Supplemental Digital Content is available in the text.
Keywords: adolescent; atherosclerosis; causality; diabetes mellitus, type 2; hyperglycemia; metabolic syndrome; young adult
Background:
We investigated the temporal causal longitudinal associations of carotid-femoral pulse wave velocity (cfPWV), a measure of arterial stiffness, and carotid intima-media thickness (cIMT) progression with the risk of dysglycemia, insulin resistance, and dyslipidemia.
Methods:
We included 3862, 17.7-year-old, participants from the Avon Longitudinal Study of Parents and Children, followed up for 7 years. cfPWV, cIMT, and fasting plasma samples were repeatedly measured. We computed homeostatic model assessment (HOMA) of insulin resistance and percent pancreatic beta-cell function. Data were analyzed using logistic regression, linear mixed-effect, and cross-lagged structural equation models.
Results:
A higher cfPWV at 17.7 years was associated with higher insulin at age 24.5 years (odds ratio, 1.25 [CI, 1.08–1.44]; P=0.003), which slightly attenuated after covariates adjustment. Higher cIMT at 17.7 years was associated with lower insulin (odds ratio, 0.06 [0.01–0.95]; P=0.046) at 24.5 years, after covariate adjustments. In mixed-effect models, the 7-year progression in cfPWV (predictor) was directly associated with the increase in triglyceride (outcome). cIMT progression was associated with the 7-year increase in LDL (low-density lipoprotein), triglyceride, and glucose. In cross-lagged models, higher cfPWV at 17.7 years was associated with higher insulin (β=0.06, SE, 0.12, P=0.014), HOMA of insulin resistance, and HOMA-percent pancreatic beta-cell function at 24.5 years. However, insulin, HOMA of insulin resistance, and HOMA-percent pancreatic beta-cell function at 17.7 years were not associated with cfPWV at 24.5 years. Higher cIMT at 17.7 years was associated with reduced insulin, HOMA of insulin resistance, and HOMA-percent pancreatic beta-cell function at 24.5 years, but not vice versa. Higher glucose at 17.7 years was associated with higher cfPWV and cIMT at 24.5 years only.
Conclusions:
Arterial stiffness in adolescence may be a causal risk factor for hyperinsulinemia and insulin resistance in young adulthood.
The global prevalence of young-onset type 2 diabetes, that is, type 2 diabetes diagnosed before the age of 40 years, is on the increase and cardiometabolic morbidities and mortality related to young-onset type 2 diabetes are driven by its long duration.1 Young-onset type 2 diabetes is characterized by rapid deterioration of beta-cell function on the background of insulin resistance2 and altered metabolic milieu.3 In a randomized controlled trial, metformin monotherapy or lifestyle modification failed to treat young-onset type 2 diabetes, warranting further research on ways to ameliorate the risk in early life.3–5 Other risk factors that are strongly associated with young-onset type 2 diabetes are high blood pressure, high LDL (low-density lipoprotein) cholesterol, obesity, and a family history of diabetes.1,3,4 It is also known that altered vascular function and structure, such as stiffened arteries, is a complication of young-onset type 2 diabetes.3
However, emerging evidence among apparently healthy adults aged 48.3±12.0 years followed up for 3.72 years revealed that higher arterial stiffness may precede higher fasting blood glucose and could be a novel risk factor in the causal pathway of incident type 2 diabetes.6 To date, among adolescents and young adults, no study has investigated whether altered arterial function and structure, independent of known risk factors,3 precede the risk of dysglycemia, hyperinsulinemia, and insulin resistance that are precursors of young-onset type 2 diabetes and its comorbidities, such as dyslipidemia. Such insight is important and timely as the Lancet Commission on diabetes has recently recommended that young-onset diabetes requires improved risk stratification and disease classification.1 Therefore, we examined the temporal causal longitudinal associations of carotid-femoral pulse wave velocity (cfPWV), a measure of arterial stiffness, and carotid intima-media thickness (cIMT) with fasting LDL, HDL (high-density lipoprotein) cholesterol, triglyceride, glucose, insulin, insulin resistance, and pancreatic beta-cell function among 3862 adolescents followed up for 7 years, using data from the ALSPAC (Avon Longitudinal Study of Parents and Children) birth cohort, England, United Kingdom.
Methods
Data Availability Statement
The informed consent obtained from ALSPAC participants does not allow the data to be made freely available through any third-party maintained public repository. However, data used for this submission can be made available on request to the ALSPAC Executive. The ALSPAC data management plan describes in detail the policy regarding data sharing, which is through a system of managed open access. Full instructions for applying for data access can be found here: http://www.bristol.ac.uk/alspac/researchers/access/. The ALSPAC study website contains details of all the data that are available (http://www.bristol.ac.uk/alspac/researchers/our-data/).
Study Cohort
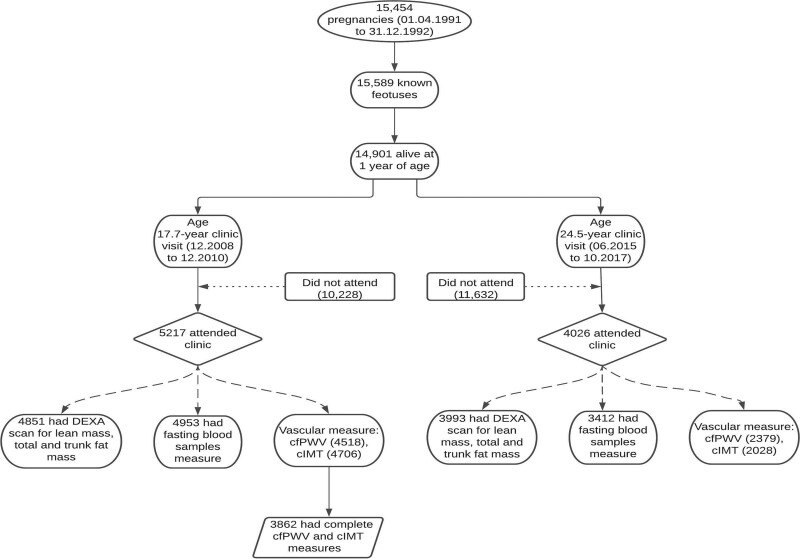
Details of the ALSPAC birth cohort have been published earlier7–9 and are summarized in the Supplemental Material. The ALSPAC birth cohort investigates factors that influence childhood development and growth. Altogether, 14 541 pregnancies from women residing in Avon, southwestern England, United Kingdom, who had a total of 14 676 fetuses, were enrolled between April 1, 1991, and December 31, 1992. When the oldest children were ≈7 years of age, an attempt was made to bolster the initial sample with eligible cases who had failed to join the study originally resulting in 913 additional pregnancies. The total sample size for analyses using any data collected after 7 years of age was 15 454 pregnancies, resulting in 15 589 fetuses. Of these 14 901 were alive at 1 year of age. Regular clinic visits of the children commenced at 7 years of age and are still ongoing. Study data at 24.5 years were collected and managed using REDCap electronic data capture tools.10 For our analysis, we included participants who had both cfPWV and cIMT measurements at age 17.7 years (Figure 1). The demographic characteristics of excluded participants were similar to those included in this study (Table S1 in the Supplemental Material). Ethical approval for the study was obtained from the ALSPAC Ethics and Law Committee and the Local Research Ethics Committees. Consent for biological samples has been collected in accordance with the Human Tissue Act (2004). Informed consent for the use of data collected via questionnaires and clinics was obtained from participants following the recommendations of the ALSPAC Ethics and Law Committee at the time.
