Abstract
We previously culture isolated a strain of Ehrlichia canis, the causative agent of canine ehrlichiosis, from a human in Venezuela. In the present study, we examined whether dogs and ticks are infected with E. canis in Venezuela and, if so, whether this is the same strain as the human isolate. PCR analysis using E. canis-specific primers revealed that 17 of the 55 dog blood samples (31%) and all three pools of four Rhipicephalus sanguineus ticks each were positive. An ehrlichial agent (Venezuelan dog Ehrlichia [VDE]) was isolated and propagated in cell culture from one dog sample and was further analyzed to determine its molecular and antigenic characteristics. The 16S rRNA 1,408-bp sequence of the new VDE isolate was identical to that of the previously reported Venezuelan human Ehrlichia isolate (VHE) and was closely related (99.9%) to that of E. canis Oklahoma. The 5′ (333-bp) and 3′ (653-bp) sequences of the variable regions of the 16S rRNA genes from six additional E. canis-positive dog blood specimens and from three pooled-tick specimens were also identical to those of VHE. Western blot analysis of serum samples from three dogs infected with VDE by using several ehrlichial antigens revealed that the antigenic profile of the VDE was similar to the profiles of VHE and E. canis Oklahoma. Identical 16S rRNA gene sequences among ehrlichial organisms from dogs, ticks, and a human in the same geographic region in Venezuela and similar antigenic profiles between the dog and human isolates suggest that dogs serve as a reservoir of human E. canis infection and that R. sanguineus, which occasionally bites humans residing or traveling in this region, serves as a vector. This is the first report of culture isolation and antigenic characterization of an ehrlichial agent from a dog in South America, as well as the first molecular characterization of E. canis directly from naturally infected ticks.
Ehrlichia canis is a gram-negative obligatory intracellular bacterium with a tropism of canine monocytes and macrophages. It is transmitted by the brown dog tick Rhipicephalus sanguineus in the United States (12, 19). E. canis causes canine monocytic ehrlichiosis (CME), which was first described in Algeria in 1935 (6). CME is currently reported throughout the world but at higher frequencies in tropical and subtropical regions (7, 13, 15, 28–30).
After the bite of an infected tick, CME may be manifested in dogs by such symptoms as fever, depression, dyspnea, anorexia, and a slight weight loss in the acute phase, with laboratory findings of thrombocytopenia, leukopenia, mild anemia, and hypergammaglobulinemia. The subclinical phase of persistent ehrlichial infection and mild thrombocytopenia follows the acute phase and may last 40 to 120 days or years. The chronic phase is characterized by hemorrhages, epistaxis, and edema in addition to the clinical signs and laboratory findings of the acute phase, which are often complicated by superinfection with other microorganisms (3, 5, 14, 17, 24, 28). Without or sometimes even with doxycycline treatment, dogs infected with E. canis remain infected (14, 32).
Although E. canis previously was not considered a human pathogen, we isolated a strain from a man in Venezuela and found that it is genetically and antigenically most closely related to E. canis Oklahoma (23). Recently, a dog infection with E. canis was demonstrated by PCR in Venezuela (29). However, there had been no previous report of any Ehrlichia isolate from dogs in South America, and the prevalence rates of E. canis and vector tick species had never been shown in this region. Furthermore, whether humans and dogs are infected with the same strain of E. canis is unknown.
We report here the first culture isolation of E. canis from a dog in Venezuela and molecular and antigenic characterization of this isolate, especially in comparison to another E. canis specimen isolated from a human in Venezuela. We also report the genetic determination of an Ehrlichia sp. in ticks removed from dogs in Venezuela. The results suggest the potential of dog-to-human transmission of the E. canis strain in this region of South America.
MATERIALS AND METHODS
Dog blood samples and DNA isolation.
Blood specimens were collected in Lara State, Venezuela, from 23 military training dogs, during December 1999, and 10 civilian and 22 military dogs during April 2000. Heparinized 5- to 10-ml blood specimens were collected from each dog. After centrifugation of blood samples, plasma was collected and saved for serology. The peripheral blood mononuclear cells (PBMCs) were isolated by overlaying the buffy coat on Histopaque 1077 (Sigma, St. Louis, Mo.), and the interface fraction containing mononuclear cells was collected. The cells were washed with phosphate-buffered saline (137 mM NaCl, 10 mM Na2HPO4, 2.7 mM KCl, 1.8 mM KH2PO4 [pH 7.2]), and DNA was isolated from half of the PBMCs with a QIAamp blood kit (Qiagen, Inc., Valencia, Calif.) according to the manufacturer's instructions. DNA concentrations were determined by measuring the absorbance at 260 nm (A260) with a GeneQuant II RNA and DNA calculator (Pharmacia Biotech, Inc., Cambridge, England).
Tick samples and cDNA synthesis.
