Abstract
Infections remain a common complication of solid-organ transplantation. Most infections in the first month after transplant are typically health care–associated infections, whereas late infections, beyond 6–12 months, are community-acquired infections. Opportunistic infections most frequently present in the first 12 months post-transplant and can be modulated on prior exposures and use of prophylaxis. In this review, we summarize the current epidemiology of postkidney transplant infections with a focus on key viral (BK polyomavirus, cytomegalovirus, Epstein-Barr virus, and norovirus), bacterial (urinary tract infections and Clostridioides difficile colitis), and fungal infections. Current guidelines for safe living post-transplant are also summarized. Literature supporting prophylaxis and vaccination is also provided.
Keywords: cytomegalovirus, polyomavirus, norovirus, urinary tract infection, histoplasmosis, blastomycosis, coccidioidomycosis, vaccination, kidney transplantation, kidney transplantation series
Introduction
The field of transplantation has been transformed since the first kidney transplant was performed (1). Significant advances in surgical technique and induction and maintenance immunosuppression regimens have improved allograft outcomes. Nonetheless, infections remain a leading cause of complications after kidney transplantation (2,3). Over time, the field of transplant infectious diseases has grown, and discovery and implementation of modern antimicrobial prophylaxis has contributed to delaying and reducing the incidence of post-transplant infections (3). This review summarizes the timing of infectious complications and discusses common post-transplant infections and tactics to minimize infectious risk, as well as approaches to safe living.
Timing of Infectious Complications in Kidney Transplantation
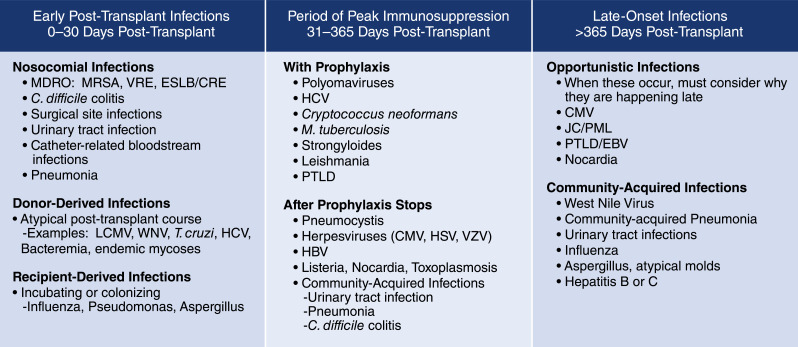
Infectious complications are categorized as occurring in one of three time periods post-transplant: early post-transplant infections, infections during peak immunosuppression, and late-onset infections (Figure 1) (3,4). A number of factors affect the timing of the infections, including specific donor and recipient factors such as a preexisting infection or immunity, the use of antimicrobial prophylaxis, and the net state of immunosuppression. Of these, the net state of immunosuppression is harder to assess because there are no direct measures. Instead, the clinician must assess a variety of factors, including current and past immunosuppression; underlying immunodeficiency; neutropenia; lymphopenia; a variety of complex metabolic conditions, such as presence of uremia, malnutrition, poorly controlled diabetes mellitus, and cirrhosis; and replication of immunomodulatory viruses. Net state of immunosuppression not only reflects the medications the patient is currently taking, but also agents, such as alemtuzumab or rituximab, which may be used as part of induction or in the treatment of rejection and may have long-standing effects on components of the immune system. Further, recent changes in immunosuppression may alter the assessment of the net state of immunosuppression. Taken together, these considerations allow a clinician to estimate if the patient is more or less immunosuppressed than the usual patient.
Figure 1.
Common infections associated with time since kidney transplantation. C. difficile, Clostideroides difficile; CMV, cytomegalovirus; EBV, Epstein-Barr virus; ESLB/CRE, extended spectrum beta-lactamase/carbapenem resistant enterobacteria; HBV, hepatitis B virus; HCV, hepatitis C virus; HSV, herpes simplex virus; JC/PML, JC virus/progressive multifocal leukoencephalopathy; LCMV, lymphocytic choriomeningitis virus; MDRO, multi-drug resistant organism; MRSA, methicillin-resistant staphylococcus aureus; M. tuberculosis, mycobacterium tuberulosis; PTLD, post-transplant lymphoproliferative disease; T. cruzi, Trypanosoma cruzi; VRE, vancomycin-resistant enterococcus; VZV, varicella zoster virus; WNV, West Nile virus.
Early post-transplant infections are infections that occur in the first 30 days post-transplant (5). The majority of such infections (approximately 98%) are common post-surgical infections, including surgical site infections, pneumonias, urinary tract infections (UTIs), bacteremias, and Clostridioides difficile colitis. Management approaches for such infections are consistent with the local epidemiology and susceptibility of predicted pathogens and published guidelines (5).
Recipient-origin infections may manifest in the first 30 days. Examples of recipient-origin infections include respiratory viral infections or occult bacteremias that were incubating in the candidates at the time they present for their transplant procedures.
Donor-derived infections, although rare (approximately 0.2%), may present during the first 30 days post-transplant (6). Donor-derived infections are defined as any infection present in the donor that is transmitted to the recipient with the transplanted organ or vessels (6–9). Such infections can be categorized as either expected, where the pathogen is known to be present in the donor at the time of procurement regardless of whether steps are taken to mitigate the disease transmission (e.g., cytomegalovirus [CMV] and Epstein-Barr virus [EBV]), or unexpected, when the donor infection is not recognized and is identified after clinical disease presents in one or more of the transplant recipients. Most (76%) unexpected donor-derived infections present within 30 days of transplantation (6).
Clinicians should have a high index of suspicion for donor-derived infections in any patient with atypical early post-transplant course, unexplained sepsis, fever, or alteration in mental status in the first 30–45 days post-transplant. Those with early infections should always prompt review of donor cultures and history. Recognition and reporting of potential donor-derived infections are essential because they may potentially affect all of the recipients of organs from the same donor. In the United States, any documented or suspected unexpected donor-derived disease transmissions need to be reported to the Organ Procurement and Transplantation Network (OPTN) as soon as possible, but not >24 hours after initially suspecting transmission (OPTN Policy 15.4), through the Patient Safety Portal. Timely reporting of suspected transmissions is essential to facilitate communication and rapidly allow screening and treatment of recipients of organs from the same donor (10).
The second period of post-transplant infections occurring during peak immunosuppression are typically opportunistic infections or pathogens that reactivate from latent infection in the recipient, such as BK virus, CMV, herpes simplex virus (HSV), varicella zoster virus, hepatitis B virus, hepatitis C virus (HCV), tuberculosis, listeria, strongyloidiasis, and Chagas disease, and generally occur between 30 days and 6 months post-transplant or within 3 months of treatment of rejection. Patients with potent induction therapy, particularly those with persistent lymphopenia, have an extended period of risk (3,4). Use of prophylactic antimicrobials may delay the infection onset, resulting in later than typical onset.
Late-onset infections typically present >6–12 months post-transplant or >3 months after treatment for a rejection episode (3,4). Most late-onset infections are community acquired, such as community-acquired pneumonia, respiratory viral infections, and UTIs. Patients may acquire infections from exposure to the environment or travel, which increases over time as the patient returns to normal function. Patients may also become less cautious, which can lead to a higher risk of community-acquired infections, as we have seen with coronavirus disease 2019 (COVID-19) (11). Rarely, opportunistic infections may present in this late period, including progressive multifocal leukoencephalopathy or Pneumocystis jirovecii (12).
Viral Infections
Although the remainder of this virology review will focus on some of the more common viral infections complicating kidney transplant, there are others that warrant discussion but already have excellent recent reviews. For example, there is a growing body of literature about HCV in kidney transplantation. Most patients are treated before transplantation, making post-transplant HCV management a rare issue, with the exception of intentional transmission of HCV from nucleic acid test-positive donors (13). Recent studies suggest the ability to treat HCV in this setting, with shorter course of therapy, but optimal approaches are yet to be defined (14).
