Objectives:
We aimed to determine the feasibility of virtual reality (VR) distraction for children with cancer undergoing subcutaneous port (SCP) access. We also aimed to estimate preliminary treatment effects of VR compared with an active distraction control (iPad).
Materials and Methods:
A single-site pilot randomized controlled trial comparing VR to iPad distraction was conducted. Eligible children and adolescents were aged 8 to 18 years undergoing treatment for cancer with upcoming SCP needle insertions. Intervention acceptability was evaluated by child, parent, and nurse self-report. Preliminary effectiveness outcomes included child-reported pain intensity, distress, and fear. Preliminary effectiveness was determined using logistic regression models with outcomes compared between groups using preprocedure scores as covariates.
Results:
Twenty participants (mean age 12 y) were randomized to each group. The most common diagnosis was acute lymphocytic leukemia (n=23, 58%). Most eligible children and adolescents (62%) participated, and 1 withdrew after randomization to the iPad group. Nurses, parents, and children reported the interventions in both groups to be acceptable, with the VR participants reporting significantly higher immersion in the distraction environment (P=0.0318). Although not statistically significant, more VR group participants indicated no pain (65% vs. 45%) and no distress (80% vs. 47%) during the procedure compared with the iPad group. Fear was similar across groups, with ~60% of the sample indicating no fear.
Discussion:
VR was feasible and acceptable to implement as an intervention during SCP access. Preliminary effectiveness results indicate that VR may reduce distress and distress compared with iPad distraction. These data will inform design of a future full-scale randomized controlled trial.
Key Words: pain, randomized controlled trial, virtual reality, pediatric
Pain is a frequent and significant problem related to cancer-directed treatment in children.1,2 Children with cancer often cite needle procedures as the most distressing experience caused by cancer and its treatment.2 Specifically, the insertion of a needle into an subcutaneous port (SCP) has been shown to be painful, frightening, and distressing to children, even when the skin is numbed with a topical anesthetic.3,4
Distraction is a well-established method to decrease pain and distress in children with cancer during medical procedures.5 Distraction may reduce both conditioned (responses to avoid pain) and unconditioned (pain associated with the procedure) pain responses by diverting attention away from the pain-eliciting stimuli.6,7 The immersive nature of virtual reality (VR) interventions mean that they may be particularly well-suited to distract children with cancer during their often repeated exposures to painful procedures.
Some studies have shown the success of VR in reducing acute pain (including related to skin-breaking procedures) in both adults and children.8–10 However, the strength of the evidence has been limited by study quality. Existing studies rarely consider the unique circumstances of children with cancer undergoing repeated painful procedures, and have not evaluated the most modern and immersive VR software.11 Our research group recently evaluated the usability (ease of use and understanding) of a VR platform for children with cancer and found VR to be safe and easy to use.12
Our next step and the primary objective of this pilot randomized controlled trial (RCT) was to determine the feasibility of implementing a VR intervention for children and adolescents with cancer undergoing SCP needle insertion. The secondary objective was to estimate preliminary treatment effects of VR compared with an active distraction control (iPad). This pilot RCT was designed to inform effectiveness testing in a future full-scale RCT. Our criteria for demonstrating study feasibility were based on previous studies.13,14 Criteria were: accrual rate of >70%, retention rate of >85%, minimal technical difficulties (ie, reported on <10% of procedures), minimal missing outcome measures (ie, <5% per measure), high acceptability based on poststudy questionnaire responses from the child, parent, nurse, and research assistant (RA), and documented adverse events.
MATERIALS AND METHODS
Trial Design
A 2-arm pilot RCT was used. Initial trial design and recruitment included a cross-over design with participants randomly allocated to receive either the VR intervention or active control (iPad) during SCP needle insertion. At the subsequent needle insertion participants would receive the alternative intervention. Due to logistic challenges outlined below, the trial was subsequently modified to a parallel 2-arm design with participants randomly allocated to study group for 1 SCP needle insertion only. The cross-over trial was registered on clinicaltrials.gov with the identifier NCT02929771. The study was approved by the locally responsible Research Ethics Board.
Participants
Eligibility
Children and adolescents were eligible for study participation if they were: (1) aged 8 to 18 years; (2) able to speak and understand English; (3) actively undergoing treatment for cancer; (4) at least 1 month, but <3 years, from initial diagnosis; and (5) requiring at least 2 SCP needle insertions for cancer-related treatment over the following 8 weeks. This last inclusion criterion was removed once the trial design changed to a parallel design. Participating parents needed to be able to speak and understand English.
Children and adolescents were excluded if they: (1) had visual, auditory or cognitive impairments precluding interaction with the intervention (VR) or control (iPad) equipment; (2) had major comorbid medical or psychiatric conditions (including needle-phobia) as reported by their health care provider or parent; (3) were receiving end-of-life care; (4) had a methicillin-resistant Staphylococcus aureus infection or symptoms of respiratory or gastrointestinal infection as reported by any member of their health care team which could contaminate the intervention or control equipment; or (5) had participated in the prior study examining usability of the VR intervention.12
Sample Size
A convenience sample of 40 children and adolescents with cancer (20 participants per treatment arm) was recruited. As pilot RCTs are not powered to be hypothesis-testing trials, formal sample size calculations were not recommended.15 Instead, sample size was informed by general recommendations for feasibility trials made by Hertzog16 which state that samples of 15 per group may be sufficient for feasibility pilot studies.
