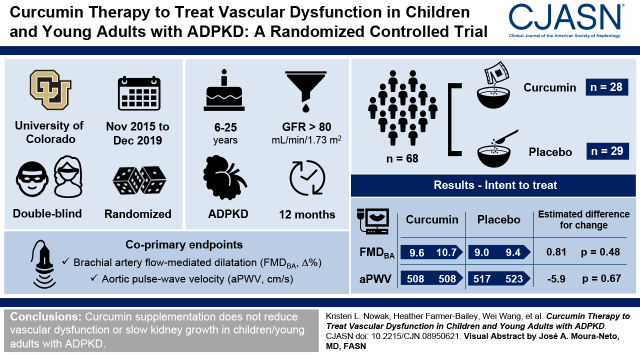
Visual Abstract
Keywords: oxidative stress, pulse wave velocity, aging, endothelial cells, ADPKD
Abstract
Background and objectives
Clinical manifestations of autosomal dominant polycystic kidney disease (ADPKD), including evidence of vascular dysfunction, can begin in childhood. Curcumin is a polyphenol found in turmeric that reduces vascular dysfunction in rodent models and humans without ADPKD. It also slows kidney cystic progression in a murine model of ADPKD. We hypothesized that oral curcumin therapy would reduce vascular endothelial dysfunction and arterial stiffness in children/young adults with ADPKD.
Design, setting, participants, & measurements
In a randomized, placebo-controlled, double-blind trial, 68 children/young adults 6–25 years of age with ADPKD and eGFR>80 ml/min per 1.73 m2 were randomized to either curcumin supplementation (25 mg/kg body weight per day) or placebo administered in powder form for 12 months. The coprimary outcomes were brachial artery flow-mediated dilation and aortic pulse-wave velocity. We also assessed change in circulating/urine biomarkers of oxidative stress/inflammation and kidney growth (height-adjusted total kidney volume) by magnetic resonance imaging. In a subgroup of participants ≥18 years, vascular oxidative stress was measured as the change in brachial artery flow-mediated dilation following an acute infusion of ascorbic acid.
Results
Enrolled participants were 18±5 (mean ± SD) years, 54% were girls, baseline brachial artery flow-mediated dilation was 9.3±4.1% change, and baseline aortic pulse-wave velocity was 512±94 cm/s. Fifty-seven participants completed the trial. Neither coprimary end point changed with curcumin (estimated change [95% confidence interval] for brachial artery flow-mediated dilation [percentage change]: curcumin: 1.14; 95% confidence interval, −0.84 to 3.13; placebo: 0.33; 95% confidence interval, −1.34 to 2.00; estimated difference for change: 0.81; 95% confidence interval, −1.21 to 2.84; P=0.48; aortic pulse-wave velocity [centimeters per second]: curcumin: 0.6; 95% confidence interval, −25.7 to 26.9; placebo: 6.5; 95% confidence interval, −20.4 to 33.5; estimated difference for change: −5.9; 95% confidence interval, −35.8 to 24.0; P=0.67; intent to treat). There was no curcumin-specific reduction in vascular oxidative stress or changes in mechanistic biomarkers. Height-adjusted total kidney volume also did not change as compared with placebo.
Conclusions
Curcumin supplementation does not improve vascular function or slow kidney growth in children/young adults with ADPKD.
Clinical Trial registry name and registration number
Curcumin Therapy to Treat Vascular Dysfunction in Children and Young Adults with ADPKD, NCT02494141.
Podcast
This article contains a podcast at https://www.asn-online.org/media/podcast/CJASN/2022_02_07_CJN08950621.mp3
Introduction
In autosomal dominant polycystic kidney disease (ADPKD), the development and continued growth of multiple kidney cysts result in ultimate loss of kidney function in the majority of patients (1); however, cardiovascular complications are the leading cause of mortality (2). Evidence of cardiovascular abnormalities can manifest in childhood, including elevated BP and increased left ventricular mass index (3,4). Additionally, vascular dysfunction, reflected by impaired endothelium-dependent dilation and increased arterial stiffness, is evident in children and young adults (5). Thus, this age group may represent an optimal therapeutic window in the prevention of future cardiovascular complications.
Impaired endothelium-dependent dilation and increased large elastic artery stiffness are commonly assessed by brachial artery flow-mediated dilation (FMDBA) and aortic pulse-wave velocity (aPWV), respectively. Both measurements are independently associated with incident cardiovascular events and mortality in an adult population (6,7), and increased systemic oxidative stress and inflammation appear to be involved (8,9).
Curcumin is a polyphenol AQ4 found in the Indian spice turmeric that has long been used in traditional Indian medicine and has a unique ability to activate transcription of key antioxidants, suppress inflammation, and reduce proliferation (10). Administration of curcumin reduces vascular dysfunction in non-ADPKD animal models (11–13) and also reduces age- (14,15) and diabetes-associated (16) vascular dysfunction in humans. These improvements are associated with increased vascular nitric oxide bioavailability and reduced oxidative stress (13,14). Notably, curcumin also improves kidney histology and reduces proliferative index, cystic index, and kidney weight:body weight in the iKSp-PKD1del murine model of ADPKD (17).
Accordingly, the primary aim of this randomized placebo-controlled trial was to determine the efficacy of curcumin for improving FMDBA and reducing aPWV in children and young adults with ADPKD. A key secondary goal was to obtain insight into the mechanisms by which curcumin may reduce vascular dysfunction. As an exploratory aim, we evaluated changes in height-corrected total kidney volume (htTKV) measured by magnetic resonance imaging. We hypothesized that oral curcumin therapy would improve FMDBA and reduce aPWV in children and young adults with ADPKD.
Materials and Methods
Detailed methods have been previously described (18), and they are provided in brief with additional details in Supplemental Material.
Study Participants
The study design and baseline participant characteristics of this clinical trial were recently reported (18). Eligible participants were recruited nationally and were enrolled at the University of Colorado Anschutz Medical Campus between November 2015 and December 2019. Briefly, participants eligible for inclusion were children and young adults 6–25 years of age with a diagnosis of ADPKD and a baseline eGFR >80 ml/min per 1.73 m2 (19,20).
Study Design
The trial was a 12-month, randomized, placebo-controlled (1:1 allocation), parallel-group, double-blind study with the polyphenol curcumin (25 mg/kg body weight per day) delivered in powder form mixed with food (to improve adherence as compared with pills, particularly among young children). The coprimary outcomes were change in FMDBA and aPWV at 12 months as indices of vascular function.
Vascular Measurements.
All vascular measurements were made as a single measurement per study visit following standard recommendations, including an overnight fast (21). Additional details are provided in Supplemental Material. FMDBA was determined using duplex ultrasonography with electrocardiogram-gated end diastolic ultrasound images analyzed by a single blinded analyst using a commercially available software package (5,22,23). Brachial artery endothelium-independent dilation (to 0.4 mg of sublingual nitroglycerin) was assessed in participants ≥18 years of age without contraindications (22,23). Pulse-wave velocity (PWV) was measured using a transcutaneous custom tonometer to noninvasively assess aPWV (measured as carotid-femoral PWV) as well as carotid-radial PWV (22,23). Carotid artery compliance, carotid artery β-stiffness index, carotid intimal medial thickness, carotid augmentation index, and carotid systolic BP were also measured (5,22). The influence of oxidative stress on FMDBA was assessed by infusing a supraphysiologic dose of ascorbic acid in a subgroup of participants ≥18 years of age (n=12 per group baseline; n=10 per group 12 months) as described previously (22,23).
