Abstract
Background
SARS-CoV-2 infected patients present thrombotic complications caused by direct endothelial cells injury of the microvessels. Pulmonary thromboembolism (PE) has been reported by Computed Tomography pulmonary angiogram (CTPA) in patients with COVID-19 pneumonia with high D-dimer levels.
Objectives
We present the characteristics of SARS-CoV-2 infected patients diagnosed of PE by CTPA in our hospital. We also present the comparison of these findings with non-infected patients with PE data.
Methods
Retrospective observational cohort study that included patients over 18 years of age hospitalised consecutively between 26th February and 20th May 2020 in an European Hospital with SARS-CoV2 virus infection, and with suspected infection at beginning of admission but with negative PCR, who were studied with CTPA for suspicion of VTE, during their hospitalization.
Results
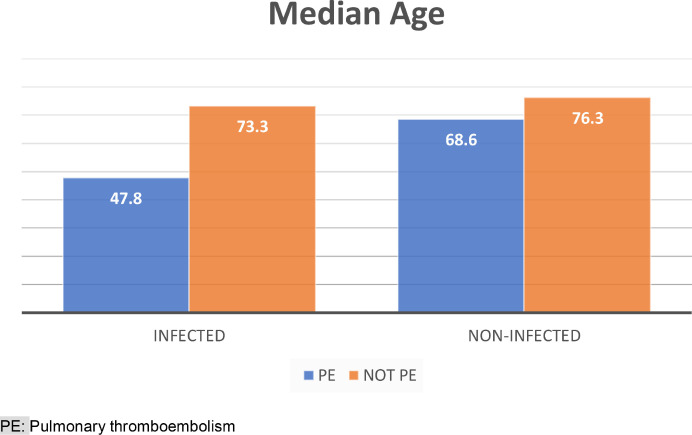
During the study period, 52 CTPA were performed in our hospital, sixteen in SARS-CoV-2 infected patients, with 4 cases (33%) of PE in the infected group, and 11 (44%) in the non-infected group. No significant differences in age (p = 0.43) and sex (p = 0.31) were found between the two groups, infected and non-infected patients. In the infected group, the patients who had PE had a much lower median age (47.8 years) than those without PE (73.3 years). No differences between infected and non-infected patients were detected in the diagnosis of PE with CTPA, 28.6% versus 27.8% (p = 1.00). Overall patient mortality was 1.9%; one patient died (6.3%) in the infected group, and none in the non-infected group (p = 0.31).
Conclusion
A considerable incidence of PE diagnosed by CTPA in SARS-CoV-2 infected patients has been observed, despite thrombo-prophylaxis.
Keywords: Coronavirus, COVID-19, SARS-CoV-2, Coagulation, Thrombosis, Thromboembolism
Introduction
The outbreak of the COVID-19 pandemic, caused by the novel coronavirus Severe Acute Respiratory Syndrome-CoronaVirus-2 (SARS-CoV-2), in the city of Wuhan, Hubei province of China, was declared by the World Health Organization (WHO) on March 21st. Thrombotic complications are an important issue in patients infected with COVID-19. Pulmonary thromboembolism (PE) has been reported by computed tomography pulmonary angiogram (CTPA) in patients with COVID-19 pneumonia with high D-dimer levels1 and in COVID-19 patient with normal D-dimer level, without strong predisposing risk factors for venous thrombo-embolism (VTE).2
SARS-CoV-2 binds Angiotensin-converting enzyme 2 receptors through the viral surface spike protein and gain entry to cells. This protein, higher in individuals with cardiovascular disease, is linked by plasmin, and potentially making them more susceptible to worse outcomes.3 This pattern of prothrombotic coagulopathy is different from what noticed in sepsis, where thrombocyte count is usually decreased, and of disseminated intravascular coagulation, where the exhausted coagulation system shows a prolongation of the prothrombin time and aPTT (activated Partial Thromboplastin Time), and a haemorrhagic tendency.4 SARS-CoV-2 infection is suspected of producing deregulation of the coagulation system, with formation of intra-alveolar or systemic fibrin clots, associated with severe respiratory disease.5 SARS-CoV-2 produces direct endothelial cells injury of the microvessels and then releases these damaged endothelial cells into the circulation.6 PE has been reported in patients suffering from SARS-CoV-2 infection (2). Moreover, microthrombosis of small pulmonary vessels has been observed in the postmortem inspection of lungs.7 Immobilization due to prolonged intensive care unit (ICU) admission in severely infected patients as well as a hypercoagulable state caused by SARS-CoV-2, may be relevant factors.