Study Setting and Recruitment
Participants were recruited from a large metropolitan pediatric hematology/oncology outpatient clinic. Potentially eligible participants were initially approached by a member of their health care team. If the child and adolescent and parent expressed interest, a RA explained the study, confirmed eligibility, and obtained written informed consent from the parents and/or the child or adolescent as appropriate in the clinic waiting room. For children unable to consent for themselves, their parents provided informed consent and assent was obtained from the child or adolescent.
Usual Care
As per the standard of care at the institution, all children had adhesive topical local anaesthetic patches placed over their SCP site one hour before their scheduled needle insertion. For the SCP needle insertion, children were situated in front of the nurse in a supine position or sat beside or on the lap of a parent (according to child preference).
Interventions
VR Headset Distraction
In addition to usual care, participants in the experimental group wore the VR head-mounted display, noise-cancelling headphones (to deliver sound) and held a wireless Bluetooth controller (to interact with the VR environment). The same equipment was previously used by our research group to evaluate the usability of the VR platform.12 The VR headset was placed on the participant and they were given instructions for how to use the controller to interact with the underwater VR environment. Once the participant was able to navigate the game, headphones were placed over their ears. The VR intervention used auditory and visual stimuli (a game which consisted of aiming rainbow balls at sea creatures as they explored an underwater environment in search of treasure) to distract the participant before, during, and after the SCP needle insertion.
iPad Distraction
In addition to usual care, participants in the active control group watched a video on an iPad while wearing the same headphones as in the experimental group. The active control participants watched the same video of an underwater environment with sea creatures and listened to the same music. The iPad was held by the RA and positioned within a meter of the participant. This distance was selected to allow the iPad to be easily seen without interfering with the clinical procedure. The use of an active distraction as control was selected due to the known effectiveness of existing of such distraction interventions in improving outcomes related to procedural pain.17,18
Procedures
Random allocation (1:1) to study group was centrally controlled and concealed using a secure, web-based service (Research Electronic Data Capture; REDCap). An RA was present in the room to ensure the technology in each of the study conditions functioned as intended, document any other interventions used (ie, parents offering consolation, topical anesthetics, child life specialist involvement), record whether the needle insertion was successful on first attempt, measure the time required for procedure set-up and conduct, and measure the time between procedure completion and completing endpoint measures.
Blinding
Participants completed preprocedure measures before being allocated to study condition (ie, before randomization). Due to the nature of the study conditions, the RA and participants could not be blinded to study condition once randomized. The statistician was blinded to group identification during the analysis. Pre-procedure measures were also completed in the waiting area, with parent and child reporting separately and out of earshot of one another. Postprocedure measures were completed separately in the treatment room.
Outcome Measures
Outcome measures were selected based on existing research recommendations and clinical relevance. Preliminary effectiveness outcomes of pain intensity, distress, and fear were selected. In studies evaluating interventions for procedural pain, it is recommended to measure multiple outcomes beyond self-reported pain intensity, such as distress, as treatment effects have been shown to differ.17,18 Additional key domains included patient satisfaction, and mechanisms known to impact VR effectiveness, such as immersion.11,18 Self-report measures were administered on paper to respondents.
Pain Intensity
Children and adolescents self-reported their pain while parents, nurses, and the RA rated the participants pain at 2 time points: current pain before the procedure, and pain during the procedure, both before and immediately following the procedure. All respondents used a well validated 11-point Numeric Rating Scale (NRS19,20) with verbal anchors of “no pain at all” at 0 and “the most pain you can imagine (this child) having” at 10.
Fear
Participants reported fear both before and following the procedure using the Child Fear Scale, which is a visual scale with established psychometrics in children as young as 5 years.21 Inter-rater reliability of the tool is 0.51 and test-retest reliability is 0.76. Convergent validity of the Child Fear Scale with another fear scale is 0.73.21
Distress
Children and adolescents, as well as parents, nurses, and the RA rated the child’s distress both before and following the procedure using an 11-point NRS, with verbal anchors of “no distress at all” at 0 and “the most distress you can imagine (this child) having” at 10. Parents reported on their own level of distress following the child’s procedure using the Parent Distress Questionnaire. Predictive validity for children’s pain and distress, and parent behaviors during medical procedures was demonstrated (0.49 to 0.50) with strong (α=0.90) internal consistency.22
Immersiveness
Immersiveness, or the degree to which one feels present in a virtual environment is a factor believed to influence the effectiveness of VR-based analgesia.23 This measure is a version of what has been used in a prior study assessing the effectiveness of VR during IV placement in children.24 It consists of 12 items on a 3-point scale (0=no, 1=a little, 2=a lot) and generates an aggregated score ranging from 0 to 24 with a higher score indicating deeper immersion. The measure was administered to children and adolescents following the procedure in both groups.
Pain Catastrophizing
Pain catastrophizing is a factor with potential to influence the effectiveness of distraction interventions for pain.6 Children and adolescents reported preprocedure their tendency to catastrophize about pain during the needle procedure using the 6-item state version of the Pain Catastrophizing Scale for Children (PCS-C), which is a self-report measure of pain catastrophizing with established psychometrics in children aged 8 years and older. Validity has been established in children aged 8 to 18 years during acute pain.25,26 Parents reported preprocedure their state tendency to catastrophize about their child’s pain during the needle procedure using the state version of the Pain Catastrophizing Scale for Parents (PCS-P), which is a 6-item self-report measure of pain catastrophizing with established psychometrics in parents of children aged 8 years and older.25 Scores on the pain catastrophizing measures ranged from 0 to 60 with higher scores indicating greater catastrophizing.
