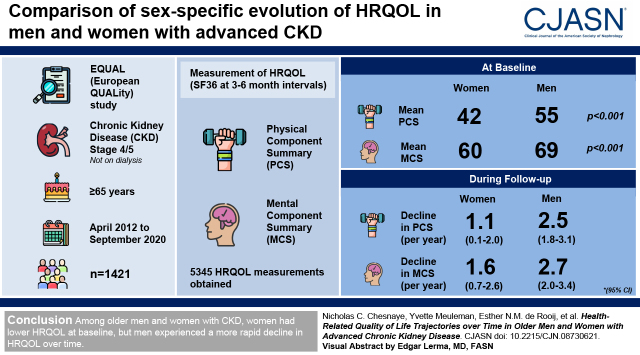
Visual Abstract
Keywords: chronic kidney disease, sex differences, quality of life, aged
Abstract
Background and objectives
The effect of sex on longitudinal health-related quality of life remains unknown in CKD. Here we assess differences in the sex-specific evolution of health-related quality of life in older men and women with advanced CKD.
Design, setting, participants, & measurements
The European Quality Study on Treatment in Advanced Chronic Kidney Disease is a European observational prospective cohort study in referred patients with CKD and an incident eGFR<20 ml/min per 1.73 m2 who are ≥65 years of age not on dialysis. Health-related quality of life was measured using the 36-Item Short Form Survey at 3- to 6-month intervals between April 2012 and September 2020, providing Physical Component Summary and Mental Component Summary scores. Trajectories were modeled by sex using linear mixed models, and sex differences in health-related quality-of-life slope were explored.
Results
We included 5345 health-related quality-of-life measurements in 1421 participants. At baseline, women had considerably lower mean Physical Component Summary (42) and Mental Component Summary (60) compared with men (Physical Component Summary: 55; Mental Component Summary: 69; P<0.001). However, during follow-up, Physical Component Summary and Mental Component Summary scores declined approximately twice as fast in men (Physical Component Summary: 2.5 per year; 95% confidence interval, 1.8 to 3.1; Mental Component Summary: 2.7 per year; 95% confidence interval, 2.0 to 3.4) compared with in women (Physical Component Summary: 1.1 per year; 95% confidence interval, 0.1 to 2.0; Mental Component Summary: 1.6 per year; 95% confidence interval, 0.7 to 2.6). This difference was partly attenuated after adjusting for important covariates, notably eGFR decline. Higher serum phosphate, lower hemoglobin, and the presence of preexisting diabetes were associated with lower Physical Component Summary and Mental Component Summary scores in men but to a lesser extent in women.
Conclusions
Among older men and women with advanced CKD, women had lower health-related quality of life at baseline, but men experienced a more rapid decline in health-related quality of life over time.
Introduction
Differences between the sexes are apparent in the epidemiology of CKD. The prevalence of CKD, especially the earlier stages, is higher in women, whereas paradoxically, more men progress to KRT (1). Patient outcomes also differ by sex, with men at a higher risk of mortality throughout the earlier stages of CKD, although this difference declines progressively with decreasing kidney function and is reduced to zero on dialysis (2). These differences may arise due to biologic or physiologic differences (“sex”) or due to culturally and socially constructed attributes of women and men (“gender”). Although acknowledging this distinction, we use the term “sex” throughout the manuscript to encompass both the biologic and gender differences between men and women.
It is increasingly being accepted that the patient's health-related quality of life (HRQOL) is equally as important as other clinical outcomes when assessing a patient’s health status (3). It has been established that women consistently report a poorer HRQOL than men in the general population (4–7) as well as in various chronic disease populations (8–11). Sex differences regarding patient-reported outcomes are also apparent in the CKD population (1,12). In patients with severely low kidney function, several studies report a poorer HRQOL in women across both mental and physical HRQOL domains (13,14), although this finding is not universal (15–17).
Few studies have investigated the interdependence of HRQOL and sex over time in older patients with advanced CKD (18). CKD is highly prevalent in this age group, and given the rising life expectancy, efforts to improve HRQOL in the elderly should remain in focus. An understanding of sex-specific HRQOL over the course of predialysis CKD and the potential mechanisms underlying any differences may provide insights into a patient’s health and needs and aid sex-specific clinical monitoring and the KRT decision-making process. In this paper, we aim to (1) describe the sex-specific evolution of HRQOL in referred patients with CKD of older age, (2) determine which factors explain the difference in HRQOL between the sexes, and (3) explore the sex-specific determinants of HRQOL.
Materials and Methods
Study Design and Population
The European Quality Study on Treatment in Advanced Chronic Kidney Disease (EQUAL) is an ongoing observational cohort study including patients with CKD of 65 years of age and older with an incident eGFR <20 ml/min per 1.73 m2 (calculated by the Modification of Diet in Renal Disease equation) not on dialysis receiving routine medical care in Germany, Italy, The Netherlands, Poland, Sweden, and the United Kingdom. Participants were excluded if the drop in eGFR resulted from an acute event or if they had previously received dialysis or a kidney transplant. Approval was obtained from the medical ethical committees in each country. Informed consent was obtained from all participants. A full description of the study has been published elsewhere (19).
Data Collection
Clinical data were collected between April 2012 and September 2020 on demographics, primary kidney disease, laboratory data, medication, cardiovascular risk factors, and cardiovascular comorbid conditions (Supplemental Table 1). Study visits and data collection of routine biochemistry and HRQOL were scheduled at 3- to 6-month intervals, and participants were followed until dialysis initiation, kidney transplantation, death, refusal of further participation, loss to follow-up, or end of follow-up at 4 years. eGFR was calculated from serum creatinine level standardized to isotope dilution mass spectrometry using the Chronic Kidney Disease Epidemiology Collaboration equation. Albumin-creatinine ratio was also determined following routine 24-hour urine collection or a single sample if 24-hour urinary collection was unavailable. Hyper-polypharmacy was defined as the use of ten or more medications. Primary kidney disease was classified using the codes of the European Renal Association–European Dialysis and Transplantation Association.
HRQOL is a multidimensional concept commonly defined as an individual’s perceived physical, mental, emotional, and social health (20). HRQOL was collected through self-administered paper questionnaires. HRQOL was measured using the Short-Form 36 (SF-36), a 36-item questionnaire measuring HRQOL on eight domains, resulting in an overall Physical Component Summary (PCS) score and a Mental Component Summary (MCS) score. The PCS is composed of the domains of physical functioning, role limitations due to physical problems, general health, and bodily pain, reflecting physical HRQOL, whereas the MCS is composed of social functioning, role limitations due to emotional problems, vitality, and mental health, reflecting mental HRQOL. PCS and MCS scores range from zero to 100. The questionnaire showed good internal consistency for both PCS and MCS in our population measured by Cronbach α-values (0.86 and 0.90, respectively).
