Abstract
Faster techniques are needed for the early diagnosis of dengue fever and dengue hemorrhagic fever during the acute viremic phase of infection. An isothermal nucleic acid sequence-based amplification (NASBA) assay was optimized to amplify viral RNA of all four dengue virus serotypes by a set of universal primers and to type the amplified products by serotype-specific capture probes. The NASBA assay involved the use of silica to extract viral nucleic acid, which was amplified without thermocycling. The amplified product was detected by a probe-hybridization method that utilized electrochemiluminescence. Using normal human plasma spiked with dengue viruses, the NASBA assay had a detection threshold of 1 to 10 PFU/ml. The sensitivity and specificity of the assay were determined by testing 67 dengue virus-positive and 21 dengue virus-negative human serum or plasma samples. The “gold standard” used for comparison and evaluation was the mosquito C6/36 cell culture assay followed by an immunofluorescent assay. Viral infectivity titers in test samples were also determined by a direct plaque assay in Vero cells. The NASBA assay was able to detect dengue viral RNA in the clinical samples at plaque titers below 25 PFU/ml (the detection limit of the plaque assay). Of the 67 samples found positive by the C6/36 assay, 66 were found positive by the NASBA assay, for a sensitivity of 98.5%. The NASBA assay had a specificity of 100% based on the negative test results for the 21 normal human serum or plasma samples. These results indicate that the NASBA assay is a promising assay for the early diagnosis of dengue infections.
Dengue viruses are transmitted by the Aedes aegypti mosquito and are among the most important arboviruses because of the high morbidity they cause to humans who inhabit urban communities in the tropical and subtropical regions of the world. It is estimated that two billion people live in areas at risk for dengue virus transmission and that as many as 100 million infections occur annually (13, 17). There are four antigenically related serotypes of dengue viruses (dengue-1, -2, -3, and -4). A primary infection with any of the four serotypes of dengue viruses usually results in subclinical or self-limited febrile disease. The more severe forms of the disease, dengue hemorrhagic fever (DHF) and dengue shock syndrome (DSS), have been reported in up to 5 to 10% of secondary infections, with case fatality rates as high as 10% (4, 13, 17).
The development of a more rapid diagnostic assay for dengue virus detection with high sensitivity and specificity will be very useful for the management and treatment of patients and for epidemiological surveillance. Dengue viruses frequently can be isolated from the blood of patients during the early phase of acute dengue illness, when immunoglobulin M (IgM) antibodies may not be detectable (7). However, virus isolation requires the use of tissue culture assays, involving incubation periods of a week or longer. Molecular diagnostic systems using reverse transcriptase PCR (RT-PCR) for detecting dengue viral RNA in human serum or plasma samples have been shown to be faster assays than cell culture and are highly effective for diagnosing dengue fever cases. A number of these studies using RT-PCR have used a time-consuming two-step nested amplification approach to achieve increased sensitivity. However, this method also increases the likelihood of false-positive reactions due to cross-contamination with dengue virus PCR products in the laboratory (5, 10, 15). The recent development of the TaqMan RT-PCR assay should lead to some improvements such as the real-time detection of an increase in dengue virus-specific DNA during amplification by simultaneous monitoring of a fluorescence signal in tightly sealed tubes (8, 11); however, the reverse transcription step and the thermal cycling remain.
Nucleic acid sequence-based amplification (NASBA) is a single-step isothermal RNA-specific amplification process that avoids these steps (9). The NASBA assay involves the use of silica to extract nucleic acid (2), which is then amplified without thermocycling. The amplified product is then detected by electrochemiluminescence (ECL). The NASBA assay has been successfully used for the detection of viral (9) and bacterial (12) RNA in clinical samples. The objective of this study was to evaluate the NASBA assay as a potential alternative to the tedious tissue culture methods for rapid detection of dengue virus in clinical specimens obtained during the acute viremic phase of illness. The sensitivity and specificity of the NASBA assay was determined using known dengue virus isolation-positive and -negative serum or plasma samples, respectively.
MATERIALS AND METHODS
Human serum or plasma samples.
Anonymous human serum samples from patients with acute dengue infections were received from existing collections in Indonesia, Peru, and Taiwan. A total of 67 serum samples that were reconfirmed with a double-blinded approach by virus isolation from C6/36 cells in our laboratory were used for evaluation of the NASBA assay. These virus-positive serum samples were collected 0 to 6 days after the onset of illness, during the acute phase of dengue infections prior to antibody development, with the exception of four samples that had very low levels of antibodies to dengue virus. Among these 67 samples, 30 were positive for dengue-1, 10 for dengue-2, 23 for dengue-3, and four for dengue-4. A total of 21 normal human serum or plasma samples collected from healthy donors in the United States, an area where dengue virus is not endemic, were purchased from PanBio Index Inc. (Baltimore, Md.) or were obtained from the Walter Reed Army Medical Center (Washington, D.C.) and were used as true negative controls. All positive and negative samples were thawed and tested in a randomized, blinded fashion for dengue virus in a C6/36 cell culture assay and in a Vero cell plaque assay, and RNA was extracted simultaneously for testing in the NASBA assay.