Intervention Satisfaction and Acceptability
The intervention satisfaction questionnaire was completed by children and adolescents, nurses, and parents following the procedure. The investigator-developed form collected data on acceptability, perceived utility of pain reducing procedures, and recommendations for changes related to the needle insertion experience. Acceptability questions were captured on a 4-point scale from “not at all” to “very much.” Child-reported motion sickness or dizziness was assessed preprocedure and postprocedure. If children and adolescents responded “yes” to motion sickness or dizziness, symptoms were rated on a 4-point scale from “not bad at all” to “very bad.”
Statistical Methods
Feasibility outcomes and demographic data were analyzed with descriptive statistics for each study group. Preliminary effectiveness outcomes were analyzed following an intent-to-treat approach, with all available data analyzed for all participants assigned to the group they were randomized. Due to the nonlinear distribution of effectiveness outcomes, linear mixed models as initially planned were not used. Outcomes of pain, fear, and distress were dichotomized to present or absent based on a value of 0 (absent) or greater (present). Histograms of the outcome distributions are presented in the results. A logistic model was used to analyze each outcome (pain, distress, fear) score with pretreatment values used as covariates. Intraclass correlations were calculated to determine agreement between child-reported and parent, nurse, and researcher observer-reported outcomes. Results are presented as odds ratios with corresponding 95% confidence intervals and P-values. All data were analyzed using Stata 15.1.
RESULTS
Primary Analyses: Feasibility
Participant Flow and Recruitment
Recruitment began in June 2017 and ended August 2019, with removal of the crossover design component occurring in September 2017. Recruitment was stopped when the specified sample size goal was met. Study enrolment and follow-up data are provided in the Consolidated Standards of Reporting Trials (CONSORT) flow diagram in Figure 1. The sample consisted of 40 children and adolescents with an average age of 12 years who were majority male (n=25, 63%) and most diagnosed with acute lymphoblastic leukemia (n=23, 58%). Participant demographics by study group are provided in Table 1.
FIGURE 1.
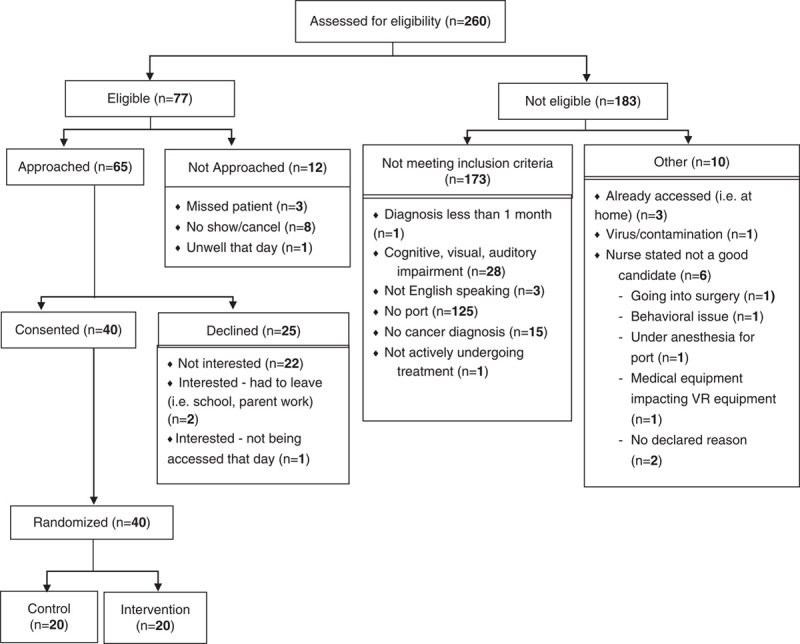
CONSORT participant flow diagram.
TABLE 1.
Demographic and Disease Characteristics of the Study Sample, N=40
| Characteristic | VR Headset, n=20 | iPad, n=20 | Total, N=40 |
|---|---|---|---|
| Age, mean (SD) (y) | 12.1 (3.0) | 12.6 (3.6) | 12.4 (3.2) |
| Sex, n (%) | |||
| Female | 7 (35) | 8 (40) | 15 (37) |
| Male | 13 (65) | 12 (60) | 25 (63) |
| Ethnicity | |||
| Arab or West Asian | 3 (15) | 0 | 3 (8) |
| Black | 2 (10) | 2 (11) | 4 (10) |
| Chinese | 1 (5) | 2 (11) | 3 (8) |
| Filipino | 0 | 1 (5) | 1 (3) |
| Korean | 1 (5) | 0 | 1 (3) |
| Latin American | 2 (10) | 0 | 2 (5) |
| South Asian | 1 (5) | 2 (11) | 3 (8) |
| White | 10 (50) | 12 (63) | 22 (56) |
| Diagnosis, n (%) | |||
| Acute lymphoblastic leukemia | 14 (70) | 9 (45) | 23 (58) |
| Brain tumor | 3 (15) | 7 (35) | 10 (25) |
| Lymphoma | 2 (10) | 1 (5) | 3 (7) |
| Other | 1 (5) | 3 (15) | 4 (10) |
| Time since diagnosis at procedure (mo), mean (SD) | 9.9 (10.3) | 11.6 (10.5) | 10.7 (10.3) |
VR indicates virtual reality.