Figure 1.
Flowchart of study participants. Participants that had complete predictor and or outcome of interest at 17.7 y were included in the analyses. cfPWV indicates carotid-femoral pulse wave velocity; cIMT, carotid intima-media thickness; and DEXA, dual-energy X-ray absorptiometry.
All blood biochemical values were repeatedly measured from fasting samples at age 17.7 and 24.5 years as detailed in the Supplemental Material. Total fat mass and lean mass were assessed using a dual-energy X-ray absorptiometry scanner. At age 17.7 years, cfPWV was assessed from pressure waveforms obtained using the Vicorder device (Skidmore Medical, Bristol, United Kingdom).11–13 At 24.5 years, cfPWV was measured, after five minutes of resting in a semi-prone position, using a Vicorder instrument (Skidmore Medical, Bristol, United Kingdom) which has 2 blood pressure measurement channels and 2 Velcro pressure sensor cuffs applied over each of the carotid and femoral arteries.13 At age of 17.7 years, cIMT was assessed by ultrasound using a linear 12-MHz transducer (Vivid7, GE Medical, Chicago, IL). The average of cIMT at 17.7 years serially measured at 3 different cardiac cycles was computed. Interobserver variability for cIMT was assessed in a separate sample of 25 young adults (coefficient of variation: 4.4±2.2%).11,13 The right and left common carotid arteries at age 24.5 years were imaged using an ultrasound machine (CardioHealth Panasonic and a 13.5 MHz linear array broadband transducer (probe; center frequency 9.0 MHz) in line with standard protocols and detailed in the Supplemental Material.13 Participants with fasting HDL <1.0 mmol/L, LDL >3.0 mmol/L, triglyceride >2.0 mmol/L, were categorized as at risk of dyslipidemia14 and glucose >6.1 mmol/L, and insulin >11.78 mU/L, were categorized at risk of hyperglycemia and hyperinsulinemia.2,15,16 We calculated homeostatic model assessment of insulin resistance (HOMA-IR) and HOMA-percent pancreatic beta-cell function (HOMA-%β) from (fasting insulin×fasting glucose/22.5) and ([20×fasting insulin]/[fasting glucose−3.5]), respectively.17 Participants had young-onset type 2 diabetes when clinic fasting glucose was ≥7 mmol/L or reported physician diagnosis at 17.7 or 24.5 years clinic visit.2 Further details of the measurement procedure for fasting lipids, glucose, insulin, cfPWV, cIMT, and other covariates such as heart rate and blood pressure are contained in the Supplemental Material. Questionnaires to assess smoking behavior were administered at the 17.7-year and 24.5-year clinic visits. At the 17.7-year clinic visit, participants were briefly asked about their personal and family (mother, father, and siblings) medical history, such as a history of hypertension, diabetes, high cholesterol, and vascular disease. Moderate to vigorous physical activity at age 15.5 years was assessed with ActiGraphTM accelerometer worn for 7 days whereas at 24.5 years moderate to vigorous physical activity was assessed using ActiGraph GT3X+ accelerometer device worn for four consecutive days, ideally starting the day after the clinic visit. Missing data were handled with multiple imputations11–13 (see the Appendix in the Supplemental Material and Tables S2 and S3).
Statistical Analysis
Participant’s descriptive characteristics were summarized as means and standard deviation, medians, and interquartile ranges, or frequencies and percentages. We explored sex differences using independent t tests, Mann-Whitney U tests, or χ2 tests for normally distributed, skewed or dichotomous variables, respectively. We assessed the normality of variables and logarithmically or reciprocally transformed skewed variables before further analyses.
We investigated the separate longitudinal associations of cfPWV and cIMT (predictors) at 17.7 years with each of fasting LDL, HDL, triglyceride, insulin, and glucose categories (outcomes) at 24.5 years using binary logistic regression models (Supplemental Material). We also examined the separate associations of the 7-year progression in cfPWV and cIMT with the longitudinal progression in each of the metabolic outcomes from ages 17.7 to 24.5 years using linear mixed-effect models for repeated measures. Analyses were adjusted for sex, age at 17.7 years, and covariates repeatedly measured at ages 17.7 and 24.5 years, viz, resting heart rate, systolic blood pressure, fat mass, lean mass, high-sensitivity C-reactive protein, smoking status, family history of hypertension, diabetes, high cholesterol or vascular disease and moderate to vigorous physical activity at 15.5 and 24.5 years as well as fasting plasma samples; LDL, HDL, insulin, triglyceride, or glucose, depending on the outcome.
Lastly, we used structural equation modeling with autoregressive cross-lagged path analysis (detailed in the Supplemental Material and published earlier13) to examine the separate temporal causal associations of cfPWV and cIMT with metabolic outcomes, adjusting for covariates listed above. All covariates were selected based on previous studies.3,11–13,18,19 We examined sex interactions and presented sex-stratified results. We also presented body mass index–weight stratified results, cross-sectional results, and age- and sex-adjusted partial correlation analyses in Tables S4 through S7. Differences and associations with a 2-sided P<0.05 were considered statistically significant with conclusions based on effect estimates and their CI or SE. Analyses involving 40% of a sample of 10 000 ALSPAC children at 0.8 statistical power, 0.05 alpha, and 2-sided P value would show a minimum detectable effect size of 0.049 standard deviations if they had relevant exposure for a normally distributed quantitative variable.20 All statistical analyses were performed using SPSS statistics software, Version 27.0 (IBM Corp, Armonk, NY), and structural equation modeling were conducted using IBM AMOS version 27.0.