Statistical Analyses
Participant characteristics were reported by sex. Linear mixed models were used to model the participant’s physical and mental HRQOL trajectories. A random intercept was included to capture the variation in HRQOL baseline values between individuals, and a random slope for time was included to capture variability in the individual's HRQOL trajectory. The model included time, sex, and their interaction, and it describes the sex-specific trajectories of HRQOL over time. In subsequent models, we investigated to what extent the association of sex on the HRQOL trajectory is explained by various groups of a priori–defined (time-varying) covariates (from repeated measurements). The difference between the covariate-adjusted estimates and the estimates from the unadjusted model for the sex-specific HRQOL slopes represents the proportion of the association between sex and HRQOL that is explained by each covariate (21). The variables eGFR, urine albumin-creatinine ratio, albumin, calcium, phosphate, potassium, cholesterol, hemoglobin, and BP were included in the models as time-varying covariates. In addition, we explored sex-specific determinants of HRQOL using interaction analyses (22). We first explored unadjusted interaction effects between participant sex, demographics, medication, cardiovascular risk factors, blood chemistry, kidney function, and comorbidities using an interaction term between sex and the variable of interest, including the random effects described above. On the basis of expert opinion and identification of univariable statistical significance of the interaction term, variables were selected for further investigation and adjusted for confounders following the criteria for confounding (i.e., not in the causal pathway, common cause of both exposure and outcome) (Supplemental Table 2) (23). Nonlinear associations were assessed using natural cubic splines.
We followed participants until death or dialysis initiation. Consequently, missing values may be introduced when participants drop out of the study due to mortality or are censored due to dialysis initiation. As HRQOL is related to these events, dropout may be deemed informative (24–26). As a sensitivity analysis, we applied joint models for longitudinal and time-to-event data to avoid biased estimates of HRQOL trajectory because of dropout due to mortality or dialysis initiation (27). The joint model links the linear mixed model described above to a Cox survival model, which captures the risk of the combined event of either mortality or dialysis. In this manner, the joint model informs the longitudinal QoL trajectory on missingness caused by either of these events. The joint model estimates may then be interpreted as the longitudinal QoL trajectory in the hypothetical situation that none of the participants died or started dialysis (28).
Participants who completed at least one HRQOL questionnaire were included in this study. A flow diagram is included in Supplemental Figure 1. In this group, 30% of the longitudinally collected questionnaires were missing. Missing values (Supplemental Table 3) were imputed using the MICE package. A complete case analysis of the main results provided similar estimates (Supplemental Table 4). All analyses were performed with SAS version 9.4 and R version 3.4.1.
Results
Participant Characteristics at Baseline
Table 1 describes the baseline characteristics of the 1421 included participants by sex. On average, participants were 76 years old at inclusion, two thirds were men, and the eGFR at baseline was 17 ml/min per 1.73 m2. Women were older; were more likely to be widowed; had lower levels of education; had a higher body mass index; and had higher values of serum calcium, cholesterol, and potassium, but they had lower levels of hemoglobin. Women were more likely to have a prescription for antidepressants. Women had higher baseline eGFR and a lower albumin-creatinine ratio. In comparison with those included in this study, excluded participants (n=320) had a different primary kidney disease distribution, were prescribed more medications, and had higher levels of serum potassium (Supplemental Table 5).
Table 1.
Baseline characteristics of participants in the European Quality Study on Treatment in Advanced Chronic Kidney Disease
| Characteristics | Overall, n=1421 | Women, n=485 | Men, n=936 |
|---|---|---|---|
| Demographics | |||
| Age, yr | 76 (7) | 77 (7) | 76 (6) |
| Education level, n (%) | |||
| Low education | 437 (31) | 184 (38) | 253 (27) |
| Intermediate education | 767 (54) | 257 (53) | 510 (55) |
| High education | 217 (15) | 44 (9) | 173 (19) |
| Marital status, n (%) | |||
| Married | 913 (64) | 203 (42) | 710 (76) |
| Divorced | 103 (7) | 45 (9) | 58 (6) |
| Widowed | 346 (24) | 209 (43) | 137 (15) |
| Never married | 59 (4) | 28 (6) | 31 (3) |
| One or more children, n (%) | 1256 (88) | 429 (89) | 827 (88) |
| Primary kidney disease, n (%) | |||
| Glomerular disease | 146 (10) | 39 (8) | 107 (11) |
| Tubulointerstitial disease | 127 (9) | 54 (11) | 73 (8) |
| Diabetes | 286 (20) | 83 (17) | 203 (22) |
| Hypertension | 516 (36) | 185 (38) | 331 (35) |
| Miscellaneous kidney disorders | 346 (24) | 124 (26) | 222 (24) |
| Weight, kg | 80 (17) | 73 (17) | 83 (17) |
| Height, cm | 168 (10) | 159 (7) | 173 (8) |
| BMI, kg/m2 | 28 (5) | 29 (6) | 28 (5) |
| Medication, n (%) | |||
| Hyper-polypharmacy | 333 (23) | 115 (24) | 218 (23) |
| Antidepressant prescription | 96 (7) | 51 (11) | 45 (5) |
| Cardiovascular | |||
| Systolic BP, mm Hg | 143 (22) | 141 (22) | 144 (21) |
| Diastolic BP, mm Hg | 74 (11) | 74 (11) | 74 (11) |
| Hb, g/dl | 11.6 (1.5) | 11.5 (1.4) | 11.7 (1.5) |
| Smoking status, n (%) | |||
| Current smoker | 123 (9) | 38 (9) | 85 (9) |
| Ex-smoker | 770 (54) | 170 (35) | 600 (64) |
| Never | 528 (37) | 277 (57) | 251 (27) |
| Blood chemistry | |||
| Albumin, g/dl | 3.8 (5.9) | 3.8 (5.8) | 3.8 (5.9) |
| Calcium, mg/dl | 9.2 (0.8) | 9.2 (0.8) | 8.8 (0.8) |
| Cholesterol, mg/dl | 174 (50) | 190 (54) | 166 (4.6) |
| PO4, mg/dl | 4.0 (0.9) | 4.0 (0.9) | 4.0 (0.9) |
| Potassium, mEq/L | 4.6 (0.6) | 4.6 (0.6) | 4.7 (0.6) |
| Kidney function | |||
| eGFR, ml/min per 1.73 m2 | 17 (5) | 18 (6) | 17 (5) |
| UACR, mg/g | 292 [44–1221] | 177 [27–1027] | 363 [62–1292] |
| Comorbidities, n (%) | |||
| Diabetes | 588 (41) | 177 (37) | 411 (44) |
| Chronic heart failure | 262 (18) | 84 (17) | 178 (19) |
| Cerebrovascular disease | 210 (15) | 70 (14) | 140 (15) |
| Peripheral vascular disease | 242 (17) | 61 (13) | 181 (19) |
| Myocardial infarction | 249 (18) | 52 (11) | 197 (21) |
| Angina pectoris | 217 (15) | 48 (10) | 169 (18) |
| Left ventricular hypertrophy | 347 (24) | 101 (21) | 246 (26) |
| Atrial fibrillation | 253 (18) | 88 (18) | 165 (18) |
| Hypertension | 1272 (90) | 432 (89) | 840 (90) |
| Short-Form 36 | |||
| Mental Component Summary | 65.6 (22.7) | 59.5 (22.7) | 68.8 (22.1) |
| Physical Component Summary | 50.3 (22.5) | 42.0 (21.2) | 54.6 (22.0) |
| Physical functioning | 51.2 (30.2) | 39.2 (28.9) | 57.5 (28.9) |
| Physical role functioning | 42.6 (43.1) | 33.5 (41.4) | 47.2 (43.3) |
| Emotional role functioning | 65.9 (42.8) | 58.6 (45.6) | 69.7 (40.7) |
| Bodily pain | 61.6 (29.8) | 50.3 (28.8) | 67.4 (28.6) |
| Social role functioning | 72.8 (27.9) | 67.2 (29.4) | 75.8 (26.7) |
| Mental health | 73.7 (20.3) | 68.6 (21.3) | 76.3 (19.3) |
| Vitality | 50.6 (23.4) | 44.1 (22.7) | 54.0 (23.0) |
| General health perceptions | 46.6 (18.2) | 46.1 (18.0) | 46.8 (18.3) |
Continuous variables are expressed as mean (SD) except for UACR, which is presented as median [interquartile range]. BMI, body mass index; Hb, hemoglobin; PO4, phosphate; UACR, urine albumin-creatinine ratio; Short-Form 36, 36-Item Short Form Survey.