Viral isolation and IFA.
The serum samples were diluted 1:10 in culture medium and inoculated into each of the 25-cm2 tissue culture flasks containing Aedes albopictus mosquito (C6/36) cell monolayers for confirmation of viral isolation results as described previously (16). After a 1-h adsorption of the inoculum onto the cells at 28°C, cell cultures were incubated for 7 days at 28°C. Cells were harvested for identification of virus by an immunofluorescence assay (IFA) as previously described (18). The serotype-specific monoclonal antibodies used were 15F3 for dengue-1, 3H5 for dengue-2, 5D4 for dengue-3, and 1H10 for dengue-4 (6). Fluorescein isothiocyanate (FITC)-conjugated goat anti-mouse antibodies were used as the detector.
Direct plaque assay in Vero cells.
Three dilutions (1:5, 1:10, and 1:100 dilutions) were made for each of the human serum samples and were inoculated into duplicate wells of six-well tissue culture plates containing Vero cell monolayers. The cell cultures were incubated for 7 days at 37°C in a 5% CO2 incubator and then overlaid with agar and neutral red to determine the PFU as described elsewhere (3).
Dengue viruses and negative-control viruses.
Seed stocks of all four serotypes of dengue viruses were prepared in Vero cells, and virus titers were determined by the plaque assay. These viruses, including dengue-1 (Hawaii strain), dengue-2 (New Guinea C strain), dengue-3 (CH53489), and dengue-4 (341750), were used to spike normal human plasma samples to determine the detection limits of the dengue NASBA assays and to develop the dengue NASBA assays. Two other flaviviruses, yellow fever virus (17D, vaccine strain) and Japanese encephalitis virus (SA14-14-2, live attenuated vaccine strain), and a non-dengue-related virus, human immunodeficiency virus (HIV), were used as negative-control viruses for cross-reactivity testing of the dengue serotype-specific and dengue group-specific NASBA assays.
Amplification of dengue viral RNA.
Nucleic acid was extracted from dengue virus stocks and from human serum or plasma test samples using the method of Boom et al. (2). This procedure utilized 100 μl of plasma or serum as the starting input material. Final nucleic acid extracts were obtained in a total volume of 50 μl of elution buffer. Dengue viral RNA was amplified by NASBA using a modified version of the procedure of Romano et al. (14). Basically, 5 μl of the nucleic acid extract was brought up to a 20-μl final reaction volume containing 40 mM Tris (pH 8.5), 12 mM MgCl2, 70 mM KCl, 5 mM dithiothreitol, 1 mM each dATP, dCTP, dGTP, and dTTP, 2 mM each ATP, CTP, and UTP, 1.5 mM GTP, 0.5 mM ITP, 0.1 μg of bovine serum albumin (BSA)/μl, 1.5 M sorbitol, 0.08 U of RNase H, 32 U of T7 RNA polymerase, 6.4 U of avian myeloblastosis virus reverse transcriptase (AMV-RT), 0.2 μM each of the two amplification oligonucleotides, and 15% dimethyl sulfoxide (DMSO). The amplification oligonucleotides used to target the dengue virus RNA genome are given in Table 1. These amplification oligonucleotides were derived from the 3′ noncoding region of the dengue virus genome; P1 was complementary to bases 10,632 to 10,653 of the dengue-1 genome (GenBank accession number M87512), and P2 corresponded to bases 10,497 to 10,516 of the dengue-1 genome. Although the precise map positions of these two oligonucleotides were not identical in all four dengue serotypes, the target sequences for both oligonucleotides were completely maintained in each serotype (i.e., 100% complementarity). Reactions were conducted at 41°C for 90 min. The amplification reaction product was single-stranded antisense RNA that corresponded to the region defined by the amplification oligonucleotides. Each amplicon also included a detector probe overhang sequence, which was part of the P2 amplification oligonucleotide (Fig. 1).
TABLE 1.
