Abstract
It is unclear whether SARS-CoV-2 VOCs differentially escape Fc effector functions of antibodies in addition to neutralization. In this issue of Cell Reports Medicine, Richardson et al.1 show that VOCs differ both in their ability to evade as well as elicit cross-reactive Fc-effector functions.
It is unclear whether SARS-CoV-2 VOCs differentially escape Fc effector functions of antibodies in addition to neutralization. In this issue of Cell Reports Medicine, Richardson et al. show that VOCs differ both in their ability to evade as well as elicit cross-reactive Fc-effector functions.
Main text
Like most pathogens, SARS-CoV-2 infection triggers host innate, humoral, and cell-mediated immune responses that can contribute to protection. SARS-CoV-2 neutralizing antibodies (nAbs) have taken the centerstage in defining protective immunity as they can directly prevent virus from entering susceptible cells. In the face of nAb pressure, SARS-CoV-2 evolves to robustly escape neutralization resulting in variants of concern (VOCs) like Alpha, Beta, Delta, and Omicron. Reductions in nAb titers increase the risk of symptomatic SARS-CoV-2 infection.2 However, immunity conferred by natural infection and vaccination has remained efficacious against severe COVID-19 illness despite a significant drop in neutralizing titers over time.3 Moreover, mRNA immunization protects from infection before nAbs are induced.4 These data argue for additional correlates of protection. T cell-mediated immunity5 certainly remains at play, but one cannot discount the additional modes of antibody-mediated protection. Antibodies complexed with target antigen can use their Fc domain to mediate effector functions through interactions with Fc receptors (FcRs) expressed on effector leukocytes. FcR engagement on natural killer cells, neutrophils, and monocytes facilitates pleiotropic functions, including the clearance of virus-infected cells through phagocytosis (antibody-dependent cellular phagocytosis [ADCP]) cytotoxic killing of virus-infected cells (antibody-dependent cellular cytotoxicity [ADCC]), and nibbling of infected cell membranes (antibody-dependent cellular trogocytosis [ADCT]). The Fc domain can also induce complement deposition (antibody-dependent complement deposition [ADCD]) and lyse opsonized cells or viruses. Unlike nAb that must bind to limited regions within the SARS-CoV-2 spike (RBD, NTD, and S2) for neutralization, 80%–96% of spike-binding antibodies are non-neutralizers that can potentially bind to the entire surface of the spike.6,7 These non-neutralizing antibodies (non-nAbs) can hypothetically bind and successfully target VOCs through Fc-effector functions. Therefore, testing VOC sensitivity to antibody-mediated Fc-effector responses elicited by natural infection or vaccination is crucial for understanding immune correlates of protection against VOCs and guiding future vaccine design.
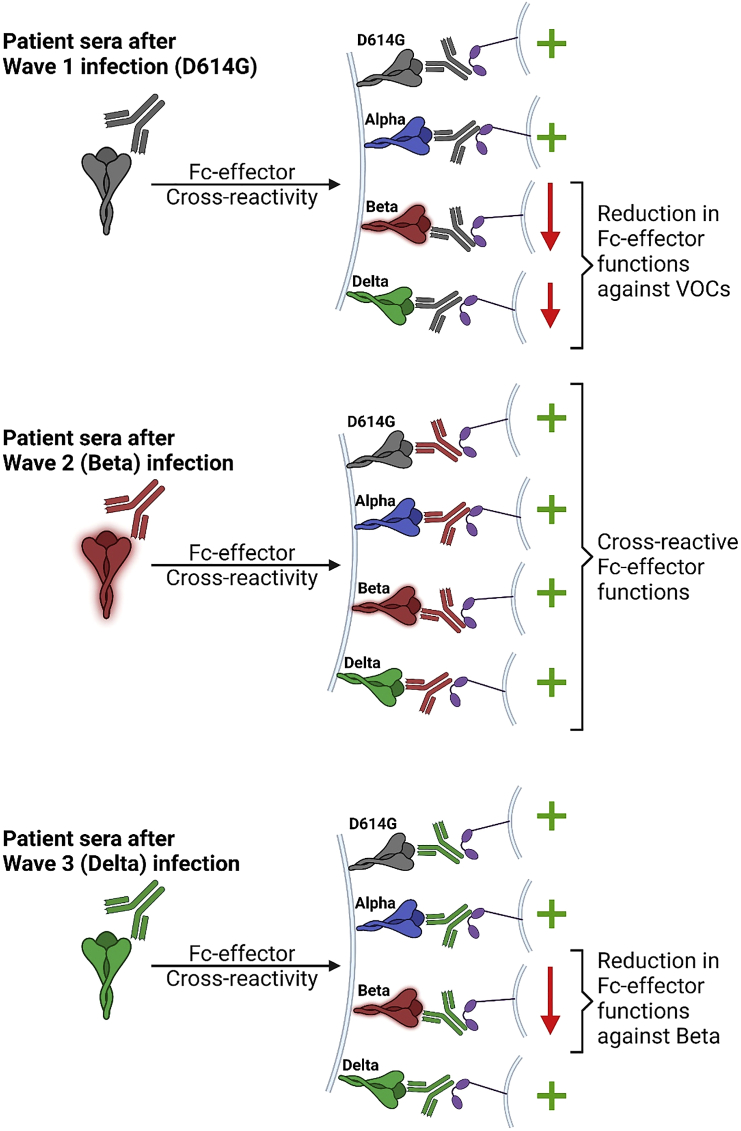
In this issue of Cell Reports Medicine, Richardson et al.1 investigated these Fc-effector responses using plasma samples from convalescent donors infected during the three distinct waves of COVID-19 infection in South Africa (Figure 1). Each wave was dominated by a different variant (wave 1, D614G; wave 2, Beta; and wave 3, Delta). This allowed the authors to compare both resistance and cross-susceptibility of VOCs to elicited Fc-effector responses (ADCC, ADCP, ADCT, and ADCD) in plasma of convalescent and Ad26.COV2.S-vaccinated individuals. While wave 1 plasma showed a significant decrease in neutralizing activity against Beta, the amount of total spike-binding IgA