Results
Cohort Study Characteristics
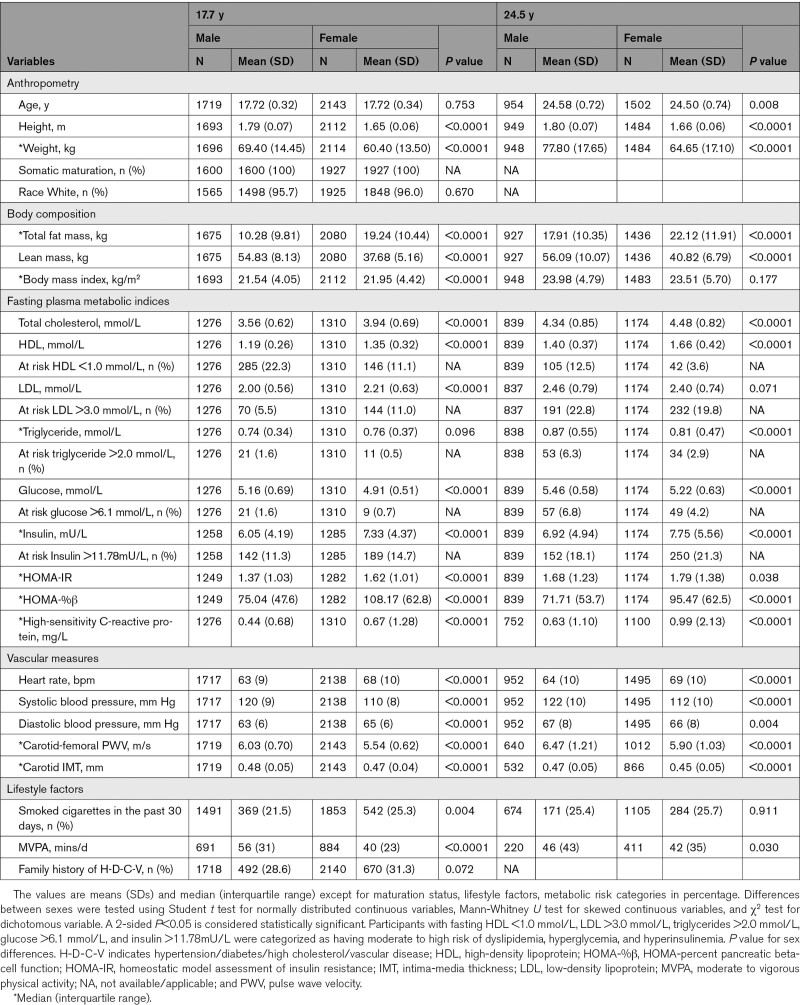
Of the 14 901 children in the ALSPAC birth cohort who were alive at 1 year of age, 5217 adolescents participated in the 17.7-year follow-up clinic visit and 4026 young adults participated in the 24.5-year follow-up clinic visit (Figure 1). We studied 3862 participants who had complete cfPWV and cIMT measurements at age 17.7 years. Females had higher fasting total cholesterol, HDL, and insulin but lower glucose compared to males at both 17.7 and 24.5 years. At 24.5 years, 9.2%, 41.6%, 24.5%, 7.5%, and 10.3% of 3862 participants were at risk of high glucose, insulin, LDL, triglyceride, and low HDL, respectively. Fewer than 8 participants had youth-onset type 2 diabetes at ages 17.7 and 24.5 years. Pancreatic beta-cell function decreased, and insulin resistance increased from ages 17.7 through 24.5 years. Other participants’ characteristics are shown in Table 1 and Tables S1–S3.
Table 1.
Descriptive Characteristics of Cohort Participants
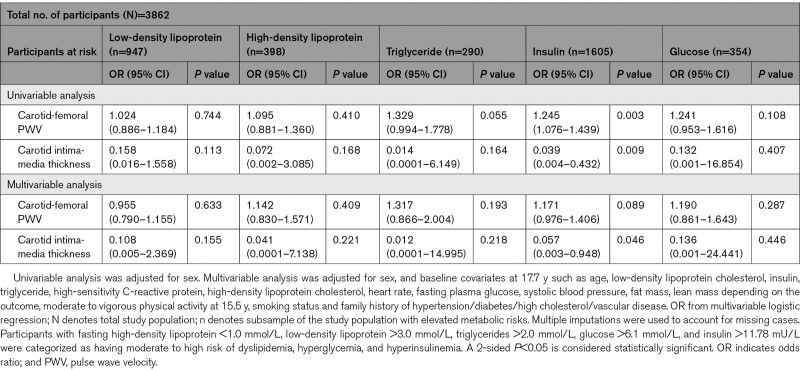
Longitudinal Associations of cfPWV and cIMT at 17.7 Years With Risk Categories of Metabolic Derangements at Age 24.5 Years
A higher cfPWV at 17.7 years was associated with the risk of high insulin at age 24.5 years, although the effect was attenuated and no longer significant, after adjusting for cardiometabolic and lifestyle factors (Table 2). A higher cIMT at 17.7 years was associated with the risk of low insulin at 24.5 years (odds ratio, 0.06 [0.01–0.95]; P=0.046). cfPWV and cIMT were not associated with other metabolic risks. In the cross-sectional analyses, cfPWV at 17.7 years was unrelated to measures of metabolic risks at 17.7 years, but higher cfPWV at 24.5 years was associated with higher insulin and glucose at 24.5 years (Table S4). cIMT at either age 17.7 or 24.5 years was not associated with elevated metabolic alterations at age 17.7 or 24.5 years, respectively (Table S4).
Table 2.
Carotid-Femoral Pulse Wave Velocity and Carotid Intima-Media Thickness at 17.7 Years in Association With Moderate to High-Risk Categories of Altered Metabolic and Lipid Indices at 24.5 Years
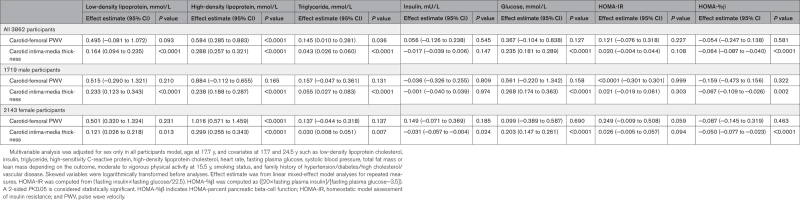
Effect of cfPWV and cIMT Progression on Metabolic and Lipid Progression From Ages 17.7 to 24.5 Years
A 7-year cfPWV progression was directly associated with the 7-year increase in HDL: (effect estimate 0.58 mmol/L [0.29–0.88]; P<0.0001) and triglyceride: (0.15 mmol/L [0.01–0.28]; P=0.036), but not with LDL, glucose, HOMA-IR, and HOMA-%β after adjustment for cardiometabolic and lifestyle factors (Table 3). The sex-stratified results were similar to the combined results except that among males, increased cfPWV was not associated with HDL and triglyceride (Table 3). According to body mass index–weight categories, cfPWV progression was associated with the increase in HDL in both normal-weight and overweight/obese participants. However, cfPWV progression was associated with increased beta-cell function only among overweight/obese participants (0.53 [0.06–1.01]; P=0.027; Table S5).