Accrual and Retention
The cross-over design was modified to a parallel 2-arm trial following recruitment of the first 4 study participants. The change in design occurred because: (1) participants were not returning to clinic for subsequent SCP needle insertions within the specified 8-week time window or (2) identifying returning participants and implementing research procedures within the short time period children spend in the clinic was problematic. Data from all participants recruited within the cross-over design was retained and included in all analyses. These participants completed only 1 SCP needle insertion within the study and all study procedures and data collection matched those of the remaining 36 participants.
Over the 2-year and 2-month study period a total of 40 participants consented and were randomized (1.5 per month) out of the 65 eligible and approached participants (62%). The remaining 25 participants declined to participate, with the majority (n=22) reporting not being interested in participating. Reasons children were not interested included thinking that the study would add additional stress to the anxiety-provoking procedure and feeling content with their current SCP needle insertion coping strategies. Retention was high with only 1 participant in the active control group withdrawing following randomization when their group assignment was revealed.
Outcome Completion
Child-reported pain, distress, and fear, and pain catastrophizing data were available for 39 (100% of participants who did not withdraw) participants preprocedure and 38 participants (97%) postprocedure. The response rate for parent, nurse, and RA pain and distress measures ranged from n=30 (77%) to n=38 (97%). At times respondents were unavailable postprocedure to complete the measures, for example if the nurse had to provide additional care to a patient. See Table 2 for response rates at all time points for all raters.
TABLE 2.
Participant, Parent, Nurse, and Research Staff Reported Pain, Distress, and Fear (Child) Pre and During Subcutaneous Port Needle Insertion Procedures
| VR | iPad | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Outcome and Respondent | N | Mean | SD | Med. | Min | Max | n | Mean | SD | Med. | Min | Max |
| Child and adolescent | ||||||||||||
| Pain, pre | 20.0 | 0.9 | 1.6 | 0.0 | 0.0 | 5.0 | 19.0 | 0.3 | 0.7 | 0.0 | 0.0 | 2.0 |
| Pain, during | 20.0 | 0.9 | 1.5 | 0.0 | 0.0 | 4.0 | 18.0 | 1.3 | 2.3 | 1.0 | 0.0 | 10.0 |
| Distress, pre | 20.0 | 1.1 | 2.1 | 0.0 | 0.0 | 8.0 | 19.0 | 1.2 | 2.0 | 0.0 | 0.0 | 7.0 |
| Distress, during | 20.0 | 0.3 | 0.8 | 0.0 | 0.0 | 3.0 | 18.0 | 1.8 | 2.8 | 0.0 | 0.0 | 8.0 |
| Fear, pre | 20.0 | 0.8 | 1.1 | 1.0 | 0.0 | 4.0 | 19.0 | 0.9 | 1.2 | 0.0 | 0.0 | 4.0 |
| Fear, during | 19.0 | 0.4 | 0.6 | 0.0 | 0.0 | 2.0 | 18.0 | 0.8 | 1.3 | 0.0 | 0.0 | 4.0 |
| Parent | ||||||||||||
| Pain, pre | 16.0 | 3.5 | 3.2 | 3.0 | 0.0 | 10.0 | 14.0 | 2.8 | 2.7 | 2.0 | 0.0 | 8.0 |
| Pain, during | 20.0 | 1.6 | 2.4 | 1.0 | 0.0 | 9.0 | 15.0 | 2.0 | 2.3 | 1.0 | 0.0 | 7.0 |
| Distress, pre | 16.0 | 5.4 | 3.2 | 5.0 | 0.0 | 10.0 | 14.0 | 5.0 | 3.0 | 5.0 | 0.0 | 10.0 |
| Distress, during | 20.0 | 2.0 | 2.7 | 1.0 | 0.0 | 9.0 | 15.0 | 2.7 | 3.0 | 2.0 | 0.0 | 8.0 |
| Nurse | ||||||||||||
| Pain, pre | 16.0 | 1.7 | 1.6 | 1.0 | 0.0 | 5.0 | 16.0 | 2.3 | 2.1 | 2.0 | 0.0 | 8.0 |
| Pain, during | 20.0 | 1.7 | 1.9 | 1.0 | 0.0 | 6.0 | 18.0 | 2.9 | 2.7 | 2.0 | 0.0 | 10.0 |
| Distress, pre | 16.0 | 3.8 | 2.9 | 3.5 | 0.0 | 8.0 | 16.0 | 3.6 | 2.2 | 3.0 | 1.0 | 8.0 |
| Distress, during | 20.0 | 1.6 | 2.2 | 0.5 | 0.0 | 7.0 | 18.0 | 3.4 | 2.9 | 3.0 | 0.0 | 10.0 |
| Research staff | ||||||||||||
| Pain, pre | 16.0 | 1.4 | 1.9 | 0.5 | 0.0 | 5.0 | 16.0 | 1.6 | 2.4 | 0.5 | 0.0 | 7.0 |
| Pain, during | 20.0 | 1.3 | 1.6 | 0.5 | 0.0 | 5.0 | 18.0 | 1.4 | 2.4 | 0.0 | 0.0 | 8.0 |
| Distress, pre | 16.0 | 2.3 | 2.0 | 2.0 | 0.0 | 6.0 | 16.0 | 1.3 | 2.1 | 0.5 | 0.0 | 8.0 |
| Distress, during | 20.0 | 1.6 | 2.5 | 0.0 | 0.0 | 7.0 | 18.0 | 2.3 | 3.4 | 0.0 | 0.0 | 10.0 |
Outcomes obtained for participant pain, distress, and fear during the procedure were not normally distributed. Most children reported no pain, distress, and fear (ie, value of 0 on each measure) during the procedure in both groups. The distribution of all responses is presented in Figure 2 with descriptive statistics reported in Table 2.