Systemic Markers of Oxidative Stress and Inflammation.
IL-6, TNF-α, IFN-γ, and C-reactive protein levels were measured by ELISA. Targeted liquid chromatography-tandem mass analysis of a marker of oxidative stress (8-iso-prostaglandin F2α) was also performed on spot urine samples using a validated assay and normalized by urine creatinine (22,24).
Kidney Magnetic Resonance Imaging.
A Siemens Skyra 3.0-T system was used to obtain magnetic resonance imaging for volumetric measurements of the kidneys (25). Total kidney volume adjusted for height to account for normal growth in children (htTKV) (26) was measured by a single blinded analyst by stereology using Analyze software. Total cyst volume, fractional cyst volume, and kidney parenchyma were also measured. Disease severity was categorized according to the Mayo Imaging classification system for participants ≥15 years of age (27).
Adherence, Safety, and Study Monitoring
In brief, participants were monitored with regular phone inquiries (months 1, 3, 6, and 9) regarding medication supply, safety/adverse events, and adherence questions. A comprehensive metabolic panel was performed at months 1, 6, and 12. For comparison of eGFR using the same equation across all ages (children and adults), the new full age spectrum eGFR equation was used (28). During phone safety calls, suggestions were made to help increase adherence as needed. Percentage of adherence was calculated at the final visit on the basis of the total powder consumed relative to expected. The study was monitored with annual meetings by an independent data and safety monitoring board.
Study Approval
All procedures were approved by the institutional review board of the University of Colorado Anschutz Medical Campus and adhere to the Declaration of Helsinki. The nature, benefits, and risks of the study were explained to the volunteers, and their written informed consent/assent was obtained prior to participation.
Statistical Analyses
All statistical analyses were performed using SAS version 9.4. A linear regression model was fit to assess the curcumin effect versus placebo on the outcomes by regressing each outcome variable at 12 months on study group with adjustment for baseline values. For outcome variables with more than two time points, a linear mixed effects model with random intercept and random slope was used. The interaction of sex and group on the primary outcome variables as well as htTKV was examined. The differences in flow-mediated dilation within each group during saline infusion versus vitamin C infusion on the same day were analyzed by a paired t test. Study drug adherence was compared between groups using an independent samples t test.
All participants were included in the final analysis using multiple imputation for the primary outcomes with the expectation-maximization method (29) on the basis of a prespecified intent-to-treat analysis. A secondary complete case analysis was performed, including all participants with end of study data regardless of the level of study adherence. A complete case analysis was also used for all secondary and other outcomes. A sample size of 27 participants per group was calculated on the basis of 90% power and a two-side type 1 error rate of 0.025, adjusted for two primary end points, in order to detect a mean increase in FMDBA of 1.5% given an SD of 2.0 (30) and a mean decrease of aPWV of 100 cm/s given an SD of 125 (31). To account for a potential dropout of 20%, 34 participants per group were enrolled.
Results
Enrollment and Baseline Clinical Characteristics
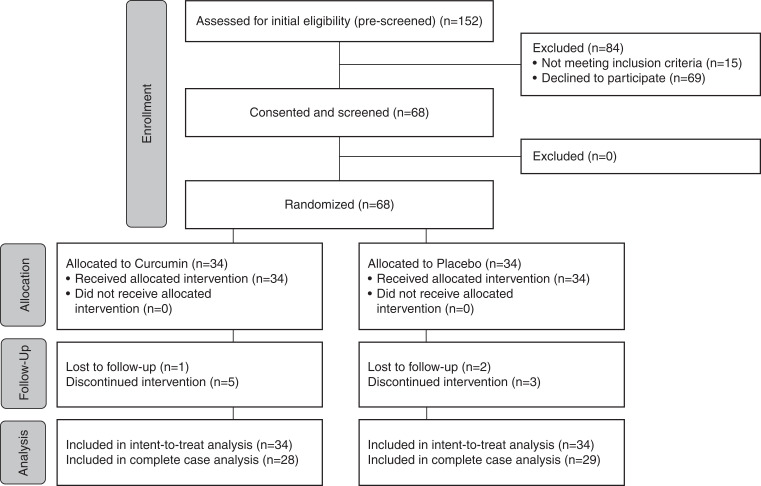
Of the 68 participants who were screened for participation, all 68 were randomized to receive either curcumin or placebo (Figure 1). An additional 84 participants were prescreened for enrollment, but they were determined to be ineligible or not interested. Participants were 18±5 (mean ± SD) years, 54% were women, 82% were non-Hispanic White participants, and 38% were children <18 years of age. Groups had similar baseline characteristics (Table 1).
Figure 1.
Patient enrollment, randomization, and completion Consolidated Standards of Reporting Trials (CONSORT) flow diagram. This flow diagram describes enrollment, randomization, and study completion.
Table 1.
Baseline characteristics of all randomized participants
| Variable | Curcumin, n=34 | Placebo, n=34 |
|---|---|---|
| Age, yr | 18±6 | 19±5 |
| Sex, women, n (%) | 19 (56%) | 18 (53%) |
| Race and ethnicity, n (%) | ||
| Non-Hispanic White | 28 (82%) | 28 (82%) |
| Hispanic or other | 6 (14%) | 6 (18%) |
| eGFR, ml/min per 1.73 m2 | 115±16 | 118±19 |
| BMI category | ||
| Normal weight or underweight | 23 (68%) | 24 (71%) |
| Overweight | 6 (18%) | 8 (24%) |
| Obese | 5 (15%) | 2 (6%) |
| Systolic BP, mm Hg | 116±12 | 118±19 |
| Diastolic BP, mm Hg | 71±9 | 73±10 |
| Hypertension | 13 (38%) | 16 (47%) |
| Medications, n (%) | ||
| ACEi/ARB | 9 (27%) | 12 (35%) |
| Any antihypertensive | 10 (29%) | 13 (3%) |
| Statin | 5 (15%) | 7 (21%) |
| Oral contraceptive or IUDa | 7 (21%) | 4 (12%) |
| Psychiatric medications | 5 (15%) | 3 (9%) |
| Mayo classification, n (%) b | ||
| 1A | 0 (0%) | 4 (12%) |
| 1B | 2 (6%) | 5 (15%) |
| 1C | 7 (21%) | 2 (6%) |
| 1D | 9 (27%) | 6 (18%) |
| 1E | 7 (21%) | 10 (29%) |
| Not applicable | 9 (27%) | 7 (21%) |
Data are mean ± SD or percentage. eGFR is by the full age spectrum equation. BP was the mean office reading in the seated position. BMI, body mass index; ACEi, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; IUD, intrauterine device.
Percentage of women.
Mayo classification is only applicable in participants ≥15 years of age (n=44). Those in the not applicable category were <15 years of age.