Respiratory viral infections, including influenza, respiratory syncytial virus, and COVID-19, can result in severe infections in kidney transplant recipients, but frequently are self-limited (15). Vaccines for influenza are recommended in all transplant recipients and their close contacts. Antiviral therapy with neuraminidase inhibitors are recommended for the treatment of influenza in kidney transplant recipients; baloxavir marboxil, a new anti-influenza antiviral, is approved but generally not recommended for transplant recipients because of the concern for emergence of resistance. Although the optimal therapy for COVID-19 has yet to be defined for kidney transplant recipients, most current guidelines recommend considering remdesivir, dexamethasone, and/or convalescent plasma (11).
Epstein-Barr Virus
EBV is a human herpesvirus infecting about 90% of adults. It is transmitted mainly via oropharyngeal secretions, and primary infection is commonly asymptomatic. EBV remains latent within the B lymphocytes, but can reactivate post-transplant. This can manifest as asymptomatic viremia, infectious mononucleosis syndrome, or other organ involvement such as hepatitis, myocarditis, and pancreatitis. The majority of symptomatic infections in kidney transplant recipients are primary infection, likely related to reactivation of donor virus. The most concerning presentation of EBV is post-transplant lymphoproliferative disorder (PTLD) (16,17).
Current guidelines recommend routine screening for EBV in high-risk kidney transplant recipients (donor EBV seropositive [D+]/recipient EBV seronegative [R−]) by nucleic acid testing (18). Monitoring is performed at regular intervals in the first year post-transplant and after treatment of acute rejection. Reduction of immunosuppression should be considered in EBV-naïve recipients with an increasing EBV viral load (19). Subclinical EBV DNAemia has been reported in up to 40% of patients in the first post-transplant year and is associated with worse graft outcomes and increased opportunistic infections (20).
PTLD represents 21% of all cancers in solid-organ transplants recipients (21,22). Early-onset PTLD, occurring within the first post-transplant year, is commonly seen in younger individuals and is more frequently associated with EBV positivity and allograft involvement (22). The most common risk factors for PTLD are EBV D+/R− status and the degree of immunosuppression, with T cell-depleting induction a strong factor. A recent meta-analysis concluded that antiviral prophylaxis had no effect on PTLD incidence (23). Belatacept, a costimulation blocker approved in kidney transplant recipients, is contraindicated in EBV-seronegative recipients because of an estimated ten-fold higher risk of PTLD reported during the phase 3 studies (24,25). If there is a clinical suspicion of PTLD, the quantitative blood EBV viral load should be assessed, although there are no specific diagnostic levels.
The cornerstone of EBV/PTLD management is immunosuppression reduction, which leads to disease regression in 20%–80% of cases. PTLD management should be done in consultation with an oncologist, who may recommend anti-B cell therapy (rituximab), chemotherapy, and/or radiation. Adoptive immunotherapy with EBV-specific T lymphocytes is an emerging therapeutic option (26). In the largest series of patients retransplanted after PTLD, only one of the 52 patients developed recurrent PTLD (27).
Cytomegalovirus
CMV is a ubiquitous human herpesvirus, with a seroprevalence rate of 30%–97% (28,29). The risk of CMV infection or disease after transplant is determined primarily by the donor and recipient CMV serostatus (from highest to lowest risk: D+/R−, D+/R+, D−/R+, D−/R−) (29). Use of lymphocyte-depleting agents, as induction or for allograft rejection, has been shown to significantly increase the risk of CMV disease (28,29). Of the newer immunosuppressive agents, belatacept has been associated with an increased risk of CMV primary infection and a prolonged course of viral replication in patients at high risk of CMV (30). In the absence of preventive measures, CMV infection and disease develop in 40%–100% and 67% in kidney transplant recipients, respectively. With the current preventive strategies, the incidence is about 17%–37%, with the highest risk in the first 100 days (28,29).
Immune monitoring of CMV-specific T cell responses is another strategy to assess post-transplant CMV risk. IFN-γ release assays (QuantiFERON-CMV, ELISpot) and intracellular cytokine staining for IFN-γ have been shown to predict both CMV viremia and disease (31). Emerging data suggest that detection of CMV-specific immunity is associated with a lower risk of infection and may be helpful in determining duration of prophylaxis and preemptive monitoring (28).
The key strategies for prevention of CMV are universal prophylaxis, preemptive therapy, and a hybrid approach known as “surveillance after prophylaxis” (29). There are data to support each strategy, and current guidelines suggest any approach is acceptable. Prophylaxis is easier in the outpatient setting, protects against HSV and varicella zoster virus, and is associated with rare instances of early CMV infection and lower rates of graft rejection. It is, however, associated with risk of late-onset CMV, resistance development, higher drug costs, and side effects. In a single-center study of 176 patients with CMV D+/R− serostatus, 29% of patients developed CMV disease at a median of 61 days after stopping antiviral prophylaxis (32). Preemptive therapy lacks some of the benefits listed above, but results in lower rates of late-onset CMV and less drug toxicity. Preemptive therapy has higher laboratory costs and has also been associated with resistance development.
Oral valganciclovir is the most commonly used prophylaxis medication, with a recommended dose of 900 mg daily, and dose reduction for kidney dysfunction (28,29). Most guidelines recommend 100 days of prophylaxis for intermediate-risk patients and 200 days of prophylaxis for high-risk patients (33). Letermovir, a novel viral terminase inhibitor, is being evaluated for prophylaxis in CMV D+/R− kidney recipients, but does not have HSV coverage. Patients with CMV D−/R− serostatus have a very low risk of CMV disease, and acyclovir prophylaxis can be used to prevent HSV (29).
CMV can present as asymptomatic DNAemia, CMV syndrome (viremia, constitutional symptoms, cytopenias without organ involvement), or tissue invasive disease. CMV disease can affect many organs; most commonly, the gastrointestinal tract, liver, pancreas, and lung. CMV also has a predilection to cause allograft nephritis. CMV has been described to have immunomodulatory effects and can increase risk of activation of other herpes viruses, EBV-mediated PTLD, allograft rejection, and other opportunistic infections. CMV infection and disease have been associated with higher risk of mortality and graft loss (34).
All patients with a clinical suspicion for CMV infection or disease should be tested by PCR of blood or serum, which are less sensitive for certain organ-invasive diseases like gastrointestinal disease and retinitis (35). Histopathologic examination of tissue biopsy specimens may be needed to diagnose invasive CMV disease (36).
CMV management involves immunosuppression reduction and antiviral therapy. Antimetabolites may be stopped or reduced, depending upon the immunologic profile of the recipient. First-line therapy for CMV is valganciclovir or intravenous ganciclovir. Intravenous therapy is preferred in life-threatening illness, CMV pneumonitis, and colitis. Both agents require monitoring of blood counts and kidney function, with dose reduction for kidney dysfunction. Treatment is continued until there is clinical improvement and CMV viral loads are undetectable. Although secondary prophylaxis has previously been widely used, recent data suggest it may not be required for most patients (28,29). Genotypic assays for resistance should be performed if DNAemia persists despite 2 weeks of antiviral therapy (37). Second-line agents used for treatment of resistant CMV include foscarnet, high-dose ganciclovir, cidofovir, and CMV Ig. Letermovir is not approved for CMV treatment. Off-label use for ganciclovir-resistant CMV has been complicated by emergence of the letermovir-resistant virus (38). Adoptive transfer of CMV‐specific T cells may be considered as adjunctive therapies for resistant CMV, in collaboration with transplant infectious disease experts (29).
Polyomaviruses
The BK polyomavirus is a human polyomavirus, first identified in the urine of a kidney transplant recipient with ureteral stenosis (39). Primary BK polyomavirus infection occurs during childhood, with 80%–90% adults being exposed (40). The virus remains latent in the kidney tubules and uroepithelium (41).