Health-Related Quality-of-Life Trajectory over Time by Sex
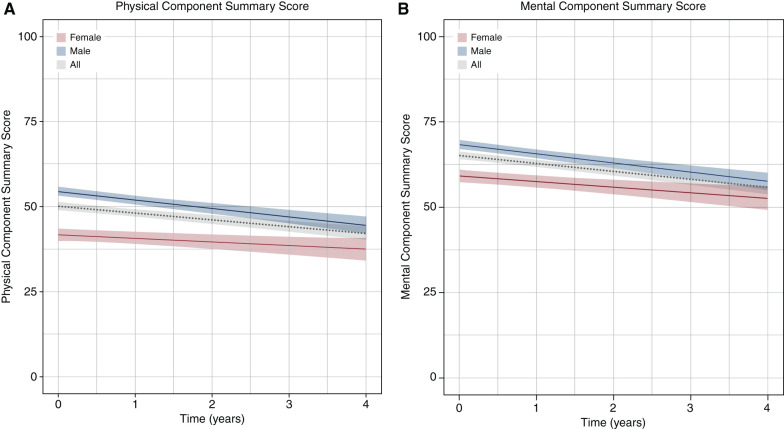
At baseline, women had considerably lower mean PCS (42) and MCS (60) as compared with men (PCS: 55; MCS: 69). During follow-up, we included 5345 HRQOL measurements over a total of 2047 person-years, with a median of three (interquartile range, 2–5) measurements per participant and a median follow-up time of 14 months (interquartile range, 5–27). Overall, PCS declined by 1.9 (95% confidence interval [95% CI], 1.3 to 2.4) points each year, and MCS declined by 2.1 (95% CI, 1.6 to 2.7) points each year. Although women had overall lower HRQOL scores, Figure 1 and Table 2 demonstrate that PCS and MCS declined approximately twice as fast in men (PCS: 2.5 per year; 95% CI, 1.8 to 3.1; MCS: 2.7 per year; 95% CI, 2.0 to 3.4) compared with women (PCS: 1.1 per year; 95% CI, 0.1 to 2.0; MCS: 1.6 per year; 95% CI, 0.7 to 2.6). Examination of the separate SF-36 domains revealed that “role functioning emotional” contributed most to the MCS difference in annual decline found between men and women and that “role functioning physical” and “bodily pain” contributed the most to the PCS difference (Supplemental Table 6). The association of sex with HRQOL trajectories remained similar after adjusting for potential informative censoring due to either mortality (men 23%; women 22%) or dialysis initiation (men 38%; women 31%) (Supplemental Table 7). In a subset of 1032 participants with at least two HRQOL measurements and >6 months of follow-up, trajectories remained similar in men (PCS: −2.8 per year; 95% CI, −3.5 to −2.1; MCS: −3.0 per year; 95% CI, −3.7 to −2.3) and women (PCS: −1.1 per year; 95% CI, −2.1 to −0.2; MCS: −1.8 per year; 95% CI, −2.8 to −0.9).
Figure 1.
Population average (A) Physical Component Summary and (B) Mental Component Summary trajectories and 95% confidence intervals for men (blue), women (red), and overall (gray).
Table 2.
Annual decline in health-related quality of life in men and women sequentially adjusted for an expanding set of covariates
| Annual Change for Physical Component Summary (95% Confidence Interval) | P Value | Annual Change for Mental Component Summary (95% Confidence Interval) | P Value | |
|---|---|---|---|---|
| Unadjusted change | ||||
| Women | −1.1 (−2.0 to −0.1) | −1.6 (−2.6 to −0.7) | ||
| Men | −2.5 (−3.1 to −1.8) | −2.7 (−3.4 to −2.0) | ||
| Difference in change comparing women with men | ||||
| Unadjusted model | 1.4 (0.3 to 2.5) | 0.01 | 1.1 (−0.1 to 2.2) | 0.07 |
| + Demographics | 1.2 (0.0 to 2.4) | 0.06 | 0.8 (−0.4 to 2.1) | 0.19 |
| + Medication | 1.1 (−0.1 to 2.3) | 0.07 | 0.8 (−0.5 to 2.0) | 0.23 |
| + Cardiovascular | 1.3 (0.1 to 2.6) | 0.04 | 0.8 (−0.5 to 2.1) | 0.22 |
| + Blood chemistry | 1.2 (0.0 to 2.5) | 0.06 | 0.7 (−0.7 to 2.0) | 0.32 |
| + Kidney function | 1.2 (−0.1 to 2.5) | 0.08 | 0.5 (−0.9 to 1.8) | 0.48 |
| + Comorbidities | 1.2 (−0.1 to 2.5) | 0.08 | 0.5 (−0.8 to 1.8) | 0.46 |
The adjusted estimates for the difference between the sex-specific slopes for health-related quality of life (HRQOL) can be compared with the estimates from the unadjusted model. The difference between the two estimates then represents the proportion of the association of sex on HRQOL slope that is explained through the covariates. Demographics include participant age, educational level, marital status, having children, primary kidney disease, and country. Medication covariates include hyper-polypharmacy and antidepressant prescription. Cardiovascular covariates include both systolic and diastolic BP, hemoglobin, smoking status, and body mass index. Blood chemistry covariates include serum albumin, calcium, cholesterol, phosphate, and potassium. Kidney function covariates include eGFR and UACR. Comorbidity covariates include diabetes, chronic heart failure, cerebrovascular disease, peripheral vascular disease, myocardial infarction, angina pectoris, left ventricular hypertrophy, atrial fibrillation, and hypertension. UACR, urine albumin-creatinine ratio.
