Visual Abstract
Keywords: TNFR-1, TNFR-2, KIM-1, biomarkers, clinical trial design, risk prediction, normoalbuminuria, kidney outcomes, prognosis, hepatitis a virus cellular receptor 1
Abstract
Background and objectives
Clinical trials in nephrology are enriched for patients with micro- or macroalbuminuria to enroll patients at risk of kidney failure. However, patients with normoalbuminuria can also progress to kidney failure. TNF receptor-1, TNF receptor-2, and kidney injury marker-1 (KIM-1) are known to be associated with kidney disease progression in patients with micro- or macroalbuminuria. We assessed the value of TNF receptor-1, TNF receptor-2, and KIM-1 as prognostic biomarkers for CKD progression in patients with type 2 diabetes and normoalbuminuria.
Design, setting, participants, & measurements
TNF receptor-1, TNF receptor-2, and KIM-1 were measured using immunoassays in plasma samples from patients with type 2 diabetes at high cardiovascular risk participating in the Canagliflozin Cardiovascular Assessment Study trial. We used multivariable adjusted Cox proportional hazards analyses to estimate hazard ratios per doubling of each biomarker for the kidney outcome, stratified the population by the fourth quartile of each biomarker distribution, and assessed the number of events and event rates.
Results
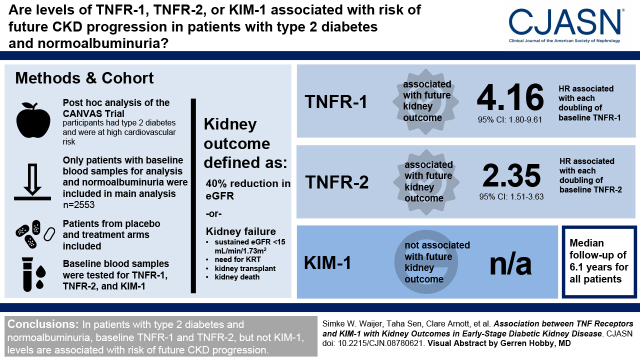
In patients with normoalbuminuria (n=2553), 51 kidney outcomes were recorded during a median follow-up of 6.1 (interquartile range, 5.8–6.4) years (event rate, 3.5; 95% confidence interval, 2.6 to 4.6 per 1000 patient-years). Each doubling of baseline TNF receptor-1 (hazard ratio, 4.2; 95% confidence interval, 1.8 to 9.6) and TNF receptor-2 (hazard ratio, 2.3; 95% confidence interval, 1.5 to 3.6) was associated with a higher risk for the kidney outcome. Baseline KIM-1, urinary albumin-creatinine ratio, and eGFR were not associated with kidney outcomes. The event rates in the highest quartile of TNF receptor-1 (≥2992 ng/ml) and TNF receptor-2 (≥11,394 ng/ml) were 5.6 and 7.0 events per 1000 patient-years, respectively, compared with 2.8 and 2.3, respectively, in the lower three quartiles.
Conclusions
TNF receptor-1 and TNF receptor-2 are associated with kidney outcomes in patients with type 2 diabetes and normoalbuminuria.
Clinical Trial registry name and registration number:
CANagliflozin cardioVascular Assessment Study (CANVAS), NCT01032629
Introduction
Traditional clinical end points in clinical trials in patients with CKD are kidney failure and doubling of serum creatinine. However, these end points are late events in the progression of CKD and take a long time to manifest. To ensure that sufficient end points occur within the duration of a clinical trial, patients at advanced stages of CKD, those with high albuminuria and low eGFR, are enrolled. Patients at early stages of CKD are usually excluded from CKD clinical trials because of low event rates. However, although event rates are lower, some patients without traditional risk markers still progress and experience clinical kidney outcomes. Moreover, because the prevalence of early-stage CKD is much higher than later stages of CKD, the total number of kidney outcomes was similar when comparing patients at early and advanced stages of CKD in past cardiovascular outcome trials (1,2). For example, in the Dapagliflozin Effect on Cardiovascular Events–Thrombolysis in Myocardial Infarction 58 trial, 69% of patients had normoalbuminuria and 7% had macroalbuminuria; the numbers of kidney outcomes in both subgroups were 145 and 106, respectively (1). Identifying patient characteristics or biomarkers that associate with kidney disease progression in early stages of CKD will enable better tailoring of treatment to those at risk of progressive kidney function loss and may facilitate clinical trial design and conduct.
Biomarkers, including tumor necrosis factor receptor-1 (TNFR-1), TNFR-2, and kidney injury molecule-1 (KIM-1), are associated with kidney disease progression (3). TNFR-1 and TNFR-2 are circulating receptors of the proinflammatory cytokine TNF-α. Studies in patients with established CKD demonstrated that higher concentrations of TNFR-1 and TNFR-2 are associated with a higher risk of progressive kidney function loss and kidney failure (4–6). KIM-1 is also associated with the progression of kidney disease. Elevated plasma and urine levels of KIM-1 are associated with progression to kidney failure (5,7–9). However, the association of these biomarkers with kidney disease progression in early stages of CKD and their utility to enrich clinical trials for kidney outcomes in patients with normoalbuminuria are unknown.
We performed a post hoc analysis of the Canagliflozin Cardiovascular Assessment Study (CANVAS) randomized controlled trial that assessed the efficacy and safety of canagliflozin in patients with type 2 diabetes and higher cardiovascular risk. We assessed the performance of TNFR-1, TNFR-2, and KIM-1 as prognostic biomarkers to identify patients with normoalbuminuria at risk of CKD progression.
Materials and Methods
Patients and Study Design
The CANVAS program was a double-blind, randomized, placebo-controlled trial that assessed the cardiovascular efficacy and safety of the sodium-glucose cotransporter 2 inhibitor canagliflozin in patients with type 2 diabetes at higher cardiovascular risk. The CANVAS program consists of two trials: the CANVAS trial and the Canagliflozin Cardiovascular Assessment Study Renal (CANVAS-R) trial. Baseline blood samples of the CANVAS trial only were stored for exploratory biomarker analyses. No blood samples from the CANVAS-R trial were stored for biomarker analyses. For our analysis, we included all patients with available blood samples for measurement at baseline.