FIGURE 2.
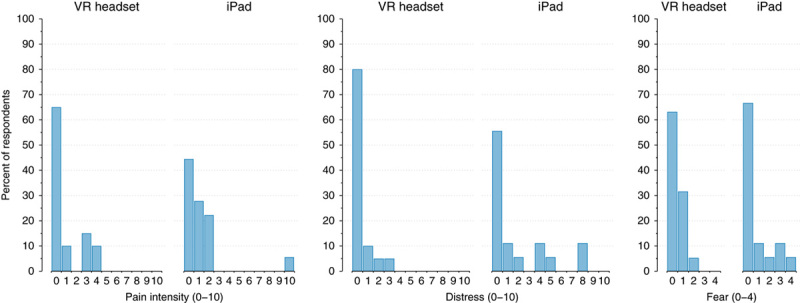
Child and adolescent reported pain, distress, and fear during subcutaneous port needle insertion procedures.
Adverse Events
No serious adverse events or harms were reported in either group. No difference in dizziness or motion sickness postprocedure or between the study groups was seen. Two (10%) participants in each group reported motion sickness or dizziness prior to the procedure, with 2 VR (10%) and 3 iPad group (17%) participants reporting motion sickness or dizziness during the procedure.
Intervention Implementation and Technical Issues
Technical and other issues were minimal. In the active control group, 1 participant stopped the iPad intervention before the SCP needle insertion due to anxiety around the procedure. Another active control group participant did not receive the active control intervention as the SCP needle insertion was conducted before the iPad could be provided. One participant in the VR group requested headphones be removed during the procedure.
Acceptability and Immersiveness
Participant-reported and parent-reported acceptability was high in both study groups. Mean acceptability scores for parents and children and adolescents are provided in Table 3. Nurse-reported acceptability of the VR intervention was also high. Thirteen (65%) nurses reported that the VR headset had no negative impact on clinical workflow during SCP needle insertion and no nurses reporting that it “completely” negatively impacted clinic workflow. For all VR procedures, 20 nurses (100%) indicated that it was “as easy” or “easier” to conduct the SCP needle insertion with the VR intervention compared with without. In the iPad group, nurses indicated on 17 (95%) procedures that it was “as easy” or “easier” to conduct the SCP needle insertion with the iPad compared with without.
TABLE 3.
Child and Adolescent and Parent Reported Acceptability of the VR and iPad Interventions
| Child | Parent | |||
|---|---|---|---|---|
| Acceptability Item | VR, n=20 | iPad, n=18 | VR, n=17 | iPad, n=13 |
| How much did you like having the VR headset or iPad during your (child’s) port access (SCP needle insertion)? | 3.3 (1.0) | 3.3 (0.8) | 3.6 (0.8) | 3.3 (0.9) |
| How helpful was the VR headset or iPad during your (child’s) port access (SCP needle insertion)? | 2.9 (1.1) | 2.9 (1.0) | 3.4 (1.0) | 3.0 (1.2) |
| How much did the VR headset or iPad decrease hurt/pain during your (child’s) port access (SCP needle insertion)? | 2.3 (1.1) | 2.3 (1.1) | 3.1 (1.1) | 2.6 (1.2) |
| If you need a port access next time, how much would you want the VR headset or iPad for distraction? | 3.0 (0.9) | 3.0 (1.0) | 3.5 (0.9) | 2.9 (1.2) |
SCP indicates subcutaneous port; VR, virtual reality.
Participant-reported immersiveness with the intervention (VR or iPad) was significantly higher (P=0.0318) in the VR group (M=16.4, SD=5.4) compared with the control group (M=14.5, SD=5.4).
Secondary Analyses
Pain and Distress Interrater Agreement (Self-report vs. Observer)
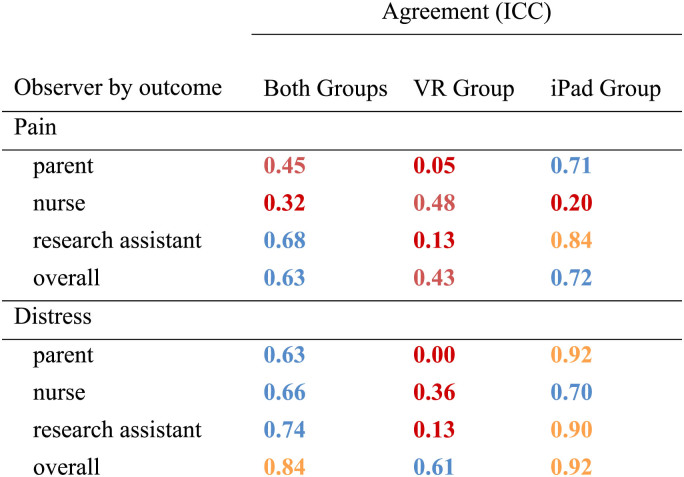
Agreement with participant-reported pain and distress was examined for parent, nurse, and RA observer reports. Results are shown in Table 4. Agreement for all respondents and outcomes were broadly “poor” in the VR group.27 In the iPad group, agreement ranged from “poor” to “excellent”. Given the low observer reported reliability with child reported outcomes, as well as the difference in reliability between study groups, preliminary effectiveness results focus on participant-report only.