In immunocompromised hosts, the disease can progress from asymptomatic viruria to viremia and organ-invasive disease. It usually presents as BK polyomavirus-associated nephropathy in kidney transplant recipients (42). Asymptomatic viruria, detected on routine screening, is the earliest manifestation and is seen in 25%–40% of patients in the first year. Of those with persistent viruria and high urinary viral loads, 10%–20% develop viremia after a few weeks. BK polyomavirus-associated nephropathy occurs in patients with persistent high-titer viremia, typically >10,000 copies/ml, and is seen in 1%–10% of all kidney transplant recipients (40,43). It most commonly occurs in the first post-transplant year, when the degree of immunosuppression is the highest. The most significant risk is the degree of immunosuppression, but other factors are donor related (viruria, female sex, deceased donor), recipient related (male sex, highly sensitized status, AB0 incompatibility, HLA mismatch, low BK polyomavirus-specific neutralizing antibody or T cell activity), or transplant related (ureteric stent, treatment for acute rejection, tacrolimus exposure) (40,44).
Current guidelines recommend routine post-transplant screening for BK viremia monthly for 9 months, and then every 3 months until 2 years’ post-transplant. Screening is also recommended when evaluating for graft dysfunction and with a kidney allograft biopsy (40). Early detection of BK polyoma viremia combined with reduction in immunosuppression can prevent progression to BK polyomavirus-associated nephropathy and preserve graft function (45).
Viremia has a 50%–60% positive predictive value for diagnosis of BK polyomavirus-associated nephropathy, and patients with sustained viral loads of ≥10,000 copies/ml are presumed to have BK polyomavirus-associated nephropathy (40,43). Kidney biopsy is the gold standard for diagnosis and is helpful in assessing disease severity, chronicity, and concurrent rejection. Given the patchy involvement of the kidney, guidelines recommend two biopsy cores containing medulla and immunohistochemistry for SV40 T antigen (46).
The cornerstone of management of BK viremia and nephropathy is immunosuppression reduction (45). The various strategies for reduction have not been compared in randomized controlled trials, and center-specific, individualized protocols are used. Worsening kidney allograft function after reduction of immunosuppression should prompt evaluation for possible graft rejection with a biopsy (19). Acute rejection has been reported in 8%–12% after reduction of immunosuppression for BK viremia or nephropathy (45,47). In a retrospective cohort of patients with BK polyomavirus-associated nephropathy, 14% developed de novo donor-specific antibodies, which was a risk factor for subsequent antibody-mediated rejection and graft loss (48).
Other adjunctive treatments used with varying degree of success include intravenous Ig, leflunomide, and cidofovir (40). Data from randomized controlled trials do not demonstrate superiority of one or more of these therapies over immunosuppression reduction alone (49). Intravenous IG may be considered in patients with severe hypogammaglobulinemia, concomitant rejection, or those at high immunologic risk. Leflunomide has both immunosuppressive and antiviral activity and has been used to replace the antimetabolites in recipients with a higher risk of rejection. It is, however, associated with hematologic and hepatotoxicity, and therapeutic drug monitoring is difficult (50). Cidofovir is associated with a dose-dependent nephrotoxicity, and its use is not recommended in patients with significant kidney dysfunction or proteinuria. Quinolones are no longer recommended for treatment of BK polyomavirus-associated nephropathy (51). Adoptive T cell therapy is a novel therapeutic option in BK polyomavirus-associated nephropathy and has been shown to reduce viral load in the kidney tubules when used early in the course of the disease (52).
Unfortunately, graft loss occurs in 15%–50% of BK polyomavirus-associated nephropathy cases. Retransplantation in these patients has been successful, with 5-year death-censored graft survival of 90.6% (53). Undetectable levels of viremia at time of retransplantation were associated with absence of BK viremia at 1 year post-transplant. In patients with persistent viremia, a decline of at least 2 log10 copies/ml after reduction of immunosuppression indicates an antiviral immune response, and retransplant may be considered (54). The role of transplant nephrectomy before a second transplant is not well defined, but can be considered in those with persistent viremia (55).
Norovirus
Norovirus infections typically present as an acute infection characterized by severe nausea; vomiting; watery, nonbloody diarrhea; abdominal cramps; and, occasionally, low-grade fever, muscle aches, chills, and headache in immunocompetent hosts (56). Immunocompromised patients can develop chronic norovirus infections, associated with relapsing and remitting episodes of watery diarrhea that may last for months to years (57). Norovirus is the second most common documented cause of diarrhea, after C. difficile, among solid-organ transplant recipients (58). A total of 30% of patients with chronic norovirus have a ≥20% increase in creatinine within 1 year of the diagnosis, as a result of recurrent dehydration and supratherapeutic tacrolimus levels during periods of diarrhea (58). Given the high prevalence of norovirus, kidney transplant recipients with diarrhea, particularly chronic or relapsing diarrhea, should be screened for norovirus by PCR or antigen testing of stool (59).
The current mainstay of therapy for norovirus is supportive, with antimotility agents and hydration (57–59). Reduction of immunosuppression is commonly practiced, although there is no clear evidence that it is associated with viral clearance (57). Several agents, including oral and intravenous Igs and nitazoxanide, are used off-label, with variable evidence to support their use, and a clinical trial of nitazoxanide is ongoing (57).
Bacterial Infections
Clostridioides difficile
C. (formerly Clostridium) difficile, an anaerobic, spore-forming, Gram-positive bacterium, causes C. difficile infection, which is five times more likely in hospitalized solid-organ transplant recipients compared with the general population (60). C. difficile infection affects 3%–16% of kidney transplant recipients, often early post-transplant (60,61). Severe presentation with fulminant colitis (5.3%) and need for colectomy (2.7%) appear to be higher than in other patient populations, and C. difficile infection has been associated with graft loss in at least one study (61,62). Recurrences of C. difficile infection have been reported in nearly 20% of solid-organ transplant recipients, comparable with other hospitalized patients (62). Risk factors for C. difficile infection include those reported in nontransplant patients such as recent antibiotic exposure, age >65 years, acid suppression medications, and hospitalization (60). Additionally, transplant-specific risks include induction with antithymocyte globulin and hypogammaglobulinemia (60,63). Diagnosis relies on presence of three or more unformed stools in a 24-hour period, and the demonstration of C. difficile toxin or PCR testing of the stool. Unexplained abdominal pain with fever and leukocytosis in a patient with ileus should prompt C. difficile infection testing (60). Primary therapy with oral vancomycin or fidaxomicin is suggested for both severe and nonsevere events (60,64). High-dose oral vancomycin with intravenous metronidazole is recommended for fulminant cases, with consideration for surgical intervention (60,64). In addition, bezlotoxumab, a human mAb against toxin B, can be considered in solid-organ transplant recipients at higher risk for recurrence of C. difficile infection (60,65). For recurrences, treatment options include either fidaxomicin or prolonged, tapered, or pulsed oral vancomycin (64). Additionally, fecal microbiota transplantation should be considered with multiple recurrences and has been shown to be safe and potentially helpful in some, but not all, solid-organ transplant recipients (66).
Urinary Tract Infection
UTIs are the most common infections in kidney transplant recipients. They occur most commonly in the first year post-transplant, with a prevalence that ranges widely from 7%–80% (67). Similar to nontransplant patients, the incidence of UTIs is higher in female kidney transplant recipients because of anatomic predisposition (68). Gram-negative bacteria cause up to 90% of cases, and Escherichia coli was most commonly reported (69).
Perioperative and prophylactic antibiotics during the early post-transplant period are standard-of-care measures adopted to prevent UTIs. Trimethoprim-sulfamethoxazole is recommended for 6–12 months post-transplant to prevent P. jirovecii pneumonia, but it also serves as an effective UTI prophylaxis and lowers the risk of both UTI and bacteremia (12). Current guidelines recommend that patients who cannot take trimethoprim-sulfamethoxazole for prophylaxis receive an additional antibiotic for UTI prevention, at least until the ureteral stent is removed (70). Minimizing the stent duration is associated with the lowest risk of early post-transplant UTIs, but needs to be balanced against risk of urological complications (71).