Adjustment for various groups of covariates attenuated the rate of decline of PCS and MCS in both men and women and reduced the difference in slopes between the sexes (Table 2). The difference in rate of HRQOL decline adjusted for individual covariates and covariate groups is presented in Supplemental Table 8. The largest reduction in difference in annual decline in PCS and MCS between men and women was observed after adjusting for (nonlinear) eGFR over time. Full adjustment for all covariates reduced the difference in annual decline in PCS and MCS between men and women to 1.2 (95% CI, −0.1 to 2.5) and 0.5 (95% CI, −0.8 to 1.8), respectively.
Sex-Specific Determinants of Mean Health-Related Quality of Life
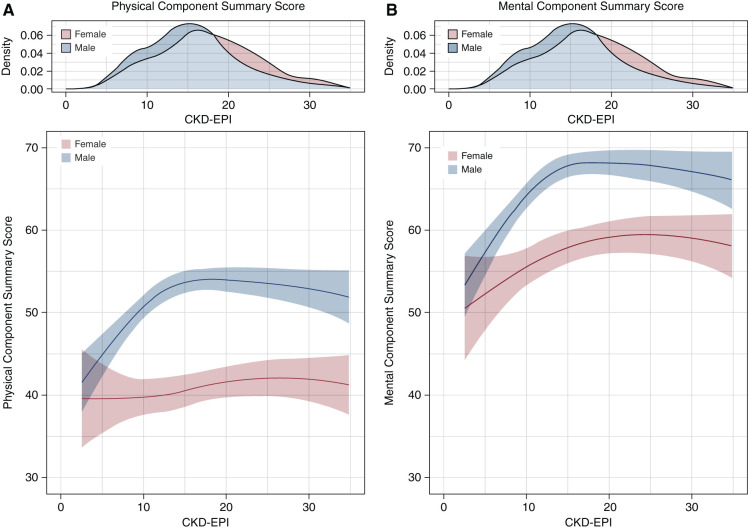
Interaction effects between participant characteristics and sex on mean MCS and PCS were explored univariably (Supplemental Table 9), and they were subsequently selected and adjusted for confounders (Table 3). In adjusted analyses, higher serum phosphate showed a strong inverse association on both mean PCS (per millimoles per liter: −4.1) and mean MCS (−5.3) in men but not in women (PCS: 2.7; MCS: 1.4). Higher serum hemoglobin was more strongly associated with improved mean HRQOL in men (per grams per deciliter; PCS: 1.3; MCS: 1.7) compared with women (PCS: 0.6; MCS: 0.7). The presence of preexisting diabetes showed a negative association with mean PCS (−6.9) and mean MCS (−6.1) in men but to a lesser extent in women (PCS: −2.6; MCS: −0.1). Importantly, we identified nonlinear sex-specific associations of eGFR with HRQOL, demonstrating an increasingly stronger negative association of lower eGFR on both mean PCS and mean MCS in men compared with women below approximately 15 ml/min per 1.73 m2 (Figure 2, Table 3).
Table 3.
Associations of clinical characteristics with health-related quality of life adjusted for confounders
| Clinical Characteristic | Physical Component Summary | Mental Component Summary | ||||
|---|---|---|---|---|---|---|
| Women | Men | P Value for Interaction | Women | Men | P Value for Interaction | |
| eGFR, per 5 ml/min per 1.73 m2, at 10 ml/min per 1.73 m2 | ||||||
| Crude | 0.4 (–2.0 to 2.9) | 4.7 (3.2 to 6.2) | <0.001 | 2.9 (0.3 to 5.5) | 5.6 (4.0 to 7.2) | 0.09 |
| Adjusted | 0.6 (–1.8 to 3.1) | 4.8 (3.3 to 6.3) | <0.001 | 2.3 (–0.4 to 4.9) | 5.0 (3.4 to 6.7) | 0.08 |
| eGFR, per 5 ml/min per 1.73 m2, at 20 ml/min per 1.73 m2 | ||||||
| Crude | 0.8 (–0.4 to 2.0) | −0.2 (–1.1 to 0.8) | 0.23 | 0.7 (–0.6 to 2.0) | −0.1 (–1.1 to 0.9) | 0.31 |
| Adjusted | 1.1 (0.0 to 2.4) | −0.1 (–1.0 to 0.9) | 0.11 | 0.8 (–0.5 to 2.1) | −0.1 (–1.1 to 0.9) | 0.27 |
| Hemoglobin, per g/dl | ||||||
| Crude | 0.7 (0.1 to 1.4) | 1.5 (1.1 to 1.9) | 0.05 | 0.9 (0.3 to 1.6) | 1.9 (1.4 to 2.3) | 0.02 |
| Adjusted | 0.6 (–0.1 to 1.2) | 1.3 (0.9 to 1.7) | 0.07 | 0.7 (0.0 to 1.4) | 1.7 (1.2 to 2.1) | 0.02 |
| Phosphate, per mg/dl | ||||||
| Crude | 0.8 (–0.1 to 1.6) | −1.5 (–2.1 to –0.9) | <0.001 | 0.2 (–0.7 to 1.2) | −2.0 (–2.6 to –1.4) | <0.001 |
| Adjusted | 0.9 (0 to 1.7) | −1.3 (–1.9 to –0.7) | <0.001 | 0.5 (–0.5 to 1.4) | −1.7 (–2.4 to –1.1) | <0.001 |
| Systolic BP, per 10 mm Hg | ||||||
| Crude | −0.2 (–0.6 to 0.2) | 0.4 (0.1 to 0.7) | 0.02 | 0.0 (–0.4 to 0.5) | 0.4 (0.0 to 0.7) | 0.21 |
| Adjusted | 0.0 (–0.4 to 0.4) | 0.5 (0.2 to 0.8) | 0.03 | 0.1 (–0.3 to 0.6) | 0.4 (0.1 to 0.8) | 0.22 |
| Cholesterol, per 25 mg/dl, at 100 mg/dl | ||||||
| Crude | 1.3 (0.1 to 2.4) | 1.2 (0.5 to 1.9) | 0.86 | 1.7 (0.4 to 2.9) | 1.4 (0.6 to 2.2) | 0.73 |
| Adjusted | 1.0 (–0.1 to 2.1) | 1.0 (0.3 to 1.7) | 0.96 | 1.5 (0.3 to 2.7) | 1.2 (0.4 to 2.0) | 0.71 |
| Cholesterol, per 25 mg/dl, at 200 mg/dl | ||||||
| Crude | 0.1 (–0.3 to 0.5) | 0.4 (0.1 to 0.8) | 0.16 | 0.6 (0.1 to 1.0) | 0.7 (0.4 to 1.1) | 0.54 |
| Adjusted | 0.0 (–0.4 to 0.4) | 0.4 (0.0 to 0.7) | 0.14 | 0.5 (0.0 to 0.9) | 0.6 (0.3 to 1.0) | 0.54 |
| Diabetes, present versus absent | ||||||
| Crude | −4.2 (–7.5 to –0.9) | −7.6 (–10.0 to –5.3) | 0.10 | −1.7 (–5.1 to 1.8) | −6.8 (–9.1 to –4.4) | 0.02 |
| Adjusted | −2.6 (–6.0 to 0.8) | −6.9 (–9.5 to –4.3) | 0.03 | −0.1 (–3.7 to 3.6) | −6.1 (–8.9 to –3.3) | <0.001 |
| Peripheral vascular disease, present versus absent | ||||||
| Crude | −4.2 (–8.8 to 0.5) | −6.5 (–9.4 to –3.6) | 0.40 | 0.05 | ||
| Adjusted | −3.7 (–8.1 to 0.8) | −5.0 (–7.8 to –2.2) | 0.61 | −1.0 (–5.7 to 3.8) | −5.7 (–8.7 to –2.6) | 0.10 |
| Myocardial infarction, present versus absent | ||||||
| Crude | −9.3 (–14.3 to –4.4) | −4.1 (–7.0 to –1.2) | 0.07 | −6.9 (–12.1 to –1.8) | −3.9 (–6.9 to –0.9) | 0.32 |
| Adjusted | −4.4 (–9.1 to 0.3) | 0.4 (–2.4 to 3.2) | 0.08 | −3.3 (–8.4 to 1.8) | −0.5 (–3.5 to 2.6) | 0.34 |
The model for eGFR was adjusted for demographic covariates, medication covariates, BP, hemoglobin, smoking status, calcium, cholesterol, PO4, potassium, UACR, and comorbidity covariates. The model for hemoglobin was adjusted for age, hyper-polypharmacy, smoking status, and kidney function covariates. The model for serum PO4 was adjusted for age, educational level, marital status, children, hyper-polypharmacy, smoking status, and kidney function covariates. The model for systolic BP was adjusted for demographic covariates, medication covariates, cardiovascular covariates, blood chemistry covariates, and comorbidity covariates (except hypertension). The model for cholesterol was adjusted for demographic covariates, hyper-polypharmacy, smoking status, albumin, and diabetes. The model for diabetes was adjusted for demographic covariates, medication covariates, and smoking status. The model for peripheral vascular disease was adjusted for demographic covariates, BP, smoking status, calcium, cholesterol, PO4, kidney function covariates, and comorbidity covariates. The model for myocardial infarction was adjusted for demographic covariates, cardiovascular covariates, calcium, cholesterol, PO4, potassium, kidney function covariates, and comorbidity covariates. Because of the nonlinear relationship with health-related quality of life, the associations for both eGFR and serum cholesterol are presented at varying values. PO4, phosphate; UACR, urine albumin-creatinine ratio.