The design and primary results of the CANVAS trial have been published previously (10,11). The CANVAS trial included 4330 patients with type 2 diabetes who were 30 years or older, had an eGFR>30 ml/min per 1.73 m2, and were at higher cardiovascular risk. Patients needed to have an glycated hemoglobin (HbA1c) level of ≥7.0% (53 mmol/mol) and ≤10.5% (91 mmol/mol). Higher cardiovascular risk was defined as a history of symptomatic atherosclerotic vascular disease or an age of ≥50 years with two or more cardiovascular risk factors, including a diabetes duration of ≥10 years, systolic BP >140 mm Hg while using greater than or equal to one antihypertensive agent, current smoking, micro- or macroalbuminuria, or an HDL cholesterol level of <1 mmol/L. Eligible patients were randomly assigned to 100 mg/d canagliflozin, 300 mg/d canagliflozin, or placebo in a 1:1:1 ratio.
The trial protocol was approved by a local or central ethics committee at all trial sites, and each participant provided written informed consent before entering the study. The trial was conducted according to the Declaration of Helsinki.
Biomarker Measurements
Plasma TNFR-1, TNFR-2, and KIM-1 were measured from baseline blood samples using high-performance electrochemiluminescence immunoassays (Mesoscale Quickplex SQ 120 platform performed by RenalytixAI, New York, NY). All immunoassays were measured between August 2019 and December 2019. A random sample of 469 blood samples was measured in duplicate, with the following mean (minimum, maximum) coefficients of variation: TNFR-1, 2% (0%, 10%); TNFR-2, 2% (0%, 12%); and KIM-1, 3% (0%, 18%). First morning void urine was collected to determine urinary albumin-creatinine ratio (UACR).
Outcomes
The kidney outcome for this post hoc analysis was defined as a composite of 40% reduction in eGFR sustained for at least two consecutive measurements, kidney failure defined as a sustained eGFR<15 ml/min per 1.73 m2, the need for KRT and kidney transplantation, or kidney death. The cardiovascular outcome for this study was defined as hospitalization for heart failure. These end points were prespecified in the study protocol, and end points were adjudicated by a blinded adjudication committee (10).
Statistical Analyses
Baseline characteristics are presented as means and SDs. For variables with skewed distribution, medians and interquartile ranges (IQRs) are reported. UACR, TNFR-1, TNFR-2, and KIM-1 were log transformed in all analyses to account for their skewed distributions. Categorical variables are presented as the percentage of observations. Baseline characteristics are presented for patients with normoalbuminuria (UACR<30 mg/g) and compared with patients with microalbuminuria (UACR≥30 and <300 mg/g) and macroalbuminuria (UACR≥300 mg/g). Body mass index was missing in four patients, LDL cholesterol was missing in three patients, and creatinine was missing in two patients. We imputed the mean values for these patients. Differences in baseline characteristics across these UACR subgroups were tested with the one-way ANOVA or chi-squared test where appropriate. eGFR was calculated from the serum creatinine measurements using the Chronic Kidney Disease Epidemiology Collaboration equation (12).
We used Cox proportional hazard regression analyses to estimate the hazard ratios (HRs) for each outcome associated with each doubling in baseline TNFR-1, TNFR-2, or KIM-1. We also assessed the association between each doubling in baseline UACR and eGFR with the kidney outcome to compare the performance of the novel biomarkers with established risk markers of CKD progression. Models were adjusted for covariates that were added in a stepwise manner in three consecutive models. In the first model, we adjusted for treatment allocation (canagliflozin or placebo). In the second model, we additionally adjusted for age, sex, race, body mass index, history of cardiovascular disease (yes or no), systolic and diastolic BP, LDL cholesterol, and HbA1c. The third model was adjusted for treatment allocation, UACR, and eGFR. The final and fourth model was adjusted for all covariates included in models 2 and 3. Estimations of the association of doubling in UACR and eGFR with the kidney outcome were performed in the final model without adjustments for UACR and eGFR, respectively. In addition, we divided the population into quartiles by TNFR-1 and TNFR-2 level and determined the HR for each quartile using the first quartile as a common reference.
To assess if the biomarkers of interest can be used as risk selection markers in patients with normoalbuminuria, we divided the population above or below the fourth quartile of baseline TNFR-1 and TNFR-2, respectively. We assessed in both subgroups the number of events and Poisson event rates, calculated as the number of events per 1000 patient-years.
The effect of using the biomarkers for prognostic enrichment on sample size, total number of screenings, and costs of a future clinical trial was evaluated using the Biomarker Prognostic Enrichment Tool (http://prognosticenrichment.com/orig/) (13). As inputs, we used the event rate of the placebo group and the performance of the association of TNFR-1, TNFR-2, and UACR with the kidney outcomes among patients with normoalbuminuria. We assumed a 90% power to detect a relative risk reduction for the kidney outcome of 40%, consistent with the observed effect of canagliflozin in the CANVAS program, and a type 1 error rate of 0.05. To estimate the implications of prognostic enrichment on trial costs, we set the screening costs per patient at $1000 and the costs per patient during the trial at $50,000.
We performed Cox proportional hazard regression analysis to assess if the treatment effect of canagliflozin relative to placebo in patients with normoalbuminuria and TNFRs above or below the fourth quartile was consistent with the main CANVAS trial findings. Heterogeneity of treatment effects across TNFR subgroups was tested by adding an interaction term between the TNFR subgroup and treatment assignment to the model.
All statistical analyses were performed in Stata 14.2 SE (StataCorp 2015, Stata Statistical Software: Release 14, StataCorp LP, College Station, TX).
Results
Of the 4330 participants of the CANVAS trial, 3532 (82%) had available blood samples for measurements at baseline. Of these, 2553 (72%) had normoalbuminuria, 778 (22%) had microalbuminuria, and 201 (6%) had macroalbuminuria. Median follow-up time was 6.1 (IQR, 5.8–6.4) years. Baseline characteristics stratified by baseline albuminuria are shown in Table 1. Patients with macroalbuminuria had a higher BP, higher HbA1c, and lower eGFR compared with patients with micro- or normoalbuminuria. TNFR-1, TNFR-2, and KIM-1 levels were also higher in those with macroalbuminuria (Table 1).
Table 1.