Table 3.
Longitudinal Progression in Arterial Stiffness and Carotid Intima-Media Thickness in Relation to Progression in Fasting Metabolic and Lipid Indices From Age 17.7 to 24.5 Years
A 7-year progression in cIMT was directly associated with the 7-year increase in LDL, HDL, triglyceride, and glucose but negatively associated with HOMA-%β (−0.06 [−0.09 to −0.04]; P<0.0001), after adjustment for cardiometabolic and lifestyle factors (Table 3). The sex-stratified results were in tandem with the combined results except that among females, cIMT progression was inversely associated with insulin (Table 3). cIMT progression was directly associated with the increase in LDL, HDL, triglyceride, glucose, but negatively associated with HOMA-%β (−0.09 [−0.11 to −0.06]; <0.0001) among normal-weight individuals (Table S5). However, cIMT progression was directly associated with the increase in LDL, HDL, and glucose among overweight/obese participants (Table S5).
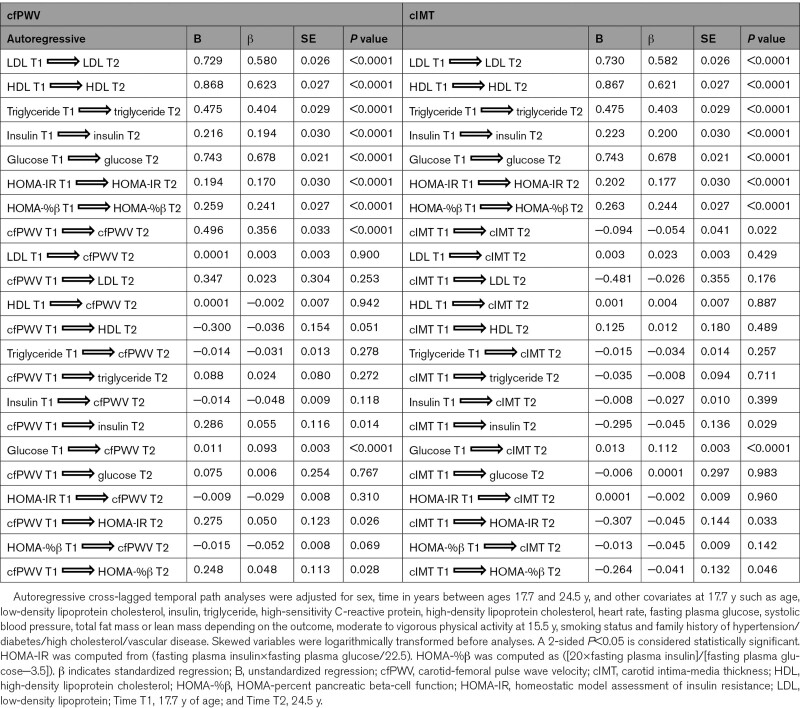
Autoregressive Cross-Lagged Temporal Causal Longitudinal Associations of cfPWV and cIMT With Metabolic and Lipid Indices
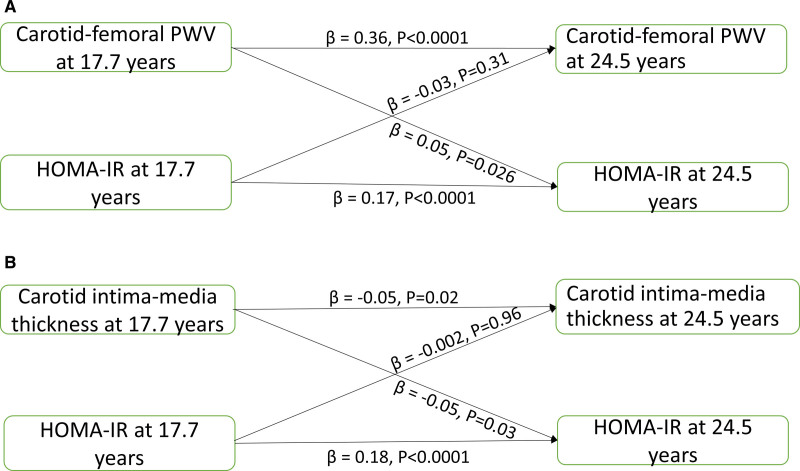
LDL, HDL, triglyceride, insulin, glucose, HOMA-IR, HOMA-%β, and cfPWV at 17.7 years were directly and independently associated with individual variables at 24.5 years; however, cIMT at 17.7 years was negatively associated with cIMT at 24.5 years (Table 4). Higher cfPWV at 17.7 years was associated with higher insulin (standardized regression coefficient (β)=0.06, SE=0.12, P=0.014), HOMA-IR (β=0.05, SE=0.12, P=0.026) and HOMA-%β (β=0.05, SE=0.11, P=0.028) at 24.5 years, but insulin, HOMA-IR and HOMA-%β at 17.7 years were not associated with cfPWV at 24.5 years, after adjustment for baseline covariates (Figure 2A and Table 4). cfPWV at 17.7 years was not associated with glucose at 24.5 years; however, higher glucose at 17.7 years was associated with higher cfPWV at 24.5 years. There were no statistically significant temporal or bidirectional associations between cfPWV at 17.7 years and LDL or triglyceride at 24.5 years. However, cfPWV at 17.7 years had a borderline negative temporal association with HDL at 24.5 years (β=−0.04, SE=0.15, P=0.051), but not vice versa (Table 4).
Table 4.
Autoregressive Cross-Lagged Temporal Path Analyses of cfPWV and cIMT With Metabolic Risks at 17.7 and 24.5 Years of Age
Figure 2.
Temporal causal relationships between arterial measures and insulin resistance. Autoregressive cross-lagged temporal causal associations of arterial stiffness (A) and carotid intima-media thickness (B) with insulin resistance from 17.7 to 24.5 y among 3862 adolescents from the ALSPAC (Avon Longitudinal Study of Parents and Children) birth cohort. Analyses were adjusted for sex, time in years between ages 17.7 and 24.5 y, and other covariates at 17.7 y such as age, low-density lipoprotein cholesterol, triglyceride, high-sensitivity C-reactive protein, high-density lipoprotein cholesterol, heart rate, systolic blood pressure, total fat mass, lean mass, moderate to vigorous physical activity at 15.5 y, smoking status, and family history of hypertension/diabetes/high cholesterol/vascular disease. Homeostatic model assessment of insulin resistance (HOMA-IR) was computed from (fasting insulin×fasting glucose/22.5). PWV indicates pulse wave velocity.
