31:1730–1734. doi: 10.1128/jcm.31.7.1730-1734.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Maurin M, Birtles R, Raoult D. Current knowledge of Bartonella species. Eur J Clin Microbiol Infect Dis. 1997;16:487–506. doi: 10.1007/BF01708232. [DOI] [PubMed] [Google Scholar]
- 41.Maurin M, Roux V, Stein A, Ferrier F, Viraben R, Raoult D. Isolation and characterization by immunofluorescence, sodium dodecyl sulfate-polyacrylamide gel electrophoresis, Western blot, restriction fragment length polymorphism-PCR, 16S rRNA gene sequencing, and pulsed-field gel electrophoresis of Rochalimaea quintana from a patient with bacillary angiomatosis. J Clin Microbiol. 1994;32:1166–1171. doi: 10.1128/jcm.32.5.1166-1171.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Minnick M F, Strange J C, Williams K F. Characterization of the 16S–23S rRNA intergenic spacer of Bartonella bacilliformis. Gene. 1994;143:149–150. doi: 10.1016/0378-1119(94)90622-x. [DOI] [PubMed] [Google Scholar]
- 43.Raoult D, Drancourt M, Carta A, Gastaut J A. Bartonella (Rochalimaea) quintana isolation in patient with chronic adenopathy, lymphopenia, and a cat. Lancet. 1994;343:977. doi: 10.1016/s0140-6736(94)90102-3. [DOI] [PubMed] [Google Scholar]
- 44.Regnery R L, Anderson B E, Clarridge III J E, Rodriguez-Barradas M, Jones D C, Carr J H. Characterization of a novel Rochalimaea species, R. henselae sp. nov., isolated from blood of a febrile human immunodeficiency virus-positive patient. J Clin Microbiol. 1992;30:265–274. doi: 10.1128/jcm.30.2.265-274.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Regnery R, Martin M, Olson J. Naturally occurring “Rochalimaea henselae” infection in domestic cat. Lancet. 1992;340:557–558. doi: 10.1016/0140-6736(92)91760-6. [DOI] [PubMed] [Google Scholar]
- 46.Relman D A, Loutit J S, Schmidt T M, Falkow S, Tompkins L S. The agent of bacillary angiomatosis: an approach to the identification of uncultured pathogens. N Engl J Med. 1990;323:1573–1580. doi: 10.1056/NEJM199012063232301. [DOI] [PubMed] [Google Scholar]
- 47.Roux V, Raoult D. The 16S–23S rRNA intergenic spacer region of Bartonella (Rochalimaea) species is longer than usually described in other bacteria. Gene. 1995;156:107–111. doi: 10.1016/0378-1119(94)00919-j. [DOI] [PubMed] [Google Scholar]
- 48.Roux V, Raoult D. Inter- and intraspecies identification of Bartonella (Rochalimaea) species. J Clin Microbiol. 1995;33:1573–1579. doi: 10.1128/jcm.33.6.1573-1579.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Roux V, Eykyn S J, Wyllie S, Raoult D. Bartonella vinsonii subsp. berkhoffii as an agent of afebrile blood culture-negative endocarditis in a human. J Clin Microbiol. 2000;38:1698–1700. doi: 10.1128/jcm.38.4.1698-1700.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sander A, Ruess M, Bereswill S, Schuppler M, Steinbruechner B. Comparison of different DNA fingerprinting techniques for molecular typing of Bartonella henselae isolates. J Clin Microbiol. 1998;36:2973–2981. doi: 10.1128/jcm.36.10.2973-2981.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Saitou N, Nei M. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol. 1987;4:406–425. doi: 10.1093/oxfordjournals.molbev.a040454. [DOI] [PubMed] [Google Scholar]
- 52.Schwartzman W. Bartonella (Rochalimaea) infections: beyond cat scratch. Annu Rev Med. 1996;47:355–364. doi: 10.1146/annurev.med.47.1.355. [DOI] [PubMed] [Google Scholar]
- 53.Sweger D, Resto-Ruiz S, Johnson D P, Schmiederer M, Hawke N, Anderson B. Conservation of the 17-kDa antigen gene within the genus Bartonella. Clin Diagn Lab Immunol. 2000;7:251–257. doi: 10.1128/cdli.7.2.251-257.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Vinson J W. In vitro cultivation of the rickettsial agent of trench fever. Bull WHO. 1966;35:155–164. [PMC free article] [PubMed] [Google Scholar]
- 55.Welch D F, Pickett D A, Slater L N, Steigerwalt A G, Brenner D J. Rochalimaea henselae sp. nov., a cause of septicemia, bacillary angiomatosis, and parenchymal bacillary peliosis. J Clin Microbiol. 1992;30:275–280. doi: 10.1128/jcm.30.2.275-280.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Welch D F, Carroll K C, Hofmeister E K, Persing D H, Robison D A, Steigerwalt A G, Brenner D J. Isolation of a new subspecies, Bartonella vinsonii subsp. arupensis, from a cattle rancher: identity with isolates found in conjunction with Borrelia burgdorferi and Babesia microti among naturally infected mice. J Clin Microbiol. 1999;37:2598–2601. doi: 10.1128/jcm.37.8.2598-2601.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zeaiter, Z., P. E. Fournier, and D. Raoult. Phylogenetic classification of Bartonella species by comparing groEL sequences. Int. J. Syst. Evol. Microbiol., in press. [DOI] [PubMed]