Baseline characteristics stratified by baseline urinary albumin-creatinine ratio
| Characteristic | Normoalbuminuria, n=2553 | Microalbuminuria, n=778 | Macroalbuminuria, n=201 |
|---|---|---|---|
| End point | |||
| Kidney outcome | 51 | 45 | 41 |
| Baseline characteristics | |||
| Age, yr | 63 (8) | 63 (8) | 63 (7) |
| Sex, n (%) | |||
| Men | 1660 (65) | 564 (72) | 143 (71) |
| Women | 893 (35) | 214 (28) | 58 (29) |
| Race, n (%) | |||
| Asian | 243 (10) | 92 (12) | 18 (9) |
| Black | 54 (2) | 18 (2) | 9 (4) |
| White | 2085 (82) | 624 (80) | 163 (81) |
| Other | 171 (7) | 44 (6) | 11 (5) |
| BMI, kg/m2a | 32.6 (6.2) | 32.9 (6.1) | 32.4 (5.8) |
| Cardiovascular disease history, n (%) | 1511 (59) | 470 (60) | 120 (60) |
| Systolic BP, mm Hg | 135 (15) | 139 (16) | 146 (18) |
| Diastolic BP, mm Hg | 77 (10) | 78 (10) | 80 (10) |
| LDL cholesterol, mg/dla | 88 (35) | 86 (35) | 97 (40) |
| HbA1c, % | 8.1 (0.9) | 8.3 (0.9) | 8.4 (0.9) |
| eGFR, ml/min per 1.73 m2a | 80 (17) | 78 (18) | 70 (21) |
| UACR, mg/g | 8.2 (5.5–13.2) | 66.8 (43.6–121.4) | 675.4 (436.8–1167.1) |
| TNFR-1, pg/ml | 2477 (2068–2990) | 2781.5 (2290–3484) | 3495 (2792–4414) |
| TNFR-2, pg/ml | 9323 (7642–11,392) | 10,475 (8403–13,185) | 12,834 (10,338–17,459) |
| KIM-1, pg/ml | 97 (64–145) | 143.5 (98–225) | 281 (186–474) |
| Treatment allocation, n (%) | |||
| Placebo | 847 (33) | 257 (33) | 81 (40) |
| Canagliflozin | 1706 (67) | 521 (67) | 120 (60) |
Data are n (percentage), mean (SD), or median (25th to 75th percentiles). BMI, body mass index; HbA1c, glycated hemoglobin; UACR, urinary albumin-creatinine ratio; TNFR-1, TNF receptor-1; TNFR-2, TNF receptor-2; KIM-1, kidney injury molecule-1.
Mean BMI (four patients), LDL cholesterol (three patients), and creatinine (two patients) values were imputed for patients with missing baseline values.
The numbers of kidney outcomes were similar across albuminuria subgroups; 51, 45, and 41 outcomes were recorded in patients with normo-, micro-, and macroalbuminuria, respectively (Table 1). The event rate was, however, markedly lower in the normo- compared with micro- or macroalbuminuria subgroups, with corresponding rates of 3.5 (95% confidence interval [95% CI], 2.6 to 4.6), 10.5 (95% CI, 7.8 to 14.1), and 43.1 (95% CI, 31.7 to 58.5) per 1000 patient-years, respectively.
Association of Biomarkers with the Kidney Outcomes in Patients with Normoalbuminuria
We observed a wide variation in TNFR-1, TNFR-2, and KIM-1 levels in patients with normoalbuminuria; the mean and 2.5th to 97.5th percentiles for each biomarker were 2600 (1439–4500) pg/ml, 9922 (5101–17,729) pg/ml, and 121 (31–360) pg/ml, respectively (Supplemental Figure 1). In unadjusted analyses, TNFR-1, TNFR-2, and KIM-1 were statistically significantly associated with the kidney outcome (Table 2). When models were adjusted for baseline characteristics and cardiovascular and kidney risk markers, each doubling of TNFR-1 and TNFR-2 was significantly associated with a higher risk of kidney outcomes, with corresponding HRs of 4.2 (95% CI, 1.8 to 9.6; P=0.001) and 2.3 (95% CI, 1.5 to 3.6; P<0.001), respectively. In the fully adjusted model, we did not observe a statistically significant association between doubling of baseline KIM-1 and the kidney outcome (HR, 1.4; 95% CI, 1.0 to 1.9; P=0.07). Baseline eGFR and UACR were also not associated with the kidney outcome in the fully adjusted model (Table 2).
Table 2.
Associations of doubling in baseline TNF receptor-1, TNF receptor-2, kidney injury molecule-1, urinary albumin-creatinine ratio, and eGFR with the kidney outcome in patients with normoalbuminuria
| Biomarker | n/N | Hazard Ratio (95% Confidence Interval) | |||
|---|---|---|---|---|---|
| Model 1 | Model 2 | Model 3 | Model 4 | ||
| TNFR-1 | 51/2553 | 2.89 (1.52 to 5.50) | 2.07 (1.04 to 4.15) | 4.07 (1.85 to 8.98) | 4.18 (1.81 to 9.64) |
| TNFR-2 | 51/2553 | 2.05 (1.40 to 3.01) | 1.92 (1.13 to 3.26) | 2.13 (1.46 to 3.10) | 2.35 (1.51 to 3.65) |
| KIM-1 | 51/2553 | 1.42 (1.06 to 1.91) | 1.32 (0.96 to 1.81) | 1.36 (1.00 to 1.86) | 1.36 (0.98 to 1.88) |
| UACR | 51/2553 | 1.39 (1.01 to 1.91) | 1.26 (0.90 to 1.75) | 1.39 (1.01 to 1.91) | 1.25 (0.89 to 1.74) |
| eGFR | 51/2553 | 0.93 (0.40 to 2.17) | 2.39 (0.84 to 6.78) | 0.93 (0.40 to 2.16) | 2.33 (0.82 to 6.59) |
Models are adjusted for the following covariates. Model 1 was adjusted for treatment allocation. Model 2 was adjusted for treatment allocation as well as age, sex, race, body mass index, history of cardiovascular disease, systolic BP, diastolic BP, LDL cholesterol, and glycated hemoglobin. Model 3 was adjusted for treatment allocation as well as UACR and eGFR. Model 4 was adjusted for all covariates in models 2 and 3. When analyzing the association of UACR or eGFR with the outcome, models 3 and 4 are not adjusted for UACR and eGFR, respectively. TNFR-1, TNFR-2, KIM-1, and UACR are log transformed for the analysis. TNFR-1, TNF receptor-1; TNFR-2, TNF receptor-2; KIM-1, kidney injury molecule-1; UACR, urinary albumin-creatinine ratio.