TABLE 4.
Agreement (ICC) Between Child and Adolescent-reported and Parent, Nurse, and Researcher Observer-reported Outcomes
Red corresponds to poor agreement (<0.40); orange corresponds to fair agreement (0.40 to 0.59); blue corresponds to good agreement (0.60 to 0.74); green corresponds to excellent agreement (0.75 to 1.00).
ICC indicates intraclass correlation coefficient; VR, virtual reality.
Preliminary Effectiveness
Estimates of preliminary effectiveness using the dichotomized participant-reported outcomes of pain, distress, and fear are presented in Table 5. None of the preliminary effectiveness outcomes were statistically significant between groups. Adjusting for pain before the procedure, the odds of having pain during the procedure among those in the active control iPad group was 3 times the odds for a participant who used VR. For distress, the odds of having distress during the procedure among those in the iPad group was 4.1 times the odds for a Participants who used VR, adjusting for distress before the procedure. Adjusting for fear before the procedure, the odds of being scared during the needle insertion was the same across interventions. Descriptively, 65% (n=13) of participants in the VR group reported no pain during the procedure, compared with 45% (n=8) in the iPad group. For distress, 80% of VR participants (n=16) reported no distress, compared to 56% of iPad participants (n=10). Scores for fear were similar in both groups with 63% (n=12) of VR participants and 67% (n=12) of iPad participants reporting no fear.
TABLE 5.
Preliminary Effectiveness Estimates for Procedural Child-reported Pain, Distress, and Fear, Adjusting for Preprocedure Ratings
| Outcome | Odds Ratio | SE | P | 95% Confidence Interval |
|---|---|---|---|---|
| Pain intensity | ||||
| Preprocedure | 3.238 | 2.657 | 0.152 | 0.648-16.174 |
| Group: iPad control | 3.063 | 2.226 | 0.123 | 0.737-12.725 |
| Distress | ||||
| Preprocedure | 6.639 | 5.460 | 0.021 | 1.325-33.275 |
| Group: iPad control | 4.148 | 3.438 | 0.086 | 0.817-21.056 |
| Fear | ||||
| Fear preprocedure | 5.545 | 4.336 | 0.029 | 1.197-25.678 |
| Group: iPad control | 0.877 | 0.653 | 0.860 | 0.204-3.774 |
Preprocedural participant-reported pain catastrophizing was low in the VR group (M=9.0, SD=11.5) and the iPad group (M=13.8, SD=14.9). Parents catastrophized more about child pain in both groups (M=19.3, SD=14.2) and (M=21.1, SD=14.2) compared with children’s and adolescents reports of their own pain catastrophizing.
DISCUSSION
The results of this pilot RCT indicate that VR is a feasible and acceptable intervention to implement within pediatric oncology for SCP needle insertion. Results indicate that children and adolescents who use VR as an intervention have a desire to continue to use VR during future SCP insertions. We show that, within the context of our study site, a cross-over study design is not feasible due to difficulties following participants over time. In contrast, a 2-arm parallel study design is feasible. While our study accrual rate (63%) was below our target of 70% or higher, retention (97%) was high, along with 95% or higher rates of outcome completion for child report. The accrual rate of 63% is also higher than rates reported in other pediatric VR pilot studies.28 Moving forward, engaging patients in the study design and recruitment strategies can be used to improve accrual rates.29 Outcome completion rates were lower for observer-reported outcomes by parents, nurses, and the RA than for children. Children and adolescents, parents, and nurses reported high satisfaction with the VR intervention. Nurses found use of the VR headset integrated well into their workflow. No serious adverse events were reported, and levels of dizziness or motion sickness were the same in the VR and iPad groups.
Low inter-rater agreement was identified when comparing child and adolescent report to all observer reports. These findings are aligned with existing research which supports child-reported pain as the reference standard.30 When considered along with the lower rates of completion for observer outcomes, collecting only child-reported primary outcomes would be the most efficient, feasible, and valid method for future research. Of note, inter-rater agreement was lower in the VR group when compared with iPad. It is possible that the VR headset impacted the ability of the other respondents to provide an observer rating of child pain and distress because facial cues relevant for observer interpretation of others’ pain experience may be obscured.31
In terms of preliminary estimates of effectiveness, overall pain, distress, and fear was low in both groups, which may indicate effectiveness of the interventions in both groups. Less pain and distress were seen in the VR group compared with the iPad group, while fear scores remained similar between study groups. The results should be interpreted conservatively due to the small sample size, correspondingly large confidence intervals, and dichotomized outcomes. In this study, VR was compared with a known effective distraction intervention (iPad) and all participants received topical anesthetic as part of standard care.32 Other studies in the same population have compared VR with no distraction.11,33,34 Such studies have also been limited by small sample sizes. A recent Cochrane review of VR for distraction in acute pediatric pain found that sample sizes were not large enough to draw strong conclusions. The review highlighted the need for future large-scale studies (minimum 200 participants per arm) comparing VR to other technology distractions (eg, iPad) to determine effectiveness in a range of clinical settings. The results of this pilot study are well positioned to guide development of a full-scale study in line with the recommendations and findings of the Cochrane review.