R. sanguineus ticks (eight males and four engorged females) were collected from military dogs in Lara State, Venezuela, during December 1999. These ticks were separated into three groups (two male groups and one female group) of four ticks each and dissected with a sterile razor blade by dividing the body along the median plane under a dissecting microscope. The body halves were homogenized with a glass homogenizer in a TRIzol reagent (GIBCO-BRL, Grand Island, N.Y.), and the total RNA was extracted according to the manufacturer's instruction. The final RNA pellet was resuspended in diethyl pyrocarbonate-treated distilled deionized sterile water, heated at 70°C for 10 min, and reverse-transcribed in a 20-μl reaction mixture (10 mM random hexamer, 0.5 mM deoxynucleoside triphosphate [dNTP] mixture, 1 U of RNase inhibitor [GIBCO-BRL], and 200 U of SuperScript II reverse transcriptase [RT] [GIBCO-BRL]) at 42°C for 50 min. The synthesized cDNAs in the final solution were used as template in the PCR.
PCR.
The nested PCR was carried out to detect E. canis DNA or cDNA from canine PBMCs or tick tissues, respectively, as described previously with primers ECC-ECB (outside pairs) and HE3-ECA (nested pairs) specific to the 16S rRNA gene of E. canis (32). Briefly, the amplification was carried out in a 50-μl reaction mixture including PCR buffer, 1.5 mM MgCl2, 10 pmol of primer pairs, 0.2 mM each of the dNTP mixture, 1.5 U of Taq DNA polymerase, and 0.5 μg of DNA or 1 μl of cDNA template, with 4 min of denaturation at 94°C followed by 40 cycles each consisting of 1 min of denaturation at 94°C, 1 min of annealing at 60°C, and 1 min of extension at 72°C. The final extension was allowed to continue for 7 min. PCR products were electrophoresed in 1.5% agarose gels and visualized with ethidium bromide.
Sequence analysis of the 16S rRNA Ehrlichia gene in samples from dogs and ticks.
Full-length 16S rRNA genes of E. canis were amplified as two fragments with primer pairs A17 (5′-GTTTGATCCTGGCTCAG-3′)-817R (5′-GAGTTTTAGTCTTGCGAC 3′) and 750F (5′-TAGTCCACGCTGTAAACG-3′)-EC3 (5′-ACCCTAGTCACTAACCCAAC-3′), respectively. PCR was performed as described above except that the annealing temperature was 54°C. Two microliters of amplicons observed at the expected sizes of 866 and 691 bp on agarose gel electrophoresis and 389-bp PCR or RT-PCR products of 16S rRNA was cloned into the PCRII vector of a TA cloning kit (Invitrogen Co., San Diego, Calif.) as described by the manufacturer. Recombinant plasmids were purified using the Concert Rapid Miniprep system (GIBCO-BRL), and the inserts were sequenced by a dideoxy chain termination method with the universal synthetic primers M13 and T7. The sequences of two clones from each sample were determined on both DNA strands. The alignment of DNA sequences, determination of evolutionary distance values, and construction of a phylogenic tree were performed by using the DNASTAR program (DNASTAR, Inc., Madison, Wis.).
Culture isolation of Ehrlichia sp.
Another half of the PBMCs from PCR-positive dogs was inoculated into DH82, a canine macrophage cell line, for culture isolation. The cultured cells were maintained in Dulbecco minimum essential medium supplemented with 10% heat-inactivated fetal bovine serum, 2 mM l-glutamine, and 10 mM N-(2-hydroxyethyl-piperazine)-N′-(4-butanesulfonic acid) buffer in a humidified 37°C incubator with 5% CO2–95% air as previously described (25). The cells were examined for infectivity every 2 to 3 days by microscopic examination of a Diff-Quick (American Scientific Product, Obetz, Ohio)-stained cytocentrifuged preparation.
Culturing and purification of organisms.
Venezuelan dog Ehrlichia (VDE), Venezuelan human Ehrlichia (VHE), E. canis Oklahoma, and Ehrlichia chaffeensis Arkansas were propagated in DH82 cells as described above. Ehrlichia organisms were purified by Sephacryl S-1000 (Pharmacia, Uppsala, Sweden) column chromatography as previously described (25). Protein concentrations of purified E. chaffeensis and E. canis were determined by bicinchoninic acid protein assay (Pierce, Rockford, Ill.), using bovine serum albumin as the standard.
IFA.
An indirect fluorescent antibody assay (IFA) was performed by the procedure described elsewhere (25). DH82 cells infected with strain Oklahoma of E. canis or strain Arkansas of E. chaffeensis were used for the preparation of antigen slides, and fluorescent isothiocyanate-conjugated goat anti-dog immunoglobulin G (IgG) (Jackson ImmunoResearch Laboratories, Inc., West Grove, Pa.) was used at a 1:200 dilution as a secondary antibody.
Purification of recombinant P30 protein.