Postkidney transplant UTIs can be categorized as asymptomatic bacteriuria, uncomplicated UTI/simple cystitis, complicated UTI/pyelonephritis, or recurrent UTI. Asymptomatic bacteriuria is diagnosed by a screening urine culture without concurrent symptoms. Although it was once thought to be associated with complications, recent data suggest that there is no benefit in treating asymptomatic bacteriuria, with treatment associated with risks of adverse events, including C. difficile infection (72). Current guidelines recommend against surveillance urine cultures or treating asymptomatic bacteriuria in most kidney transplant recipients. However, if two consecutive urine samples yield >105 of the same uropathogen in the first 2 months post‐transplant, antibiotic treatment for 5 days may be considered (70).
Uncomplicated UTIs are diagnosed in patients with lower urinary tract symptoms and a positive urine culture. Transplant recipients with clinical symptoms of cystitis can be treated with oral antibiotics based on the organism isolated for 5–7 days (70). Complicated UTIs present with systemic symptoms (fever, chills, malaise, nausea, vomiting) and/or allograft pain with a positive urine culture. Bacteremia may be present in approximately 10% of cases. Urine and blood cultures should be collected before initiation of therapy, and imaging of the urinary tract should be obtained. Management includes empirical broad-spectrum parenteral antibiotics, which can be narrowed to definitive treatment once the organism and susceptibilities are identified. Patients can be switched to oral antibiotics once the clinical condition improves, to complete a 7–14 day course (70).
Recurrent UTIs are defined as three or more episodes in 1 year, or two or more episodes in 6 months. Urinary tract obstruction owing to strictures or calculi, indwelling urinary stents, complex kidney cysts, vesico-ureteric reflux, and bladder dysfunction can result in recurrent UTIs. Suppressive antibiotic prophylaxis has limited efficacy in kidney transplant recipients (73) and poses risk of emergence of drug-resistant organisms. Methenamine hippurate has been shown to reduce frequency of UTIs, antibiotic use, and need for hospitalization in kidney transplant recipients (74,75). Although strong evidence is lacking, strategies such as behavioral education (perineal hygiene, postcoital voiding in women, frequent voiding), Lactobacillus probiotics, d-mannose, cranberry products, and vaginal estrogens and hyaluronic acid/chondroitin sulfate in postmenopausal women can also be tried (70).
UTIs caused by drug-resistant organisms, such as extended-spectrum β-lactamase-producing Gram-negative bacteria and carbapenem-resistant Enterobacteriaceae, are increasing in kidney transplant recipients. Intravenous antibiotics are frequently required, namely, carbapenems for extended-spectrum β-lactamase organisms and amikacin and colistin for carbapenem-resistant Enterobacteriaceae. Fosfomycin and nitrofurantoin are the oral agents that retain broad-spectrum antimicrobial activity, and can be used judiciously in patients with cystitis (67). Data regarding the effect of UTIs on patient and graft outcomes are conflicting. Although some studies have shown a higher risk of mortality and graft loss (up to 41% and 29% in the first year, respectively), others have not found an effect on long-term graft function and survival (76–78).
Fungal Infections
As time from transplant increases, the risk of fungal pathogens associated with early post-operative infections, such as the Candida species, can be supplanted by more indolent infections with endemic mycoses, such as histoplasmosis, blastomycosis, and coccidioidomycosis. Histoplasma capsulatum, Blastomyces dermatitidis, Coccidioides immitis, and Coccidioides posadasii are all dimorphic fungi that exist as yeast in the human body and mycelial forms in the environment. Each pathogen has regional endemicity, which emphasizes the need for local residence and travel evaluation to assess risk. Histoplasmosis and blastomycosis infections are endemic to the upper Midwest of the United States, around the Great Lakes (Histoplasma) and the Ohio and Mississippi River valleys (Histoplasma/Blastomycoses), whereas Coccidioides is predominant in the Southwest United States (79).
Among the endemic myoses in solid-organ transplant recipients, histoplasmosis is most frequent, causing 5%–9% of post-transplant invasive fungal infections when early infections with Aspergillus and Candida are included (80,81). Histoplasmosis occurs in 0.1%–0.3% of kidney transplant recipients, at median of 2–5 years post-transplant (81–83). The most common presentation in kidney transplant includes pneumonia and disseminated disease, but rare presentations occur with cutaneous lesions and hemophagocytic lymphohistocytosis (82–85). Risk factors included leukopenia, CMV, and a diagnosis of bacterial pneumonia (81). One series reported 21% graft failure and 7% (one of 14) mortality (82). Diagnosis focuses on direct visualization or culture from sputum, bronchoalveolar lavage (BAL), or tissue. Noninvasive measures include histoplasma antigen enzyme immunoassay (EIA) from both urine and serum in suspected cases, and serology is of limited assistance (79). Post-transplant prophylaxis is not recommended, and treatment most frequently includes amphotericin, itraconazole, and, more recently, voriconazole and posaconazole (79,83,86). Monitoring histoplasma antigen EIA, particularly in the blood, to assess recovery is suggested by some experts (79).
Blastomycosis is less common overall (80), occurring at a median of 2 years post-transplant (82). Pneumonia has been reported in 80% of kidney transplant with blastomycoses, and disseminated disease commonly includes cutaneous manifestations (82). Risk for blastomycosis in kidney transplant is difficult to assess outside of environmental exposure given the relatively infrequent events; one study from Wisconsin identified two of three cases developed in a minority population, the Hmong (87). Similar to histoplasmosis, diagnosis focuses on direct visualization or culture from sputum, BAL, or tissue. Blastomycosis antigen EIA from urine, serum, BAL, or cerebrospinal fluid is available but less sensitive (62%–83%), and suffers from crossreactivity with other fungi. Again, serology is of limited assistance (79). Treatment with lipid-formulation amphotericin, with transition to azole therapy after initial recovery, is recommended for severe cases, whereas primary azole therapy can be used for mild cases (79).
Coccidioidomycosis caused by C. immitis and C. posadasii can be donor-derived, newly acquired post-transplant, or a reactivation from prior recipient disease. In endemic regions, 3% of kidney transplant recipients will develop coccidioidomycosis; screening and prophylaxis of high-risk patients reduces infection frequency (79,88,89). Presentation includes cutaneous, skeletal, pulmonary, meningitis, and disseminated disease (89,90). Culture is confirmatory; however, several available serologic assays can provide additional data, especially when used in combination, and may be used for monitoring during recovery (79). Treatment with lipid-formulation amphotericin, with transition to fluconazole therapy after initial recovery, is recommended for severe pulmonary and disseminated cases, whereas primary fluconazole therapy is recommended for meningitis and mild pulmonary disease (79). Lifelong azole suppression is recommended because of the significant risk for relapse in solid-organ transplant recipients.
Safer Living
Continued health after kidney transplant relies on the identification and mitigation of risk in everyday life. Routine adherence to general infection prevention principles, such as hand washing, is paramount (91). Meticulous care with food preparation, avoidance of undercooked meat, and strict avoidance of well water can lower food-borne pathogen risk. Employment, hobbies, and pet ownership should be discussed. Behaviors that increase risk for sexually transmitted infections should be discussed, and appropriate prevention education and vaccination, such as hepatitis B and age-appropriate human papillomavirus vaccination, should be provided (91–93). Additional focus on vaccine-preventable illness, especially in those increased with travel, should be provided (see Table 1), including annual influenza vaccination, routine adult shingles vaccination, and boosters for tetanus and pertussis (92–94). As many kidney transplant recipients thrive in the post-transplant period, travel for enjoyment may increase. Safety for travel requires evaluation of travel destination, risk assessment dependent on planned activities, and preparation of emergency medication supplies (92). Infection-related risk can be mitigated with pretravel vaccination (i.e., hepatitis A), avoidance of environmental risk (i.e., mosquitos), and preparation for common travel-related diseases (i.e., diarrhea and respiratory infection) (92,93).
Table 1.
Post-transplant vaccination for kidney transplant recipients
| Routine Vaccination | Travel-Related Vaccination |
|---|---|
| Influenza | Influenza |
| Hepatitis B | Hepatitis B |
| Hepatitis A | Hepatitis A |
| Tdap (diphtheria/tetanus/pertussis) | Tdap (diphtheria/tetanus/pertussis) |
| Streptococcus pneumoniae (conjugate) | Rabies |
| Streptococcus pneumoniae (polysaccharide) | Japanese encephalitis |
| Human papillomavirus | Salmonella typhi (intramuscular, inactivated: typhoid VI polysaccharide vaccine) |
| Herpes zoster (varicella, subunit: Shingrix) |
Travel-related vaccines should be determined on the basis of anticipated destination, planned activities, prior evidence of seroprotection (hepatitis A virus/hepatitis B virus) and time since prior vaccination (Tdap).