Adherence and Adverse Events
At 12 months, total adherence, assessed by weighing the remaining powder upon bottle return, was higher in the placebo group (90% versus 68% in the curcumin group; P<0.001). The average prescribed daily doses were 1559±534 mg (curcumin) and 1569±538 mg (placebo). Two participants in the curcumin group and three participants in the placebo group had a per protocol dose reduction due to tolerability and subsequently completed the study. Of these, one participant in the curcumin group and one participant in the placebo group eventually resumed taking the full dose.
Seventeen participants experienced an adverse event related or possibly related to the drug (n=8 curcumin; n=9 placebo [includes all participants regardless of study completion and more than one adverse event per participant when applicable]) (Table 2). There were no serious adverse events in either group. Six curcumin participants and five placebo participants discontinued the intervention prior to the final study visit. Two curcumin participants and one placebo participant dropped out of the study due to powder tolerability. Four curcumin participants and four placebo participants dropped out of the study due to other/unknown reasons. One placebo participant was discontinued from the study due to a mild elevation (<2.5× the upper limit of normal) in alanine aminotransferase and aspartate aminotransferase (other outcome measure) that persisted after stopping the study medication for 1 month. The median (interquartile range [IQR]) duration of participation prior to study discontinuation for these participants was 103 (IQR, 79–160) days (curcumin: 104 [IQR, 79–160] days; placebo: 87 [IQR, 86–138] days). There was no difference in change in eGFR, any other clinical laboratory values, or BP between groups at month 12 (Supplemental Table 1). There was a decrease in full age spectrum eGFR at 1 and 6 months in both groups, likely due to a difference in laboratory (Quest versus the University of Colorado Hospital). Very few participants had changes in antihypertensive medication usage (n=0 curcumin and n=2 placebo) or dose (n=1 curcumin and n=1 placebo) throughout the duration of the study.
Table 2.
Adverse events among all randomized participants
| Adverse Event | Curcumin | Placebo |
|---|---|---|
| Related or possibly related | ||
| Flatulence, constipation, diarrhea, or nausea | 7 | 7 |
| Headache | 1 | 1 |
| Warming or redness of face | 2 | |
| Tingling of tongue/mouth | 2 | |
| Dry mouth | 1 | |
| Skin irritation | 1 | |
| Eye redness, dryness, or swelling | 2 | |
| Likely or definitely unrelated | ||
| Orthopedic injury | 3 | |
| Mildly elevated LFTs | 2 | |
| Fever | 3 | |
| Kidney infection | 1 | 2 |
| Urinary tract infection | 2 | 1 |
| Upper respiratory infection | 5 | |
| IBS, worsening or incident | 2 | |
| COVID-19 infection | 2 | |
| Sore throat | 2 | |
| Tonsillitis, tonsil infection, or tonsillectomy | 1 | 1 |
| Hand, foot, and mouth | 1 | |
| Streptococcal pharyngitis | 1 | |
| Ear infection | 1 | |
| Numbness in extremities | 1 | |
| Emesis | 2 | |
| Influenza | 1 | |
| Lightheadedness | 1 | |
| Osteomyelitis | 1 | |
| Giardia infection | 1 | |
| Conjunctivitis | 1 | |
| Cholecystectomy surgery | 1 | |
| Iron deficiency | 1 | |
| Flank pain | 1 | |
| Abdominal and pelvic pain | 1 | |
| Sinus infection | 1 | |
| Strabismus surgery | 1 | |
| Yeast infection | 1 | |
| Root canal | 1 | |
Data are n, including participants who discontinued participation. LFT, liver function tests; IBS, irritable bowel syndrome; COVID-19, coronavirus disease 2019.
Primary Outcome Measures: Effect of Curcumin on Vascular Function
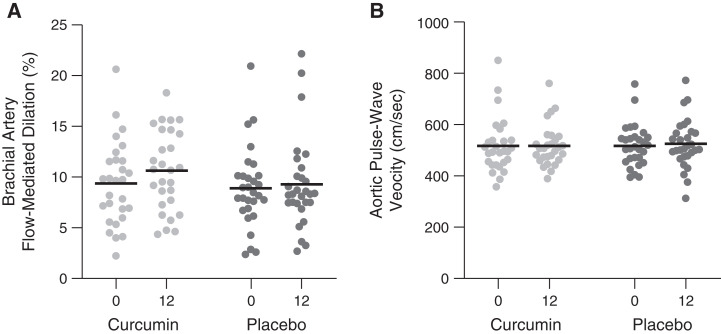
The coprimary end point, FMDBA (percentage Δ), did not change in the curcumin group (baseline: 9.6±4.0; 12 months: 10.7±4.4) as compared with the placebo group (baseline: 9.0±4.1; 12 months: 9.4±6.0; within-group change for FMDBA [percentage change]: curcumin: 1.14; 95% confidence interval [95% CI], –0.84 to 3.13; placebo: 0.33; 95% CI, –1.34 to 2.00; estimated difference in change: 0.81; 95% CI, –1.21 to 2.84; P=0.48 for comparison of change across groups) (Table 2). Results were similar using a complete case analysis including all participants completing the study regardless of adherence (P=0.23) (Figure 2A, Table 2). Results were also similar when presented as absolute change (Table 3). There was no interaction by sex (group × sex P=0.57). Shear rate and baseline diameter remained constant across the study and thus were not included as covariates (Table 3). Smooth muscle cell responsiveness to nitric oxide, measured by brachial artery endothelium-independent dilation to sublingual nitroglycerin (other outcome measure), was also unaffected by curcumin supplementation (Table 3).
Figure 2.
Changes in brachial artery flow-mediated dilation and aortic pulse-wave velocity with curcumin and placebo. Brachial artery flow-mediated dilation (A) and aortic pulse-wave velocity (measured as carotid-femoral pulse-wave velocity) (B) at baseline and following 12 months of treatment with curcumin or placebo among participants completing the study (lines represent means, with individual participants shown by dots).
Table 3.