The recombinant clone (pET29p30) that expresses the recombinant 30-kDa antigen of E. canis (rP30) (22) was cultured, and the recombinant fusion protein was purified by affinity chromatography with the His-Bind buffer kit containing 6 M urea (Novagen, Madison, Wis.) and refolded as described previously (22, 31).
Western immunoblotting.
Western immunoblotting was performed as previously described (25, 31) with a modification. A total of 12 μg of uninfected DH82 cells and pET29a-transformed Escherichia coli lysates (negative controls); purified VDE, VHE, E. chaffeensis, and E. canis; and 0.5 μg of affinity-purified rP30 protein were used for Western immunoblotting analysis. All serum samples were preabsorbed three times with pET29a-transformed E. coli at 4°C overnight before use. Sera used in Western blotting were from the following sources: Venezuelan E. canis-positive dogs 12, 33, and 35 (all from April 2000) at a 1:200 dilution; a dog experimentally infected with E. canis Oklahoma (A. Unver, T. Tajima, N. Ohashi, Y. Rikihisa, and R. G. Stich, Abstr. 100th Gen. Meet. Am. Soc. Microbiol. 2000, abstr. D-74, p. 242, 2000) at a 1:1,000 dilution; a dog experimentally infected with E. chaffeensis Arkansas (8) at a 1:300 dilution; and a PCR-confirmed human patient infected with E. chaffeensis (31; kindly provided by MRL Diagnostics, Cypress, Calif.) at a 1:1,000 dilution. Secondary antibodies for dog and human sera were peroxidase-conjugated affinity-purified anti-dog IgG+IgM and anti-human IgG+IgM+IgA (Kirkegaard & Perry Laboratories, Inc., Gaithersburg, Md.), respectively, at a 1:2,000 dilution.
Nucleotide sequence accession numbers.
The GenBank database accession numbers for the 16S rRNA nucleotide sequences of organisms used for comparison in this study are as follows: E. canis Oklahoma, M73221; E. canis Florida, M72226; E. canis Israel, U26740; E. canis Gzh982, AF162860; E. canis Germishuys, U54805; E. canis Gxht67, AF156786; E. canis Gdt3, AF156785; E. canis 95E10, U96437; E. canis Okinawa, AF308455; E. canis Venezuela, AF287154; E. chaffeensis, M73222; E. ewingii, M73227; E. platys, AF156784; Neorickettsia helminthoeca, U12457. The nucleotide sequences reported here have been assigned GenBank accession numbers AF373612 for the 16S rRNA of VHE, AF373613 for VDE, and AF373614 and AF373615 for E. canis from ticks.
RESULTS
Of 55 dog blood samples from Venezuela, 17 (31%) that were analyzed by PCR using E. canis-specific primers were positive, including 16 of 45 samples from military dogs (36%) and 1 of 10 samples from civilian dogs (10%). In addition, all three pools of four ticks each from Venezuela were positive by PCR using E. canis-specific primers. PCR-positive dog blood samples were inoculated into DH82 cells for culture isolation. An ehrlichial agent was isolated, using a DH82 cell culture system, from PBMCs of one military dog (dog 35) and was designated VDE. Twelve ticks collected from the military dogs were identified as R. sanguineus by microscopic examination by J. Keirans, Georgia Southern University, Statesboro. Three groups of ticks were analyzed by RT-PCR to detect ehrlichial RNA, since RNA is rapidly turned over, and the presence of RNA likely indicates the presence of viable organisms. We found that RT-PCR based on the 16S rRNA sequence is 100-fold more sensitive than PCR in detecting Ehrlichia organisms in ticks (8). All three groups of ticks were positive by RT-PCR, utilizing the E. canis-specific primers.
A nearly complete (1,408-bp) sequence of the 16S rRNA gene of VDE isolate was obtained. To compare the sequences from a larger number of dog and tick populations from Venezuela, PCR or RT-PCR products covering the 5′ variable region from nucleotides 49 to 437 of 16S rRNA from six additional infected dogs and three groups of four ticks were sequenced. These sequences were all identical to the corresponding full-length sequence of VDE (Table 1). Other gene fragments from nucleotide positions 743 to 1,433 of the 16S rRNA gene from the same samples were also sequenced since several different bases were found among VDE and other E. canis strains (Table 1). These sequences from six additional infected dogs and three groups of ticks were also found to be identical to the corresponding sequences of VDE.
TABLE 1.