Consequently, PE may be considered in SARS-CoV-2 infected patients with abrupt onset of oxygenation desaturation, respiratory distress, and decreased blood pressure.8 This possibility might be suspected also by elevated D-dimer values, even though D-dimer is a non-specific acute phase reactant. However, other authors argued that there is no evidence for use of biomarkers such as D-dimer to guide intensification of anticoagulant dosing despite it being a marker of poor prognosis.9
In a recent study investigating the prognostic factors of 28-day mortality of severely SARS-CoV-2 infected patients, the use of anticoagulant therapy for at least seven days, resulted in lower mortality in patients with D-dimer over six fold the upper limit of normal (Low Molecular Weight Heparin (LMWH): 32.8% vs No-LMWH: 52.4%, P = 0.017), but without overall benefit for patients on LMWH.7 A recent study examined two groups of patients: those with COVID-19 and those without COVID-19. The COVID-19 group showed lower mortality rates with heparin administration (LMWH (40–60 mg enoxaparin/day) or unfractionated heparin (UFH) (10,000–15,000 U/day)) than those without heparin. Interestingly, there was no difference in mortality in the COVID-19 negative patients with the use of heparin.10
Our goal is to analyze the characteristics of SARS-CoV-2 infected patients diagnosed of PE by CTPA in our hospital.
Methods
Study design
Retrospective observational cohort study that included patients 18 years of age or older with SARS-CoV2 virus infection confirmed by diagnostic test of reverse transcription of polymerase chain reaction (RT-qPCR) in a sample of nasopharyngeal or sputum aspirate, and patients with suspected infection at beginning of admission but with negative PCR, who were studied with CTPA for suspicion of VTE, during their ward admission to an European Hospital from February 26th- to May 20th of 2020.
The study protocol was approved definitively on 25th May 2020 by the Hospital Drug Research Ethics Committee. An exemption from requesting the written informed consent of patients was obtained, due to the retrospective observational study design, in which the difficulty of obtaining patient consent would have compromised the realization of the study.
The personal data of study participants was handled in compliance with current European legislation, ensuring its confidentiality. This data was dissociated and pseudo-anonymised in the database for subsequent statistical analysis by an independent expert.
Patients who denied consent for CTPA, and pregnant or breastfeeding women were excluded from the study. Patients who were re-admitted at the hospital for any reason were also excluded, and only the first episode of hospitalization was considered.Our routine protocol for patients with severe clinical features of COVID-19 infection was multidetector pulmonary CT angiography using 16 slice multi-detector CT (GE Healthcare, Milwaukee, WI) after intravenous injection of 60 ml iodinated contrast agent (Iohexol 350 mg/mL, GE Healthcare, Milwaukee, WI) at a flow rate of 3.5 mL/s, triggered on the main pulmonary artery.
Variables and demographic data
Demographic data, comorbidities, clinical symptoms, laboratory results, radiological tests and treatment of each patient were evaluated. A data collection sheet was created in Microsoft Excel where all variables were automatically collected. The instruments used to measure the variables were previously and constantly validated because they were used in routine clinical practice.
Statistics
Statistical analysis and processing of the data was carried out using the SPSS version 19 statistical package (IBM Corp. Released 2010. IBM SPSS Statistics for Windows, Version 19.0. Armonk, NY: IBM Corp.). Categorical variables are represented as absolute frequencies and percentages, and quantitative variables are expressed by median and Interquartile Range (IQR) calculated by Tukey's Hinges method. The difference analysis used student's T test for variables or the Mann-Whitney test if they have normal distribution or not respectively. To contrast the categorical variables, the test of chi-squared test (χ2). The statistical significance level adopted for all contrast tests was p < 0.05.
Results
During the study period, 52 CTPA were performed in our hospital, sixteen in SARS-CoV-2 infected patients with positive RT-qPCR test. The median age of all patients included in the study was 70.1 years (Interquartile range (IQR): 55.7 to 83.3) and 27 (51.9%) were male. No significant differences in age (p = 0.43) and sex (p = 0.31) were found between the two groups, infected and non-infected patients. However, in the infected group, the patients who had PE had a much lower median age (47.8 years) than those without PE (73.3 years) (see Fig. 1 ). Table 1 shows the demographic and comorbidities of the patients. The most common comorbidities were hypertension (71.2%), hyperlipidaemia (48.1%), diabetes (34.6%) and obesity (36.5%). In the non-infected group, congestive heart failure (27.8%, p = 0.02) and previous treatment with anticoagulants (22.2%, p = 0.04) were more significantly frequent than in the infected group.