Effects of curcumin on vascular parameters
| Vascular Parameters | Curcumin, n=28 | Placebo, n=29 | Difference in Change According to Treatment Assignment | ||||
|---|---|---|---|---|---|---|---|
| Baseline | 12 mo | Change within Group | Baseline | 12 mo | Change within Group | ||
| Coprimary outcomes a | |||||||
| Brachial artery FMD, % Δ | |||||||
| Intent to treat | 9.6±4.0 | 10.7±4.4 | 1.14 (–0.84 to 3.13) | 9.0±4.1 | 9.4±6.0 | 0.33 (–1.34 to 2.00) | 0.81 (–1.21 to 2.84) |
| Complete case analysis | 9.4±4.1 | 10.6±3.9 | 1.25 (–0.28 to 2.78) | 8.9±4.0 | 9.3±4.5 | 0.40 (–0.37 to 1.17) | 0.97 (–0.61 to 2.55) |
| Carotid-femoral PWV | |||||||
| Intent to treat | 508±103 | 508±92 | 0.6 (–25.7 to 26.9) | 517±85 | 523±107 | 6.5 (–20.4 to 33.5) | −5.9 (–35.8 to 24.0) |
| Complete case analysis | 517±106 | 517±81 | 0.05 (–16.50 to 16.60) | 518±82 | 525±95 | 6.8 (–10.6 to –24.1) | −6.9 (–28.9 to 15.1) |
| Secondary outcomes a | |||||||
| Brachial artery FMD, mm Δ | 0.28±0.13 | 0.33±0.11 | 0.12 (0.00 to 2.78) | 0.28±0.11 | 0.29±0.12 | 0.01 (–0.01 to 0.04) | 0.03 (–0.01 to 0.08) |
| Baseline brachial artery diameter, mm | 3.00±0.38 | 3.12±0.36 | 0.13 (0.07 to 0.17) | 3.15±0.42 | 3.17±0.40 | 0.03 (–0.03 to 0.09) | 0.08 (0.00 to 0.15) |
| Peak shear rate, s−1 | 1092±295 | 1127±302 | 24 (–87 to 136) | 1018±342 | 1004±366 | −22 (–122 to 78) | 71 (–68 to 211) |
| Brachial artery dilation to NTG, % Δb | 32±8 | 30±5 | −1.1 (–5.8 to 3.5) | 31±8 | 30±9 | −0.9 (–4.5 to 2.7) | 0.2 (–4.5 to 4.9) |
| Brachial artery dilation to NTG, mm Δb | 0.95±0.22 | 0.95±0.15 | 0.00 (–0.12 to 0.11) | 0.94±0.21 | 0.92±0.19 | −0.02 (–0.12 to 0.08) | 0.49 (0.23 to 0.76) |
| Brachial systolic BP, mm Hg | 117±13 | 117±12 | 0.0 (–2.5 to 3.2) | 114±13 | 117±13 | 2.6 (0.6 to 4.7) | −1.9 (–5.1 to 1.3) |
| Brachial diastolic BP, mm Hg | 65±7 | 65±8 | 0.0 (–3.5 to 3.8) | 62±9 | 65±11 | 1.4 (–1.4 to 4.2) | −0.6 (–4.9 to 3.7) |
| Carotid intimal medial thickness, mm | 0.41±0.05 | 0.42±0.0.05 | 0.01 (0.00 to 0.02) | 0.39±0.04 | 0.39±0.0.04 | 0.00 (–0.01 to 0.01) | 0.00 (–0.00 to 0.02) |
| Carotid-radial PWV, cm/s | 812±131 | 801±164 | −11 (–49 to 26) | 809±149 | 812±139 | 3 (–45 to 50) | −13 (–71 to 44) |
| Carotid augmentation index, % | −8.8±15.3 | −7.2±16.6 | 1.6 (–2.6 to 5.8) | −4.9±17 | −3.4±18.6 | 1.4 (–2.0 to 4.8) | −0.2 (–5.4 to 5.0) |
| Carotid artery compliance, mm/mm Hg × 10−1 | 0.16±0.04 | 0.15±0.04 | 0.00 (–0.02 to 0.02) | 0.16±0.07 | 0.15±0.08 | −0.01 (–0.02 to 0.01) | 0.01 (–0.02 to 0.03) |
| Carotid β-stiffness index, A.U. | 4.22±0.84 | 4.52±1.13 | 0.25 (–0.29 to 0.79) | 4.67±1.22 | 5.24±1.49 | 0.57 (0.07 to 1.07) | −0.51 (–1.2 to 0.17) |
| Carotid systolic BP, mm Hg | 106±14 | 108±12 | 2.1 (–2.1 to 6.3) | 105±15 | 108±15 | 2.1 (–0.5 to 4.61) | 0.2 (–4.2 to 4.6) |
Data are mean ± SD or mean (95% confidence interval) for change within group and difference in change. For intent-to-treat analyses (coprimary outcomes), values are derived from multiple imputation with the expectation-maximization method (with the exception of baseline values). For complete case analyses, the difference in change across groups is the slope (95% confidence interval) derived from linear regression models after adjustment for baseline values for given variable, with the curcumin group compared with the placebo group. BP were taken in the supine position. FMD, flow-mediated dilation; PWV, pulse-wave velocity; NTG, nitroglycerin.
Primary outcomes are presented as both intent-to-treat and complete case analyses; secondary outcomes are presented as complete case analyses.
NTG was administered to n=25 participants ≥18 years of age (n=13 curcumin and n=12 placebo).
The second coprimary end point, aPWV, also did not change with curcumin (baseline: 508±103 cm/s; 12 months: 508±92 cm/s) as compared with placebo (baseline: 517±85 cm/s; 12 months: 523±107 cm/s; within-group change for aPWV [centimeters per second]: curcumin: 0.6; 95% CI, –25.7 to 26.9; placebo: 6.5; 95% CI, –20.4 to –33.5; estimated difference in change: –5.9; 95% CI, –35.8 to 24.0; P=0.67) (Table 2). Results were similar using a complete case analysis including all participants completing the study regardless of adherence (P=0.53) (Figure 2B, Table 2). There was no interaction by sex (group × sex P=0.57). Carotid-radial PWV (other outcome measure), a measure of peripheral arterial stiffness, also did not change in either group (Table 3). Resting brachial systolic BP, carotid systolic BP, carotid augmentation index, carotid intimal medial thickness, carotid artery compliance, and carotid β-stiffness index (other outcomes measures) were also unchanged (Table 3).
Secondary Outcome Measures: Vascular Oxidative Stress and Markers of Oxidative Stress and Inflammation
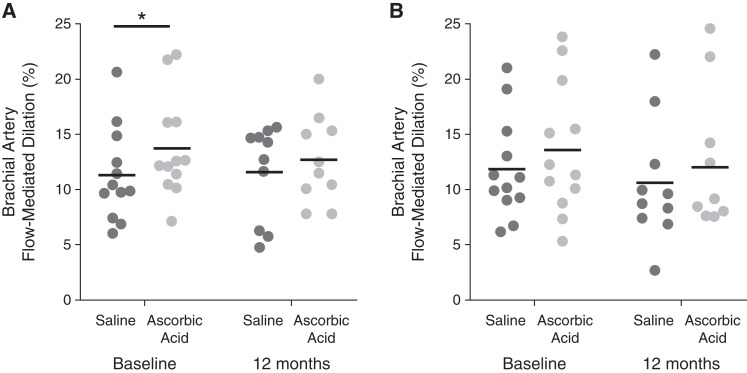
In the subgroup that participated in these AQ10 procedures, ascorbic acid infusion significantly improved FMDBA as compared with isovolumetric saline in the curcumin group (saline =10.7±4.2%; ascorbic acid =13.7±4.6%; P=0.008) as reported previously for the whole cohort (18). A similar trend was observed in the placebo group at baseline (saline =11.8±4.6%; ascorbic acid =13.5±5.9%; P=0.07). Ascorbic acid infusion did not improve FMDBA at 12 months in the curcumin group (saline =11.6±4.3%; ascorbic acid =12.7±4.0%; P=0.33) or the placebo group (saline =10.6±5.7%; ascorbic acid =12.0±6.4%; P=0.13). However, there was no difference between change in FMDBA with ascorbic acid at 12 months (comparison of change in FMDBA between groups: curcumin: 1.1±3.5%; placebo: 1.4±2.6%; P=0.85), indicating no curcumin-specific reduction in vascular oxidative stress (Figure 3).
Figure 3.
Brachial artery flow-mediated dilation in response to inhibition of vascular oxidative stress via an acute supraphysiologic infusion of ascorbic acid. Brachial artery flow-mediated dilation following an acute supraphysiologic infusion of ascorbic acid known to inhibit superoxide production or isovolumetric saline at baseline (left) and following 12 months (right) of treatment with curcumin (A) or placebo (B). These measures were performed in a subgroup of participants ≥18 years of age. *P<0.05 by paired t test comparing saline with vitamin C within a single group at a single time point.