Nucleotide differences among 16S rRNA genes of E. canis from different sources
| E. canis strains | Nucleotide at positiona:
|
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 199 | 226 | 355 | 700 | 849 | 882 | 918 | 953 | 980 | 1081 | 1239 | 1265 | 1308 | |
| VDEb | G | G | C | C | A | G | A | C | A | A | C | C | G |
| VTEc | • | • | • | / | • | • | • | • | • | • | • | • | • |
| VHEd | • | • | • | • | • | •m | • | • | • | • | • | • | • |
| Oklahomae | A | • | • | • | • | •m | • | • | • | • | • | • | • |
| Floridaf | A | • | • | • | • | − | • | • | • | • | • | T | • |
| 611g | • | • | • | • | − | • | • | • | C | • | • | • | A |
| Gzh982h | • | • | • | • | • | • | • | • | • | • | T | • | • |
| Germishuysi | • | • | − | • | • | • | • | • | • | • | • | • | • |
| Gxht67h | A | A | • | T | • | • | G | • | • | G | / | / | / |
| Gdt3h | A | • | • | • | • | • | • | • | • | • | / | / | / |
| 95E10j | / | / | / | / | • | • | • | T | • | • | • | • | • |
| Okinawak | • | • | • | / | / | / | / | / | / | / | / | / | / |
| Venezuelal | • | • | • | / | / | / | / | / | / | / | / | / | / |
Bullets represent positions conserved relative to the sequence based on VDE. The shill symbol (“/”) indicates the sequences were unavailable.
Venezuelan dog culture isolate in this study.
E. canis cDNA from R. sanguineus ticks from Venezuela in this study.
Venezuelan Human culture isolate (23).
E. canis type strain culture isolated from a dog in Oklahoma (2).
E. canis culture isolated from a dog in Florida (2).
E. canis culture isolated from a dog in Israel.
E. canis DNA from a dog in The People's Republic of China (13).
E. canis DNA from a sheep in South Africa (1).
E. canis DNA from a dog in Virginia.
E. canis DNA from a dog in Okinawa, Japan (30).
E. canis DNA from a dog in Venezuela (29).
Corrected bases based on the sequence data that were obtained by resequencing in the present study.
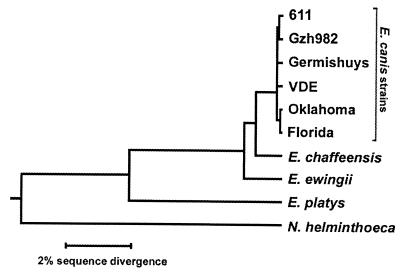
VDE and Venezuelan tick Ehrlichia (VTE) sequences were aligned with the 16S rRNA gene sequences from six almost complete and five partial GenBank-accessible sequences of E. canis and the published VHE sequence, and the positions of nucleotides that differed among these sequences are shown in Table 1. The VDE and VTE sequences were identical to that of VHE, and it differed by one base from the sequences of the Oklahoma, Gzh892, Germishuys, Gdt3, and 95E10; by three bases from the sequences of the Florida and Israel strains; and by five bases from the sequence of the Gxht67 strain of E. canis. Since the 16S rRNA gene sequence from a dog in Malacaibo, Venezuela (AF287154), is partial (302 bp) and does not include all the variable regions found among E. canis strains, we could not determine whether this isolate is different from VDE. Nucleotide sequence identities among 16S rRNA genes from VDE, VHE, E. canis strains with almost complete sequences available in the GenBank database, and representative canine ehrlichial and neorickettsial agents from three distinct groups are shown in Table 2. The phylogram obtained from the comparison of 16S rRNA gene sequences between VDE, VHE, and other canine ehrlichial and neorickettsial agents is shown in Fig. 1.
TABLE 2.
Nucleotide sequence identities between 16S rRNA genes of Venezuelan ehrlichiae and other agents
| Source | % Gene sequence identitya for:
|
||||||||
|---|---|---|---|---|---|---|---|---|---|
|
E. canis strain
|
E. chaffeensis | E. ewingii | E. platys | ||||||
| VDE | Oklahoma | Florida | 611 | Gzh982 | Germishuys | ||||
| E. canis VDEb | |||||||||
| E. canis Oklahomacd | 99.9 | ||||||||
| E. canis Floridacd | 99.5 | 99.5 | |||||||
| E. canis 611cd | 99.8 | 99.7 | 99.2 | ||||||
| E. canis Gzh982cd | 99.5 | 99.5 | 98.9 | 99.3 | |||||
| E. canis Germishuyscd | 99.6 | 99.5 | 99.0 | 99.4 | 99.1 | ||||
| E. chaffeensisd | 97.7 | 97.6 | 97.2 | 97.5 | 97.2 | 97.3 | |||
| E. ewingiid | 96.6 | 96.5 | 96.9 | 96.4 | 96.2 | 96.2 | 97.0 | ||
| E. platysd | 88.0 | 87.9 | 87.4 | 87.8 | 87.4 | 87.6 | 88.6 | 87.9 | |
| N. helminthoecad | 80.7 | 80.8 | 79.1 | 80.4 | 78.9 | 79.0 | 81.2 | 79.1 | 79.8 |
The values are percent 16S rRNA gene sequences identities for 1,304 bases which can be unambiguously aligned (bases 17 to 1,320 in the E. canis Venezuela).