Disclosures
L. Danziger-Isakov reports consultancy agreements with Merck and Takeda; research funding from Ansun Biopharma, Astellas, Merck, Takeda/Shire, and Viracor; participating in a Data Safety and Monitoring Board for Astellas; and served on an advisory board for GlaxoSmithKline. M.G. Ison reports paid consultation from Adagio, AlloVir, Celltrion, Cidara, Genentech/Roche, Janssen, Shionogi, and Viracor Eurofins; research support, paid to Northwestern University, from AiCuris, Janssen, and Shire; honoraria from Adagio, AlloVir, Celltrion, Cidara, Genentech/Roche, Janssen, Shionogi, and Viracor Eurofins; serving as an editor of American Journal of Transplantation and Transplant Infectious Diseases; and serving as a paid member of Data Safety and Monitoring Boards from Janssen, Merck, SAB Biotherapeutics, Sequiris, Takeda, and Vitaeris. The remaining author has nothing to disclose.
Funding
None.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.Barker CF, Markmann JF: Historical overview of transplantation. Cold Spring Harb Perspect Med 3: a014977, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fishman JA: Infection in organ transplantation. Am J Transplant 17: 856–879, 2017 [DOI] [PubMed] [Google Scholar]
- 3.van Delden C, Stampf S, Hirsch HH, Manuel O, Meylan P, Cusini A, Hirzel C, Khanna N, Weisser M, Garzoni C, Boggian K, Berger C, Nadal D, Koller M, Saccilotto R, Mueller NJ; Swiss Transplant Cohort Study : Burden and timeline of infectious diseases in the first year after solid organ transplantation in the Swiss transplant cohort study. Clin Infect Dis 71: e159–e169, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fishman JA: Infection in solid-organ transplant recipients. N Engl J Med 357: 2601–2614, 2007 [DOI] [PubMed] [Google Scholar]
- 5.Dorschner P, McElroy LM, Ison MG: Nosocomial infections within the first month of solid organ transplantation. Transpl Infect Dis 16: 171–187, 2014; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kaul DR, Vece G, Blumberg E, La Hoz RM, Ison MG, Green M, Pruett T, Nalesnik MA, Tlusty SM, Wilk AR, Wolfe CR, Michaels MG: Ten years of donor-derived disease: A report of the disease transmission advisory committee [published online ahead of print July 5, 2020]. Am J Transplant 10.1111/ajt.16178 [DOI] [PubMed] [Google Scholar]
- 7.Ison MG, Hager J, Blumberg E, Burdick J, Carney K, Cutler J, Dimaio JM, Hasz R, Kuehnert MJ, Ortiz-Rios E, Teperman L, Nalesnik M: Donor-derived disease transmission events in the United States: Data reviewed by the OPTN/UNOS Disease Transmission Advisory Committee. Am J Transplant 9: 1929–1935, 2009 [DOI] [PubMed] [Google Scholar]
- 8.Ison MG, Nalesnik MA: An update on donor-derived disease transmission in organ transplantation. Am J Transplant 11: 1123–1130, 2011 [DOI] [PubMed] [Google Scholar]
- 9.Wolfe CR, Ison MG; American Society of Transplantation Infectious Diseases Community of Practice : Donor-derived infections: Guidelines from the American Society of Transplantation Infectious Diseases Community of Practice. Clin Transplant 33: e13547, 2019 [DOI] [PubMed] [Google Scholar]
- 10.Miller R, Covington S, Taranto S, Carrico R, Ehsan A, Friedman B, Green M, Ison MG, Kaul D, Kubak B, Lebovitz DJ, Lyon GM, Nalesnik MA, Pruett TL, Teperman L, Vasudev B, Blumberg E: Communication gaps associated with donor-derived infections. Am J Transplant 15: 259–264, 2015 [DOI] [PubMed] [Google Scholar]
- 11.Kates OS, Haydel BM, Florman SS, Rana MM, Chaudhry ZS, Ramesh MS, Safa K, Kotton CN, Blumberg EA, Besharatian BD, Tanna SD, Ison MG, Malinis M, Azar MM, Rakita RM, Morillas JA, Majeed A, Sait AS, Spaggiari M, Hemmige V, Mehta SA, Neumann H, Badami A, Goldman JD, Lala A, Hemmersbach-Miller M, McCort ME, Bajrovic V, Ortiz-Bautista C, Friedman-Moraco R, Sehgal S, Lease ED, Fisher CE, Limaye AP; UW COVID-19 SOT Study Team : COVID-19 in solid organ transplant: A multi-center cohort study [published online ahead of print August 7, 2020]. Clin Infect Dis 10.1093/cid/ciaa1097 [DOI] [Google Scholar]
- 12.Fishman JA, Gans H; American Society of Transplantation Infectious Diseases Community of Practice : Pneumocystis jiroveci in solid organ transplantation: Guidelines from the American Society of Transplantation Infectious Diseases Community of Practice. Clin Transplant 33: e13587, 2019 [DOI] [PubMed] [Google Scholar]
- 13.Sise ME, Goldberg DS, Kort JJ, Schaubel DE, Alloway RR, Durand CM, Fontana RJ, Brown RS Jr., Friedewald JJ, Prenner S, Landis JR, Fernando M, Phillips CC, Woodle ES, Rike-Shields A, Sherman KE, Elias N, Williams WW, Gustafson JL, Desai NM, Barnaba B, Norman SP, Doshi M, Sultan ST, Aull MJ, Levitsky J, Belshe DS, Chung RT, Reese PP: Multicenter Study to Transplant Hepatitis C-Infected Kidneys (MYTHIC): An open-label study of combined glecaprevir and pibrentasvir to treat recipients of transplanted kidneys from deceased donors with hepatitis C virus infection. J Am Soc Nephrol 31: 2678–2687, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Te H, Doucette K: Viral hepatitis: Guidelines by the American Society of Transplantation Infectious Disease Community of Practice. Clin Transplant 33: e13514, 2019 [DOI] [PubMed] [Google Scholar]
- 15.Ison MG, Hirsch HH: Community-acquired respiratory viruses in transplant patients: Diversity, impact, unmet clinical needs. Clin Microbiol Rev 32: e00042-19, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Le J, Durand CM, Agha I, Brennan DC: Epstein-Barr virus and renal transplantation. Transplant Rev (Orlando) 31: 55–60, 2017 [DOI] [PubMed] [Google Scholar]
- 17.Allen UD, Preiksaitis JK; American Society of Transplantation Infectious Diseases Community of Practice : Post-transplant lymphoproliferative disorders, Epstein-Barr virus infection, and disease in solid organ transplantation: Guidelines from the American Society of Transplantation Infectious Disease Community of Practice. Clin Transplant 33: e13652, 2019 [DOI] [PubMed] [Google Scholar]
- 18.Kasiske BL, Zeier MG, Chapman JR, Craig JC, Ekberg H, Garvey CA, Green MD, Jha V, Josephson MA, Kiberd BA, Kreis HA, McDonald RA, Newmann JM, Obrador GT, Vincenti FG, Cheung M, Earley A, Raman G, Abariga S, Wagner M, Balk EM; Kidney Disease: Improving Global Outcomes : KDIGO clinical practice guideline for the care of kidney transplant recipients: A summary. Kidney Int 77: 299–311, 2010 [DOI] [PubMed] [Google Scholar]
- 19.Kidney Disease : Improving Global Outcomes: Clinical Practice Guideline for the Care of Kidney Transplant Recipients. Available at: https://kdigo.org/guidelines/transplant-recipient. Accessed November 1, 2020
- 20.Bamoulid J, Courivaud C, Coaquette A, Chalopin JM, Gaiffe E, Saas P, Ducloux D: Subclinical Epstein-Barr virus viremia among adult renal transplant recipients: Incidence and consequences. Am J Transplant 13: 656–662, 2013 [DOI] [PubMed] [Google Scholar]
- 21.Opelz G, Döhler B: Lymphomas after solid organ transplantation: A collaborative transplant study report. Am J Transplant 4: 222–230, 2004 [DOI] [PubMed] [Google Scholar]