Urinary levels of the oxidative stress marker 8-iso-prostaglandin F2α did not change in the curcumin versus placebo group (Table 4). Serum C-reactive protein, IL-6 (secondary outcomes), TNF-α, and IFN-γ (other outcomes) levels also did not change (Table 4).
Table 4.
Effects of curcumin on secondary and other outcomes TNF-α, unit
| Markers | Curcumin, n=28 | Placebo, n=29 | Difference in Change According to Treatment Assignment | ||||
|---|---|---|---|---|---|---|---|
| Baseline | 12 mo | Change within Group | Baseline | 12 mo | Change within Group | ||
| Urinary 8-iso-prostaglandin F2α, mg/d, normalized to urinary creatinine, mg/dl, e−8 | 14.1 (8.1 to 27.6) | 14.4 (8.9 to 25.9) | 0.00 (–0.34 to 0.34) | 9.3 (6.6 to 21.7) | 12.5 (6.3 to 33.4) | 0.27 (–0.14 to 0.69) | −0.14 (–0.61 to 0.32) |
| CRP, mg/L | 0.70 (0.18 to 2.45) | 0.63 (0.38 to 2.91) | 0.21 (–0.47 to 0.88) | 0.32 (0.19 to 0.68) | 0.44 (0.11 to 1.30) | 0.17 (0.36 to 0.70) | 0.49 (–0.27 to 1.25) |
| IL-6, pg/ml | 0.42 (0.32 to 0.71) | 3.6 (2.6 to 7.8) | 2.24 (1.69 to 2.80) | 0.43 (0.36 to 0.76) | 4.3 (3.7 to 5.3) | 2.33 (0.91 to 1.54) | −0.11 (–0.65 to 0.42) |
| IFN-γ, pg/ml | 3.7 (2.5 to 4.7) | 1.2 (0.93 to 2.1) | −1.05 (–1.35 to –0.75) | 4.1 (2.9 to 6.5) | 1.2 (1.1 to 1.5) | −1.14 (–1.41 to –0.87) | −0.01 (0.25 to 0.22) |
| 1.34±0.54 | 0.61±0.38 | −0.73 (–0.95 to –0.51) | 1.58±0.83 | 0.63±0.49 | −0.95 (–1.27 to –0.63) | 0.01 (–0.22 to 0.25) | |
Data are median (interquartile range) or mean (95% confidence interval) for change within group and difference in change on the basis of a complete case analysis. The difference in change across groups is the slope (95% confidence interval) derived from linear regression models after adjustment for baseline values for given variable, with the curcumin group compared with the placebo group. For all variables except TNF-α, the change within group and difference in change across groups are comparing log-transformed values. CRP, C-reactive protein.
Exploratory Outcome Measure: Kidney Magnetic Resonance Imaging
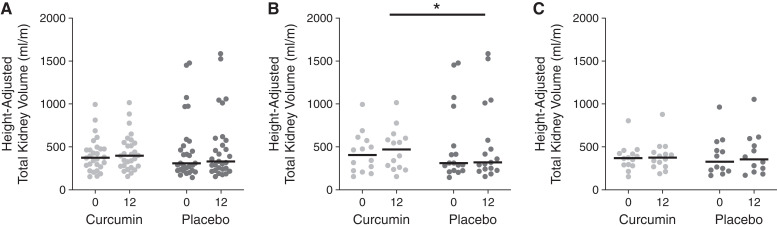
Although the trial was not powered for this exploratory end point, htTKV did not change over the 12-month study with curcumin administration (curcumin: baseline: 375; 95% CI, 275 to 474 ml/m; 12 months: 397; 95% CI, 275 to 548 ml/m) as compared with placebo (baseline: 309; 95% CI, 225 to 510 ml/m; 12 months: 331; 95% CI, 239 to 576 ml/m; P=0.24) (Figure 4A). There was a significant interaction by sex (group × sex P=0.04). Change in htTKV was greater in the curcumin group compared with the placebo group only in boys (curcumin: baseline: 402; 95% CI, 222 to 573 ml/m; 12 months: 469; 95% CI, 260 to 601 ml/m; placebo: baseline: 299; 95% CI, 222 to 743 ml/m; 12 months: 329; 95% CI, 231 to 794 ml/m; P=0.03) in contrast to our hypothesis (Figure 4B), with no difference in girls (curcumin: baseline: 375; 95% CI, 294 to 428 ml/m; 12 months: 380; 95% CI, 276 to 440 ml/m; placebo: baseline: 309; 95% CI, 234 to 463 ml/m; 12 months: 331; 95% CI, 255 to 519 ml/m; P=0.52) (Figure 4C). Change in total cyst volume, fractional cyst volume, and kidney parenchyma in the whole cohort at 12 months were also similar between groups (other outcomes measures) (Supplemental Table 2).
Figure 4.
Change in height-adjusted total kidney volume with curcumin and placebo. Height-adjusted total kidney volume at baseline and following 12 months of treatment with curcumin or placebo in the whole cohort (A), men (B), and women (C); lines represent medians, with individual participants shown by dots. *P<0.05 for group effect by linear regression with adjustment with baseline height-adjusted total kidney volume.
Discussion
In the first randomized controlled clinical trial of curcumin supplementation in ADPKD, we found no improvements in vascular dysfunction (FMDBA and aPWV) with 12 months of supplementation in a population of children and young adults. The lack of improvement in vascular function was consistent with a lack of reduction in vascular oxidative stress and circulating/urinary markers of oxidative stress and inflammation. We observed no change in kidney growth (exploratory end point) with supplementation in the overall cohort and an unexpected interaction with sex, such that men administered curcumin had a greater increase in htTKV than boys administered placebo.
The study was adequately powered to detect a mean increase in FMDBA of 1.5%, given an SD of 2.0. This is consistent with a recent small meta-analysis that observed a 1.49% (95% CI, 0.16% to 2.82%) improvement in FMDBA with curcumin supplementation across populations of healthy older adults (32). A 1-unit difference in percentage Δ FMDBA is associated with an approximately 13% reduction in cardiovascular disease risk (33). There was no change in carotid-femoral PWV (–41.59; 95% CI, –86.59 to 3.42 cm/s) in the overall meta-analysis; however, carotid-femoral PWV was slightly reduced (–36.68; 95% CI, –70.75 to –2.6 cm/s) in a subanalysis of trials that used a low dose (<500 mg/d) (32). In addition to a non-ADPKD population, notable differences of previous trials on curcumin supplementation and vascular function include a shorter duration, older (nonpediatric) populations, and pill rather than powder administration. Dosing in these trials was less than or equal to the dose prescribed in our trial. Baseline vascular function in our study was slightly better than we previously reported in children/young adults (5) as well as in slightly older young adults with ADPKD (23), possibly due to a younger study population and a larger number of children <18 years of age. Notably, FMDBA and aPWV at the end of study in the curcumin group (10.6±3.9% and 517±181 cm/s, respectively) were comparable with our previous values in healthy children/young adults (10.2±3.1% and 478±66 cm/s, respectively) and slightly older young adults (10.8±4.7% and 562±81 cm/s, respectively). It remains possible that the assumptions in our power calculations were incorrect, including the possibility that the effect size for curcumin differs in children versus adults. Additionally, our observed SD for FMDBA was higher than predicted and observed previously (5), possibly due to a more heterogeneous sample. Finally, our power calculations were on the basis of a change in percentage of FMDBA of 1.5, and this value falls within the 95% CI for the observed change in percentage of flow-mediated dilation across groups. Thus, despite a lack of detected difference in FMDBA with curcumin supplementation, a difference could still exist. In contrast, the change in aPWV used in the power calculations falls outside the observed 95% CI for the observed change in aPWV, implying there is no difference in change in aPWV when comparing the curcumin and placebo groups.