Venezuelan human, dog, and tick ehrlichias (reference 23 and the present study).
The sources of sequences are indicated in Table 1.
GenBank accession numbers are shown in Materials and Methods.
FIG. 1.
Phylogram obtained from the comparison of 16S rRNA gene sequences between VDE and other agents. Evolutionary distance values were determined and the tree was constructed by the CLUSTAL method using DNASTAR program.
In addition to the dog and the tick samples from Venezuela, the 16S rRNA genes of E. canis Oklahoma and VHE were resequenced in the present study for confirmation. One base was found to be different between previously published sequence data of E. canis Oklahoma (2) (GenBank accession number M73221) and VHE (23) and the current sequence results. At nucleotide position 882 of the 16S rRNA gene, there was an additional guanine nucleotide (G) in both strains of E. canis. These two updated sequences were used for the sequence alignment and comparison in this study (Tables 1 and 2).
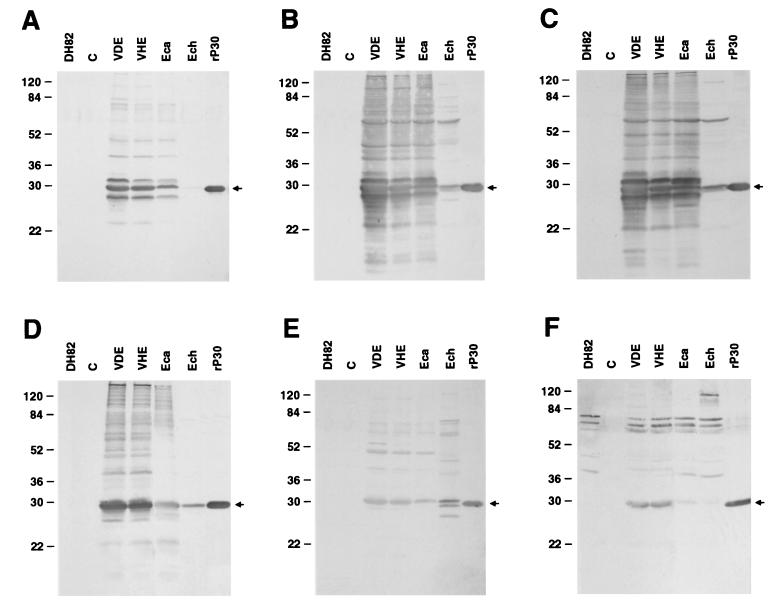
Figure 2 shows the results of Western blot analysis of serum samples from three Venezuelan dogs infected with E. canis, including dog 35 from which VDE was isolated, one dog experimentally infected with E. canis Oklahoma, and one dog experimentally infected with E. chaffeensis Arkansas, and the result for a human patient infected with E. chaffeensis (all dog and human samples were PCR confirmed). To compare the antigen profiles, two negative controls (DH82- and pET29-transformed E. coli lysates,) and four strains of purified ehrlichiae (VDE, VHE, E. canis Oklahoma, and E. chaffeensis Arkansas) and affinity-purified rP30 of E. canis were used as antigens for each Western blotting. Dog sera from three infected dogs (Fig. 2A to C) strongly reacted with major antigens with approximate molecular sizes of 110, 70, 58, 48, 43, 32, 30 to 28, and 24 kDa of VDE, VHE, and E. canis Oklahoma. However, these sera reacted strongly only to 58- and to 30- to 28-kDa antigens but weakly to 110-kDa antigens of E. chaffeensis. The reaction pattern of the same ehrlichial antigens with serum from a dog experimentally infected with E. canis Oklahoma was almost identical to the patterns with sera from three VDE-infected dogs (Fig. 2D). In addition, E. canis Oklahoma serum also reacted to the 95-, 65-, and 51-kDa antigens of VDE, VHE, and E. canis. Only the 28-kDa antigen of E. chaffeensis reacted to these serum samples. The serum from a dog experimentally infected with E. chaffeensis reacted strongly with major antigens of E. chaffeensis but weakly with VDE, VHE, and E. canis antigens (Fig. 2E). The same ehrlichial antigens reacted with the serum from a human patient infected with E. chaffeensis in the United States (Fig. 2F). In this blot, VHE and VDE 28-kDa antigens reacted but E. chaffeensis or E. canis Oklahoma 28-kDa antigens did not. This serum cross-reacted strongly with uninfected DH82 cells at a 60- to 70-kDa range. The serum samples were also analyzed by using rP30 because we have previously demonstrated that rP30 is a sensitive E. canis- and E. chaffeensis-specific antigen for immunoblot diagnosis for both human and canine sera (22, 31). All sera reacted strongly with rP30 (each panel in Fig. 2).
FIG. 2.