Figure 2.
The nonlinear relationship between eGFR and both (A) Physical Component Summary and (B) Mental Component Summary by sex. The distribution of eGFR by sex is provided in the density plot. CKD-EPI, Chronic Kidney Disease Epidemiology Collaboration.
Discussion
In this paper, we investigate the interdependence between sex, HRQOL, and important demographic and clinical factors in older patients with advanced CKD. Our findings demonstrate that women consistently report lower physical and mental HRQOL scores compared with men. Nonetheless, despite the higher overall HRQOL scores reported by men, HRQOL declined approximately twice as fast over time compared with women. This association was attenuated to some extent after accounting for sex differences in eGFR levels, suggesting an explanatory role for decreasing eGFR. However, the majority of this disparity remained unexplained, suggesting that other unidentified factors may play a role. Interestingly, lower eGFR, lower serum hemoglobin, higher phosphate levels, and preexisting diabetes were associated with lower HRQOL in men but to a lesser extent in women.
Our finding that women perceive an overall poorer HRQOL compared with men is consistent with that found in the general population (4–7), in patients on dialysis (29), and in several studies on nondialysis-dependent CKD (13,14), although the latter is not universal (15–17). Pagels et al. (15) found no association between sex and HRQOL in a cross-sectional analysis of 535 Swedish patients with CKD stages 2–5, despite a history of cardiovascular disease and higher CRP levels being more common among men. Similarly, Chow et al. (16) found that the presence of CKD (<60 ml/min per 1.73 m2) had comparable effects on HRQOL in Australian men and women, with the exceptions of women reporting more problems in the bodily pain domain and men reporting more problems in the mental health domain. Aggarwal et al. (17) also report domain-specific sex discrepancies in patients with CKD, with women reporting lower physical HRQOL compared with men but similar mental HRQOL.
Several potential mechanisms may explain the lower overall reported HRQOL found in women at baseline and throughout follow-up. First, men may be more likely than women to deny or under-report perceived signs of physical weakness due to social and cultural norms defining the concept of masculinity (30,31). Second, men and women commonly apply different coping styles to deal with the limitations imposed by CKD. Women tend to apply more emotion-focused coping than men, which has been associated with a poorer quality of life (32,33). Third, women report more symptoms compared with men and perceive these symptoms to be more intense. We previously demonstrated the negative association of symptoms with HRQOL (34). Moreover, this difference in symptom sensitivity seems to persist after excluding gynecologic and reproductive symptoms and after restriction to medically unexplained symptoms (4,35). Last, women tend to have a higher prevalence of anxiety and depression affecting both mental and physical HRQOL (36,37), which is also reflected by our data showing that women are prescribed antidepressants twice as frequently.
In our population of older participants with advanced CKD, despite the higher overall HRQOL reported by men, both their physical and mental HRQOLs declined approximately twice as fast over time compared with women. As the minimal clinically important difference on the SF-36 scale is considered to be three to five points (38), the HRQOL difference in annual decline between men and women (PCS: 1.4; MCS: 1.1) would theoretically exceed this threshold after 3 years. It should be kept in mind that our population of CKD stages 4 and 5 participants offers only a snapshot of a far lengthier disease trajectory and that rates of HRQOL decline in men and women are likely to vary in previous stages of CKD. Although we found no evidence of a floor effect in our study, a simple explanation for the faster HRQOL decline in men may be the result of women already having suffered most of their HRQOL decline during previous CKD stages, leading to their relatively low baseline HRQOL, which in turn, offers less room to decline further during follow-up in this study.
The literature on HRQOL in CKD is still dominated by cross-sectional studies. The few longitudinal studies exploring the role of sex on HRQOL trajectories over time in advanced stage CKD found, in contrast to our own results, no difference in the rate of HRQOL decline between men and women (14,39). Mujais et al. (14) studied HRQOL in a subset of 649 patients with CKD (eGFR<60 ml/min per 1.73 m2) with multiple annual HRQOL measurements, finding that although HRQOL declined progressively with more advanced stages of CKD, sex was not associated with the rate of HRQOL decline. Somewhat in line with our results, Zimbudzi et al. (40) studied the effect of an integrated care intervention on longitudinal HRQOL in 179 patients with CKD and comorbid diabetes, finding that between baseline and 12 months, physical HRQOL had improved among women but declined among men. As this study was designed to assess the effect of an integrated care model on HRQOL, the authors attributed this finding to women being more amenable to the intervention.