- 22.Ghobrial IM, Habermann TM, Macon WR, Ristow KM, Larson TS, Walker RC, Ansell SM, Gores GJ, Stegall MD, McGregor CG: Differences between early and late posttransplant lymphoproliferative disorders in solid organ transplant patients: Are they two different diseases?. Transplantation 79: 244–247, 2005 [DOI] [PubMed] [Google Scholar]
- 23.AlDabbagh MA, Gitman MR, Kumar D, Humar A, Rotstein C, Husain S: The role of antiviral prophylaxis for the prevention of Epstein-Barr virus-associated posttransplant lymphoproliferative disease in solid organ transplant recipients: A systematic review. Am J Transplant 17: 770–781, 2017 [DOI] [PubMed] [Google Scholar]
- 24.Sam T, Gabardi S, Tichy EM: Risk evaluation and mitigation strategies: A focus on belatacept. Prog Transplant 23: 64–70, 2013 [DOI] [PubMed] [Google Scholar]
- 25.Kotton CN, Fishman JA: Viral infection in the renal transplant recipient. J Am Soc Nephrol 16: 1758–1774, 2005 [DOI] [PubMed] [Google Scholar]
- 26.Burns DM, Crawford DH: Epstein-Barr virus-specific cytotoxic T-lymphocytes for adoptive immunotherapy of post-transplant lymphoproliferative disease. Blood Rev 18: 193–209, 2004 [DOI] [PubMed] [Google Scholar]
- 27.Caillard S, Cellot E, Dantal J, Thaunat O, Provot F, Janbon B, Buchler M, Anglicheau D, Merville P, Lang P, Frimat L, Colosio C, Alamartine E, Kamar N, Heng AE, Durrbach A, Moal V, Rivalan J, Etienne I, Peraldi MN, Moreau A, Moulin B; French PTLD Registry : A French cohort study of kidney retransplantation after post-transplant lymphoproliferative disorders. Clin J Am Soc Nephrol 12: 1663–1670, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kotton CN, Kumar D, Caliendo AM, Huprikar S, Chou S, Danziger-Isakov L, Humar A; The Transplantation Society International CMV Consensus Group : The third international consensus guidelines on the management of cytomegalovirus in solid-organ transplantation. Transplantation 102: 900–931, 2018 [DOI] [PubMed] [Google Scholar]
- 29.Razonable RR, Humar A: Cytomegalovirus in solid organ transplant recipients-Guidelines of the American Society of Transplantation Infectious Disease Community of Practice. Clin Transplant 33: e13512, 2019 [DOI] [PubMed] [Google Scholar]
- 30.Karadkhele G, Hogan J, Magua W, Zhang W, Badell IR, Mehta A, Lyon M, Pastan S, Pearson TC, Larsen CP: CMV high-risk status and posttransplant outcomes in kidney transplant recipients treated with belatacept. Am J Transplant 21: 208–221, 2021 [DOI] [PubMed] [Google Scholar]
- 31.Kumar D, Chin-Hong P, Kayler L, Wojciechowski D, Limaye AP, Osama Gaber A, Ball S, Mehta AK, Cooper M, Blanchard T, MacDougall J, Kotton CN: A prospective multicenter observational study of cell-mediated immunity as a predictor for cytomegalovirus infection in kidney transplant recipients. Am J Transplant 19: 2505–2516, 2019 [DOI] [PubMed] [Google Scholar]
- 32.Arthurs SK, Eid AJ, Pedersen RA, Kremers WK, Cosio FG, Patel R, Razonable RR: Delayed-onset primary cytomegalovirus disease and the risk of allograft failure and mortality after kidney transplantation. Clin Infect Dis 46: 840–846, 2008 [DOI] [PubMed] [Google Scholar]
- 33.Humar A, Lebranchu Y, Vincenti F, Blumberg EA, Punch JD, Limaye AP, Abramowicz D, Jardine AG, Voulgari AT, Ives J, Hauser IA, Peeters P: The efficacy and safety of 200 days valganciclovir cytomegalovirus prophylaxis in high-risk kidney transplant recipients. Am J Transplant 10: 1228–1237, 2010 [DOI] [PubMed] [Google Scholar]
- 34.Santos CA, Brennan DC, Fraser VJ, Olsen MA: Delayed-onset cytomegalovirus disease coded during hospital readmission after kidney transplantation. Transplantation 98: 187–194, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Eid AJ, Arthurs SK, Deziel PJ, Wilhelm MP, Razonable RR: Clinical predictors of relapse after treatment of primary gastrointestinal cytomegalovirus disease in solid organ transplant recipients. Am J Transplant 10: 157–161, 2010 [DOI] [PubMed] [Google Scholar]
- 36.Paya CV, Holley KE, Wiesner RH, Balasubramaniam K, Smith TF, Espy MJ, Ludwig J, Batts KP, Hermans PE, Krom RA: Early diagnosis of cytomegalovirus hepatitis in liver transplant recipients: Role of immunostaining, DNA hybridization and culture of hepatic tissue. Hepatology 12: 119–126, 1990 [DOI] [PubMed] [Google Scholar]
- 37.Lurain NS, Chou S: Antiviral drug resistance of human cytomegalovirus. Clin Microbiol Rev 23: 689–712, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cherrier L, Nasar A, Goodlet KJ, Nailor MD, Tokman S, Chou S: Emergence of letermovir resistance in a lung transplant recipient with ganciclovir-resistant cytomegalovirus infection. Am J Transplant 18: 3060–3064, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hirsch HH, Randhawa PS; American Society of Transplantation Infectious Diseases Community of Practice : BK polyomavirus in solid organ transplantation - Guidelines from the American Society of Transplantation Infectious Disease Community of Practice. Clin Transplant 33: e13528, 2019 [DOI] [PubMed] [Google Scholar]
- 40.Kean JM, Rao S, Wang M, Garcea RL: Seroepidemiology of human polyomaviruses. PLoS Pathog 5: e1000363, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Egli A, Infanti L, Dumoulin A, Buser A, Samaridis J, Stebler C, Gosert R, Hirsch HH: Prevalence of polyomavirus BK and JC infection and replication in 400 healthy blood donors. J Infect Dis 199: 837–846, 2009 [DOI] [PubMed] [Google Scholar]
- 42.Crowhurst T, Nolan J, Faull R, Holmes M, Holmes-Liew CL: BK virus-associated nephropathy in a lung transplant patient: Case report and literature review. BMC Infect Dis 20: 600, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hirsch HH, Knowles W, Dickenmann M, Passweg J, Klimkait T, Mihatsch MJ, Steiger J: Prospective study of polyomavirus type BK replication and nephropathy in renal-transplant recipients. N Engl J Med 347: 488–496, 2002 [DOI] [PubMed] [Google Scholar]
- 44.Mallat SG, Tanios BY, Itani HS, Lotfi T, McMullan C, Gabardi S, Akl EA, Azzi JR: CMV and BKPyV infections in renal transplant recipients receiving an mTOR inhibitor-based regimen versus a CNI-based regimen: A systematic review and meta-analysis of randomized, controlled trials. Clin J Am Soc Nephrol 12: 1321–1336, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schaub S, Hirsch HH, Dickenmann M, Steiger J, Mihatsch MJ, Hopfer H, Mayr M: Reducing immunosuppression preserves allograft function in presumptive and definitive polyomavirus-associated nephropathy. Am J Transplant 10: 2615–2623, 2010 [DOI] [PubMed] [Google Scholar]