The study was limited in terms of the ability to evaluate preliminary effectiveness due to the distribution of outcome data. This was the result of low levels of reported pain, distress, and fear in the sample, which were lower than expected based on previous research.35,36 It is possible that the study participants existing experience with the SCP procedure led to reduced scores on the outcomes, or that those with the highest levels of distress and fear chose not to consent to this research. Future studies could aim to recruit participants at their first SCP procedure to increase the availability of the intervention, reduce potential development of needle fear, and increase the heterogeneity of the sample.37 The study design also resulted in a potential limitation. The design was altered after 4 participants were recruited to remove the cross-over design component, but no participant data were removed from the analysis as a result. The study was conducted in one tertiary care pediatric center, with the VR and iPad intervention support provided by research staff versus clinical staff (eg, child life specialist). Feasibility and implementation of the interventions may differ without this dedicated support. As well, all participants received the same VR intervention (same virtual environment and gamification), which may have impacted acceptability, immersiveness, and effectiveness compared with a different VR program, and limits the generalizability of the findings related to other VR interventions.
CONCLUSION
In this sample of pediatric oncology patients presenting for SCP needle insertion, VR as a distraction intervention was feasible and acceptable to patients, their families, and clinicians. Important considerations for outcome selection and study design were identified, including the importance of patient-reported outcomes compared with proxy when using VR headsets. Preliminary effectiveness data supports evaluating VR to reduce procedural pain and distress in a future full-scale trial in this population.
ACKNOWLEDGMENTS
The authors thank all families who participated in this research.
Footnotes
Research funding was provided by the SickKids Garron Family Cancer Centre (Toronto, Canada) and the SickKids Pain Centre (Toronto, Canada). The authors declare no conflict of interest.
Contributor Information
Amos S. Hundert, Email: amos.hundert@sickkids.ca.
Kathryn A. Birnie, Email: kathryn.birnie@ucalgary.ca.
Oussama Abla, Email: oussama.abla@sickkids.ca.
Karyn Positano, Email: karyn.positano@sickkids.ca.
Celia Cassiani, Email: celiamarie.cassiani@mail.utoronto.ca.
Sarah Lloyd, Email: sarahdelloyd@gmail.com.
Petra Hroch Tiessen, Email: petra.hroch@mail.utoronto.ca.
Chitra Lalloo, Email: chitra.lalloo@sickkids.ca.
Lindsay A. Jibb, Email: lindsay.jibb@sickkids.ca.
Jennifer Stinson, Email: jennifer.stinson@sickkids.ca.
REFERENCES
- 1.Collins JJ. Cancer pain management in children. Eur J Pain. 2001;5(SA):37–41. [DOI] [PubMed] [Google Scholar]
- 2.Kestler SA, LoBiondo-Wood G. Review of symptom experiences in children and adolescents with cancer. Cancer Nurs. 2012;35:E31–E49. [DOI] [PubMed] [Google Scholar]
- 3.Lüllmann B, Leonhardt J, Metzelder M, et al. Pain reduction in children during port-à-cath catheter puncture using local anaesthesia with EMLATM. Eur J Pediatr. 2010;169:1465–1469. [DOI] [PubMed] [Google Scholar]
- 4.Liossi C, White P, Hatira P. A randomized clinical trial of a brief hypnosis intervention to control venepuncture-related pain of paediatric cancer patients. Pain. 2009;142:255–263. [DOI] [PubMed] [Google Scholar]
- 5.Jibb L, Nathan P, Stevens B, et al. Psychological and physical interventions for the management of cancer-related pain in pediatric and young adult patients: an integrative review. Oncol Nurs Forum. 2015;42:E339–E357. [DOI] [PubMed] [Google Scholar]
- 6.Birnie KA, Chambers CT, Spellman CM. Mechanisms of distraction in acute pain perception and modulation. Pain. 2017;158:1012–1013. [DOI] [PubMed] [Google Scholar]
- 7.Koller D, Goldman RD. Distraction techniques for children undergoing procedures: a critical review of pediatric research. J Pediatr Nurs. 2012;27:652–681. [DOI] [PubMed] [Google Scholar]
- 8.Law EF, Dahlquist LM, Sil S, et al. Videogame distraction using virtual reality technology for children experiencing cold pressor pain: the role of cognitive processing. J Pediatr Psychol. 2011;36:84–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Garrett B, Taverner T, Masinde W, et al. A rapid evidence assessment of immersive virtual reality as an adjunct therapy in acute pain management in clinical practice. Clin J Pain. 2014;30:1089–1098. [DOI] [PubMed] [Google Scholar]
- 10.Hua Y, Qiu R, Yao W-Y, et al. The effect of virtual reality distraction on pain relief during dressing changes in children with chronic wounds on lower limbs. Pain Manag Nurs. 2015;16:685–691. [DOI] [PubMed] [Google Scholar]
- 11.Lambert V, Boylan P, Boran L, et al. Virtual reality distraction for acute pain in children. Cochrane database Syst Rev. 2020;10:CD010686. doi: 10.1002/14651858.CD010686.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Birnie KA, Kulandaivelu Y, Jibb L, et al. Usability testing of an interactive virtual reality distraction intervention to reduce procedural pain in children and adolescents with cancer. J Pediatr Oncol Nurs. 2018;35:406–416. [DOI] [PubMed] [Google Scholar]