Because of the paucity of longitudinal studies, the mechanisms underlying the faster decline of HRQOL found in men are difficult to pinpoint. We previously demonstrated a faster kidney decline in men compared with women in the EQUAL population (12), and others have demonstrated that lower levels of eGFR correlate well with lower HRQOL (39). In addition, a faster decline in kidney function is associated with a steeper increase in symptom burden (41), which in turn, negatively affects HRQOL. Consequently, adjustment for longitudinal eGFR expectedly attenuated the sex difference in annual HRQOL decline, reflecting the detrimental effects of the faster progression of CKD in men on their HRQOL. This was paralleled by our interaction analysis showing that below an eGFR of approximately 15 ml/min per 1.73 m2, further declines in eGFR were considerably more strongly associated with HRQOL in men than in women, especially regarding physical HRQOL. We hypothesize that the faster eGFR decline in men and subsequent deterioration in health (or vice versa) leave men with less time to adapt and cope with the imposed physical and mental limitations, which may further exacerbate the negative consequences of declining eGFR on HRQOL. Looking at the specific HRQOL domains, men experienced the most rapid declines in both their emotional and physical role functioning, suggesting that the consequences of their faster kidney decline are most felt by men when it comes to the quality and quantity of their regular daily activities.
In our interaction analyses, we also identified several other sex-specific determinants of mean HRQOL that may help explain the sex disparity in the rate of HRQOL decline. Higher serum phosphate showed a strong negative association with HRQOL in men but not in women. In line with this finding, others have demonstrated sex heterogeneity in the associations of elevated serum phosphate on the risk of atherosclerosis, coronary artery disease, and mortality in the CKD population, with high phosphate affecting adverse outcomes more in men than in women (42,43).
We also demonstrate a significant interaction in the association of hemoglobin levels with HRQOL by sex, with higher levels of hemoglobin being more beneficial to HRQOL in men than women. It is known that low hemoglobin levels in CKD are associated with negative outcomes and that increases in hemoglobin are correlated with longitudinal improvements in HRQOL (14,44). In the general as well as the CKD population, women tend to have lower hemoglobin levels compared with men (45,46) and appear to tolerate these lower levels without any long-term consequences (47). Moreover, declines in eGFR lead to larger decreases in hemoglobin levels in men compared with women (48,49), which in turn, may affect HRQOL in men more than in women.
The main strength of our study is that participants in our international cohort were prospectively included when their eGFR dropped below the predefined level of 20 ml/min per 1.73 m2, thus minimizing the risk of survivor bias. Unlike most other cohort studies, we were able to measure HRQOL prospectively using the SF-36 questionnaire. Nonetheless, our study is also subject to several limitations. We were unable to capture the complex interplay between demographic, psychosocial, and biologic factors not collected by our study that are also likely responsible for sex differences related to HRQOL. We also were unable to capture life events and changes in socioeconomic environment. Nonetheless, we explore the sex-specific effect of many important clinical and nonclinical factors. We were able to include the majority of participants in the EQUAL cohort; however, 30% of the questionnaires were missing during follow-up, although this may be expected given the health status and age of the population (50). Furthermore, as residual and unmeasured confounding may play a role, we are unable to infer causality to our findings.
In summary, in our population of patients of older age with advanced CKD, we demonstrate that although women consistently report lower physical and mental HRQOLs compared with men, the rate of HRQOL decline over time was approximately twice as fast in men. The faster decline in men was explained in part by their lower eGFR, which had a stronger association with HRQOL as compared with women. By assessing HRQOL through a sex perspective, we identified that high levels of phosphate, low levels of hemoglobin, and preexisting diabetes were associated with lower HRQOL in men but to a lesser extent in women, warranting further investigation into whether men could benefit from interventions targeting the intensified treatment of anemia and a reduction in serum phosphate levels. Implications for this research also nurture speculations on the sex imbalance with respect to the proportion of individuals requiring KRT. Further research on this topic could focus on the development of CKD stage– and sex-specific multidisciplinary and psychosocial intervention strategies, as well as HRQOL thresholds that could be used to classify older patients in need of additional support. Overall, our results provide a better understanding of HRQOL over the course of advanced stage CKD, highlighting potential mechanisms underlying sex-specific differences, which in turn, will potentially help improve patient-centered care.
Disclosures
F.W. Dekker reports research funding from Astellas, Chiesi, and Vifor; collaboration with the Dutch Kidney Patients Association; and collaboration with the Dutch Quality Institue for Renal Care (Nefrovisie). C. Drechsler reports research funding from Genzyme. M. Evans reports institutional grants from Astellas Pharma and AstraZeneca; payment for lectures from Astellas, AstraZeneca, Baxter Healthcare, Fresenius Medical Care, and Vifor Pharma; serving as a scientific advisor or member of Astellas, AstraZeneca, and the Vifor Pharma advisory board; and serving as a member of steering committee of the Swedish Renal Registry and as a member the European Renal Association–European Dialysis and Transplant Association (ERA-EDTA) Registry Committee. K.J. Jager reports serving on the editorial boards of African Journal of Nephrology, Journal of Renal Nutrition, Kidney International Reports, Nephrology, and Nephrology Dialysis Transplantation. A.A. Pagels reports serving as a scientific advisor or member of the Swedish Renal Registry. C. Wanner reports consultancy agreements with Akebia, Bayer, Boehringer-Ingelheim, Gilead, GlaxoSmithKline (GSK), MSD, Sanofi-Genzyme, Triceda, and Vifor; research funding from Idorsia (grant to institution) and Sanofi-Genzyme (grant to institution); honoraria from Astellas, AstraZeneca, Bayer, Boehringer-Ingelheim, Chiesi, Eli-Lilly, FMC, Sanofi-Genzyme, and Shire-Takeda; honoraria for consultancy and lecturing from Amicus, AstraZeneca, Bayer, Boehringer-Ingelheim, Eli-Lilly, GILEAD, GSK, MSD, Sanofi-Genzyme, and Takeda; and other interests/relationships with ERA-EDTA. All remaining authors have nothing to disclose.
Funding
Main funding was received from the European Renal Association–European Dialysis and Transplant Association and contributions from the Swedish Medical Association, the Stockholm County Council Avtal om Läkarutbildning och Forskning Medicine and Center for Innovative Research, the Italian Society of Nephrology, Dutch Kidney Foundation grant SB 142, the Young Investigators Grant in Germany, and the National Institute for Health Research in the United Kingdom.
Supplementary Material
Acknowledgments
We thank all of the participants and health professionals participating in the EQUAL study.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
Supplemental Material
This article contains the following supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.08730621/-/DCSupplemental.
Supplemental Summary 1. List of EQUAL Study Investigators.
Supplemental Figure 1. Flow chart.
Supplemental Table 1. Definitions for preexisting cardiovascular comorbid conditions.
Supplemental Table 2. Confounders in the relationship between the determinant of interest and HRQOL on the basis of literature and expert opinion.