Higher cIMT at 17.7 years was associated with lower insulin (β=−0.05, SE=0.14, P=0.029), HOMA-IR (β=−0.05, SE=0.14, P=0.033) and HOMA-%β at 24.5 years, but insulin, HOMA-IR and HOMA-%β at 17.7 years were not associated with cIMT at 24.5 years, after adjustment for baseline covariates (Figure 2B and Table 4). cIMT at 17.7 years was not associated with glucose at 24.5 years; however, higher glucose at 17.7 years was associated with higher cIMT at 24.5 years. There were no statistically significant temporal associations of cIMT with any measure of lipid indices in either causal path (Table 4).
Discussion
We present novel temporal causal longitudinal results from a very large birth cohort where arterial stiffness, assessed using cfPWV, and cIMT during adolescence were examined as potential predictors of dysglycemia, insulin resistance, and dyslipidemia. First, we observed that higher cfPWV at 17.7 years was associated with the risk of hyperinsulinemia whereas higher cIMT was associated somewhat paradoxically with the risk of decreased insulin at 24.5 years. Second, we reported that cfPWV and cIMT progression were directly associated with the 7-year increase in HDL and triglyceride. Also, cIMT was directly associated with the increase in LDL and glucose and negatively associated with beta-cell function. Lastly, using cross-lagged temporal causal models, higher adolescent cfPWV temporally preceded higher fasting insulin, insulin resistance, and beta-cell function in young adulthood, whereas higher adolescent cIMT preceded lower fasting insulin, insulin resistance, and beta-cell function.
Arterial Stiffness With the Risk of Dysglycemia, Insulin Resistance, and Dyslipidemia
Young-onset type 2 diabetes has a worse prognosis than adult-onset type 2 diabetes because the beta-cell function deteriorates rapidly and the disease potentially has a long duration.1,3–5 Recent evidence among apparently healthy adults aged 48.3±12.0 years followed up for 3.72 years suggests that higher arterial stiffness measured with brachial-ankle PWV may be associated with an increased risk of incident type 2 diabetes.6 Another study conducted in adults with an average age of 71.9±5.6 years and followed up for 4.43 years reported that higher cfPWV was associated with incident type 2 diabetes.21 In the current study, we report a temporal association between cfPWV and risk for deterioration of glycemic status among adolescents followed up for 7 years. We observed that higher glucose at 17.7 years was associated with higher arterial stiffness at 24.5 years contrary to the adult’s study,6 and higher arterial stiffness at 17.7 years was associated with higher insulin concentration and insulin resistance at 24.5 years. Our present study in over 3800 adolescents overcame several limitations of the adults studies6,21 in that we utilized gold standard measure of arterial stiffness, accounted for important covariates, such as accelerometer measured physical activity, dual-energy X-ray absorptiometry–measured body composition, lipids, an inflammatory biomarker, blood pressure, resting heart rate, smoking status, family history of diabetes and cardiovascular diseases, with a longer follow-up period of 7 years. We also utilized several statistical modeling approaches including the autoregressive cross-lagged causal models6 to untangle temporal relationships between arterial stiffness and the risk factors for developing young-onset type 2 diabetes. The independent effect of arterial stiffness on the development of type 2 diabetes may be critical in disease prevention and health promotion since a randomized controlled trial of 699 adolescents assigned to metformin, metformin plus rosiglitazone, and metformin plus lifestyle intervention arm failed to improve insulin sensitivity and beta-cell function over 4 years.4,5 An observational study found no association between metformin use and decreased arterial stiffness in adults with type 2 diabetes,22 hence, future intervention on young-onset type 2 diabetes may consider a concurrent reduction of arterial stiffness in addition to diabetes therapy. This could potentially interrupt the vicious cycle of arterial stiffness and incident young-onset type 2 diabetes.
Among our participants, arterial stiffness preceded higher insulin and insulin resistance but did not precede higher glucose. This is in contrast to the adult study where increased brachial-ankle PWV temporally preceded fasting blood glucose.6 This adult study lacked information on fasting insulin and insulin resistance, and the disparity in findings may also be associated with the modality of arterial stiffness measured and participants’ age.6 For instance, the brachial-ankle PWV quantifies the muscular and peripheral arterial segment rather than the central arterial segment accessed by the cfPWV.23,24 Of note, among overweight/obese adolescents, arterial stiffness progression was associated with the 7-year increase in pancreatic beta-cell function after controlling for fat mass, cardiometabolic, and lifestyle factors. We have recently shown that higher arterial stiffness may temporally contribute to overweight and obesity and that the associations may be bidirectional, which suggests a complex intersection between hemodynamic vascular and metabolic function.13,24 Central arterial stiffness predicts hard cardiovascular events25 and may be clinically useful in predicting early risk of young-onset type 2 diabetes (hyperinsulinemia, insulin resistance, and compensatory increase in beta-cell function)2 because arterial stiffness alters blood circulation to high-flow, low-resistance organs such as the liver and pancreas.23,24 However, we could not examine whether arterial stiffness predicts incident young-onset type 2 diabetes due to the extremely low number of participants with the disease.