- 46.Nickeleit V, Singh HK, Randhawa P, Drachenberg CB, Bhatnagar R, Bracamonte E, Chang A, Chon WJ, Dadhania D, Davis VG, Hopfer H, Mihatsch MJ, Papadimitriou JC, Schaub S, Stokes MB, Tungekar MF, Seshan SV; Banff Working Group on Polyomavirus Nephropathy : The Banff Working Group classification of definitive polyomavirus nephropathy: Morphologic definitions and clinical correlations. J Am Soc Nephrol 29: 680–693, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hardinger KL, Koch MJ, Bohl DJ, Storch GA, Brennan DC: BK-virus and the impact of pre-emptive immunosuppression reduction: 5-year results. Am J Transplant 10: 407–415, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cheungpasitporn W, Kremers WK, Lorenz E, Amer H, Cosio FG, Stegall MD, Gandhi MJ, Schinstock CA: De novo donor-specific antibody following BK nephropathy: The incidence and association with antibody-mediated rejection. Clin Transplant 32: e13194, 2018 [DOI] [PubMed] [Google Scholar]
- 49.Johnston O, Jaswal D, Gill JS, Doucette S, Fergusson DA, Knoll GA: Treatment of polyomavirus infection in kidney transplant recipients: A systematic review. Transplantation 89: 1057–1070, 2010 [DOI] [PubMed] [Google Scholar]
- 50.Schneidewind L, Neumann T, Dräger DL, Kranz J, Hakenberg OW: Leflunomide in the treatment of BK polyomavirus associated nephropathy in kidney transplanted patients - A systematic review. Transplant Rev (Orlando) 34: 100565, 2020 [DOI] [PubMed] [Google Scholar]
- 51.Lee BT, Gabardi S, Grafals M, Hofmann RM, Akalin E, Aljanabi A, Mandelbrot DA, Adey DB, Heher E, Fan PY, Conte S, Dyer-Ward C, Chandraker A: Efficacy of levofloxacin in the treatment of BK viremia: A multicenter, double-blinded, randomized, placebo-controlled trial. Clin J Am Soc Nephrol 9: 583–589, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jahan S, Scuderi C, Francis L, Neller MA, Rehan S, Crooks P, Ambalathingal GR, Smith C, Khanna R, John GT: T-cell adoptive immunotherapy for BK nephropathy in renal transplantation. Transpl Infect Dis 22: e13399, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Leeaphorn N, Thongprayoon C, Chon WJ, Cummings LS, Mao MA, Cheungpasitporn W: Outcomes of kidney retransplantation after graft loss as a result of BK virus nephropathy in the era of newer immunosuppressant agents. Am J Transplant 20: 1334–1340, 2020 [DOI] [PubMed] [Google Scholar]
- 54.Hirsch HH, Ramos E: Retransplantation after polyomavirus-associated nephropathy: Just do it?. Am J Transplant 6: 7–9, 2006 [DOI] [PubMed] [Google Scholar]
- 55.Geetha D, Sozio SM, Ghanta M, Josephson M, Shapiro R, Dadhania D, Hariharan S: Results of repeat renal transplantation after graft loss from BK virus nephropathy. Transplantation 92: 781–786, 2011 [DOI] [PubMed] [Google Scholar]
- 56.Glass RI, Parashar UD, Estes MK: Norovirus gastroenteritis. N Engl J Med 361: 1776–1785, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lee LY, Ison MG: Diarrhea caused by viruses in transplant recipients. Transpl Infect Dis 16: 347–358, 2014 [DOI] [PubMed] [Google Scholar]
- 58.Echenique IA, Penugonda S, Stosor V, Ison MG, Angarone MP: Diagnostic yields in solid organ transplant recipients admitted with diarrhea. Clin Infect Dis 60: 729–737, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Angarone M, Snydman DR; American Society of Transplantation Infectious Diseases Community of Practice : Diagnosis and management of diarrhea in solid-organ transplant recipients: Guidelines from the American Society of Transplantation Infectious Disease Community of Practice. Clin Transplant 33: e13550, 2019 [DOI] [PubMed] [Google Scholar]
- 60.Mullane KM, Dubberke ER; American Society of Transplantation Infectious Diseases Community of Practice : Management of Clostridioides (formerly Clostridium) difficile infection (CDI) in solid organ transplant recipients: Guidelines from the American Society of Transplantation Community of Practice. Clin Transplant 33: e13564, 2019 [DOI] [PubMed] [Google Scholar]
- 61.Cusini A, Béguelin C, Stampf S, Boggian K, Garzoni C, Koller M, Manuel O, Meylan P, Mueller NJ, Hirsch HH, Weisser M, Berger C, van Delden C; Swiss Transplant Cohort Study : Clostridium difficile infection is associated with graft loss in solid organ transplant recipients. Am J Transplant 18: 1745–1754, 2018 [DOI] [PubMed] [Google Scholar]
- 62.Paudel S, Zacharioudakis IM, Zervou FN, Ziakas PD, Mylonakis E: Prevalence of Clostridium difficile infection among solid organ transplant recipients: A meta-analysis of published studies. PLoS One 10: e0124483, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Boutros M, Al-Shaibi M, Chan G, Cantarovich M, Rahme E, Paraskevas S, Deschenes M, Ghali P, Wong P, Fernandez M, Giannetti N, Cecere R, Hassanain M, Chaudhury P, Metrakos P, Tchervenkov J, Barkun JS: Clostridium difficile colitis: Increasing incidence, risk factors, and outcomes in solid organ transplant recipients. Transplantation 93: 1051–1057, 2012 [DOI] [PubMed] [Google Scholar]
- 64.McDonald LC, Gerding DN, Johnson S, Bakken JS, Carroll KC, Coffin SE, Dubberke ER, Garey KW, Gould CV, Kelly C, Loo V, Shaklee Sammons J, Sandora TJ, Wilcox MH: Clinical practice guidelines for Clostridium difficile infection in adults and children: 2017 update by the Infectious Diseases Society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA). Clin Infect Dis 66: 987–994, 2018 [DOI] [PubMed] [Google Scholar]
- 65.Gerding DN, Kelly CP, Rahav G, Lee C, Dubberke ER, Kumar PN, Yacyshyn B, Kao D, Eves K, Ellison MC, Hanson ME, Guris D, Dorr MB: Bezlotoxumab for prevention of recurrent Clostridium difficile infection in patients at increased risk for recurrence. Clin Infect Dis 67: 649–656, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cheng YW, Phelps E, Ganapini V, Khan N, Ouyang F, Xu H, Khanna S, Tariq R, Friedman-Moraco RJ, Woodworth MH, Dhere T, Kraft CS, Kao D, Smith J, Le L, El-Nachef N, Kaur N, Kowsika S, Ehrlich A, Smith M, Safdar N, Misch EA, Allegretti JR, Flynn A, Kassam Z, Sharfuddin A, Vuppalanchi R, Fischer M: Fecal microbiota transplantation for the treatment of recurrent and severe Clostridium difficile infection in solid organ transplant recipients: A multicenter experience. Am J Transplant 19: 501–511, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hollyer I, Ison MG: The challenge of urinary tract infections in renal transplant recipients. Transpl Infect Dis 20: e12828, 2018 [DOI] [PubMed] [Google Scholar]
- 68.Ariza-Heredia EJ, Beam EN, Lesnick TG, Kremers WK, Cosio FG, Razonable RR: Urinary tract infections in kidney transplant recipients: Role of gender, urologic abnormalities, and antimicrobial prophylaxis. Ann Transplant 18: 195–204, 2013 [DOI] [PubMed] [Google Scholar]
- 69.Senger SS, Arslan H, Azap OK, Timurkaynak F, Cağir U, Haberal M: Urinary tract infections in renal transplant recipients. Transplant Proc 39: 1016–1017, 2007 [DOI] [PubMed] [Google Scholar]
- 70.Goldman JD, Julian K: Urinary tract infections in solid organ transplant recipients: Guidelines from the American Society of Transplantation Infectious Disease Community of Practice. Clin Transplant 33: e13507, 2019 [DOI] [PubMed] [Google Scholar]
- 71.Liu S, Luo G, Sun B, Lu J, Zu Q, Yang S, Zhang X, Dong J: Early removal of double-J stents decreases urinary tract infections in living donor renal transplantation: A prospective, randomized clinical trial. Transplant Proc 49: 297–302, 2017 [DOI] [PubMed] [Google Scholar]