and IgG antibodies were only marginally different. These data implied that convalescent plasma contains non-nAbs that can bind epitopes beyond those mutated in VOCs and potentially mediate Fc-effector functions. Indeed, Fc-effector functions mediated by wave 1 plasma against Beta spike were affected to a lesser extent than the large drop in neutralization. Interestingly Beta VOC-specific ADCD activity was decreased substantially in wave 1 plasma compared to other Fc effector functions. Several factors including antibody isotype, glycosylation, and distance of the antibody-binding epitope from the membrane govern complement deposition activity. However, these aspects were not investigated in the current study. Subsequent domain-mapping revealed that despite harboring neutralization-resistant mutations, wave 1 plasma could bind Beta RBD and NTD to elicit ADCC activities. In contrast to wave 1 plasma samples, wave 2 plasma mediated ADCP, ADCD, and ADCT to similar levels against D614G and Beta spikes, demonstrating that infection with Beta VOC elicits broader Fc-effector responses. Again, not all Fc-effector functions were similar, with wave 2 plasma demonstrating significantly higher ADCC activity against the autologous infecting Beta spike.
Figure 1.
SARS-CoV-2 Beta VOC elicits broadly reactive Fc-effector functions
Fc-effector functions of antibodies against SARS-CoV-2 are of increasing interest for their protective roles during infection. Richardson et al.1 test Fc-effector functions mediated by patient plasma samples collected during distinct waves of infection in South Africa. Their data shows that prior infection with the Beta variant elicits the broadest Fc effector functions—a finding which may guide future vaccine design.
Richardson et al. next expanded the ADCC analyses to include a larger panel of VOCs (Alpha, Beta, Gamma, Delta) in addition to plasma samples for wave 3 and Ad26.COV2.S vaccinees. Plasma samples for wave 1 and vaccinees were similar and maintained robust Fc-effector activity against autologous D614G Spike while showing reduced ADCC activity against all VOCs except Alpha. In contrast, wave 2 plasma maintained ADCC activity against all VOCs whereas wave 3 plasma mediated ADCC that was more cross-reactive than the wave 1 but less cross-reactive than wave 2. Interestingly, ADCC activity in wave 3 plasma was significantly reduced against Beta spike. Thus, this study showed that the Beta variant is unique in not only evading Fc-effector functions among the VOCs tested but also elicits antibodies with better Fc-effector cross-reactivity against all VOCs.
Kaplonek et al.8 recently also tested the cross-reactivity of Fc-effector functions against VOCs. Their results showed that despite robust binding to VOC spike proteins, antibodies generated after natural infection showed compromised Fc-effector responses, whereas those induced by mRNA-1273 vaccine were more resistant to escape mutations in VOC spike. The divergence in Fc-effector cross-reactivities of convalescent plasma between the two studies could be due to recruitment of subjects with asymptomatic or mild symptoms8 versus hospitalized patients.1 Overall, both studies have provided evidence that cross-reacting non-nAbs continue to interact with emerging VOCs and have the potential to provide immunity against disease despite reductions in neutralizing titers. Further experiments in animal models that focus on protection offered by plasma samples from vaccinated and convalescent individuals against VOCs will clarify the effective contribution of residual Fc-effector activities toward VOC-specific immunity.