Because KIM-1 was not significantly associated with the kidney outcome after multivariable adjustment, we restricted further analyses to TNFR-1 and TNFR-2. Analyses of quartiles of baseline TNFR-1 and TNFR-2 showed that in the upper quartile of the TNFR-1 and TNFR-2 distribution, the largest number of events occurred. Accordingly, the HR was larger at higher quartiles of TNFR-1 and TNFR-2, with HRs of 5.2 (95% CI, 1.7 to 15.5) for TNFR-1 and 3.6 (95% CI, 1.5 to 8.8) for TNFR-2 in the highest compared with the first quartile in the fully adjusted model (Supplemental Figure 2).
Performance of Tumor Necrosis Factor Receptor-1 and Tumor Necrosis Factor Receptor-2 as Risk Selection Markers for Future Clinical Trials
To identify patients with normoalbuminuria at highest risk for kidney outcomes for a future clinical trial and assess the performance of the TNFRs to enrich clinical trial populations with participants likely to experience events, we selected patients with a TNFR-1 or TNFR-2 level in the highest (fourth) quartile (Supplemental Figure 3). Because of the strong correlation between TNFR-1 and TNFR-2, the combination of these biomarkers was not tested (Pearson correlation of 0.7) (Supplemental Figure 4).
Patients with normoalbuminuria and TNFR-1 or TNFR-2 in the fourth quartile had a median TNFR-1 level of 3448 (IQR, 3181–3855) pg/ml and a median TNFR-2 of 13,144 (IQR, 12,080–14,923) pg/ml. The kidney end point occurred in 20 of 638 patients (3%) in the fourth quartile of TNFR-1, corresponding to an event rate of 5.6 (95% CI, 3.6 to 8.6) per 1000 patient-years. In the fourth quartile of TNFR-2, 25 of 638 patients (4%) experienced the kidney outcome; the corresponding event rate was 7.0 (95% CI, 4.7 to 10.3) per 1000 patient-years. In comparison, in the lower three quartiles of TNFR-1 and TNFR-2, the event rates were 2.8 (95% CI, 2.0 to 4.0) and 2.3 (95% CI, 1.6 to 3.4), respectively (Table 3).
Table 3.
Number of patients, events, and event rate for the kidney outcome in patients with normoalbuminuria stratified by the fourth quartile of TNF receptor-1 or TNF receptor-2 and in patients with normoalbuminuria, microalbuminuria, and macroalbuminuria
| Subgroup | Cutoff Value | No. of Patients | No. of Events | Event Rate, per 1000 Patient-yr |
|---|---|---|---|---|
| TNFR-1<4th quartile + normoalbuminuria | <2992 pg/ml | 1915 | 31 | 2.80 (1.97 to 3.98) |
| TNFR-1≥4th quartile + normoalbuminuria | ≥2992 pg/ml | 638 | 20 | 5.57 (3.59 to 8.63) |
| TNFR-2<4th quartile + normoalbuminuria | <11,394 pg/ml | 1915 | 26 | 2.35 (1.60 to 3.45) |
| TNFR-2≥4th quartile + normoalbuminuria | ≥11,394 pg/ml | 638 | 25 | 6.98 (4.72 to 10.33) |
| Normoalbuminuriaa | <30 mg/g | 2553 | 51 | 3.48 (2.64 to 4.58) |
| Microalbuminuria | <300 mg/g | 778 | 45 | 10.49 (7.83 to 14.05) |
| Macroalbuminuria | ≥300 mg/g | 201 | 41 | 43.06 (31.71 to 58.48) |
TNFR-1, TNF receptor-1; TNFR-2, TNF receptor-2.
Event rate was 5.44 (95% confidence interval, 3.70 to 7.99) per 1000 patient-years in the placebo group and 2.53 (95% confidence interval, 1.71 to 3.75) per 1000 patient-years in the canagliflozin group.
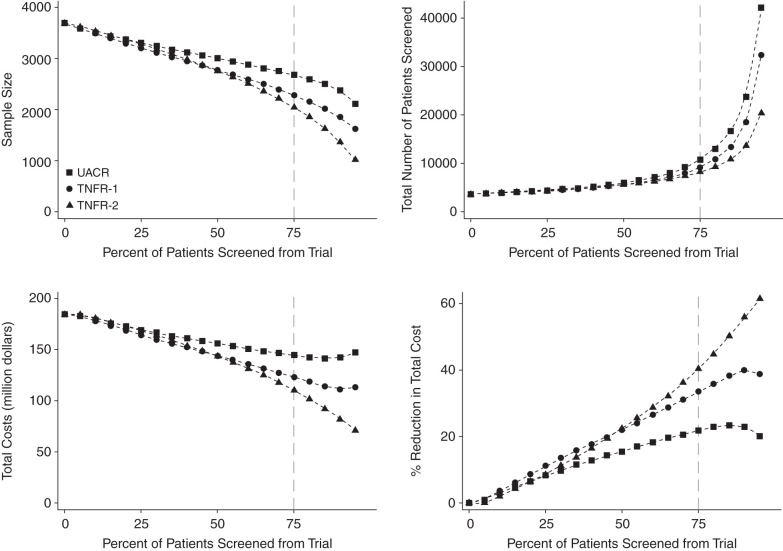
We evaluated the effect of using TNFR concentrations as a prognostic enrichment marker on sample size requirements and costs for a hypothetical future clinical trial. Enrichment with TNFR-1 or TNFR-2 resulted in a higher event rate and a decrease in clinical trial sample size. Although enrichment with either TNFR resulted in a larger number of patients required to be screened, the overall clinical trial costs decreased (Figure 1). Specifically, using a threshold to include patients with the 25% highest TNFR-2 concentrations (i.e., fourth quartile) would reduce the sample size of the hypothetical trial from 3692 to 2046 (a 45% reduction) and increase the number of patients needed to screen for the trial from 3692 to 8183, but it would reduce costs by 40% (Supplemental Table 1). Similar results are observed when using TNFR-1 for prognostic enrichment (Figure 1, Supplemental Table 1). Prognostic enrichment with KIM-1 did not lead to a reduction in sample size or a reduction in costs in comparison with UACR (Supplemental Figure 5).
Figure 1.
Prognostic enrichment with TNF receptor-1 (TNFR-1) or TNFR-2 resulted in a decrease in sample size, an increase in the number of patients required to be screened, and an overall reduction in clinical trial costs. Figure shows the effect of prognostic enrichment with TNFR-1 or TNFR-2 on sample size, number of screenings, and costs for a future trial in the normoalbuminuria population. UACR, urinary albumin-creatinine ratio.