Supplemental Table 3. Proportion of missing values.
Supplemental Table 4. A complete case analysis of the annual decline in HRQOL in men and women.
Supplemental Table 5. Participant characteristics at baseline for excluded and included participants.
Supplemental Table 6. Annual decline for SF-36 domain scores in men and women.
Supplemental Table 7. Annual decline in QoL in men and women adjusted for informative censoring due to dropout caused by death or dialysis.
Supplemental Table 8. Annual decline in HRQOL in men and women and subsequent adjustment for (groups of) covariates.
Supplemental Table 9. Exploratory univariable sex-specific determinants of mean physical and mental QoL.
References
- 1.Carrero JJ, Hecking M, Chesnaye NC, Jager KJ: Sex and gender disparities in the epidemiology and outcomes of chronic kidney disease. Nat Rev Nephrol 14: 151–164, 2018 [DOI] [PubMed] [Google Scholar]
- 2.Nitsch D, Grams M, Sang Y, Black C, Cirillo M, Djurdjev O, Iseki K, Jassal SK, Kimm H, Kronenberg F, Oien CM, Levey AS, Levin A, Woodward M, Hemmelgarn BR; Chronic Kidney Disease Prognosis Consortium : Associations of estimated glomerular filtration rate and albuminuria with mortality and renal failure by sex: A meta-analysis. BMJ 346: f324, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fukuhara S, Yamazaki S, Hayashino Y, Green J: Measuring health-related quality of life in patients with end-stage renal disease: Why and how. Nat Clin Pract Nephrol 3: 352–353, 2007 [DOI] [PubMed] [Google Scholar]
- 4.Barsky AJ, Peekna HM, Borus JF: Somatic symptom reporting in women and men. J Gen Intern Med 16: 266–275, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fryback DG, Dunham NC, Palta M, Hanmer J, Buechner J, Cherepanov D, Herrington SA, Hays RD, Kaplan RM, Ganiats TG, Feeny D, Kindet al. P: US norms for six generic health-related quality-of-life indexes from the National Health Measurement study. Med Care 45: 1162–1170, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Burström K, Johannesson M, Diderichsen F: Swedish population health-related quality of life results using the EQ-5D. Qual Life Res 10: 621–635, 2001 [DOI] [PubMed] [Google Scholar]
- 7.Loge JH, Kaasa S: Short form 36 (SF-36) health survey: Normative data from the general Norwegian population. Scand J Soc Med 26: 250–258, 1998 [PubMed] [Google Scholar]
- 8.Ong L, Irvine J, Nolan R, Cribbie R, Harris L, Newman D, Mangat I, Dorian P: Gender differences and quality of life in atrial fibrillation: The mediating role of depression. J Psychosom Res 61: 769–774, 2006 [DOI] [PubMed] [Google Scholar]
- 9.Riedinger MS, Dracup KA, Brecht ML, Padilla G, Sarna L, Ganz PA: Quality of life in patients with heart failure: Do gender differences exist? Heart Lung 30: 105–116, 2001 [DOI] [PubMed] [Google Scholar]
- 10.Vigneshwaran E, Padmanabhareddy Y, Devanna N, Alvarez-Uria G: Gender differences in health related quality of life of people living with HIV/AIDS in the era of highly active antiretroviral therapy. N Am J Med Sci 5: 102–107, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Di Marco F, Verga M, Reggente M, Maria Casanova F, Santus P, Blasi F, Allegra L, Centanni S: Anxiety and depression in COPD patients: The roles of gender and disease severity. Respir Med 100: 1767–1774, 2006 [DOI] [PubMed] [Google Scholar]
- 12.Chesnaye NC, Dekker FW, Evans M, Caskey FJ, Torino C, Postorino M, Szymczak M, Ramspek CL, Drechsler C, Wanner C, Jager KJ: Renal function decline in older men and women with advanced chronic kidney disease-results from the EQUAL study. Nephrol Dial Transplant 36: 1656–1663, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kefale B, Alebachew M, Tadesse Y, Engidawork E: Quality of life and its predictors among patients with chronic kidney disease: A hospital-based cross sectional study. PLoS One 14: e0212184, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mujais SK, Story K, Brouillette J, Takano T, Soroka S, Franek C, Mendelssohn D, Finkelstein FO: Health-related quality of life in CKD patients: Correlates and evolution over time. Clin J Am Soc Nephrol 4: 1293–1301, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pagels AA, Söderkvist BK, Medin C, Hylander B, Heiwe S: Health-related quality of life in different stages of chronic kidney disease and at initiation of dialysis treatment. Health Qual Life Outcomes 10: 71, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chow FYF, Briganti EM, Kerr PG, Chadban SJ, Zimmet PZ, Atkins RC: Health-related quality of life in Australian adults with renal insufficiency: A population-based study. Am J Kidney Dis 41: 596–604, 2003 [DOI] [PubMed] [Google Scholar]
- 17.Aggarwal HK, Jain D, Pawar S, Yadav RK: Health-related quality of life in different stages of chronic kidney disease. QJM 109: 711–716, 2016 [DOI] [PubMed] [Google Scholar]
- 18.Neugarten J, Golestaneh L: Influence of sex on the progression of chronic kidney disease. Mayo Clin Proc 94: 1339–1356, 2019 [DOI] [PubMed] [Google Scholar]
- 19.Jager KJ, Ocak G, Drechsler C, Caskey FJ, Evans M, Postorino M, Dekker FW, Wanner C: The EQUAL study: A European study in chronic kidney disease stage 4 patients. Nephrol Dial Transplant 27[Suppl 3]: iii27–iii31, 2012 [DOI] [PubMed] [Google Scholar]
- 20.Ferrans CE, Zerwic JJ, Wilbur JE, Larson JL: Conceptual model of health-related quality of life. J Nurs Scholarsh 37: 336–342, 2005 [DOI] [PubMed] [Google Scholar]
- 21.VanderWeele TJ: Mediation analysis: A practitioner’s guide. Annu Rev Public Health 37: 17–32, 2016 [DOI] [PubMed] [Google Scholar]
- 22.VanderWeele TJ: On the distinction between interaction and effect modification. Epidemiology 20: 863–871, 2009 [DOI] [PubMed] [Google Scholar]
- 23.Jager KJ, Zoccali C, Macleod A, Dekker FW: Confounding: What it is and how to deal with it. Kidney Int 73: 256–260, 2008 [DOI] [PubMed] [Google Scholar]
- 24.Henderson R, Diggle P, Dobson A: Joint modelling of longitudinal measurements and event time data. Biostatistics 1: 465–480, 2000 [DOI] [PubMed] [Google Scholar]
- 25.Hu C, Sale ME: A joint model for nonlinear longitudinal data with informative dropout. J Pharmacokinet Pharmacodyn 30: 83–103, 2003 [DOI] [PubMed] [Google Scholar]