- 13.Stinson JN, Jibb LA, Nguyen C, et al. Development and testing of a multidimensional iPhone pain assessment application for adolescents with cancer. J Med Internet Res. 2013;15:e51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stinson JN, McGrath PJ, Hodnett ED, et al. An internet-based self-management program with telephone support for adolescents with arthritis: a pilot randomized controlled trial. J Rheumatol. 2010;37:1944–1952. [DOI] [PubMed] [Google Scholar]
- 15.Arain M, Campbell MJ, Cooper CL, et al. What is a pilot or feasibility study? A review of current practice and editorial policy. BMC Med Res Methodol. 2010;10:67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hertzog MA. Considerations in determining sample size for pilot studies. Res Nurs Health. 2008;31:180–191. [DOI] [PubMed] [Google Scholar]
- 17.Birnie KA, Noel M, Chambers CT, et al. Psychological interventions for needle-related procedural pain and distress in children and adolescents. Cochrane database Syst Rev. 2018;10:CD005179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McGrath PJ, Walco GA, Turk DC, et al. Core outcome domains and measures for pediatric acute and chronic/recurrent pain clinical trials: PedIMMPACT recommendations. J Pain. 2008;9:771–783. [DOI] [PubMed] [Google Scholar]
- 19.Cohen LL. Reducing infant immunization distress through distraction. Health Psychol. 2002;21:207–211. [PubMed] [Google Scholar]
- 20.Birnie KA, Hundert AS, Lalloo C, et al. Recommendations for selection of self-report pain intensity measures in children and adolescents: a systematic review and quality assessment of measurement properties. Pain. 2019;160:5–18. [DOI] [PubMed] [Google Scholar]
- 21.McMurtry CM, Noel M, Chambers CT, et al. Children’s fear during procedural pain: preliminary investigation of the Children’s Fear Scale. Health Psychol. 2011;30:780–788. [DOI] [PubMed] [Google Scholar]
- 22.Caes L, Vervoort T, Devos P, et al. Parental distress and catastrophic thoughts about child pain: implications for parental protective behavior in the context of child leukemia-related medical procedures. Clin J Pain. 2014;30:787–799. [DOI] [PubMed] [Google Scholar]
- 23.Triberti S, Repetto C, Riva G. Psychological factors influencing the effectiveness of virtual reality-based analgesia: a systematic review. Cyberpsychol Behav Soc Netw. 2014;17:335–345. [DOI] [PubMed] [Google Scholar]
- 24.Gold JI, Kim SH, Kant AJ, et al. Effectiveness of virtual reality for pediatric pain distraction during i.v. placement. Cyberpsychol Behav. 2006;9:207–212. [DOI] [PubMed] [Google Scholar]
- 25.Durand H, Birnie KA, Noel M, et al. State versus trait: validating state assessment of child and parental catastrophic thinking about children’s acute pain. J Pain. 2017;18:385–395. [DOI] [PubMed] [Google Scholar]
- 26.Birnie KA, Chambers CT, Chorney J, et al. Dyadic analysis of child and parent trait and state pain catastrophizing in the process of children’s pain communication. Pain. 2016;157:938–948. [DOI] [PubMed] [Google Scholar]
- 27.Cicchetti DV. Guidelines, criteria, and rules of thumb for evaluating normed and standardized assessment instruments in psychology. Psychol Assess. 1994;6:284–290. [Google Scholar]
- 28.Goldman RD, Behboudi A. Virtual reality for intravenous placement in the emergency department-a randomized controlled trial. Eur J Pediatr. 2021;180:725–731. [DOI] [PubMed] [Google Scholar]
- 29.Fern LA, Taylor RM. Enhancing accrual to clinical trials of adolescents and young adults with cancer. Pediatr Blood Cancer. 2018;65:e27233. [DOI] [PubMed] [Google Scholar]
- 30.Zhou H, Roberts P, Horgan L. Association between self-report pain ratings of child and parent, child and nurse and parent and nurse dyads: meta-analysis. J Adv Nurs. 2008;63:334–342. [DOI] [PubMed] [Google Scholar]
- 31.Craig KD, Prkachin KM, Grunau RE.Turk DC, Melzack R. The facial expression of pain. Handbook of Pain Assessment. New York: The Guilford Press; 2011:117–133. [Google Scholar]
- 32.Ali S, Ma K, Dow N, et al. A randomized trial of iPad distraction to reduce children’s pain and distress during intravenous cannulation in the paediatric emergency department. Paediatr Child Health. 2020;26:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nilsson S, Finnström B, Kokinsky E, et al. The use of virtual reality for needle-related procedural pain and distress in children and adolescents in a paediatric oncology unit. Eur J Oncol Nurs. 2009;13:102–109. [DOI] [PubMed] [Google Scholar]
- 34.Windich-Biermeier A, Sjoberg I, Dale JC, et al. Effects of distraction on pain, fear, and distress during venous port access and venipuncture in children and adolescents with cancer. J Pediatr Oncol Nurs. 2007;24:8–19. [DOI] [PubMed] [Google Scholar]
- 35.Ljungman G, Gordh T, Sörensen S, et al. Pain variations during cancer treatment in children: a descriptive survey. Pediatr Hematol Oncol. 2000;17:211–221. [DOI] [PubMed] [Google Scholar]
- 36.Ljungman G, Kreuger A, Gordh T, et al. Treatment of pain in pediatric oncology: a Swedish nationwide survey. Pain. 1996;68:385–394. [DOI] [PubMed] [Google Scholar]
- 37.Taddio A, Ipp M, Thivakaran S, et al. Survey of the prevalence of immunization non-compliance due to needle fears in children and adults. Vaccine. 2012;30:4807–4812. [DOI] [PubMed] [Google Scholar]