Subgroup analyses by the baseline biomarker levels were performed to ascertain that the benefit of canagliflozin in reducing the risk of clinical outcomes was preserved in patients in the fourth quartile of baseline TNFR-1 and TNFR-2. We observed that the treatment effect of canagliflozin on the kidney outcome and hospitalization for heart failure is consistent in patients with a baseline TNFR-1 or TNFR-2 level above or below the fourth quartile as well as in patients with micro- or macroalbuminuria (both P for interaction >0.39) (Supplemental Table 2).
Discussion
In this post hoc analysis of the CANVAS trial, we demonstrated that baseline TNFR-1 and TNFR-2 levels can be used to identify patients with normoalbuminuria at elevated risk of CKD progression. In these individuals, there was a strong association between TNFR-1 and TNFR-2 with the kidney outcome. The association between KIM-1 and the kidney outcome did not reach significance after multivariable adjustment. These data highlight the potential use of TNFR-1 or TNFR-2 as biomarkers to identify individuals with normoalbuminuria at risk of CKD progression.
TNFR-1 and TNFR-2 are receptors of the proinflammatory cytokine TNF-α and have been shown to be associated with kidney outcomes in patients with and without diabetes at various stages of CKD (4–6,14–16). Here, we confirm and extend these findings in a large, heterogeneous population with type 2 diabetes, normoalbuminuria, and high cardiovascular risk treated according to contemporary treatment guidelines. In our study, participants in the highest quartile of TNFR-1 and TNFR-2 had a five-fold higher risk compared with the lowest quartile of developing the kidney outcome. This finding is in accordance with an earlier study demonstrating that the vast majority of kidney events occurred in the highest quartile of the TNFR-1 distribution (4). Taken together, TNFRs are associated with kidney disease progression across a range of patients and in patients without obvious signs of kidney damage, such as those with normal eGFR and normoalbuminuria.
Prior studies have also evaluated the applicability of TNFR-1 to improve clinical trial design (17). A study by Yamanouchi et al. (17) evaluated the use of TNFR-1 to enrich the clinical trial cohort for patients with diabetes and CKD stage 3 or 4 more likely to reach kidney failure. This study showed that TNFR-1 levels >4300 pg/ml or between 2900 and 4300 pg/ml in combination with UACR>1900 mg/g are the optimal thresholds for high-risk selection (17). Here, we extend these criteria and show that in people with type 2 diabetes and normoalbuminuria, TNFR-1 (≥2992 pg/ml) and TNFR-2 (≥11,394 pg/ml) could be used for enrichment in a clinical trial. In addition, because the prevalence of early-stage CKD is much higher than that of later stages of CKD, the use of biomarkers, such as TNFR-1 and TNFR-2, would significantly increase the number of potential eligible clinical trial participants at risk of kidney function decline beyond the commonly used selection criteria, such as the presence of macroalbuminuria.
KIM-1 is a marker for damage to the proximal tubule and has been proposed as a risk marker for progression of kidney disease (5,7–9). In our study, we did not observe a clear association between plasma KIM-1 and kidney outcomes in patients with normoalbuminuria. Previous studies in patients with type 1 and type 2 diabetes showed strong associations between plasma KIM-1 levels and kidney outcomes, including eGFR decline and progression of CKD (5,8,9). A recent trial confirmed this association in a general population cohort with preserved kidney function. Compared with our findings, in this study, baseline plasma KIM-1 was associated with kidney outcomes, whereas we found no significant association (18). These differences may be explained by differences in design and analytical methods of the studies. Specifically, the longer follow-up and difference in outcome definition (incident CKD defined as eGFR<60 ml/min per 1.73 m2) in the study by Schulz et al. (18) may have increased statistical power to detect a significant association in their study. Perhaps more importantly, no data on albuminuria were collected in the study by Schulz et al. (18), and therefore, associations were not adjusted for albuminuria.
Albuminuria is a well-known risk marker for kidney failure. Multiple studies in varying patient populations have shown a strong and continuous association between higher degrees of albuminuria and risk for kidney outcomes without a threshold below which this association disappears (19,20). This indicates that even subtle increases in albuminuria, even within the currently considered normoalbuminuric range, are already associated with a higher risk of CKD progression and clinical events. In this study, we did not observe a statistically significant association between albuminuria and the kidney outcome in participants with normoalbuminuria. The relatively low number of kidney outcomes may have resulted in insufficient statistical power to detect and confirm this association. An increase in albuminuria usually precedes the development of severe kidney damage (19,20). However, recent studies show that the prevalence of nonalbuminuric CKD is increasing (21–23). Patients with high GFR and low albuminuria have been excluded from nearly all recent clinical trials because of concerns of low event rates and large sample size requirements. TNFR-1 and TNFR-2 could be considered as risk markers for these patients for whom evidence-based treatments are unavailable (24).
Canagliflozin reduces the relative risks of kidney failure and heart failure hospitalization in patients with type 2 diabetes at high cardiovascular risk and with established kidney disease (11,25). In this study, we demonstrated that canagliflozin reduced the kidney outcome and hospitalization for heart failure consistently across patients with normoalbuminuria irrespective of baseline TNFR-1 or TNFR-2 levels. Thus, no effect modification occurred when selecting patients on the basis of TNFR-1 or TNFR-2 levels, supporting the utility of these markers for future trials. Using TNFR-1 or TNFR-2 as a biomarker for prognostic enrichment for a clinical trial decreases the sample size and costs, leading to an increase in feasibility of a clinical trial. However, the disadvantage of using enrichment criteria is that it reduces generalizability.
The strength of this study includes that CANVAS is a large trial with a heterogeneous population of patients with type 2 diabetes at higher cardiovascular risk with a long follow-up. Also, the CANVAS trial had clear, prespecified end points that were assessed by a blinded end point adjudication committee. This study also has some limitations. First, it is a post hoc analysis prone to chance findings. Second, there is only a small number of events in the normoalbuminuria group, limiting the statistical power. However, the coherence with prior data suggests that our findings are unlikely the result of chance. Because of day-to-day variability in albuminuria, guidelines recommend assessment of albuminuria in three consecutively collected first morning void urine samples. In the CANVAS trial, urinary albumin and creatinine were measured in single first morning void urine samples, which may have led to misclassification of the albuminuria status. Finally, the CANVAS trial enrolled patients with type 2 diabetes with established cardiovascular disease or high cardiovascular risk. The results cannot be generalized to patients without type 2 diabetes.