Higher adolescent arterial stiffness did not appear to predict an elevated risk of dyslipidemia in young adulthood, probably due to the limited number of participants at risk of dyslipidemia. Nonetheless, the 7-year increase in cfPWV was independently associated with the increase in triglyceride and HDL during the 7-year follow-up period. Previous epidemiological and intervention studies in which elevated cfPWV predicted dyslipidemia have been inconsistent.23 However, the cross-lagged causal findings suggest a slight possibility in which higher arterial stiffness in adolescence may temporally precede lower HDL in young adulthood. Consistent with the foregoing, the independent associations of arterial stiffness progression with the 7-year increase in HDL may reflect a physiologic adaptation to progressively stiffening elastic arteries at young age. Given the convergence of hemodynamic arterial function with liver and pancreatic function,13,23,24 these findings in an apparently healthy young cohort may suggest that higher arterial stiffness contributes to dyslipidemia. Hence, the mechanism which explains temporal causal associations of arterial stiffness with dyslipidemia and metabolic organ damage warrants further research.13,23,24,26
Carotid Intima-Media Thickness With the Risk of Dysglycemia, Insulin Resistance, and Dyslipidemia
cIMT is predictive of cardiovascular events27 and increased cIMT has been reported in persons with type 2 diabetes and impaired glucose tolerance.28 We now report the temporal relationships between cIMT and metabolic factors associated with the risk factors for developing young-onset type 2 diabetes. We observed that higher glucose in adolescence was associated with higher cIMT in young adulthood, in consonance with a previous study among middle-aged adults.29 However, higher cIMT in adolescence was not associated with higher glucose at 24.5 years. In contrast to arterial stiffness findings, higher cIMT at 17.7 years was associated with a risk of low insulin at 24.5 years, which is consistent with the negative association with fasting insulin, insulin resistance, and beta-cell function in the cross-lagged temporal findings. The 7-year cIMT progression was associated with a 7-year increase in glucose in both sexes and weight categories, the decrease in beta-cell function in both sexes, and the decrease in insulin concentration among females. These consistent findings across different models suggest that higher cIMT may precede pancreatic beta-cell failure, insulin insufficiency, and dysglycemia, which are also precursors of young-onset type 2 diabetes.1
Similarly, cIMT progression was associated with the increase in LDL, HDL, and triglyceride in both sexes and across weight categories, but there were no temporal causal relationships. Taken together, a single cIMT measure may be limited in detecting a subtle increase in dysglycemia and dyslipidemia risk in an apparently healthy young population, but cIMT progression, that is, repeated measures over time, may better predict increased risk, independent of cardiovascular, metabolic, inflammatory, and lifestyle risk factors. Lifestyle modification due to repeated arterial measurements (Hawthorne effect) might not influence our findings because the proportion of cigarette smokers, involvement in physical activity, and overweight status increased across the 7 years.13 We observed that neither cfPWV nor cIMT progression had statistically significant associations with the increase in insulin and insulin resistance among overweight/obese participants, partly due to <25% prevalence of overweight and <5% prevalence of obesity at 17.7 years. Besides, obesity seems to minimally influence the relationship of vascular function and structure with the risk of youth-onset type 2 diabetes.3 Clinically, our findings could enhance new targets for intervention on reducing arterial stiffness and carotid wall thickness from adolescence for an early attenuation of risks for the development of young-onset type 2 diabetes and dyslipidemia.6,24 From a public health perspective, repeated measures of arterial stiffness and cIMT may be a simple screening tool for identifying at-risk individuals rather than expensive investigations or treatment.13,23,24
Strengths and Limitations
Using an extensively phenotyped large birth cohort (ALSPAC) with repeated measures of variables during adolescence and young adulthood we investigated temporal causal associations of cfPWV and cIMT with metabolic risk factors associated with the development of young-onset type 2 diabetes and dyslipidemia. The application of advanced statistical tools, such as autoregressive cross-lagged models, provides an attempt at understanding causal path although clinical trials are gold standard for inferring causality. Available ALSPAC data enabled us to control for objectively or directly measured variables such as physical activity, fat mass, lean mass. We lacked dietary data at the studied time points, but we controlled for participants’ body composition and metabolic indices, which partly reflect participants’ diet.30 Ambulatory blood pressure data was unavailable; however, we controlled for repeated systolic blood pressure measurements. Few (<0.4%) adolescents developed incident young-onset type 2 diabetes during the 7-year follow-up; hence, we could not investigate whether arterial stiffness predicts incident young-onset type 2 diabetes. Therefore, a longer follow-up is warranted when investigating disease incidence in a healthy young cohort. The diagnosis of young-onset type 2 diabetes was based on fasting plasma glucose >7mmol/L or reported physician diagnosis since glycated hemoglobin measures were unavailable. However, the American Diabetes Association advised that fasting plasma glucose is as equally appropriate as glycated hemoglobin in diagnosing traits for type 2 diabetes.2 Our participants were mostly Caucasian with minimal geographic variations; thus, our findings may not be generalizable to other racial groups. We could not exclude the possibility of residual confounding of other unmeasured variables, such as the level of uric acid, which could potentially affect both peripheral hemodynamic and metabolic indices.31 Moreover, the likelihood of an asymmetric time course in vascular alteration and metabolic derangement may partly explain the difference in trajectories. However, a recent study among middle-aged healthy adults concluded that peripheral hemodynamic abnormalities may temporally precede incident type 2 diabetes in the causal path.6
Perspectives
In a 7-year prospective assessment of the temporal causal relationships of arterial stiffness and carotid thickness with risk factors for developing young-onset type 2 diabetes and dyslipidemia, we demonstrate that higher cfPWV in adolescence was associated with risk of hyperinsulinemia in young adulthood, whereas higher cIMT in adolescence was associated with the risk of low insulin concentration at the end of the observation period. cfPWV and cIMT progressions were directly associated with the progressive increase in HDL and triglyceride across the 7-year follow-up. Adolescent arterial stiffness may be a precursor of hyperinsulinemia, insulin resistance, and low HDL in young adulthood rather than a consequence. However, carotid thickness in adolescence may be a precursor of insulin insufficiency and decreased beta-cell function in young adulthood. Future interventions aimed at improving insulin sensitivity and beta-cell function may consider a concurrent reduction of arterial stiffness and carotid thickness with diabetic therapy thus interrupting the vicious cycle of arterial stiffness or carotid thickness and type 2 diabetes. Also, future studies are warranted to examine the potential pathological role of vascular stiffness in the development of metabolic diseases in the young population.
Article Information
Acknowledgments
We are extremely grateful to all the families who took part in this study, the midwives for their help in recruiting them, and the whole ALSPAC (Avon Longitudinal Study of Parents and Children) team, which includes interviewers, computer and laboratory technicians, clerical workers, research scientists, volunteers, managers, receptionists and nurses. Graphic abstract was created with BioRender.com. A.O. Agbaje and T.-P. Tuomainen had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. A.O. Agbaje participated in concept and design. A.O. Agbaje, A.R. Barker, G.F. Mitchell, and T-P. Tuomainen participated in acquisition, analysis, or interpretation of data. A.O. Agbaje contributed to drafting of the article. A.O. Agbaje, A.R. Barker, G.F. Mitchell, and T-P. Tuomainen contributed to critical revision of the article for important intellectual content. A.O. Agbaje contributed to statistical analysis. A.O. Agbaje obtained funding. This publication is the work of the authors, and A.O. Agbaje, A.R. Barker, G.F. Mitchell, and T-P. Tuomainen will serve as guarantors for the contents of this article.
Sources of Funding
The UK Medical Research Council and Wellcome (Grant ref: 217065/Z/19/Z; 076467/Z/05/Z) and the University of Bristol provide core support for ALSPAC (Avon Longitudinal Study of Parents and Children). The British Heart Foundation grant (CS/15/6/31468) funded blood pressure, carotid intima-media thickness, carotid-femoral pulse wave velocity, and Actigraph activity monitoring device measurement at 24 years clinic visit. The Medical Research Council grant (MR/M006727/1) supported smoking data collection. A comprehensive list of grants funding is available on the ALSPAC website (http://www.bristol.ac.uk/alspac/external/documents/grant-acknowledgements.pdf); This research (A.O. Agbaje) was specifically funded by the Doctoral Program in Clinical Research, Institute of Public Health and Clinical Nutrition, School of Medicine, Faculty of Health Sciences, University of Eastern Finland; the Jenny and Antti Wihuri Foundation (Grant no: 00180006); the North Savo regional and central Finnish Cultural Foundation (Grants no: 65191835 and 00200150); the Orion Research Foundation sr; the Aarne Koskelo Foundation; the Antti and Tyyne Soininen Foundation; the Paulo Foundation; the Paavo Nurmi Foundation; and the Yrjö Jahnsson Foundation (Grant no: 20217390). The University of Eastern Finland funded open access publishing. The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the article; and decision to submit the article for publication.