Western immunoblot analysis of sera from dog 35 (military dog, 1:160 E. canis IFA titer) (A), dog 12 (civilian dog, 1:80 E. canis IFA titer) (B), dog 33 (military dog, 1:160 E. canis IFA titer) (C), dog 10184 experimentally infected with E. canis Oklahoma (1:5,120 E. canis IFA titer) (D), dog 30133 experimentally infected with E. chaffeensis Arkansas (1:640 E. chaffeensis IFA titer) (E), and PCR-confirmed human patient (patient 42) infected with E. chaffeensis (1:1,280 E. chaffeensis IFA titer) (F). Lanes: DH82, dog macrophage cell line DH82 (negative control); C, pET29-transformed E. coli (negative control); VDE, purified VDE; VHE, purified VHE; Eca, purified E. canis; Ech, purified E. chaffeensis; Eca, rP30, affinity-purified recombinant fusion protein of E. canis (27 kDa). The numbers on the left of each panel indicate molecular masses in kilodaltons based on the broad-range prestained standards (Bio-Rad). The arrowheads show the position of rP30.
DISCUSSION
We previously reported the isolation and antigenic and genetic characterization of an E. canis strain culture isolated from a human in Venezuela (23), definitely proving human infection with an E. canis strain for the first time. However, it was unclear whether in Venezuela humans and dogs are infected with the same strain of E. canis. It was also unknown which species of tick transmit VHE. Based on the 16S rRNA gene sequence comparison and Western immunoblot analysis, VDE was found to be identical to VHE and closely related to the type strain of E. canis Oklahoma. Furthermore, only a single 16S rRNA gene sequence was found in several dogs and ticks in this study. This suggests that the same E. canis strain is responsible for both canine and human monocytic ehrlichiosis in Venezuela.
R. sanguineus is known to be primarily responsible for transmission of E. canis in North America (12, 19). Rhipicephalus ticks have a wide distribution in South America (18). Our data demonstrate that R. sanguineus is infected with a strain of E. canis identical to VHE and VDE. The presence of E. canis RNA in ticks suggests that E. canis is viable. Although R. sanguineus rarely bites humans in North America (21), human infestation with brown dog ticks has been reported in the Mediterranean region and in Central and North America (9, 10). VHE was isolated from a human who had a close relationship with dogs and ticks (23). Taken together, our findings suggest that chronically infected dogs serve as a reservoir of human E. canis infection in this region of South America and that R. sanguineus serves as a vector.
E. canis has a worldwide distribution along with its vector tick, R. sanguineus. High E. canis seroprevalence rates were frequently reported among dogs in North America, Europe, the Middle East, and North and South Africa. The reports of E. canis prevalence rates among dogs detected by molecular techniques such as PCR are, however, limited. Kordick et al. reported that 15 of 27 dogs (56%) in a kennel in North Carolina were E. canis positive as determined by PCR (16). Murphy et al. detected E. canis DNA from 2 of 65 dogs (3%) in Oklahoma (20). In the present study, 17 of 55 Venezuelan dogs (31%) were found to be positive by PCR specific to E. canis. This high infection rate shows that E. canis is a common pathogen among the dog population in Venezuela and attention needs to be paid by both veterinarians and public health professionals in this area. Since most of PCR-positive dogs did not show significant clinical signs compared to PCR-negative dogs, the infection rates of dogs in this region may be greater than the currently recognized rate based on the manifestation of severe clinical diseases.
Western blot analysis of serum samples from VDE-infected dogs, obtained using several ehrlichial antigens as well as VDE, revealed that antigenic profiles of VDE are almost identical to those of VHE and E. canis Oklahoma. This result supports our 16S rRNA sequence results at multiple protein levels, which suggest that VDE and VHE are the same organism parasitizing different host species. Our study supports the idea that humans are at risk of being infected with the Ehrlichia spp. infecting domestic animals when ticks bite both these animals and humans. E. ewingii was shown to infect both dogs and humans, most likely by the bite of Amblyomma americanum ticks (4). Furthermore, E. canis was reported to infect sheep with the clinical signs indistinguishable from Heartwater in South Africa (1). Thus, E. canis infection may not be limited to Canidae as previously thought (27).
In Mexico and Argentina, human ehrlichial infections were reported based on serologic data using E. chaffeensis as antigen (11, 26). Since E. chaffeensis serologically cross-reacts with E. canis and E. ewingii (4, 25, 31), it is important to perform species-specific PCR and sequence analysis to determine which Ehrlichia sp. and strain is infecting humans in these regions. Culture isolation helps further characterization of the agent and provides the geographic-area-specific antigen for a sensitive serologic test.
ACKNOWLEDGMENTS
This research was supported by a grant RO1AI47407 from the National Institutes of Health, an Ohio State University canine research grant, and an Ohio State University Faculty International Travel grant. A. Unver is a recipient of a scholarship from the National Ministry of Education in Turkey.