In conclusion, higher plasma concentrations of TNFR-1 and TNFR-2 are associated with a higher risk of kidney disease progression in patients with type 2 diabetes and normoalbuminuria.
Disclosures
C. Arnott reports honoraria from Amgen and research funding supported by an NSW (New South Wales) Health Early and Mid-Career Researchers Grant and an Medical Research Future Fund Investigator Grant. S.G. Coca reports employment with the Icahn School of Medicine at Mount Sinai (Mount Sinai owns part of Renalytix); consultancy agreements with 3ive, Axon, Bayer, CHF Solutions, Renalytix, Reprieve Cardiovascular, Takeda, and Vifor; ownership interest in pulseData and Renalytix; fees for advisory boards or steering committee roles from Akebia, Bayer, Boehringer-Ingelheim, CHF Solutions, Takeda, ProKidney, Quark, Relypsa (Vifor), and RenalytixAI; research funding from ProKidney, the Renal Research Institute, Renalytix, and XORTX; salary support and research funds from National Institutes of Health (NIH) grants U01DK106962, R01DK115562, R01DK112258, R01DK126477, R01DK118222-03S1, R01DK093770 09S1, and UH3DK114920; patents and inventions with Renalytix; scientific advisor or membership with Renalytix and Reprieve Cardiovascular; and other interests/relationships as an associate editor for Kidney360 and with the editorial boards of CJASN, JASN, and Kidney International. D. de Zeeuw reports consultancy agreements with Abbvie, Bayer, Boehringer-Ingelheim, Fresenius, Janssen, Mitsubishi Tanabe, and Travere Pharmaceuticals; honoraria from Bayer, Boehringer-Ingelheim, Fresenius, Janssen, Mitsubishi Tanabe, and Travere Pharmaceuticals; being part of the steering committees and/or a speaker for AbbVie and Janssen; being a part of data safety and monitoring committees for Bayer; and receiving honoraria paid to the institution as a consultant/speaker. R.T. Gansevoort reports consultancy agreements with AstraZeneca, Bayer, Galapagos, Otsuka Pharmaceutical, and Sanofi-Genzyme; research funding from AstraZeneca, Bayer, Galapagos, Otsuka Pharmaceuticals, and Sanofi-Genzyme; honoraria from Bayer, Galapagos, Mironid, Otsuka Pharmaceuticals, and Sanofi-Genzyme; fees for acting on advisory boards or as a consultant for and grants from Abbvie, AstraZeneca, Baxter, Bayer, Mundipharma, and Sanofi-Genzyme (all money was paid to his employing institution); and scientific advisor or membership with American Journal of Kidney Diseases, CJASN, Journal of Nephrology, Kidney360, Nephrology Dialysis Transplantation, and Nephron Clinical Practice. M.K. Hansen reports employment with Janssen Research & Development and ownership interest in Johnson & Johnson. H.J.L. Heerspink reports consultancy agreements with AbbVie, Astellas, AstraZeneca, Bayer, Boehringer-Ingelheim, Chinook, CSL Pharma, Dimerix, Fresenius, Gilead, Janssen, Merck, Mitsubishi Tanabe, Mundi Pharma, Novo Nordisk, Retrophin, and Travere Pharmaceuticals; research funding from AbbVie, AstraZeneca, Boehringer-Ingelheim, and Janssen research support (grant funding directed to the employer); support from a VIDI (917.15.306) grant from The Netherlands Organisation for Scientific Research; and being a member of the speakers bureau for AstraZeneca. K.W. Mahaffey reports consultancy agreements with Amgen, Anthos, Applied Therapeutics, AstraZeneca, Bayer, CSL Behring, Elsevier, Inova, Intermountain Health, Johnson & Johnson, Medscape, Mount Sinai, Mundi Pharma, Myokardia, Novartis, Novo Nordisk, Otsuka, Portola, Sanofi, SmartMedics, and Theravance; being a consultant (speaker fees for continuing medical education events only) for Abbott, Ablynx, AstraZeneca, Baim Institute, Boehringer-Ingelheim, Bristol-Myers Squibb, Elsevier, GlaxoSmithKline, Johnson & Johnson, MedErgy, Medscape, Mitsubishi Tanabe, Myokardia, NIH, Novartis, Novo Nordisk, Portola, Radiometer, Regeneron, Springer Publishing, and the University of California, San Francisco; research funding from Afferent, AHA, Amgen, Apple, Inc., AstraZeneca, Bayer, Cardiva Medical, Inc., Daiichi, Eidos, Ferring, Gilead, Google (Verily), Johnson & Johnson, Luitpold, Medtronic, Merck, NIH, Novartis, Sanifit, Sanofi, St. Jude, and Tenax; and honoraria from Amgen, Anthos, Applied Therapeutics, AstraZeneca, Bayer, CSL Behring, Elsevier, Inova, Intermountain Health, Johnson & Johnson, Medscape, Mount Sinai, Mundi Pharma, Myokardia, Novartis, Novo Nordisk, Otsuka, Portola, Sanofi, SmartMedics, and Theravance. B. Neal reports consultancy agreements with Janssen Research & Development, LLC; research funding from the Australian National Health and Medical Research Council Principal Research Fellowship; grant support for CANVAS (CANagliflozin cardioVascular Assessment Study) and CREDENCE (Canagliflozin and Renal Events in Diabetes with Established Nephropathy Clinical Evaluation) from Janssen; honoraria from Janssen Research & Development, LLC (all paid to the institution); and scientific advisor or membership for serving on advisory boards, steering committees, or scientific presentations for AstraZeneca, Janssen, Merck, and Mundipharma, with any consultancy, honoraria, or travel support paid to his institution. B.L. Neuen reports consultancy agreements with AstraZeneca, Bayer, and Janssen; honoraria as personal fees for advisory boards, scientific presentations, steering committee roles, and travel support from AstraZeneca, Bayer, and Janssen, with all honoraria paid to the institution; and support by an Australian NHMRC postgraduate scholarship and a university postgraduate award from the University of New South Wales. C.R. Parikh reports consultancy agreements with Genfit Biopharmaceutical Company and Novartis; ownership interest in Renaltix AI; research funding from National Institute of Diabetes and Digestive and Kidney Diseases, National Heart, Lung and Blood Institute, and NIH grants R01HL085757, UH3DK114866, U01DK106962, and R01DK093770; and scientific advisor or membership with Genfit Biopharmaceutical Company and Renalytix. V. Perkovic reports consultancy agreements with AbbVie, Astellas, AstraZeneca, Baxter, Bayer, Boehringer-Ingelheim, Bristol-Myers Squibb, Dimerix, Durect, Eli Lilly, Gilead, GlaxoSmithKline, Janssen, Merck, Metavant, Mitsubishi Tanabe, Mundipharma, Novartis, Novo Nordisk, Pfizer, PharmaLink, Relypsa, Retrophin, Roche, Sanofi, Servier, Travere, Tricida, UpToDate, and Vitae; research funding from the Australian National Health and Medical Research Council (senior research fellowship and program grant); honoraria from AbbVie, Astellas, AstraZeneca, Baxter, Bayer, Boehringer-Ingelheim, Bristol-Myers Squibb, Dimerix, Durect, Eli Lilly, Gilead, GlaxoSmithKline, Janssen, Merck, Metavant, Mitsubishi Tanabe, Mundipharma, Novartis, Novo Nordisk, Pfizer, PharmaLink, Relypsa, Retrophin, Roche, Sanofi, Servier, Travere, Tricida, UpToDate, and Vitae; and scientific advisor or membership on the steering committees for AbbVie, Bayer, Boehringer-Ingelheim, GlaxoSmithKline, Janssen, Novartis, Pfizer, and Travere. T. Sen was supported by the BEAt-DKD (Biomarker Enterprise to Attack Diabetic Kidney Disease) project. The BEAt-DKD project has received funding from Innovative Medicines Initiative 2 Joint Undertaking grant 115974. This joint undertaking receives support from the European Union's Horizon 2020 Research and Innovation Programme and EFPIA (European Federation of Pharmaceutical Industries and Associations). All remaining authors have nothing to disclose.
