Race has long been used in the calculation of eGFR, contributing to health care disparities affecting Black patients with CKD. To address this inequality, a new equation to calculate eGFR independent of race was recently developed and is recommended by the NKF-ASN Task Force for clinical practice (1,2). We sought to determine the impact of the 2021 creatinine-based CKD-EPI eGFR equation on the number of patients with CKD stages 3 to 4 seen in the Veterans Affairs (VA) health care system nationally and by VA location, as well as the number of persons with unidentified race who newly met CKD stages 3 to 4 criteria for research purposes, as eGFR could not be calculated for such persons using a race-based equation.
US veterans with new CKD stages 3 to 4 (eGFR 15–59 ml/min per 1.73 m2) in the VA system from 2005 to 2019 using the 2009 CKD-EPI equation (3) and the 2021 CKD-EPI creatinine equation (1) were identified by demographic and laboratory values from the VA Corporate Data Warehouse. CKD stages 3 to 4 was defined as two outpatient eGFR measurements <60 ml/min per 1.73 m2 at least 90 days apart with no intervening values ≥60 ml/min per 1.73 m2. The second of these two eGFR values determined CKD stage. Participants had at least one face-to-face primary care or general internal medicine encounter in the VA system. Individuals requiring maintenance dialysis or kidney transplantation were excluded using previously published methods (4). The demographic makeup of patients with CKD stages 3 to 4 was compared across VA locations when calculated using the 2021 CKD-EPI creatinine equation versus the 2009 CKD-EPI equation (1,3).
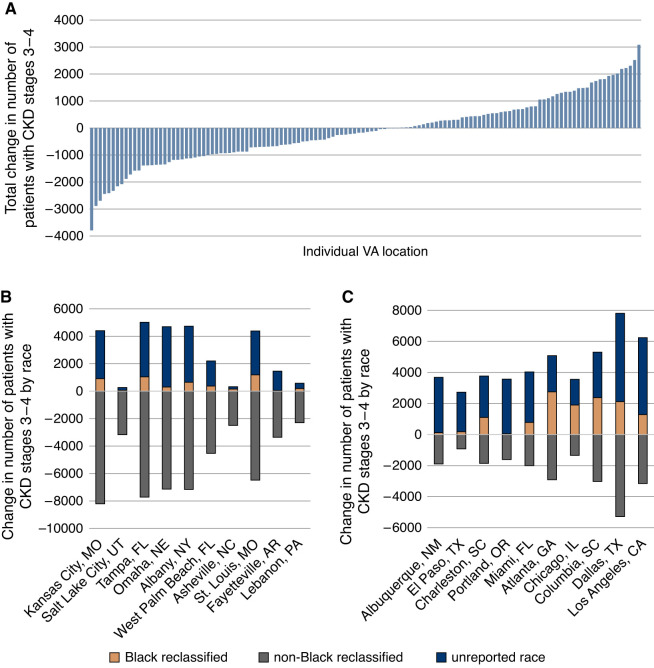
The 2009 CKD-EPI eGFR equation identified 1,780,960 individuals with CKD stages 3 to 4 compared with 1,764,054 with the 2021 CKD-EPI creatinine equation, totaling 16,906 fewer individuals nationwide identified by the 2021 equation. There were 66,190 more Black individuals with CKD stages 3 to 4 identified by the 2021 CKD-EPI creatinine equation compared with the 2009 CKD-EPI equation. An additional 206,146 individuals with unreported race were identified as having CKD stages 3 to 4 when using the 2021 CKD-EPI creatinine equation. A total of 289,242 non-Black individuals were reclassified from CKD stages 3 to 4 by the 2009 CKD-EPI equation to eGFR ≥60 ml/min per 1.73 m2 by the 2021 CKD-EPI creatinine equation. The change in the number of patients with CKD stages 3 to 4 was not consistent across VA locations nationally, with a median (interquartile range) of –171.5 (–927, 578) and a range of –3790 to 3081 change in patients per location (Figure 1A). By race, the median (interquartile range) change in CKD stages 3 to 4 per location was 288.5 (60, 798) for Black individuals, 1297.5 (493, 2307) for individuals of unidentified race, and –1886.5 (–2760, –1246) for non-Black individuals. The change by race is shown for the locations with the greatest decrease (Figure 1B) and greatest increase (Figure 1C) in total CKD stages 3 to 4.
Figure 1.
Changes in the number of patients with new CKD stages 3 to 4 per VA location identified using the 2021 CKD-EPI creatinine eGFR equation. The total change in the number of patients with CKD stages 3 to 4 using the 2021 CKD-EPI creatinine eGFR equation compared with the 2009 CKD-EPI equation varied across VA locations (A). Each bar represents an individual VA location. The increase in CKD stages 3 to 4 among Black persons (shown in orange) and persons of unidentified race whose eGFR was not calculable using a race-based equation (shown in dark blue), and the decrease in CKD stages 3 to 4 among non-Black persons (shown in gray) are shown for the ten VA locations with the greatest total decrease (B) and the greatest total increase (C) in patients with CKD stages 3 to 4.