Fig. 1.
Differences in age between infected and no-infected grouped by presence of PE
PE: Pulmonary thromboembolism.
Table 1.
Demographic characteristics and comorbidities of the patients stratified by negative or positive infection.
| N (%) | TOTAL (n = 52) | Infected Group (n = 16) |
Non-infected Group (n = 36) |
||
|---|---|---|---|---|---|
| PE (n = 4) | Not PE (n = 12) | PE (n = 11) | Not PE (n = 25) | ||
| Age in years, median (range) | 70.1 (55.7–83.3) | 47.8 (43.6–63.4) | 73.3 (57.5–84.0) | 68.6 (59.9–77.2) | 76.3 (62.3–83.1) |
| Sex Male Female |
27 (51.9) 25 (48.1) |
1 (25.0) 3 (75.0) |
5 (41.7) 7 (58.3) |
5 (45.5) 6 (54.5) |
14 (51.9) 13 (48.1) |
| Hypertension | 37 (71.2) | 2 (50.0) | 9 (75.0) | 7 (63.6) | 21 (77.8) |
| Diabetes | 18 (34.6) | 1 (25.0) | 3 (25.0) | 4 (36.4) | 11 (40.7) |
| Coronary heart disease | 8 (15.4) | 0(0.0) | 2 (16.7) | 2 (18.2) | 4 (14.8) |
| Chronic kidney disease | 10 (19.2) | 0 (0.0) | 2 (16.7) | 2 (18.2) | 6 (22.2) |
| COPD | 4 (7.7) | 1 (25.0) | 0(0.0) | 1 (9.1) | 2 (7.4) |
| Asthma | 5 (9.6) | 0 (0.0) | 1 (8.3) | 2 (18.2) | 2 (7.4) |
| Other Chronic Lung Diseases | 8 (15.4) | 0 (0.0) | 1 (8.3) | 1 (9.1) | 6 (22.2) |
| Congestive heart failure | 10 (19.2) | – | – | 1 (9.1) | 9 (33.3) |
| Malignancy tumor | 12 (23.1) | 1 (25.0) | 2 (16.7) | 2 (18.2) | 7 (25.9) |
| Cardiovascular disease | 17 (32.7) | 0 (0.0) | 3 (25.0) | 2 (18.2) | 12 (44.4) |
| Cerebrovascular disease | 4 (7.7) | 0 (0.0) | 1 (8.3) | 0 (0.0) | 3 (11.1) |
| Hyperlipidemia | 25 (48.1) | 1 (25.0) | 5 (41.7) | 5 (45.5) | 15 (55.6) |
| Smoking History: Never smoker Current smoker Former smoker |
34 (65.4) 9 (17.3) 9 (17.3) |
2 (50.0) 1 (25.0) 1 (25.0) |
6 (50.0.) 3 (25.0) 3 (25.0) |
7 (63.6) 1 (9.1) 3 (27.3) |
20 (74.1) 4 (14.8) 3 (11.1) |
| Obesity (IMC≥30 Kg/m2) | 19 (36.5) | 2 (50.0) | 3 (25.0) | 6 (54.5) | 9 (33.3) |
| Dementia | 3 (5.8) | 0 (0.0) | 1 (8.3) | 1 (9.1) | 1 (3.7) |
| Anticoagulant treatment | 8 (15.4) | – | – | 1 (9.1) | 8 (29.6) |
| ACEI or ARB | 29 (55.8) | 3 (75.0) | 8 (66.7) | 5 (45.5) | 13 (48.1) |
*ACE: Angiotensin-converting enzyme inhibitors, ARB: Angiotensin II receptor-blockers, PE: Pulmonary thromboembolism.
The median of total comorbidities was 2.5 (IQR:1.0–3.5) and 4.0 (IQR: 2.0 to 6.0) for infected and non-infected patients, respectively (p = 0.07), and for all the patients was 3 (IQR: 2.0–5.5). According to the Charlson Comorbidity Index abbreviated, 53.8% of the patients (n = 28) do not have comorbidities.
The most common symptoms were fever, dyspnea, malaise, and cough (see Table 2 ). Fever was more common in the infected group with statistically significant difference (75.0% versus 41.7%, p = 0.03).
Table 2.