In ADPKD, oxidative stress and inflammation are increased and contribute to a decline in nitric oxide bioavailability (8,9,34), a common mechanism of both impaired endothelium-dependent dilation and increased arterial stiffness (35). Curcumin can suppress both oxidative stress and inflammation in vitro and in vivo (13,36,37). However, in this trial, we observed no reduction in circulatory or urinary markers of oxidative stress or inflammation. Likewise, there was no reduction in vascular oxidative stress, as reflected by change in FMDBA following acute infusion of ascorbic acid. A reduction in resistance vessel oxidative stress was observed following curcumin supplementation in healthy older adults, without a concomitant reduction in circulating markers of oxidative stress and inflammation (14). Notable differences include vascular bed, population, study duration, and route of administration.
Curcumin has also been of interest in nephrology for over two decades as a potential treatment of AKI or chronic kidney impairment. In addition to reducing histologic evidence of kidney damage (38,39), curcumin reduces oxidative stress, increases antioxidant activity (40,41), and reduces inflammation (39) in these models. In an in vitro Madin–Darby canine kidney cell model, curcumin significantly inhibited cyst formation and enlargement (42). Similarly, curcumin treatment reduces cystic index, kidney weight:body weight, and rate of kidney function decline in pkd1-deletion mice (17). More recently, curcumin reduced cystic growth in human-derived ADPKD epithelial cells and mouse pkd1−/− cells (43). In this trial, we observed no difference in change in htTKV with curcumin in the overall cohort. Contrary to animal and cell culture models, htTKV unexpectantly increased in men in the curcumin group versus placebo. The mechanism for this observation, as well as the sex difference, is unclear. Given the small subgroup sample size and exploratory analysis, this could be a difference due to chance. Curcumin adherence was comparable in men (65±15%) as compared with women (72±24%; P=0.35).
Despite a relatively similar profile of adverse events and dropouts in each group, study drug adherence was notably lower in the curcumin group. The main reported adverse event associated with curcumin supplementation was transitory gastrointestinal distress in a small percentage of individuals (44,45); we did not observe this adverse event with curcumin in this study. Of note, several participants reported warming or redness of the face as well as tingling of the tongue or mouth, which have not been described previously. Our dose (25 mg/kg body weight) was on the basis of a 2000-mg/d dose in an average-sized adult, which has shown efficacy for improving FMDBA in adults without ADPKD (14). Notably, inadequate adherence in the curcumin group may have attenuated our power to detect an improvement in vascular function and is thus a confounding factor in assessing the effect of the intervention. It is also important to highlight that the solid lipid curcumin particle (Longvida; Verdure Sciences) that we administered was formulated to prevent glucuronidation, increasing plasma levels of curcumin significantly compared with traditional formulations (46).
Our lack of measurement of circulating curcumin levels is a notable limitation in our study, which was hindered by coronavirus disease 2019 laboratory restrictions. An additional limitation is that we could not examine the longitudinal trajectory of individual participants. Although we had planned to include locally recruited participants at an additional 6-month time point for vascular function, we did not recruit a sufficient number of local participants to perform such an analysis (only two curcumin and four placebo participants). Additionally, the end point of htTKV was exploratory; thus, we were not powered for this end point, and the duration of 1 year was relatively short. Our results may not be generalizable to other populations with ADPKD, including the broader population of adults.
There are also several important strengths of this trial. The population studied was unique, as few trials have been completed in children and young adults with ADPKD. We also performed a comprehensive assessment of vascular function, including assessment of vascular oxidative stress in a subgroup of young adult participants, and evaluated the potential role of sex as a biologic variable. Notably, our study was the first clinical trial to assess changes in vascular function in children and young adults with ADPKD.
In conclusion, curcumin failed to improve vascular function or reduce oxidative stress and inflammation in children and young adults with ADPKD. There was also no change in kidney growth in the overall cohort and an unexpected accelerated growth in boys with curcumin supplementation. Collectively, these results do not support curcumin supplementation over a year-long period as a viable treatment strategy in ADPKD; however, it remains possible that curcumin may show benefit for other end points, for a different ADPKD population, or in a larger trial.
Disclosures
M.A. Cadnapaphornchai reports consultancy agreements with Otsuka Pharmaceutical, honoraria from Otsuka Pharmaceutical, and serving as a scientific advisor or member of Otsuka tolvaptan pediatric steering committee. M. Chonchol reports consultancy agreements with Amgen, Corvidia, Otsuka, Reata, Tricidia, and Vifor; research funding from Corvidia, the National Institutes of Health, Otsuka, Reata, and Sanofi; honoraria from Amgen, Corvidia, Reata, Tricidia, and Vifor; and serving as a Deputy Editor of CJASN. B. Gitomer reports honoraria from the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) for study section participation and from the Department of Defense urological diseases study section and reports serving as a CJASN editorial board member and a Polycystic Kidney Disease Foundation member of the Scientific Advisory Council. A. Jovanovich reports research funding as a site investigator for AstraZeneca; receiving study drug free of charge from Shire; and serving on the editorial board for CJASN, as a section editor for Clinical Nephrology, as an American Heart Association Council for Kidney in Cardiovascular Disease Leadership committee member, and as Chair of the Early Investigators Committee. J. Klawitter reports research funding from NIDDK and the PKD Foundation. K.L. Nowak reports research funding from Corvidia Therapeutics, Otsuka Pharmaceutical Development and Commercialization (data analysis), and Verdure Sciences and reports interacting with the PKD Foundation. D.E. Soranno reports consultancy agreements with Levin & Perconti Attorneys at Law. All remaining authors have nothing to disclose.
Funding
This trial was supported by National Institute of Diabetes and Digestive and Kidney Diseases grant K01DK103678. Additional support was provided by National Center for Advancing Translational Sciences Colorado Clinical and Translational Science award UL1 TR002535 and National Institutes of Health high-end instrumentation grant S10OD018435. This work was also supported by the Zell Family Foundation. K.L. Nowak was also supported by the Baltimore PKD Research Clinical Core Center Pilot and Feasibility Program through National Institute of Diabetes and Digestive and Kidney Diseases grant P30DK090868. D.E. Soranno is supported by National Institute of Diabetes and Digestive and Kidney Diseases grant K08DK109226. C. Steele is supported by National Institute of Diabetes and Digestive and Kidney Diseases grant 5T32DK007135-46.
Supplementary Material
Acknowledgments
The curcumin and placebo were provided by Verdure Sciences (Noblesville, IN).