Disclosures
G.F. Mitchell is the owner of Cardiovascular Engineering, Inc, a small business that designs and manufactures devices that measure vascular stiffness. The company uses these devices in clinical trials that evaluate the effects of diseases and interventions on vascular stiffness. He also reports receiving grants from the National Institutes of Health and Novartis and consulting fees from Novartis, Bayer, Merck, and Servier. The other authors report no conflicts.
Supplementary Material
Nonstandard Abbreviations and Acronyms
- ALSPAC
- Avon Longitudinal Study of Parents and Children
- cfPWV
- carotid-femoral pulse wave velocity
- cIMT
- carotid intima-media thickness
- HDL
- high-density lipoprotein
- HOMA
- homeostatic model assessment
- HOMA-%β
- HOMA-percent pancreatic beta-cell function
- HOMA-
- homeostatic model assessment of insulin resistance
- IR
- homeostatic model assessment of insulin resistance
- LDL
- low-density lipoprotein
Supplemental Material is available at https://www.ahajournals.org/doi/suppl/10.1161/HYPERTENSIONAHA.121.18754.
For Sources of Funding and Disclosures, see page 677.
Contributor Information
Alan R. Barker, Email: A.R.Barker@exeter.ac.uk.
Gary F. Mitchell, Email: garyfmitchell@gmail.com.
Tomi-Pekka Tuomainen, Email: tomi-pekka.tuomainen@uef.fi.
Novelty and Relevance
What Is New?
In the largest adolescent longitudinal study to date, we examined for the first time the impact of arterial stiffness (carotid-femoral pulse wave velocity) and carotid intima-media thickness progression on the risk of dysglycemia, insulin resistance, and dyslipidemia.
We utilized hierarchical modeling for repeated measures and autoregressive cross-lagged structural equation models to untangle possible temporal causal paths.
What Is Relevant?
Adolescent arterial stiffness may be a precursor of insulin resistance in young adulthood rather than a consequence, whereas carotid thickness may be a precursor of insulin insufficiency and decreased beta-cell function.
Adolescent arterial stiffness appears to temporally precede low HDL, albeit with borderline significance, but arterial stiffness and carotid intima-media thickness progression were positively associated with the 7-year increase in triglyceride.
Clinical/Pathophysiological Implications?
Temporal path analysis in this study suggests that arterial stiffness in adolescence may be a novel causal risk factor for insulin resistance, hyperinsulinemia, and low HDL (high-density lipoprotein) in young adulthood. The prevention and reduction of insulin resistance and hyperinsulinemia, which are risk factors for young-onset type 2 diabetes, and dyslipidemia from adolescence may require developing novel approaches to mitigate arterial stiffness. Nonetheless, future studies are warranted to examine the biological and pathological processes through which vascular alterations contribute to metabolic diseases.
References
- 1.Chan JCN, Lim LL, Wareham NJ, Shaw JE, Orchard TJ, Zhang P, Lau ESH, Eliasson B, Kong APS, Ezzati M, et al. The Lancet Commission on diabetes: using data to transform diabetes care and patient lives. Lancet. 2021;396:2019–2082. doi: 10.1016/S0140-6736(20)32374-6 [DOI] [PubMed] [Google Scholar]
- 2.American Diabetes Association. 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes-2020. Diabetes Care. 2020;43(suppl 1):S14–S31. doi: 10.2337/dc20-S002 [DOI] [PubMed] [Google Scholar]
- 3.Dabelea D, Stafford JM, Mayer-Davis EJ, D’Agostino R, Jr, Dolan L, Imperatore G, Linder B, Lawrence JM, Marcovina SM, Mottl AK, et al. ; SEARCH for Diabetes in Youth Research Group. Association of type 1 diabetes vs type 2 diabetes diagnosed during childhood and adolescence with complications during teenage years and young adulthood. JAMA. 2017;317:825–835. doi: 10.1001/jama.2017.0686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.TODAY Study Group. Postintervention effects of varying treatment arms on glycemic failure and β-cell function in the TODAY Trial. Diabetes Care. 2021;44:75–80. doi: 10.2337/dc20-0622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.TODAY Study Group. Effects of metformin, metformin plus rosiglitazone, and metformin plus lifestyle on insulin sensitivity and β-cell function in TODAY. Diabetes Care. 2013;36:1749–1757. doi: 10.2337/dc12-2393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zheng M, Zhang X, Chen S, Song Y, Zhao Q, Gao X, Wu S. Arterial stiffness preceding diabetes: A Longitudinal Study. Circ Res. 2020;127:1491–1498. doi: 10.1161/CIRCRESAHA.120.317950 [DOI] [PubMed] [Google Scholar]
- 7.Boyd A, Golding J, Macleod J, Lawlor DA, Fraser A, Henderson J, Molloy L, Ness A, Ring S, Davey Smith G. Cohort profile: the ‘children of the 90s’–the index offspring of the Avon Longitudinal Study of Parents and Children. Int J Epidemiol. 2013;42:111–127. doi: 10.1093/ije/dys064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fraser A, Macdonald-Wallis C, Tilling K, Boyd A, Golding J, Davey Smith G, Henderson J, Macleod J, Molloy L, Ness A, et al. Cohort profile: the Avon Longitudinal Study of Parents and Children: ALSPAC mothers cohort. Int J Epidemiol. 2013;42:97–110. doi: 10.1093/ije/dys066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Northstone K, Lewcock M, Groom A, Boyd A, Macleod J, Timpson N, Wells N. The Avon Longitudinal Study of Parents and Children (ALSPAC): an update on the enrolled sample of index children in 2019. Wellcome Open Res. 2019;4:51. doi: 10.12688/wellcomeopenres.15132.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)–a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377–381. doi: 10.1016/j.jbi.2008.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chiesa ST, Charakida M, Georgiopoulos G, Dangardt F, Wade KH, Rapala A, Bhowruth DJ, Nguyen HC, Muthurangu V, Shroff R, et al. Determinants of intima-media thickness in the young: The ALSPAC Study. JACC Cardiovasc Imaging. 2021;14:468–478. doi: 10.1016/j.jcmg.2019.08.