Signs and symptoms of the patients on admission.
| N (%) | TOTAL (n = 52) | Infected Group (n = 16) |
Non-infected Group (n = 36) |
||
|---|---|---|---|---|---|
| PE (n = 4) | Not PE (n = 12) | PE (n = 11) | Not PE (n = 25) | ||
| Fever | 27 (51.9) | 3 (75.0) | 9 (75.0) | 4 (36.4) | 11 (40.7) |
| Dyspnea | 28 (53.8) | 2 (50.0) | 6 (50.0) | 5 (45.5) | 15 (55.6) |
| Cough | 18 (34.6) | 2 (50.0) | 4 (33.3) | 4 (36.4) | 9 (33.3) |
| Expectoration | 5 (9.6) | 0 (0.0) | 1 (8.3) | 1 (9.1) | 4 (14.8) |
| Sore throat | 3 (5.8) | – | – | 0 (0.0) | 3 (11.1) |
| Myalgia | 9 (17.3) | 1 (25.0) | 2 (16.7) | 4 (36.4) | 3 (11.1) |
| Headache | 2 (3.8) | – | – | 1 (9.1) | 1 (3.7) |
| Dizziness | 6 (11.5) | 0 (0.0) | 2 (16.7) | 0 (0.0) | 4 (14.8) |
| Diarrhea | 7 (13.5) | 1 (25.0) | 2 (16.7) | 0 (0.0) | 4 (14.8) |
| Chest pain | 6 (11.5) | 0 (0.0) | 1 (8.3) | 1 (9.1) | 4 (14.8) |
| Malaise | 21 (40.4) | 2 (50.0) | 7 (58.3) | 3 (27.3) | 9 (33.3) |
| Anosmia | 2 (3.8) | 1 (25.0) | 1 (8.3) | – | – |
| Dysgeusia | 3 (5.8) | 1 (25.0) | 1 (8.3) | 0 (0.0) | 1 (3.7) |
*PE: Pulmonary thromboembolism.
Abnormal chest X-ray was observed in most patients at admission. Radiological findings are showed in Table 3 . Chest CT scan was significantly different between infected and non-infected patients (p < 0.01). The most common abnormality was ground-glass opacities in both groups, being more frequent in the infected group than in the non-infected (50% vs 11%). See Fig. 2 . The patients with COVID-19 pneumonia in the infected group were 13/16 (81,25%). No differences between infected and non-infected patients were detected in the diagnosis of PE with CTPA, 28.6% versus 27.8% (p = 1.00). Bilateral affectation (p = 0.01), and the parenchymal lesion p < 0.01) were significantly more frequent in the infected group (The median of hospitalization days before CTPA was performed, was 3.5 days (IQR: 1.0–9.0); being 2 (IQR: 0.5–6.0) and 9.5 (IQR: 3.5–13.0) for the non-infected group and infected group, respectively.
Table 3.
Radiological findings.
| N (%) | TOTAL (N = 52) | Infected Group (n = 16) |
Non-infected Group (n = 36) |
||
|---|---|---|---|---|---|
| PE (n = 4) | Not PE (n = 12) | PE (n = 11) | Not PE (n = 25) | ||
| Chest X-ray | |||||
| Normal | 16 (30.8) | 2 (50.0) | 1 (8.3) | 7 (63.6) | 9 (33.4) |
| Opacities | 12 (23.1) | 1 (25.0) | 7 (58.3) | 1 (9.1) | 3 (11.1) |
| Interstitial pattern | 9 (17.3) | 1 (25.0) | 4 (33.3) | 1 (9.1) | 3 (11.1) |
| No COVID Injuries | 14 (26.9) | 0 (0.0) | 0 (0.0) | 2 (18.2) | 12 (44.4) |
| CTPA | |||||
| Bilateralism | 32 (61.5) | 4 (100.0) | 10 (83.3) | 10 (90.9) | 9 (33.3) |
| Parenchymal injury | 14 (26.9) | 2 (50.0) | 7 (58.3) | 3 (27.3) | 2 (7.4) |
| Pleural effusion | 19 (36.5) | 0 (0.0) | 5 (41.7) | 0 (0.0) | 14 (51.9) |
| Pericardial effusion | 4 (7.7) | 0 (0.0) | 1 (8.3) | 0 (0.0) | 3 (11.1) |
| Metastasis | 3 (5.8) | 0 (0.0) | 1 (8.3) | 1 (9.1) | 1 (3.7) |
PE: Pulmonary thromboembolism.