Funding
Biomarker measurements were funded by Janssen Research & Development.
Supplementary Material
Acknowledgments
The authors thank all CANVAS participants and investigators for their support in the trial.
The CANVAS trial was conducted in collaboration with the sponsor, an academic steering committee, and an academic research organization, George Clinical, and it was sponsored by Janssen Research & Development. The funder was involved in the study design, data collection, data analysis, data interpretation, and writing of the report.
H.J.L. Heerspink conceptualized the study; H.J.L. Heerspink and S.W. Waijer were responsible for data curation; K.W. Mahaffey, B.L. Neuen, V. Perkovic, and T. Sen were responsible for investigation; H.J.L. Heerspink and S.W. Waijer were responsible for formal analysis; H.J.L. Heerspink and S.W. Waijer wrote the original draft; and all authors reviewed and edited the manuscript.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
Data Sharing Statement
Data from the CANVAS program are available in the public domain via the Yale University Open Data Access Project (http://yoda.yale.edu/).
Supplemental Material
This article contains the following supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.08780621/-/DCSupplemental.
Supplemental Material. Sites and investigators in the CANVAS trial.
Supplemental Figure 1. Histograms of TNFR-1, TNFR-2, KIM-1, UACR, and eGFR in patients with normoalbuminuria.
Supplemental Figure 2. Hazard ratios per quartile of TNFR-1 and TNFR-2 for the kidney outcome in patients with normoalbuminuria.
Supplemental Figure 3. Kaplan–Meier curve for the kidney outcome in patients with normoalbuminuria below or above the fourth quartile for TNFR-1 and TNFR-2.
Supplemental Figure 4. Associations between TNFR-1 and TNFR-2 in patients with normoalbuminuria.
Supplemental Figure 5. Effect of prognostic enrichment with UACR or KIM-1 on sample size, number of screenings, and costs for a future trial in the normoalbuminuria population.
Supplemental Table 1. Effect of prognostic enrichment with TNFR-1 or TNFR-2 on sample size, number of screenings, and costs for a future trial in the normoalbuminuria population.
Supplemental Table 2. Treatment effect of canagliflozin in patients with normoalbuminuria and TNFR-1 or TNFR-2 biomarker level above or below the fourth quartile compared with micro- or macroalbuminuria.
References
- 1.Mosenzon O, Wiviott SD, Cahn A, Rozenberg A, Yanuv I, Goodrich EL, Murphy SA, Heerspink HJL, Zelniker TA, Dwyer JP, Bhatt DL, Leiter LA, McGuire DK, Wilding JPH, Kato ET, Gause-Nilsson IAM, Fredriksson M, Johansson PA, Langkilde AM, Sabatine MS, Raz I: Effects of dapagliflozin on development and progression of kidney disease in patients with type 2 diabetes: An analysis from the DECLARE-TIMI 58 randomised trial. Lancet Diabetes Endocrinol 7: 606–617, 2019 [DOI] [PubMed] [Google Scholar]
- 2.Neuen BL, Ohkuma T, Neal B, Matthews DR, de Zeeuw D, Mahaffey KW, Fulcher G, Li Q, Jardine M, Oh R, Heerspink HL, Perkovic V: Effect of canagliflozin on renal and cardiovascular outcomes across different levels of albuminuria: Data from the CANVAS Program. J Am Soc Nephrol 30: 2229–2242, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Colhoun HM, Marcovecchio ML: Biomarkers of diabetic kidney disease. Diabetologia 61: 996–1011, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Niewczas MA, Gohda T, Skupien J, Smiles AM, Walker WH, Rosetti F, Cullere X, Eckfeldt JH, Doria A, Mayadas TN, Warram JH, Krolewski AS: Circulating TNF receptors 1 and 2 predict ESRD in type 2 diabetes. J Am Soc Nephrol 23: 507–515, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Coca SG, Nadkarni GN, Huang Y, Moledina DG, Rao V, Zhang J, Ferket B, Crowley ST, Fried LF, Parikh CR: Plasma biomarkers and kidney function decline in early and established diabetic kidney disease. J Am Soc Nephrol 28: 2786–2793, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Skupien J, Warram JH, Niewczas MA, Gohda T, Malecki M, Mychaleckyj JC, Galecki AT, Krolewski AS: Synergism between circulating tumor necrosis factor receptor 2 and HbA(1c) in determining renal decline during 5-18 years of follow-up in patients with type 1 diabetes and proteinuria. Diabetes Care 37: 2601–2608, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Han WK, Bailly V, Abichandani R, Thadhani R, Bonventre JV: Kidney Injury Molecule-1 (KIM-1): A novel biomarker for human renal proximal tubule injury. Kidney Int 62: 237–244, 2002 [DOI] [PubMed] [Google Scholar]
- 8.Nowak N, Skupien J, Niewczas MA, Yamanouchi M, Major M, Croall S, Smiles A, Warram JH, Bonventre JV, Krolewski AS: Increased plasma kidney injury molecule-1 suggests early progressive renal decline in non-proteinuric patients with type 1 diabetes. Kidney Int 89: 459–467, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sabbisetti VS, Waikar SS, Antoine DJ, Smiles A, Wang C, Ravisankar A, Ito K, Sharma S, Ramadesikan S, Lee M, Briskin R, De Jager PL, Ngo TT, Radlinski M, Dear JW, Park KB, Betensky R, Krolewski AS, Bonventre JV: Blood kidney injury molecule-1 is a biomarker of acute and chronic kidney injury and predicts progression to ESRD in type I diabetes. J Am Soc Nephrol 25: 2177–2186, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Neal B, Perkovic V, de Zeeuw D, Mahaffey KW, Fulcher G, Stein P, Desai M, Shaw W, Jiang J, Vercruysse F, Meininger G, Matthews D: Rationale, design, and baseline characteristics of the Canagliflozin Cardiovascular Assessment Study (CANVAS)—A randomized placebo-controlled trial. Am Heart J 166: 217–223.e11, 2013 [DOI] [PubMed] [Google Scholar]