Verdure Sciences had no influence on the design, execution, and analysis of the results of the study. The funding agencies had no direct role in the conduct of the study; the collection, management, analyses, and interpretation of the data; or the preparation or approval of the manuscript.
Because Dr. Michel Chonchol is a Deputy Editor of CJASN, he was not involved in the peer review process for this manuscript. Another editor oversaw the peer review and decision-making process for this manuscript.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
Data Sharing Statement
Data obtained through this study and presented in this manuscript may be provided to qualified researchers with academic interest in ADPKD. Data shared will be coded, with no personal health information included. The data dictionary and study protocol (with statistical analysis plan) will also be available.
Supplemental Material
This article contains supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.08950621/-/DCSupplemental.
Supplemental Material. Detailed materials and methods.
Supplemental Table 1. Changes in vital signs and clinical laboratory values.
Supplemental Table 2. Additional kidney parameters.
References
- 1.Gabow PA, Johnson AM, Kaehny WD, Kimberling WJ, Lezotte DC, Duley IT, Jones RH: Factors affecting the progression of renal disease in autosomal-dominant polycystic kidney disease. Kidney Int 41: 1311–1319, 1992 [DOI] [PubMed] [Google Scholar]
- 2.Fick GM, Johnson AM, Hammond WS, Gabow PA: Causes of death in autosomal dominant polycystic kidney disease. J Am Soc Nephrol 5: 2048–2056, 1995 [DOI] [PubMed] [Google Scholar]
- 3.Ivy DD, Shaffer EM, Johnson AM, Kimberling WJ, Dobin A, Gabow PA: Cardiovascular abnormalities in children with autosomal dominant polycystic kidney disease. J Am Soc Nephrol 5: 2032–2036, 1995 [DOI] [PubMed] [Google Scholar]
- 4.Cadnapaphornchai MA, McFann K, Strain JD, Masoumi A, Schrier RW: Increased left ventricular mass in children with autosomal dominant polycystic kidney disease and borderline hypertension. Kidney Int 74: 1192–1196, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nowak KL, Farmer H, Cadnapaphornchai MA, Gitomer B, Chonchol M: Vascular dysfunction in children and young adults with autosomal dominant polycystic kidney disease. Nephrol Dial Transplant 32: 342–347, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Covic A, Gusbeth-Tatomir P, Goldsmith DJ: Arterial stiffness in renal patients: An update. Am J Kidney Dis 45: 965–977, 2005 [DOI] [PubMed] [Google Scholar]
- 7.Yeboah J, Crouse JR, Hsu FC, Burke GL, Herrington DM: Brachial flow-mediated dilation predicts incident cardiovascular events in older adults: The Cardiovascular Health Study. Circulation 115: 2390–2397, 2007 [DOI] [PubMed] [Google Scholar]
- 8.Kocyigit I, Kaya MG, Orscelik O, Kaya C, Akpek M, Zengin H, Sipahioglu MH, Unal A, Yilmaz MI, Tokgoz B, Oymak O, Axelsson J: Early arterial stiffness and inflammatory bio-markers in normotensive polycystic kidney disease patients. Am J Nephrol 36: 11–18, 2012 [DOI] [PubMed] [Google Scholar]
- 9.Wang D, Strandgaard S, Borresen ML, Luo Z, Connors SG, Yan Q, Wilcox CS: Asymmetric dimethylarginine and lipid peroxidation products in early autosomal dominant polycystic kidney disease. Am J Kidney Dis 51: 184–191, 2008 [DOI] [PubMed] [Google Scholar]
- 10.Miriyala S, Panchatcharam M, Rengarajulu P: Cardioprotective effects of curcumin. Adv Exp Med Biol 595: 359–377, 2007 [DOI] [PubMed] [Google Scholar]
- 11.Nakmareong S, Kukongviriyapan U, Pakdeechote P, Kukongviriyapan V, Kongyingyoes B, Donpunha W, Prachaney P, Phisalaphong C: Tetrahydrocurcumin alleviates hypertension, aortic stiffening and oxidative stress in rats with nitric oxide deficiency. Hypertens Res 35: 418–425, 2012 [DOI] [PubMed] [Google Scholar]
- 12.Rungseesantivanon S, Thenchaisri N, Ruangvejvorachai P, Patumraj S: Curcumin supplementation could improve diabetes-induced endothelial dysfunction associated with decreased vascular superoxide production and PKC inhibition. BMC Complement Altern Med 10: 57, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fleenor BS, Sindler AL, Marvi NK, Howell KL, Zigler ML, Yoshizawa M, Seals DR: Curcumin ameliorates arterial dysfunction and oxidative stress with aging. Exp Gerontol 48: 269–276, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Santos-Parker JR, Strahler TR, Bassett CJ, Bispham NZ, Chonchol MB, Seals DR: Curcumin supplementation improves vascular endothelial function in healthy middle-aged and older adults by increasing nitric oxide bioavailability and reducing oxidative stress. Aging (Albany NY) 9: 187–208, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Akazawa N, Choi Y, Miyaki A, Tanabe Y, Sugawara J, Ajisaka R, Maeda S: Curcumin ingestion and exercise training improve vascular endothelial function in postmenopausal women. Nutr Res 32: 795–799, 2012 [DOI] [PubMed] [Google Scholar]
- 16.Chuengsamarn S, Rattanamongkolgul S, Phonrat B, Tungtrongchitr R, Jirawatnotai S: Reduction of atherogenic risk in patients with type 2 diabetes by curcuminoid extract: A randomized controlled trial. J Nutr Biochem 25: 144–150, 2014 [DOI] [PubMed] [Google Scholar]
- 17.Leonhard WN, van der Wal A, Novalic Z, Kunnen SJ, Gansevoort RT, Breuning MH, de Heer E, Peters DJ: Curcumin inhibits cystogenesis by simultaneous interference of multiple signaling pathways: In vivo evidence from a Pkd1-deletion model. Am J Physiol Renal Physiol 300: F1193–F1202, 2011 [DOI] [PubMed] [Google Scholar]
- 18.Nowak KL, Farmer-Bailey H, Cadnapaphornchai MA, You Z, George D, Wang W, Jovanovich A, Soranno DE, Gitomer B, Chonchol M: Curcumin therapy to treat vascular dysfunction in children and young adults with autosomal dominant polycystic kidney disease: Design and baseline characteristics of participants. Contemp Clin Trials Commun 19: 100635, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schwartz GJ, Muñoz A, Schneider MF, Mak RH, Kaskel F, Warady BA, Furth SL: New equations to estimate GFR in children with CKD. J Am Soc Nephrol 20: 629–637, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF 3rd, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J; CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) : A new equation to estimate glomerular filtration rate. Ann Intern Med 150: 604–612, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Harris RA, Nishiyama SK, Wray DW, Richardson RS: Ultrasound assessment of flow-mediated dilation. Hypertension 55: 1075–1085, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nowak KL, Gitomer B, Farmer-Bailey H, Wang W, Malaczewski M, Klawitter J, You Z, George D, Patel N, Jovanovich A, Chonchol M: Mineralocorticoid antagonism and vascular