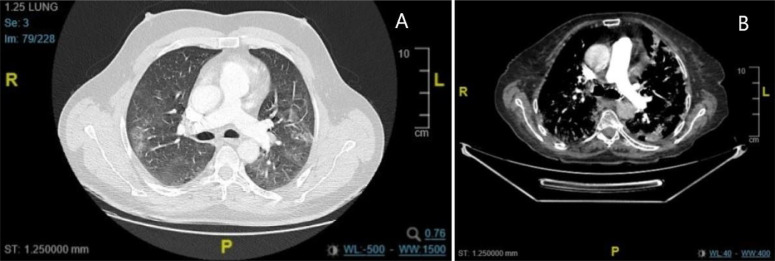
Fig. 2.
CT images of infected patients with PE (A) and without PE (B).
Table 4 shows the laboratory findings before CTPA. The most relevant laboratory findings were lymphopenia, lengthening of the prothrombin time, elevated LDH, D-dimer and ferritin. This table also shows the levels of D-dimer categorized by groups and result of PE. Among the patients who had PE, statistically significant differences were found in the levels of total CK (p = 0.01) and troponin T (p = 0.02), being higher in the infected group compared to the non-infected.
Table 4.
Laboratory results before CTPA.
| Laboratory results, median(IQR) | TOTAL(N = 52) | Infected Group(n = 16) |
Non-infected Group(n = 36) |
Reference ranges | ||
|---|---|---|---|---|---|---|
| PE(n = 4) | Not PE(n = 12) | PE(n = 11) | Not PE(n = 25) | |||
| White blood cell count, x 109/L | 7.6 (5.9–9.8) | 7.7 (6.3–9.1) | 6.7 (4.3–8.4) | 9.0 (6.8–12.1) | 7.7 (5.5–9.9) | 4.8–11 |
| Hemoglobin, g/dL | 12.7 (11.1–14.3) | 11.8 (10.9–12.6) | 12.7 (12.6–14.7) | 12.9 (10.4–14.1) | 12.9 (11.0–14.2) | 12–18 |
| Neutrophil coun,t x 109/L | 5.4 (3.3–8.0) | 5.3 (4..2–6.3) | 4.9 (1.7–5.4) | 7..2 (5.4–9.2) | 5.5 (3.1–8.0) | 1.9–8 |
| Lymphocyte count, x 109/L | 1.30 (0.95–1.65) | 1.3 (1.0–1.6) | 1.4 (1.0–1.7) | 1.0 (0.7–1.8) | 1.3 (1.0–1.6) | 0,9–4,5 |
| Platelet count, x 109/L | 204 (160–2551) | 178 (152–204) | 263 (225–276) | 199 (174–3121) | 198 (146–222) | 130–400 |
| D-dimer, μg/mL | 2323.5 (1083.0–7301.5) | 5824.5 (5120.0–6523.0) | 1573.0 (1041.0–5873.0) | 5078.5 (2258.0–12,721.0) | 1731.0 (1007.5–2494.5) | 0–500 |
| Prothrombin time, s | 12.5 (11.6–13.3) | 12.7 (12.0–13.4) | 11.7 (11.4–13.2) | 12.5 (11.2–12.9) | 13.0 (12.1–13.4) | 9–13 |
| Glucose, mg/dL | 118 (94–145) | 112 (109–115) | 102 (92–124) | 139 (118–181) | 105 (92–141) | 82–115 |
| Creatinine, mg/dL | 0.91 (0.76–1.10) | 1.40 (1.08–1.72) | 0.81 (0.68–0.87) | 0.90 (0.70–0.99) | 0.94 (0.83–1.15) | 0.7–1.2 |
| Total bilirubin, mg/dL | 0.40 (0.28–0.55) | 0.34 (0.20–0.48) | 0.47 (0.31–0.60) | 0.29 (0.22–0.45) | 0.41 (0.36–0.59) | 0.1–1.2 |
| Sodium, mmol/L | 140.2 (138.0–142.6) | 143.5 (142.2–144.8) | 142.0 (140.0–143.0) | 139.4 (138.5–142.6) | 139.7 (136.1–142.0) | 136–146 |
| Potassium, mmol/L | 4.52 (4.08–4.80) | 5.62 (4.61–6.62) | 4.40 (4.09–4.67) | 4.27 (4.01–4.47) | 4.57 (4.23–4.91) | 3.5–5.1 |
| Alanine aminotransferase (GPT), U/L |
21.5 (12.8–38.4) | 9.0 (8.3–9.6) | 36.3 (23.7–73.1) | 22.3 (17.2–28.3) | 18.7 (9.9–37.9) | 5–41 |
| Lactate dehydrogenase, U/L | 223 (199–262) | 273 (197–348) | 231 (204–260) | 223 (208–237) | 231 (164–276) | 135–225 |
| CK total, U/L | 61.0 (4.5–91.5) | 135.5 (102.0–169.0) | 61.0 (45.0–78.5) | 87 (33–99) | 50 (29–66) | 24–192 |
| C- reactive protein, mg/dL | 2.00 (0.85–5.03) | 1.87 (0.62–3.11) | 1.04 (0.45–4.95) | 2.77 (1.28–4.02) | 2.06 (1.17–5.59) | 0,1–1 |
| Procalcitonin, ng/mL | 0.068 (0.048–0.124) | 0.079 (0.073–0.084) | 0.048 (0.048–0.068) | 0.062 (0.058–0.131) | 0.081 (0.048–0.124) | 0–0.5 |
| Ferritin, ng/mL | 269.1 (179.4–955.6) | 128.1 (36.3–219.8) | 735.4 (185.4–1170.4) | 288.3 (221.5–682.7) | 269.1 (174.3–1057–9) | 30–400 |
| Troponine T, pg/mL | 15.18 (8.98–33.22) | 71.14 (50.87–91.40) | 6.37 (5.13–8.75) | 24.53 (11.77–46.18) | 14.77 (10.76–27.04) | <14 |
PE: Pulmonary thromboembolism.