We thank R. Mujica, M. Jimenez, F. Bracamonte, and R. Bastidas at Military Center for Canine Training in Venezuela for research collaboration and H. Chavier, M. Garcia, and R. Perez at the Clinical Laboratory at “Lisandro Alvarado” Centroccidental University for help in obtaining blood specimens. We thank Roger W. Stich at the Ohio State University and James E. Keirans at Georgia Southern University for the tick species identification.
REFERENCES
- 1.Allsopp M, Visser E S, du Plessis J L, Vogel S W, Allsopp B A. Different organisms associated with heartwater as shown by analysis of 16S ribosomal RNA gene sequences. Vet Parasitol. 1997;71:283–300. doi: 10.1016/s0304-4017(97)00012-5. [DOI] [PubMed] [Google Scholar]
- 2.Anderson B E, Dawson J E, Jones D C, Wilson K H. Ehrlichia chaffeensis, a new species associated with human ehrlichiosis. J Clin Microbiol. 1991;29:2838–2842. doi: 10.1128/jcm.29.12.2838-2842.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Buhles W C, Ruxsoll D L, Ristic M. Tropical canine pancytopenia: clinical, haematologic, and serologic, and serologic response of dogs to Ehrlichia canis infection, tetracycline therapy, and challenge inoculation. J Infect Dis. 1974;130:358–367. doi: 10.1093/infdis/130.4.357. [DOI] [PubMed] [Google Scholar]
- 4.Buller R S, Arens M, Hmiel S P, Paddock C, Sumner J W, Rikihisa Y, Unver A, Graudreauls-Keener M, Marinian F A, Liddell A M, Schmulewitz N, Storch G. Ehrlichia ewingii, a newly recognized agent of human ehrlichiosis. N Engl J Med. 1999;341:148–155. doi: 10.1056/NEJM199907153410303. [DOI] [PubMed] [Google Scholar]
- 5.Codner E C, Farris-Smith L L. Characterization of the subclinical phase of ehrlichiosis in dogs. J Am Vet Med Assoc. 1986;189:47–50. [PubMed] [Google Scholar]
- 6.Donatein A, Lestoquard F. Existence and algerie d'une rickettsia du chien. Bull Soc Pathol Exot. 1935;28:418–419. [Google Scholar]
- 7.Ewing S A. Geographic distribution and tick transmission of Ehrlichia canis. J Med Entomol. 1972;9:597–598. [Google Scholar]
- 8.Felek S, Unver A, Stich R W, Rikihisa Y. Sensitive detection of Ehrlichia chaffeensis in cell culture, blood, and tick specimens by reverse transcription-PCR. J Clin Microbiol. 2001;39:460–463. doi: 10.1128/JCM.39.2.460-463.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Felz M W, Durden L A, Oliver J H., Jr Ticks parasitizing humans in Georgia and South Carolina. J Parasitol. 1996;82:505–508. [PubMed] [Google Scholar]
- 10.Goddard J. Focus of human parasitism by the brown dog tick, Rhiphicephalus sanguineus (Acari:Ixodidae) J Med Entomol. 1989;26:628–629. doi: 10.1093/jmedent/26.6.628. [DOI] [PubMed] [Google Scholar]
- 11.Gongora-Biachi R A, Zavala-Velazquez J, Castro-Sansores C J, Gonzalez-Martinez P. First case of human ehrlichiosis in Mexico. Emerg Infect Dis. 1999;5:481. doi: 10.3201/eid0503.990327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Groves M G, Dennis G L, Amyx H L, Huxsoll D L. Transmission of Ehrlichia canis to dogs by ticks (Rhiphicephalus sanguineus) Am J Vet Res. 1975;36:937–940. [PubMed] [Google Scholar]
- 13.Hua P, Yuhai M, Shide T, Yang S, Bohai W, Xiangrui C. Canine ehrlichiosis caused simultaneously by Ehrlichia canis and Ehrlichia platys. Microbiol Immunol. 2000;44:737–739. doi: 10.1111/j.1348-0421.2000.tb02557.x. [DOI] [PubMed] [Google Scholar]
- 14.Iqbal Z, Rikihisa Y. Reisolation of Ehrlichia canis from blood and tissues of dogs after treatment with doxycycline. J Clin Microbiol. 1994;32:1644–1649. doi: 10.1128/jcm.32.7.1644-1649.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Keef T J, Holland C J, Salyer P E, Ristic M. Distribution of Ehrlichia canis among military working dogs in the world and selected civilian dogs in the United States. J Am Vet Med Assoc. 1982;181:236–238. [PubMed] [Google Scholar]
- 16.Kordick S K, Breitschwerdt E B, Hegarty B C, Southwick K L, Colitz C M, Hancock S I, Bradley J M, Rumbough R, Mepherson J T, MacCormack J N. Coinfection with multiple tick-borne pathogens in a Walker Hound kennel in North Carolina. J Clin Microbiol. 1999;37:2631–2638. doi: 10.1128/jcm.37.8.2631-2638.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kuehn N F, Gaunt S D. Clinical and hematologic findings in canine ehrlichiosis. J Am Vet Med Assoc. 1985;186:355–358. [PubMed] [Google Scholar]