Previous studies on viruses such as HIV-1 have highlighted the need to present the right conformation of Envelope protein for eliciting desired protective immune responses.9 The addition of two rigid proline amino acids (2P mutation) in SARS-CoV-2 spike to ensure presentation of pre-fusion conformation to the immune system played a significant role in the success of current vaccination regimens.10 Data presented by Richardson et al.1 suggest that the conformation presented by the Beta VOC spike can be further utilized to generate antibodies with broad Fc-effector function profiles for enhancing SARS-CoV-2 immunity. Moreover, with its constellation of neutralization-resistant mutations, a vaccine based on the Omicron spike may induce broader and durable immune responses to shield us from expected future viral evolution.
Acknowledgments
Declaration of interests
The authors declare no competing interests.
References
- 1.Richardson S.I., et al. SARS-CoV-2 Beta and Delta variants trigger Fc effector function with increased cross-reactivity. Cell Reports Medicine. 2022;3:100510-1–100510-8. doi: 10.1016/j.xcrm.2022.100510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Khoury D.S., Cromer D., Reynaldi A., Schlub T.E., Wheatley A.K., Juno J.A., Subbarao K., Kent S.J., Triccas J.A., Davenport M.P. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat. Med. 2021;27:1205–1211. doi: 10.1038/s41591-021-01377-8. [DOI] [PubMed] [Google Scholar]
- 3.Garcia-Beltran W.F., Lam E.C., St Denis K., Nitido A.D., Garcia Z.H., Hauser B.M., Feldman J., Pavlovic M.N., Gregory D.J., Poznansky M.C., et al. Multiple SARS-CoV-2 variants escape neutralization by vaccine-induced humoral immunity. Cell. 2021;184:2372–2383.e9. doi: 10.1016/j.cell.2021.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tauzin A., Nayrac M., Benlarbi M., Gong S.Y., Gasser R., Beaudoin-Bussières G., Brassard N., Laumaea A., Vézina D., Prévost J., et al. A single dose of the SARS-CoV-2 vaccine BNT162b2 elicits Fc-mediated antibody effector functions and T cell responses. Cell Host Microbe. 2021;29:1137–1150.e6. doi: 10.1016/j.chom.2021.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goel R.R., Painter M.M., Apostolidis S.A., Mathew D., Meng W., Rosenfeld A.M., Lundgreen K.A., Reynaldi A., Khoury D.S., Pattekar A., et al. UPenn COVID Processing Unit‡ mRNA vaccines induce durable immune memory to SARS-CoV-2 and variants of concern. Science. 2021;374:abm0829. doi: 10.1126/science.abm0829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Amanat F., Thapa M., Lei T., Ahmed S.M.S., Adelsberg D.C., Carreño J.M., Strohmeier S., Schmitz A.J., Zafar S., Zhou J.Q., et al. Personalized Virology Initiative SARS-CoV-2 mRNA vaccination induces functionally diverse antibodies to NTD, RBD, and S2. Cell. 2021;184:3936–3948.e10. doi: 10.1016/j.cell.2021.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jennewein M.F., MacCamy A.J., Akins N.R., Feng J., Homad L.J., Hurlburt N.K., Seydoux E., Wan Y.H., Stuart A.B., Edara V.V., et al. Isolation and characterization of cross-neutralizing coronavirus antibodies from COVID-19+ subjects. Cell Rep. 2021;36:109353. doi: 10.1016/j.celrep.2021.109353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kaplonek P., Fischinger S., Cizmeci D., Bartsch Y.C., Kang J., Burke J.S., Shin S.A., Dayal D., Martin P., Mann C., et al. mRNA-1273 vaccine-induced antibodies maintain Fc-effector functions across SARS-CoV-2 Variants of Concern. Immunity. 2022;55:355–365. doi: 10.1016/j.immuni.2022.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lu M., Ma X., Castillo-Menendez L.R., Gorman J., Alsahafi N., Ermel U., Terry D.S., Chambers M., Peng D., Zhang B., et al. Associating HIV-1 envelope glycoprotein structures with states on the virus observed by smFRET. Nature. 2019;568:415–419. doi: 10.1038/s41586-019-1101-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hsieh C.-L., Werner A.P., Leist S.R., Stevens L.J., Falconer E., Goldsmith J.A., Chou C.W., Abiona O.M., West A., Westendorf K., et al. Stabilized coronavirus spike stem elicits a broadly protective antibody. Cell Rep. 2021;37:109929. doi: 10.1016/j.celrep.2021.109929. [DOI] [PMC free article] [PubMed] [Google Scholar]