Fifty patients (96.2%) were treated with LMWH. Thirty-two patients (61.5%) received subcutaneous heparin at prophylactic doses, and 20 patients (38.5%) therapeutic doses. In the infected group, the use of heparin at prophylactic doses was more common than in the non-infected group (p = 0.01). The overall median duration of treatment with LMWH was 11 days (IQR: 6.5–17.0).
Overall patient mortality was 1.9%; one patient died (6.3%) in the infected group, and none in the non-infected group (p = 0.31).
Discussion
We present our case series of patients with and without SARS-CoV-2 infection, who were studied by CTPA on suspicion of pulmonary thromboembolism (PE). We have to take in account that perfusion defects among COVID19 victims are not limited to PE.11
There have been several groups that have published their results studying SARS-CoV-2 infected patients with a suspicion of pulmonary embolus. Leonard-Lorant et al. observed positive CTPA angiography for PE in thirty-two of 106 (30% [95%CI: 22–40%]),12 with higher D-dimer levels than those without pulmonary embolus. They also observed that D-dimer greater than 2660 μg/L had a sensitivity of 32/32 (100%, 95%CI: 88–100) and a specificity of 49/74 (67%, 95% CI: 52–79) for pulmonary embolism on CTPA. Stoneham et al. diagnosed 21 out of 274 (7.7%) patients with VTE, 16 with PE (5.83%).13 They found significance differences in levels of D-dimer and white cell count but not in age, gender, or presence of comorbidities between PE patients and control group. Klok et al. detected 25 symptomatic VTE events in 184 adult patients admitted in three ICU across the Netherlands.14 A similar study of 150 critical patients from four ICUs in France, found 16.7% of patients with PE despite the anticoagulant therapy.15 In the study of Middeldorp et al. 39 patients (19.6%) were diagnosed with VTE, despite routine thrombosis prophylaxis.16 Lodigiani et al. detected PE in 10 (33%) of CTPA performed.17 Grillet et al. studied one hundred patients with severe respiratory COVID disease and/or comorbidities by CTPA.18 Twenty-three (23%, [95% CI: 15–33%]) patients had acute PE. Compared with patients without PE, these patients were admitted in ICU and required mechanical ventilation more frequently.
We have detected PE in 28.6% of infected patients who were studied with CTPA. All these studies revealed the importance of early onset of anticoagulant therapy. In fact, chronic anticoagulation therapy at admission has been associated with a lower risk of the composite outcome (Hazard Ratio [HR] 0.29, 95%CI: 0.091–0.92), and patients diagnosed with thrombotic complications are at higher risk of all-cause death (HR 5.4; 95%CI: 2.4–12).19 Some authors have highlighted the possibility that some individuals infected with COVID-19 may be refractory to treatment with low molecular weight heparin, and it has been studied the use of tissue plasminogen activator in these cases.20
The main clinical symptoms of COVID-19 patients in the study of Li et al., similar as our results, were fever (88.5%), cough (68.6%), myalgia or fatigue (35.8%), expectoration (28.2%), and dyspnea (21.9%).21
We have not seen differences in mortality between both groups. However, patients with COVID-19 infection, with a high risk of venous thromboembolism, had poorer outcomes than patients with a low risk, suggesting that these patients might require increased attention in case of rapid deterioration.22 Global mortality rates of SARS-CoV-2 infection has been established in 5.7%,23 while our overall mortality was 1.9%.