function in early autosomal dominant polycystic kidney disease: A randomized controlled trial. Am J Kidney Dis 74: 213–223, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nowak KL, Wang W, Farmer-Bailey H, Gitomer B, Malaczewski M, Klawitter J, Jovanovich A, Chonchol M: Vascular dysfunction, oxidative stress, and inflammation in autosomal dominant polycystic kidney disease. Clin J Am Soc Nephrol 13: 1493–1501, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Klawitter J, Reed-Gitomer BY, McFann K, Pennington A, Klawitter J, Abebe KZ, Klepacki J, Cadnapaphornchai MA, Brosnahan G, Chonchol M, Christians U, Schrier RW: Endothelial dysfunction and oxidative stress in polycystic kidney disease. Am J Physiol Renal Physiol 307: F1198–F1206, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Grantham JJ, Torres VE, Chapman AB, Guay-Woodford LM, Bae KT, King BF Jr., Wetzel LH, Baumgarten DA, Kenney PJ, Harris PC, Klahr S, Bennett WM, Hirschman GN, Meyers CM, Zhang X, Zhu F, Miller JP; CRISP Investigators : Volume progression in polycystic kidney disease. N Engl J Med 354: 2122–2130, 2006 [DOI] [PubMed] [Google Scholar]
- 26.Cadnapaphornchai MA, George DM, McFann K, Wang W, Gitomer B, Strain JD, Schrier RW: Effect of pravastatin on total kidney volume, left ventricular mass index, and microalbuminuria in pediatric autosomal dominant polycystic kidney disease. Clin J Am Soc Nephrol 9: 889–896, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Irazabal MV, Rangel LJ, Bergstralh EJ, Osborn SL, Harmon AJ, Sundsbak JL, Bae KT, Chapman AB, Grantham JJ, Mrug M, Hogan MC, El-Zoghby ZM, Harris PC, Erickson BJ, King BF, Torres VE; CRISP Investigators : Imaging classification of autosomal dominant polycystic kidney disease: A simple model for selecting patients for clinical trials. J Am Soc Nephrol 26: 160–172, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pottel H, Hoste L, Dubourg L, Ebert N, Schaeffner E, Eriksen BO, Melsom T, Lamb EJ, Rule AD, Turner ST, Glassock RJ, De Souza V, Selistre L, Mariat C, Martens F, Delanaye P: An estimated glomerular filtration rate equation for the full age spectrum. Nephrol Dial Transplant 31: 798–806, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lin TH: A comparison of multiple imputation with EM algorithm and MCMC method for quality of life missing data. Qual Quant 44: 277–287, 2010 [Google Scholar]
- 30.Jablonski KL, Racine ML, Geolfos CJ, Gates PE, Chonchol M, McQueen MB, Seals DR: Dietary sodium restriction reverses vascular endothelial dysfunction in middle-aged/older adults with moderately elevated systolic blood pressure. J Am Coll Cardiol 61: 335–343, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jablonski KL, Fedorova OV, Racine ML, Geolfos CJ, Gates PE, Chonchol M, Fleenor BS, Lakatta EG, Bagrov AY, Seals DR: Dietary sodium restriction and association with urinary marinobufagenin, blood pressure, and aortic stiffness. Clin J Am Soc Nephrol 8: 1952–1959, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hallajzadeh J, Milajerdi A, Kolahdooz F, Amirani E, Mirzaei H, Asemi Z: The effects of curcumin supplementation on endothelial function: A systematic review and meta-analysis of randomized controlled trials. Phytother Res 33: 2989–2995, 2019 [DOI] [PubMed] [Google Scholar]
- 33.Inaba Y, Chen JA, Bergmann SR: Prediction of future cardiovascular outcomes by flow-mediated vasodilatation of brachial artery: A meta-analysis. Int J Cardiovasc Imaging 26: 631–640, 2010 [DOI] [PubMed] [Google Scholar]
- 34.Menon V, Rudym D, Chandra P, Miskulin D, Perrone R, Sarnak M: Inflammation, oxidative stress, and insulin resistance in polycystic kidney disease. Clin J Am Soc Nephrol 6: 7–13, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Santhanam L, Christianson DW, Nyhan D, Berkowitz DE: Arginase and vascular aging. J Appl Physiol (1985) 105: 1632–1642, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jain SK, Rains J, Croad J, Larson B, Jones K: Curcumin supplementation lowers TNF-alpha, IL-6, IL-8, and MCP-1 secretion in high glucose-treated cultured monocytes and blood levels of TNF-alpha, IL-6, MCP-1, glucose, and glycosylated hemoglobin in diabetic rats. Antioxid Redox Signal 11: 241–249, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Usharani P, Mateen AA, Naidu MU, Raju YS, Chandra N: Effect of NCB-02, atorvastatin and placebo on endothelial function, oxidative stress and inflammatory markers in patients with type 2 diabetes mellitus: A randomized, parallel-group, placebo-controlled, 8-week study. Drugs R D 9: 243–250, 2008 [DOI] [PubMed] [Google Scholar]
- 38.Shoskes DA: Effect of bioflavonoids quercetin and curcumin on ischemic renal injury: A new class of renoprotective agents. Transplantation 66: 147–152, 1998 [DOI] [PubMed] [Google Scholar]
- 39.Ghosh SS, Massey HD, Krieg R, Fazelbhoy ZA, Ghosh S, Sica DA, Fakhry I, Gehr TW: Curcumin ameliorates renal failure in 5/6 nephrectomized rats: Role of inflammation. Am J Physiol Renal Physiol 296: F1146–F1157, 2009 [DOI] [PubMed] [Google Scholar]
- 40.Bayrak O, Uz E, Bayrak R, Turgut F, Atmaca AF, Sahin S, Yildirim ME, Kaya A, Cimentepe E, Akcay A: Curcumin protects against ischemia/reperfusion injury in rat kidneys. World J Urol 26: 285–291, 2008 [DOI] [PubMed] [Google Scholar]
- 41.Tirkey N, Kaur G, Vij G, Chopra K: Curcumin, a diferuloylmethane, attenuates cyclosporine-induced renal dysfunction and oxidative stress in rat kidneys. BMC Pharmacol 5: 15, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gao J, Zhou H, Lei T, Zhou L, Li W, Li X, Yang B: Curcumin inhibits renal cyst formation and enlargement in vitro by regulating intracellular signaling pathways. Eur J Pharmacol 654: 92–99, 2011 [DOI] [PubMed] [Google Scholar]
- 43.Patera F, Cudzich-Madry A, Huang Z, Fragiadaki M: Renal expression of JAK2 is high in polycystic kidney disease and its inhibition reduces cystogenesis. Sci Rep 9: 4491, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lao CD, Ruffin MT 4th, Normolle D, Heath DD, Murray SI, Bailey JM, Boggs ME, Crowell J, Rock CL, Brenner DE: Dose escalation of a curcuminoid formulation. BMC Complement Altern Med 6: 10, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Suskind DL, Wahbeh G, Burpee T, Cohen M, Christie D, Weber W: Tolerability of curcumin in pediatric inflammatory bowel disease: A forced-dose titration study. J Pediatr Gastroenterol Nutr 56: 277–279, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gota VS, Maru GB, Soni TG, Gandhi TR, Kochar N, Agarwal MG: Safety and pharmacokinetics of a solid lipid curcumin particle formulation in osteosarcoma patients and healthy volunteers. J Agric Food Chem 58: 2095–2099, 2010 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.