- 72.Origüen J, López-Medrano F, Fernández-Ruiz M, Polanco N, Gutiérrez E, González E, Mérida E, Ruiz-Merlo T, Morales-Cartagena A, Pérez-Jacoiste Asín MA, García-Reyne A, San Juan R, Orellana MÁ, Andrés A, Aguado JM: Should asymptomatic bacteriuria be systematically treated in kidney transplant recipients? Results from a randomized controlled trial. Am J Transplant 16: 2943–2953, 2016 [DOI] [PubMed] [Google Scholar]
- 73.Memikoğlu KO, Keven K, Sengül S, Soypaçaci Z, Ertürk S, Erbay B: Urinary tract infections following renal transplantation: A single-center experience. Transplant Proc 39: 3131–3134, 2007 [DOI] [PubMed] [Google Scholar]
- 74.Hollyer I, Varias F, Ho B, Ison MG: Safety and efficacy of methenamine hippurate for the prevention of recurrent urinary tract infections in adult renal transplant recipients: A single center, retrospective study. Transpl Infect Dis 21: e13063, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Quintero Cardona O, Hemmige VS, Puius YA: Methenamine hippurate may have particular benefit in preventing recurrent urinary tract infections in diabetic renal transplant recipients. Transpl Infect Dis 22: e13247, 2020 [DOI] [PubMed] [Google Scholar]
- 76.Naik AS, Dharnidharka VR, Schnitzler MA, Brennan DC, Segev DL, Axelrod D, Xiao H, Kucirka L, Chen J, Lentine KL: Clinical and economic consequences of first-year urinary tract infections, sepsis, and pneumonia in contemporary kidney transplantation practice. Transpl Int 29: 241–252, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Camargo LF, Esteves AB, Ulisses LR, Rivelli GG, Mazzali M: Urinary tract infection in renal transplant recipients: Incidence, risk factors, and impact on graft function. Transplant Proc 46: 1757–1759, 2014 [DOI] [PubMed] [Google Scholar]
- 78.Papasotiriou M, Savvidaki E, Kalliakmani P, Papachristou E, Marangos M, Fokaefs E, Maroulis I, Karavias D, Goumenos DS: Predisposing factors to the development of urinary tract infections in renal transplant recipients and the impact on the long-term graft function. Ren Fail 33: 405–410, 2011 [DOI] [PubMed] [Google Scholar]
- 79.Miller R, Assi M; American Society of Transplantation Infectious Diseases Community of Practice : Endemic fungal infections in solid organ transplant recipients-Guidelines from the American Society of Transplantation Infectious Disease Community of Practice. Clin Transplant 33: e13553, 2019 [DOI] [PubMed] [Google Scholar]
- 80.Pappas PG, Alexander BD, Andes DR, Hadley S, Kauffman CA, Freifeld A, Anaissie EJ, Brumble LM, Herwaldt L, Ito J, Kontoyiannis DP, Lyon GM, Marr KA, Morrison VA, Park BJ, Patterson TF, Perl TM, Oster RA, Schuster MG, Walker R, Walsh TJ, Wannemuehler KA, Chiller TM: Invasive fungal infections among organ transplant recipients: Results of the Transplant-Associated Infection Surveillance Network (TRANSNET). Clin Infect Dis 50: 1101–1111, 2010 [DOI] [PubMed] [Google Scholar]
- 81.Leitheiser S, Harner A, Waller JL, Turrentine J, Baer S, Kheda M, Nahman NS Jr., Colombo RE: Risk factors associated with invasive fungal infections in kidney transplant patients. Am J Med Sci 359: 108–116, 2020 [DOI] [PubMed] [Google Scholar]
- 82.Parajuli S, Wick A, Pandeya S, Astor BC, Smith J, Djamali A, Mandelbrot DA: The feared five fungal infections in kidney transplant recipients: A single-center 20-year experience. Clin Transplant 32: e13289, 2018 [DOI] [PubMed] [Google Scholar]
- 83.Assi M, Martin S, Wheat LJ, Hage C, Freifeld A, Avery R, Baddley JW, Vergidis P, Miller R, Andes D, Young JA, Hammoud K, Huprikar S, McKinsey D, Myint T, Garcia-Diaz J, Esguerra E, Kwak EJ, Morris M, Mullane KM, Prakash V, Burdette SD, Sandid M, Dickter J, Ostrander D, Antoun SA, Kaul DR: Histoplasmosis after solid organ transplant. Clin Infect Dis 57: 1542–1549, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lo MM, Mo JQ, Dixon BP, Czech KA: Disseminated histoplasmosis associated with hemophagocytic lymphohistiocytosis in kidney transplant recipients. Am J Transplant 10: 687–691, 2010 [DOI] [PubMed] [Google Scholar]
- 85.Khalil S, Challener DW, Abu-Saleh O, Sohail MR: Laryngeal histoplasmosis in a kidney transplant recipient. Transpl Infect Dis 21: e13102, 2019 [DOI] [PubMed] [Google Scholar]
- 86.Cuellar-Rodriguez J, Avery RK, Lard M, Budev M, Gordon SM, Shrestha NK, van Duin D, Oethinger M, Mawhorter SD: Histoplasmosis in solid organ transplant recipients: 10 years of experience at a large transplant center in an endemic area. Clin Infect Dis 49: 710–716, 2009 [DOI] [PubMed] [Google Scholar]
- 87.Jorgenson MR, Cardinale B, Descourouez JL, Yang DY, Leverson GE, Parajuli S, Smith JA, Redfield RR: Evaluation of infectious risk and outcomes in the Hmong renal transplant population. Transpl Infect Dis 21: e13142, 2019 [DOI] [PubMed] [Google Scholar]
- 88.Braddy CM, Heilman RL, Blair JE: Coccidioidomycosis after renal transplantation in an endemic area. Am J Transplant 6: 340–345, 2006 [DOI] [PubMed] [Google Scholar]
- 89.Blair JE: Coccidioidal pneumonia, arthritis, and soft-tissue infection after kidney transplantation. Transpl Infect Dis 6: 74–76, 2004 [DOI] [PubMed] [Google Scholar]
- 90.Puing AG, Couture-Cossette A, Wang AX, Zygourakis CC, Cheng X, Stevens BA, Banaei N, Novoa RA, Ho DY, Subramanian AK: Simultaneous coccidioidomycosis and phaeohyphomycosis in a kidney transplant recipient: A case report and literature review. Transpl Infect Dis 22: e13365, 2020 [DOI] [PubMed] [Google Scholar]
- 91.Avery RK, Michaels MG; American Society of Transplantation Infectious Diseases Community of Practice : Strategies for safe living following solid organ transplantation-Guidelines from the American Society of Transplantation Infectious Disease Community of Practice. Clin Transplant 33: e13519, 2019 [DOI] [PubMed] [Google Scholar]
- 92.Buchan CA, Kotton CN; American Society of Transplantation Infectious Diseases Community of Practice : Travel medicine, transplant tourism, and the solid organ transplant recipient-Guidelines from the American Society of Transplantation Infectious Disease Community of Practice. Clin Transplant 33: e13529, 2019 [DOI] [PubMed] [Google Scholar]
- 93.Danziger-Isakov L, Kumar D;American Society of Transplantation Infectious Diseases Community of Practice: Vaccination of solid organ transplant candidates and recipients: Guidelines from the American Society of Transplantation Infectious Disease Community of Practice. Clin Transplant 33: e13563, 2019 [DOI] [PubMed] [Google Scholar]
- 94.Rubin LG, Levin MJ, Ljungman P, Davies EG, Avery R, Tomblyn M, Bousvaros A, Dhanireddy S, Sung L, Keyserling H, Kang I; Infectious Diseases Society of America : 2013 IDSA clinical practice guideline for vaccination of the immunocompromised host [published correction appears in Clin Infect Dis 59: 144, 2014]. Clin Infect Dis 58: 309–318, 2014 [DOI] [PubMed] [Google Scholar]