- 11.Neal B, Perkovic V, Mahaffey KW, de Zeeuw D, Fulcher G, Erondu N, Shaw W, Law G, Desai M, Matthews DR; CANVAS Program Collaborative Group : Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med 377: 644–657, 2017 [DOI] [PubMed] [Google Scholar]
- 12.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF 3rd, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J; CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) : A new equation to estimate glomerular filtration rate. Ann Intern Med 150: 604–612, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kerr KF, Roth J, Zhu K, Thiessen-Philbrook H, Meisner A, Wilson FP, Coca S, Parikh CR: Evaluating biomarkers for prognostic enrichment of clinical trials. Clin Trials 14: 629–638, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gohda T, Niewczas MA, Ficociello LH, Walker WH, Skupien J, Rosetti F, Cullere X, Johnson AC, Crabtree G, Smiles AM, Mayadas TN, Warram JH, Krolewski AS: Circulating TNF receptors 1 and 2 predict stage 3 CKD in type 1 diabetes. J Am Soc Nephrol 23: 516–524, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lopes-Virella MF, Baker NL, Hunt KJ, Cleary PA, Klein R, Virella G; DCCT/EDIC Research Group : Baseline markers of inflammation are associated with progression to macroalbuminuria in type 1 diabetic subjects. Diabetes Care 36: 2317–2323, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gohda T, Nishizaki Y, Murakoshi M, Nojiri S, Yanagisawa N, Shibata T, Yamashita M, Tanaka K, Yamashita Y, Suzuki Y, Kamei N: Clinical predictive biomarkers for normoalbuminuric diabetic kidney disease. Diabetes Res Clin Pract 141: 62–68, 2018 [DOI] [PubMed] [Google Scholar]
- 17.Yamanouchi M, Skupien J, Niewczas MA, Smiles AM, Doria A, Stanton RC, Galecki AT, Duffin KL, Pullen N, Breyer MD, Bonventre JV, Warram JH, Krolewski AS: Improved clinical trial enrollment criterion to identify patients with diabetes at risk of end-stage renal disease. Kidney Int 92: 258–266, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schulz CA, Engström G, Nilsson J, Almgren P, Petkovic M, Christensson A, Nilsson PM, Melander O, Orho-Melander M: Plasma kidney injury molecule-1 (p-KIM-1) levels and deterioration of kidney function over 16 years. Nephrol Dial Transplant 35: 265–273, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group : KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl 3: 1–150, 2013 [Google Scholar]
- 20.Astor BC, Matsushita K, Gansevoort RT, van der Velde M, Woodward M, Levey AS, Jong PE, Coresh J, Astor BC, Matsushita K, Gansevoort RT, van der Velde M, Woodward M, Levey AS, de Jong PE, Coresh J, El-Nahas M, Eckardt KU, Kasiske BL, Wright J, Appel L, Greene T, Levin A, Djurdjev O, Wheeler DC, Landray MJ, Townend JN, Emberson J, Clark LE, Macleod A, Marks A, Ali T, Fluck N, Prescott G, Smith DH, Weinstein JR, Johnson ES, Thorp ML, Wetzels JF, Blankestijn PJ, van Zuilen AD, Menon V, Sarnak M, Beck G, Kronenberg F, Kollerits B, Froissart M, Stengel B, Metzger M, Remuzzi G, Ruggenenti P, Perna A, Heerspink HJ, Brenner B, de Zeeuw D, Rossing P, Parving HH, Auguste P, Veldhuis K, Wang Y, Camarata L, Thomas B, Manley T; Chronic Kidney Disease Prognosis Consortium : Lower estimated glomerular filtration rate and higher albuminuria are associated with mortality and end-stage renal disease. A collaborative meta-analysis of kidney disease population cohorts. Kidney Int 79: 1331–1340, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Afkarian M, Zelnick LR, Hall YN, Heagerty PJ, Tuttle K, Weiss NS, de Boer IH: Clinical manifestations of kidney disease among US adults with diabetes, 1988-2014. JAMA 316: 602–610, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pavkov ME, Hanson RL, Knowler WC, Bennett PH, Krakoff J, Nelson RG: Changing patterns of type 2 diabetes incidence among Pima Indians. Diabetes Care 30: 1758–1763, 2007 [DOI] [PubMed] [Google Scholar]
- 23.Alicic RZ, Rooney MT, Tuttle KR: Diabetic kidney disease: Challenges, progress, and possibilities. Clin J Am Soc Nephrol 12: 2032–2045, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tuttle KR: The landscape of diabetic kidney disease transformed. Nat Rev Nephrol 16: 67–68, 2020 [DOI] [PubMed] [Google Scholar]
- 25.Perkovic V, de Zeeuw D, Mahaffey KW, Fulcher G, Erondu N, Shaw W, Barrett TD, Weidner-Wells M, Deng H, Matthews DR, Neal B: Canagliflozin and renal outcomes in type 2 diabetes: Results from the CANVAS Program randomised clinical trials. Lancet Diabetes Endocrinol 6: 691–704, 2018 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.