Sequences of the primers and probes used in the dengue NASBA assays
| Primer or probe | Sequencea | Genome positionb (GenBank accession no.) |
|---|---|---|
| P1 primer | 5′AATTCTAATACGACTCACTATAGGGGAGACAGCAGGATCTCTGGTCT3′ | 10,632–10,653 (M87512) |
| P2 primer | 5′GATGCAAGGTCGCATATGAGGGTTAGAGGAGACCCCTCCC3′ | 10,497–10,516 (M87512) |
| Ru2+-labeled detector probe | 5′GATGCAAGGTCGCATATGAG3′ | |
| Type 1 capture probe | 5′GGGAAGCTGTATCCTGGTGGTAAGG3′ | 10,550–10,574 (M87512) |
| Type 2 capture probe | 5′ATGAAGCTGTAGTCTCACTGGAAGG3′ | 10,557–10,581 (M20558) |
| Type 3 capture probe | 5′AGGGAAGCTGTACCTCCTTGCAAAG3′ | 10,530–10,554 (M93130) |
| Type 4 capture probe | 5′GAGGAAGCTGTACTCCTGGTGGAAG3′ | 10,483–10,507 (M14931) |
| Conserved capture probe | 5′AAACAGCATATTGACGCTGGG3′ | 10,609–10,629 (M87512) |
Italicized bases represent overhang sequences of the primers: a T7 promoter sequence on P1, and a sequence homologous to the detector probe on P2.
In bases.
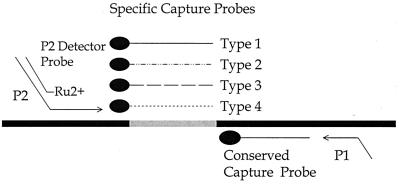
FIG. 1.
NASBA-based dengue virus subtyping assay strategy. NASBA is achieved with the P1 (antisense)–P2 (sense) oligonucleotide set. The overhang on P1 encodes the promoter sequence for the T7 RNA polymerase; the overhang on P2 is a potato leaf virus sequence that is homologous to the ruthenium-labeled detector probe. The four serotype-specific capture probes and the conserved sequence capture probe are all in the sense orientation and are immobilized onto the surface of a magnetic bead by means of a streptavidin-biotin linkage.
Detection of amplification products.
Amplification reaction products were detected using an ECL system (1, 14). The procedure involved a single hybridization reaction with two oligonucleotide probes that were specific for independent regions on the NASBA amplicon. The capture probe was immobilized onto the surface of a streptavidin-coated M280 magnetic bead (Dynal, Inc., Lake Success, N.Y.) by means of a biotin group at the 5′ end. Four independent capture probes were used in the assay, each corresponding to a specific sequence present in only one of the four serotype amplification products (Table 1). The dengue-1 capture probe corresponded to base positions 10,550 to 10,574 of the dengue-1 Singapore isolate (GenBank accession number M87512); the dengue-2 capture probe corresponded to positions 10,557 to 10,581 of the dengue-2 Jamaica isolate (accession number M20558); the dengue-3 probe corresponded to positions 10,530 to 10,554 of the dengue-3 H87 isolate (accession number M93130); and the dengue-4 capture probe corresponded to positions 10,483 to 10,507 of a recent dengue-4 isolate (accession number M14931). A conserved capture probe, which was complementary to a common region in all four serotype amplicons, was also used (Table 1). The position of the conserved probe corresponded to map positions 10,609 to 10,629 of the dengue-1 Singapore isolate. A common detector probe, labeled at the 5′ end with ruthenium (Ru2+) (1), was used in the assays and was responsible for the ECL signal. The detector probe was complementary to the overhang target sequence incorporated onto each amplicon by means of the overhang present on the P2 amplification oligonucleotide. Since amplification reaction products are antisense to the targeted region, the capture probes and the ruthenium-labeled detector probe are all in the sense orientation. Therefore, after amplification by NASBA using the P1 and P2 oligonucleotides, the amplicons were detected by hybridization with the ruthenium-labeled P2 overhang probe and either the conserved capture probe or one of the specific capture probes.
Hybridization was conducted in a 25-μl reaction mixture containing 5 μl of 1:20-diluted amplification reaction product in a solution containing 0.75 M NaCl, 75 mM sodium citrate, 0.8 mg of BSA/ml, 2 × 1012 copies of the capture probe on magnetic beads, and 2 × 1012 copies of the Ru2+-labeled detector probe. Unlabeled versions of certain capture probes were included in some of the serotype-specific hybridization reactions in order to maintain specificity. These “free” probes (i.e., not bound to the surfaces of magnetic beads) were incorporated into the hybridization reactions as follows: 2.0 × 1013 copies of free serotype 4 probe with the serotype 1-specific hybridization, 2.0 × 1012 copies of free serotype 4 probe with the serotype 3-specific hybridization, and 2.0 × 1012 copies of free serotype 1 probe with the serotype 4-specific hybridization. Hybridization reaction mixtures were incubated at 60°C for 5 min and then at 41°C for 30 min, and resulting ECL signals were measured using the NASBA QR System ECL reader (Organon Teknika, Inc., Durham, N.C.).