In this analysis, we showed that the 2021 CKD-EPI creatinine eGFR equation led to a variable change in the total burden of new CKD stages 3 to 4 across VA locations, with a marked change in the composition of this patient population. When compared with removal of the race coefficient from the 2009 CKD-EPI equation, which decreases eGFR among Black patients and generates no change among non-Black patients, the 2021 CKD-EPI creatinine equation identified a smaller increase in CKD stages 3 to 4 among Black patients and a decrease among non-Black individuals (1). Our results are consistent with the analysis by Inker et al. (1), and we further demonstrated that the 2021 CKD-EPI creatinine eGFR equation identified a substantial number of individuals with undefined race whose eGFR could not previously be calculated for the purposes of outcomes research.
Adoption of the 2021 CKD-EPI creatinine eGFR equation may have several effects that merit further studies. In the clinical setting, individuals newly determined to have CKD may need nephrology referral and eGFR-based medication dosing adjustment (5), while other individuals previously considered to have CKD may not require close follow-up in a nephrology clinic. These shifts are not uniformly distributed across VA facilities, leading to unequal burden on providers based on the racial makeup of the patients they treat. In the research setting, the demographic characteristics of individuals eligible for enrollment in prospective CKD studies will change, with implications for the generalizability of historical studies not reflective of this population, and individuals without documented race can be included in retrospective research using real-world datasets. This study has limitations. Billing codes may miss some individuals who receive dialysis outside the VA system. Methods for identifying patients’ race are likely non-standard (e.g., patient-reported versus a staff member’s judgment). This analysis lacked albuminuria to identify CKD stages 1 and 2.
Adoption of the 2021 CKD-EPI creatinine eGFR equation will shift the demographic makeup of individuals with CKD, increasing the number of Black individuals and decreasing the number of non-Black individuals defined as having CKD stages 3 and 4. While we strive to achieve equitable clinical outcomes for Black individuals with kidney disease, it is paramount that VA locations support clinicians in reassessing which patients may qualify for and most greatly benefit from receiving nephrology care.
Disclosures
L.P. Gregg reports employment with Michael E. DeBakey VA Medical Center, Houston, Texas, and VA HSR&D Center for Innovations in Quality, Effectiveness and Safety, Houston, Texas. M.E. Matheny reports employment with the Department of Veterans Affairs and consultancy agreements with NIH-VA-DoD Pain Management Grant Consortium (PMC3). M.E. Matheny also reports serving as a scientific advisor or member of SMRB Study Section, VA HSR&D, Informatics & Methods Section; Steering Committee–Indianapolis VA HSR&D COIN Center; and Steering Committee–VA HSR&D VIREC. S.D. Navaneethan reports employment with Michael E. DeBakey VA Medical Center; consultancy agreements with Bayer, Boehringer Ingelheim, REATA, Tricida, and Vifor; receiving research funding from Keryx; receiving honoraria from Bayer, Boehringer Ingelheim, REATA, Tricida, and Vifor; and serving as a scientific advisor or member of American Journal of Kidney Diseases, American Journal of Nephrology, CardioRenal Medicine, CJASN, and Current Opinion in Nephrology and Hypertension; and serving as a KDIGO: Guideline writing committee member. P. Richardson reports employment with the US Department of Veterans Affairs. S.S. Virani reports receiving honoraria from the American College of Cardiology as Associate Editor for Innovations, ACC.ORG, and receiving grant support from the Department of Veterans Affairs, World Heart Federation, Tahir, and Jooma Family. The remaining author has nothing to disclose.
Funding
This work is supported by research funding from the Department of Veterans Affairs Health Services Research & Development (1I01HX002917-01A1). S.S. Virani is also supported by a grant from the World Heart Federation, NHLBI, and Tahir and Jooma Family. This work was also supported in part by the Center for Innovations in Quality, Effectiveness and Safety (CIN 13-413), Michael E. DeBakey VA Medical Center, Houston, Texas.
Acknowledgments
Funding agencies did not have any role in study design, data collection, analysis, reporting, or the decision to submit for publication. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH or Veterans Administration.
L.P. Gregg, P. Richardson, M.E. Matheny, S.D. Navaneethan, and S.S. Virani are employees of the US Department of Veterans Affairs. The interpretation and reporting of these data are the responsibility of the authors and in no way should be viewed as official policy or interpretation of the Department of Veterans Affairs or the United States government.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.Inker LA, Eneanya ND, Coresh J, Tighiouart H, Wang D, Sang Y, Crews DC, Doria A, Estrella MM, Froissart M, Grams ME, Greene T; Chronic Kidney Disease Epidemiology Collaboration: . New creatinine- and cystatin c-based equations to estimate GFR without race. N Engl J Med: 385: 1737–1749, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Delgado C, Baweja M, Crews DC, Eneanya ND, Gadegbeku CA, Inker LA, Mendu ML, Miller WG, Moxey-Mims MM, Roberts GV, St Peter WL, Warfield C, Powe NR: A unifying approach for GFR estimation: Recommendations of the NKF-ASN Task Force on Reassessing the Inclusion of Race in Diagnosing Kidney Disease. J Am Soc Nephrol 32: 2994–3015, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF 3rd, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J; CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) : A new equation to estimate glomerular filtration rate. Ann Intern Med 150: 604–612, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Norton JM, Ali K, Jurkovitz CT, Kiryluk K, Park M, Kawamoto K, Shang N, Navaneethan SD, Narva AS, Drawz P: Development and validation of a pragmatic electronic phenotype for CKD. Clin J Am Soc Nephrol 14: 1306–1314, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Walther CP, Winkelmayer WC, Navaneethan SD: Black race coefficient in GFR estimation and diabetes medications in CKD: National estimates. J Am Soc Nephrol 32: 1319–1321, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]