- 18.Leeson H S. The recorded distribution of the tick Rhiphicephalus sanguineus (Latreille) Bull Entomol Res. 1951;42:123–124. [Google Scholar]
- 19.Lewis G E, Ristic M, Smith R D, Lincoln T, Stephenson E H. The brown dog tick Rhiphicephalus sanguineus and the dog as experimental hosts of Ehrlichia canis. Am J Vet Res. 1977;38:1953–1955. [PubMed] [Google Scholar]
- 20.Murphy G L, Ewing S A, Whitworth L C, Fox J C, Kocan A A. A molecular and serologic survey of Ehrlichia canis, E. chaffeensis, and E. ewingii in dogs and ticks from Oklahoma. Vet Parasitol. 1998;79:325–339. doi: 10.1016/s0304-4017(98)00179-4. [DOI] [PubMed] [Google Scholar]
- 21.Nelson V A. Human parasitism by the brown dog tick. J Econ Entomol. 1969;62:710–712. [Google Scholar]
- 22.Ohashi N, Unver A, Zhi N, Rikihisa Y. Cloning and characterization of multigenes encoding immunodominant 30-kDa major outer membrane protein of Ehrlichia canis and application of the recombinant protein for serodiagnosis. J Clin Microbiol. 1998;36:2671–2680. doi: 10.1128/jcm.36.9.2671-2680.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Perez M, Rikihisa Y, Wen B. Ehrlichia canis-like agent isolated from a man in Venezuela: antigenic and genetic characterization. J Clin Microbiol. 1996;34:2133–2139. doi: 10.1128/jcm.34.9.2133-2139.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rikihisa Y. The tribe Ehrlichieae and ehrlichial diseases. Clin Microbiol Rev. 1991;4:286–308. doi: 10.1128/cmr.4.3.286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rikihisa Y, Ewing S A, Fox J C. Western blot analysis of Ehrlichia chaffeensis, E. canis or E. ewingii infection of dogs and human. J Clin Microbiol. 1994;32:2107–2112. doi: 10.1128/jcm.32.9.2107-2112.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ripoll C M, Remondegui C E, Ordonez G, Arazamendi R, Fusaro H, Hyman M J, Paddock C D, Zaki S R, Olson J G, Santos-Buch C A. Evidence of rickettsial spotted fever and ehrlichial infections in a subtropical territory of Jujuy, Argentina. Am J Trop Med Hyg. 1999;61:350–354. doi: 10.4269/ajtmh.1999.61.350. [DOI] [PubMed] [Google Scholar]
- 27.Ristic M, Huxsoll D. Tribe II: Ehrlichiae. In: Krieg N R, Holt J G, editors. Bergey's manual of systematic bacteriology. Vol. 1. Baltimore, Md: The Williams & Wilkins Co.; 1984. pp. 704–711. [Google Scholar]
- 28.Ristic M, Holland C J. Canine ehrlichiosis. In: Woldehiwet Z, Ristic M, editors. Rickettsial and chlamydial diseases of domestic animals. Oxford, United Kingdom: Pergamon Press; 1993. pp. 169–186. [Google Scholar]
- 29.Suksawat J, Pitulle C, Arraga-Alvarado C, Madrigal K, Hancock S I, Breitschwerdt E B. Coinfection with three Ehrlichia species in dogs from Thailand and Venezuela with emphasis on consideration of 16S ribosomal DNA secondary structure. J Clin Microbiol. 2001;39:90–93. doi: 10.1128/JCM.39.1.90-93.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Suto, Y., A. Suto, H. Inokuma, H. Obayashi, and T. Hayashi. The first confirmed canine case of Ehrlichia canis infection in Japan. Vet. Rec., in press. [DOI] [PubMed]
- 31.Unver A, Rikihisa Y, Ohashi N, Cullman L C, Buller R, Storch G A. Western and dot blotting analysis of Ehrlichia chaffeensis indirect fluorescent-antibody assay-positive and -negative human sera by using native and recombinant E. chaffeensis and E. canis antigens. J Clin Microbiol. 1999;37:3888–3895. doi: 10.1128/jcm.37.12.3888-3895.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wen B, Rikihisa Y, Mott J M, Greene R, Kim H Y, Zhi N, Couto G C, Unver A, Bartsch R. Comparison of nested PCR with immunofluorescent-antibody assay for detection of Ehrlichia canis infection in dogs treated with doxycycline. J Clin Microbiol. 1997;35:1852–1855. doi: 10.1128/jcm.35.7.1852-1855.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]