RESULTS
The detection limits of the NASBA assay for the four serotypes of dengue viruses ranged from 1 to 10 PFU/ml using normal human plasma spiked with a known PFU dose of each of these four serotypes. Typically, detection of NASBA amplicons from all four serotypes was up to 10 times more sensitive with the conserved capture probe than with any of the four serotype-specific capture probes (data not shown). There was no cross-reactivity either with the two other flaviviruses (yellow fever virus and Japanese encephalitis virus) that were tested or with the non-dengue-related virus (HIV). This observed specificity was further supported by a BLAST analysis of the NASBA amplification primers, which revealed no homology with alternative targets. The specificity of the dengue serotyping capability of the assay was ensured through the inclusion of unlabeled competitor probes in the individual hybridization reactions. For example, hybridization of the ruthenium-labeled dengue-1 probe to the amplification product obtained from 102 PFU of dengue-4 had a background ECL signal of 464,419 U (significantly above background). By including an unlabeled dengue-4 probe in the dengue-1 ruthenium probe hybridization with type-4 template, the background ECL signal was reduced to 584 U. Importantly, including an unlabeled dengue-4 probe in the labeled dengue-1 probe mix had no effect on the detection of dengue-1 target.
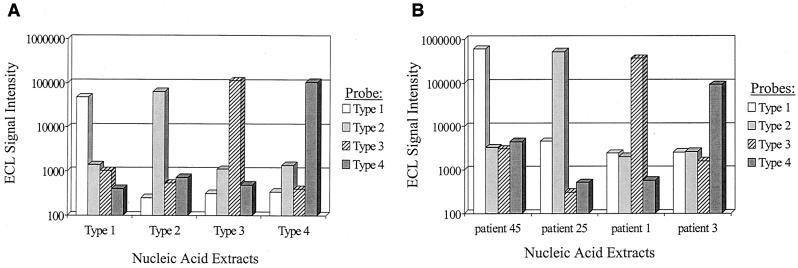
Typical ECL results generated from ruthenium-labeled probe hybridization to virus stock controls, as well as clinical samples, are provided in Fig. 2. The ECL signals depicted in Fig. 2 are raw values; consequently, the background signals produced from serotype-specific probes hybridized to alternative serotypes are shown. For the calculation of the ECL cutoff value, we determined the ECL signal for each serotype-specific probe generated from the analysis of 10 normal human plasma samples. The ECL cutoff value for each serotype was calculated as the mean plus 5 standard deviations derived from the analysis of the 10 normal human plasma samples, or 10,000 ECL units (whichever value was higher). This approach was used to score the four hybridization results obtained in the analysis of each sample (virus stock or clinical samples). Figure 2A demonstrates that background signals generated during the analysis of control virus samples were below 10,000 ECL units. Similar results were observed in the analysis of clinical samples (Fig. 2B).
FIG. 2.
ECL analysis of NASBA based analysis of dengue virus samples. (A) Nucleic acid extracts were obtained from duplicate samples of normal human plasma spiked with 1 PFU of each dengue virus serotype. Ten percent of each extract was amplified by NASBA, and the reaction products were subjected to hybridization analysis as described in Materials and Methods. The resulting raw ECL signals are provided on a log scale and are the mean values from the duplicate analysis. (B) Analysis of representative patient samples. Individual nucleic acid extracts were analyzed as described for panel A, and the resulting raw ECL signals are provided on a log scale. In each sample, the individual positive probe signal was always significantly above the background cutoff values derived for each probe (see Results).