The statistically significant differences detected in infected and non-infected PE patients in total CK and troponin T levels should lead us to consider the presence of myocardial damage caused by SARS-CoV-2, which is not found in uninfected PE patients.
Based on our data and experience, LMWH therapy should be considered, with at least prophylactic dose, in all patients with COVID-19 pneumonia for a longer period than required for clinical resolution of the disease, considering the high risk of thromboembolic complications of SARS-CoV-2 infection. Additionally, CTPA is the cornerstone in both the diagnostic workup and follow-up of SARS-CoV-2 infection (Fig. 2).
The main limitations of our study are the small sample size, and the lack of generalized study with CTPA in all the admitted COVID patients at hospital. It is possible that our incidence of PE, as in other studies, is probably highly underestimated due to the paucity of CTPA performed for PE during hospitalization, and most of the studies included only critical patients in Intensive Care Unit (ICU). In fact, the risk for VTE in COVID-19 patients is high, particularly in ICU patients. We therefore cannot establish a realistic prevalence for PE in all our COVID patients. Another limitation is the possible bias of patient selection by incorporating only the most severe or expected diagnosis of PE results, but this has been attempted to control by consecutive incorporation of all CTPA performed.
A considerable incidence of PE diagnosed by CTPA in SARS-CoV-2 infected patients in our hospital has been observed, despite thrombo-prophylaxis. The infected patients with PE should be examined for assessing myocardial damage.
Ethical approval
The local ethics committee approved this study (approved May 25, 2020) and waived the need of informed consent. Personal data were dissociated and pseudoanonymized in the database for further statistical analysis by an independent expert.
Funding
The authors do not declare any funding sources for this article.
Availability of data and materials
Data and material cannot be showed.
CRediT authorship contribution statement
D. El-Qutob: Data curation, Formal analysis, Writing – review & editing. L. Alvarez-Arroyo: Visualization, Data curation, Formal analysis, Writing – review & editing. I. Barreda: Writing – review & editing. M. Nieto: Visualization, Data curation, Formal analysis, Writing – review & editing. M. Pin: Writing – review & editing. José Luis Poveda-Andrés: Writing – review & editing. F.J. Carrera-Hueso: Data curation, Formal analysis, Writing – review & editing.
Declaration of Competing Interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Acknowledgment
This paper is part of thesis doctoral of Laura Álvarez in University of Granada (Spain), Doctoral Pharmacy Program at Pharmacy Department.
A sincere thank you to Juan Pablo Fernandez for his diligent proofreading of this paper.
References
- 1.Darzi A.J., Karam S.G., Charide R., et al. Prognostic factors for VTE and bleeding in hospitalized medical patients: a systematic review and meta-analysis. Blood. 2020;135(20):1788–1810. doi: 10.1182/blood.2019003603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anderson D.R., Morgano G.P., Bennett C., et al. American society of hematology 2019 guidelines for management of venous thromboembolism: prevention of venous thromboembolism in surgical hospitalized patients. Blood Adv. 2019;3(23):3898–3944. doi: 10.1182/bloodadvances.2019000975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Khan I.H., Savarimuthu S., Tsun Leung M.S., Harky A. The need to manage the risk of thromboembolism in COVID-19 patients. J. Vasc. Surg. 2020;72(3):799–804. doi: 10.1016/j.jvs.2020.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ranucci M., Ballotta A., Di Dedda U., et al. The procoagulant pattern of patients with COVID-19 acute respiratory distress syndrome. J. Thromb. Haemost. JTH. 2020;18(7):1747–1751. doi: 10.1111/jth.14854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Giannis D., Ziogas I.A., Gianni P. Coagulation disorders in coronavirus infected patients: COVID-19, SARS-CoV-1, MERS-CoV and lessons from the past. J. Clin. Virol. Off. Publ. Pan Am. Soc. Clin. Virol. 2020;127 doi: 10.1016/j.jcv.2020.104362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.An P., Ye Y., Chen M., Chen Y., Fan W., Wang Y. Management strategy of novel coronavirus (COVID-19) pneumonia in the radiology department: a Chinese experience. Diagn. Interv. Radiol. 