- 26.Wu MC, Carroll RJ: Estimation and comparison of changes in the presence of informative right censoring by modeling the censoring process. Biometrics 44: 175–188, 1988 [Google Scholar]
- 27.Rizopoulos D: The R package JMbayes for fitting joint models for longitudinal and time-to-event data using MCMC. J Stat Softw 72: 1–46, 2016 [Google Scholar]
- 28.Chesnaye NC, Tripepi G, Dekker FW, Zoccali C, Zwinderman AH, Jager KJ: An introduction to joint models-applications in nephrology. Clin Kidney J 13: 143–149, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lopes AA, Bragg-Gresham JL, Goodkin DA, Fukuhara S, Mapes DL, Young EW, Gillespie BW, Akizawa T, Greenwood RN, Andreucci VE, Akiba T, Held PJ, Port FK: Factors associated with health-related quality of life among hemodialysis patients in the DOPPS. Qual Life Res 16: 545–557, 2007 [DOI] [PubMed] [Google Scholar]
- 30.Courtenay WH: Constructions of masculinity and their influence on men’s well-being: A theory of gender and health. Soc Sci Med 50: 1385–1401, 2000 [DOI] [PubMed] [Google Scholar]
- 31.Gijsbers van Wijk CMT, van Vliet KP, Kolk AM, Everaerd WT: Symptom sensitivity and sex differences in physical morbidity: A review of health surveys in the United States and the Netherlands. Women Health 17: 91–124, 1991 [DOI] [PubMed] [Google Scholar]
- 32.Kristofferzon ML, Lindqvist R, Nilsson A: Relationships between coping, coping resources and quality of life in patients with chronic illness: A pilot study. Scand J Caring Sci 25: 476–483, 2011 [DOI] [PubMed] [Google Scholar]
- 33.Gemmell LA, Terhorst L, Jhamb M, Unruh M, Myaskovsky L, Kester L, Steel JL: Gender and racial differences in stress, coping, and health-related quality of life in chronic kidney disease. J Pain Symptom Manage 52: 806–812, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Voskamp PWM, van Diepen M, Evans M, Caskey FJ, Torino C, Postorino M, Szymczak M, Klinger M, Wallquist C, van de Luijtgaarden MWM, Chesnaye NC, Wanner C, Jager KJ, Dekker FW: The impact of symptoms on health-related quality of life in elderly pre-dialysis patients: Effect and importance in the EQUAL study. Nephrol Dial Transplant 34: 1707–1715, 2019 [DOI] [PubMed] [Google Scholar]
- 35.van de Luijtgaarden MWM, Caskey FJ, Wanner C, Chesnaye NC, Postorino M, Janmaat CJ, Rao A, Torino C, Klinger M, Drechsler C, Heimburger O, Szymczak M, Evans M, Dekker FW, Jager KJ; EQUAL study investigators : Uraemic symptom burden and clinical condition in women and men of ≥65 years of age with advanced chronic kidney disease: results from the EQUAL study. Nephrol Dial Transplant 34: 1189–1196, 2019 [DOI] [PubMed] [Google Scholar]
- 36.Ford DE, Erlinger TP: Depression and C-reactive protein in US adults: Data from the Third National Health and Nutrition Examination Survey. Arch Intern Med 164: 1010–1014, 2004 [DOI] [PubMed] [Google Scholar]
- 37.McLean CP, Asnaani A, Litz BT, Hofmann SG: Gender differences in anxiety disorders: Prevalence, course of illness, comorbidity and burden of illness. J Psychiatr Res 45: 1027–1035, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Leaf DE, Goldfarb DS: Interpretation and review of health-related quality of life data in CKD patients receiving treatment for anemia. Kidney Int 75: 15–24, 2009 [DOI] [PubMed] [Google Scholar]
- 39.Gorodetskaya I, Zenios S, McCulloch CE, Bostrom A, Hsu CY, Bindman AB, Go AS, Chertow GM: Health-related quality of life and estimates of utility in chronic kidney disease. Kidney Int 68: 2801–2808, 2005 [DOI] [PubMed] [Google Scholar]
- 40.Zimbudzi E, Lo C, Ranasinha S, Teede H, Usherwood T, Polkinghorne KR, Fulcher G, Gallagher M, Jan S, Cass A, Walker R, Russell G, Johnson G, Kerr PG, Zoungas S: Health-related quality of life among patients with comorbid diabetes and kidney disease attending a codesigned integrated model of care: A longitudinal study. BMJ Open Diabetes Res Care 8: e000842, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Janmaat CJ, van Diepen M, Meuleman Y, Chesnaye NC, Drechsler C, Torino C, Wanner C, Postorino M, Szymczak M, Evans M, Caskey FJ, Jager KJ, Dekker FW; EQUAL Study Investigators : Kidney function and symptom development over time in elderly patients with advanced chronic kidney disease: results of the EQUAL cohort study. Nephrol Dial Transplant 36: 862–870, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bellasi A, Mandreoli M, Baldrati L, Corradini M, Di Nicolò P, Malmusi G, Santoro A: Chronic kidney disease progression and outcome according to serum phosphorus in mild-to-moderate kidney dysfunction. Clin J Am Soc Nephrol 6: 883–891, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Martín M, Valls J, Betriu A, Fernández E, Valdivielso JM: Association of serum phosphorus with subclinical atherosclerosis in chronic kidney disease. Sex makes a difference. Atherosclerosis 241: 264–270, 2015 [DOI] [PubMed] [Google Scholar]
- 44.Ross SD, Fahrbach K, Frame D, Scheye R, Connelly JE, Glaspy J: The effect of anemia treatment on selected health-related quality-of-life domains: A systematic review. Clin Ther 25: 1786–1805, 2003 [DOI] [PubMed] [Google Scholar]
- 45.Rushton DH, Barth JH: What is the evidence for gender differences in ferritin and haemoglobin? Crit Rev Oncol Hematol 73: 1–9, 2010 [DOI] [PubMed] [Google Scholar]
- 46.Ifudu O: Patient characteristics determining rHuEPO dose requirements. Nephrol Dial Transplant 17[Suppl 5]: 38–41, 2002 [DOI] [PubMed] [Google Scholar]
- 47.Duncan JA, Levin A: Sex, haemoglobin and kidney disease: New perspectives. Eur J Clin Invest 35[Suppl 3]: 52–57, 2005 [DOI] [PubMed] [Google Scholar]
- 48.Hsu CY, McCulloch CE, Curhan GC: Epidemiology of anemia associated with chronic renal insufficiency among adults in the United States: Results from the Third National Health and Nutrition Examination Survey. J Am Soc Nephrol 13: 504–510, 2002 [DOI] [PubMed] [Google Scholar]
- 49.Hsu CY, Bates DW, Kuperman GJ, Curhan GC: Relationship between hematocrit and renal function in men and women. Kidney Int 59: 725–731, 2001 [DOI] [PubMed] [Google Scholar]
- 50.Mallinson S: The Short-Form 36 and older people: Some problems encountered when using postal administration. J Epidemiol Community Health 52: 324–328, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.