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dangardt F, Charakida M, Georgiopoulos G, Chiesa ST, Rapala A, Wade KH, Hughes AD, Timpson NJ, Pateras K, Finer N, et al. Association between fat mass through adolescence and arterial stiffness: a population-based study from The Avon Longitudinal Study of Parents and Children. Lancet Child Adolesc Health. 2019;3:474–481. doi: 10.1016/S2352-4642(19)30105-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Agbaje AO, Barker AR, Tuomainen TP. Effects of arterial stiffness and carotid intima-media thickness progression on the risk of overweight/obesity and elevated blood pressure/hypertension: a Cross-Lagged Cohort Study. Hypertension. 2022;79:159–169. doi: 10.1161/HYPERTENSIONAHA.121.18449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mach F, Baigent C, Catapano AL, Koskinas KC, Casula M, Badimon L, Chapman MJ, De Backer GG, Delgado V, Ference BA, et al. ; ESC Scientific Document Group. 2019 ESC/EAS Guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. Eur Heart J. 2020;41:111–188. doi: 10.1093/eurheartj/ehz455 [DOI] [PubMed] [Google Scholar]
- 15.American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2011;34suppl 1(suppl 1):S62–S69. doi: 10.2337/dc11-S062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ascaso JF, Pardo S, Real JT, Lorente RI, Priego A, Carmena R. Diagnosing insulin resistance by simple quantitative methods in subjects with normal glucose metabolism. Diabetes Care. 2003;26:3320–3325. doi: 10.2337/diacare.26.12.3320 [DOI] [PubMed] [Google Scholar]
- 17.Wallace TM, Levy JC, Matthews DR. Use and abuse of HOMA modeling. Diabetes Care. 2004;27:1487–1495. doi: 10.2337/diacare.27.6.1487 [DOI] [PubMed] [Google Scholar]
- 18.Ness AR, Leary SD, Mattocks C, Blair SN, Reilly JJ, Wells J, Ingle S, Tilling K, Smith GD, Riddoch C. Objectively measured physical activity and fat mass in a large cohort of children. PLoS Med. 2007;4:e97. doi: 10.1371/journal.pmed.0040097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Agbaje AO, Barker AR, Tuomainen TP. Cardiorespiratory fitness, fat mass, and cardiometabolic health with endothelial function, arterial elasticity, and stiffness. Med Sci Sports Exerc. 2022;54:141–152. doi: 10.1249/MSS.0000000000002757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Golding J, Pembrey M, Jones R; ALSPAC Study Team. ALSPAC–the Avon Longitudinal Study of Parents and Children. I. Study methodology. Paediatr Perinat Epidemiol. 2001;15:74–87. doi: 10.1046/j.1365-3016.2001.00325.x [DOI] [PubMed] [Google Scholar]
- 21.Muhammad IF, Borné Y, Östling G, Kennbäck C, Gottsäter M, Persson M, Nilsson PM, Engström G. Arterial stiffness and incidence of diabetes: A Population-Based Cohort Study. Diabetes Care. 2017;40:1739–1745. doi: 10.2337/dc17-1071 [DOI] [PubMed] [Google Scholar]
- 22.Driessen JHM, de Vries F, van Onzenoort HAW, Schram MT, van der Kallen C, Reesink KD, Sep S, Stehouwer CDA, Schaper N, Kroon AA, et al. Metformin use in type 2 diabetic patients is not associated with lower arterial stiffness: the Maastricht Study. J Hypertens. 2019;37:365–371. doi: 10.1097/HJH.0000000000001892 [DOI] [PubMed] [Google Scholar]
- 23.Boutouyrie P, Chowienczyk P, Humphrey JD, Mitchell GF. Arterial Stiffness and Cardiovascular Risk in Hypertension. Circ Res. 2021;128:864–886. doi: 10.1161/CIRCRESAHA.121.318061 [DOI] [PubMed] [Google Scholar]
- 24.Chirinos JA, Segers P, Hughes T, Townsend R. Large-artery stiffness in health and disease: JACC state-of-the-art review. J Am Coll Cardiol. 2019;74:1237–1263. doi: 10.1016/j.jacc.2019.07.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mitchell GF, Hwang SJ, Vasan RS, Larson MG, Pencina MJ, Hamburg NM, Vita JA, Levy D, Benjamin EJ. Arterial stiffness and cardiovascular events: the Framingham Heart Study. Circulation. 2010;121:505–511. doi: 10.1161/CIRCULATIONAHA.109.886655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lacolley P, Regnault V, Laurent S. Mechanisms of arterial stiffening: from mechanotransduction to epigenetics. Arterioscler Thromb Vasc Biol. 2020;40:1055–1062. doi: 10.1161/ATVBAHA.119.313129 [DOI] [PubMed] [Google Scholar]
- 27.Lorenz MW, Markus HS, Bots ML, Rosvall M, Sitzer M. Prediction of clinical cardiovascular events with carotid intima-media thickness: a systematic review and meta-analysis. Circulation. 2007;115:459–467. doi: 10.1161/CIRCULATIONAHA.106.628875 [DOI] [PubMed] [Google Scholar]
- 28.Brohall G, Odén A, Fagerberg B. Carotid artery intima-media thickness in patients with Type 2 diabetes mellitus and impaired glucose tolerance: a systematic review. Diabet Med. 2006;23:609–616. doi: 10.1111/j.1464-5491.2005.01725.x [DOI] [PubMed] [Google Scholar]
- 29.Kozakova M, Natali A, Dekker J, Beck-Nielsen H, Laakso M, Nilsson P, Balkau B, Ferrannini E; RISC Investigators. Insulin sensitivity and carotid intima-media thickness: relationship between insulin sensitivity and cardiovascular risk study. Arterioscler Thromb Vasc Biol. 2013;33:1409–1417. doi: 10.1161/ATVBAHA.112.300948 [DOI] [PubMed] [Google Scholar]
- 30.Ortega RM, Requejo AM, Andrés P, López-Sobaler AM, Redondo R, González-Fernández M. Relationship between diet composition and body mass index in a group of Spanish adolescents. Br J Nutr. 1995;74:765–773. doi: 10.1079/bjn19950004 [DOI] [PubMed] [Google Scholar]
- 31.Johnson RJ, Nakagawa T, Sanchez-Lozada LG, Shafiu M, Sundaram S, Le M, Ishimoto T, Sautin YY, Lanaspa MA. Sugar, uric acid, and the etiology of diabetes and obesity. Diabetes. 2013;62:3307–3315. doi: 10.2337/db12-1814 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The informed consent obtained from ALSPAC participants does not allow the data to be made freely available through any third-party maintained public repository. However, data used for this submission can be made available on request to the ALSPAC Executive. The ALSPAC data management plan describes in detail the policy regarding data sharing, which is through a system of managed open access. Full instructions for applying for data access can be found here: http://www.bristol.ac.uk/alspac/researchers/access/. The ALSPAC study website contains details of all the data that are available (http://www.bristol.ac.uk/alspac/researchers/our-data/).