2020;26(3):200–203. doi: 10.5152/dir.2020.20167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tang N., Bai H., Chen X., Gong J., Li D., Sun Z. Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. J. Thromb. Haemost. 2020;18(5):1094–1099. doi: 10.1111/jth.14817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Scialpi M., Scialpi S., Piscioli I., Battista Scalera G., Longo F. Pulmonary thromboembolism in critical ill COVID-19 patients. Int. J. Infect. Dis. IJID. 2020;95:361–362. doi: 10.1016/j.ijid.2020.04.056. official publication of the International Society for Infectious Diseases. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barnes G.D., Burnett A., Allen A., et al. Thromboembolism and anticoagulant therapy during the COVID-19 pandemic: interim clinical guidance from the anticoagulation forum. J. Thromb. Thrombolysis. 2020;50(1):1–10. doi: 10.1007/s11239-020-02138-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yin S., Huang M., Li D., Tang N. Difference of coagulation features between severe pneumonia induced by SARS-CoV2 and non-SARS-CoV2. J. Thromb. Thrombolysis. 2020;51(4):1–4. doi: 10.1007/s11239-020-02105-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Idilman I.S., Telli Dizman G., Ardali Duzgun S., et al. Lung and kidney perfusion deficits diagnosed by dual-energy computed tomography in patients with COVID-19-related systemic microangiopathy. Eur. Radiol. 2021;31(2):1090–1099. doi: 10.1007/s00330-020-07155-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Leonard-Lorant I., Delabranche X., Severac F., et al. Acute pulmonary embolism in COVID-19 patients on CT angiography and relationship to D-dimer levels. Radiology. 2020;296(3) doi: 10.1148/radiol.2020201561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stoneham S.M., Milne K.M., Nuttal E., et al. Thrombotic risk in COVID-19: a case series and case-control study. Clin. Med. 2020;20(4):e76–e81. doi: 10.7861/clinmed.2020-0228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Klok F.A., Kruip M., van der Meer N.J.M., et al. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb. Res. 2020;91:145–147. doi: 10.1016/j.thromres.2020.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Helms J., Tacquard C., Severac F., et al. High risk of thrombosis in patients with severe SARS-CoV-2 infection: a multicenter prospective cohort study. Intensive Care Med. 2020;46(6):1089–1098. doi: 10.1007/s00134-020-06062-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Middeldorp S., Coppens M., van Haaps T.F., et al. Incidence of venous thromboembolism in hospitalized patients with COVID-19. J. Thromb. Haemost. JTH. 2020;18(8):1995–2002. doi: 10.1111/jth.14888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lodigiani C., Iapichino G., Carenzo L., et al. Venous and arterial thromboembolic complications in COVID-19 patients admitted to an academic hospital in Milan, Italy. Thromb. Res. 2020;191:9–14. doi: 10.1016/j.thromres.2020.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grillet F., Behr J., Calame P., Aubry S., Delabrousse E. Acute pulmonary embolism associated with COVID-19 pneumonia detected by pulmonary CT angiography. Radiology. 2020;296(3) doi: 10.1148/radiol.2020201544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Klok F.A., Kruip M., van der Meer N.J.M., et al. Confirmation of the high cumulative incidence of thrombotic complications in critically ill ICU patients with COVID-19: an updated analysis. Thromb. Res. 2020;191:148–150. doi: 10.1016/j.thromres.2020.04.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang J., Hajizadeh N., Moore E.E., et al. Tissue plasminogen activator (tPA) treatment for COVID-19 associated acute respiratory distress syndrome (ARDS): a case series. J. Thromb. Haemost. JTH. 2020;18(7):1752–1755. doi: 10.1111/jth.14828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li L.Q., Huang T., Wang Y.Q., et al. COVID-19 patients' clinical characteristics, discharge rate, and fatality rate of meta-analysis. J. Med. Virol. 2020;92(6):577–583. doi: 10.1002/jmv.25757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang T., Chen R., Liu C., et al. Attention should be paid to venous thromboembolism prophylaxis in the management of COVID-19. Lancet Haematol. 2020;7(5):e362–e363. doi: 10.1016/S2352-3026(20)30109-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Baud D., Qi X., Nielsen-Saines K., Musso D., Pomar L., Favre G. Lancet Infect. Dis. 7th. Vol. 20. 773; 2020. Real estimates of mortality following COVID-19 infection. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data and material cannot be showed.