To evaluate the NASBA assay for the detection of dengue viral RNA in clinical samples, we tested 67 dengue virus-positive serum samples collected from dengue patients in Peru, Indonesia, and Taiwan, as well as 21 normal human serum or plasma samples collected from healthy donors in the United States. All samples were tested in C6/36 cell culture for dengue virus, and simultaneously aliquots were solubilized with a lysis buffer for testing by the NASBA assay. Of the 67 isolation-positive samples, the serotype-specific NASBA methods detected dengue viral RNA in 66 samples, for a sensitivity of 98.5%. We also developed a group-specific NASBA assay that was applied to a subset of the 67 samples. Of the 13 samples tested with this “conserved” probe, 12 were positive. The negative sample was the same sample that was found to be negative with the serotype-specific NASBA analysis. When the sensitivity was calculated for each serotype, the dengue-3 serotype-specific NASBA assay had a lower sensitivity, 95.7%, while the other three serotype-specific assays had a sensitivity of 100% (Table 2). The NASBA assay had a specificity of 100% (21 of 21) based on the negative test results for the 21 normal human serum or plasma samples. The serotype concordance for the NASBA assay with the cell culture method was 100% (66 of 66). Except for 8 serum samples that did not have sufficient volume, the remaining 80 test samples (including the 59 dengue virus-positive serum samples and the 21 normal human serum or plasma samples) also were directly titrated in Vero cells. Dengue viral titers ranged from undetectable to more than 105 PFU/ml for the 59 dengue virus-positive samples (Table 3). The NASBA assay was able to detect dengue viral RNA in the clinical samples at plaque titers below 25 PFU/ml (the detection limit of the plaque assay).
TABLE 2.
Sensitivity of the NASBA assays for each dengue virus serotype compared with the C6/36 cell culture assaya
| Serotype | No. of samples positive by:
|
% Sensitivityb | |
|---|---|---|---|
| NASBA assay | C6/36 assay | ||
| Dengue-1 | 30 | 30 | 100 |
| Dengue-2 | 10 | 10 | 100 |
| Dengue-3 | 22 | 23 | 95.7 |
| Dengue-4 | 4 | 4 | 100 |
| Total | 66 | 67 | 98.5 |
Aliquots of samples were isolated simultaneously by the C6/36 cell culture assay and the NASBA assay.
The sensitivity for each serotype-specific or group-specific NASBA assay equals the number of test samples that were positive by the NABSA assay divided by the number of test samples that were positive by the standard culture assay in C6/36 cells.
TABLE 3.
Comparison of NASBA results with dengue virus plaque titers for clinical serum samples by a direct plaque assay in Vero cells
| Plaque titer range (PFU/ml) | No. of samples positive by:
|
|
|---|---|---|
| C6/36 assay | NASBA | |
| <25 | 9 | 9 |
| 25–99 | 4 | 4 |
| 102–<103 | 11 | 10 |
| 103–<104 | 6 | 6 |
| 104–<105 | 21 | 21 |
| ≥105 | 8 | 10 |
| Total | 59 | 58 |
DISCUSSION
In this study, both dengue serotype- and group-specific NASBA assays were developed for detection and serotyping of dengue viral RNA in clinical samples. These NASBA assays were specific to dengue virus, as there was no cross-reaction with the other flaviviruses tested or with a non-dengue-related virus. Dengue serotype-specific NASBA assays had excellent overall sensitivity and specificity compared with the results of a C6/36 cell culture assay based on 67 isolation-positive serum samples and 21 isolation-negative samples. Serotype concordance was excellent compared with the viral isolation method. Since the NASBA assay was able to detect dengue viral RNA in the clinical samples at plaque titers below 25 PFU/ml (the detection limit of the plaque assay), the detection threshold for the clinical serum samples probably was similar to the detection thresholds for the spiked sera (1 to 10 PFU/ml).
The plaque titer of the dengue-positive serum sample that was false negative in the NASBA assay was 250 PFU/ml; this was a sample from Indonesia identified as dengue-3 by the C6/36 cell culture method followed by IFA. We considered the possibility that the NASBA assay failed to detect dengue virus in this sample because of potential sequence variations within the primer target sites. However, comparison of the NASBA assay primers to GenBank sequence data for dengue virus isolates by BLAST analysis revealed a very high degree of homology with all identified sequences. Moreover, the relatively low temperature of the NASBA process (41°C) allows for the annealing of primers with less than 100% homology. Further, since NASBA does not rely on primer extension for amplification, it does not need to be the case that the 3′-terminal primer nucleotide is annealed to the template. Thus, it is more likely that the assay missed this one sample for some other technical reason related to the conduct of the assay.
Using the TaqMan RT-PCR assay, Laue and coworkers (11) showed that dengue viral RNA was found in 94.4% (17 of 18) of patients, if the samples were taken soon after the onset of symptoms and before dengue virus antibody was detectable. The detection limit of their assay was 500 RNA molecules/ml, which was difficult to compare with our detection limit expressed as PFU/ml. It is difficult to estimate the sensitivity of this TaqMan assay because there was no comparison of the TaqMan PCR results with the results of the standard viral isolation assay in their study. Houng and coworkers (8) reported that the detection limit for their TaqMan PCR assay for dengue-2 using spiked sera was approximately 6.4 to 10 PFU/ml, while the detection limits for our NASBA assays for four serotypes of dengue viruses ranged from 1 to 10 PFU/ml using spiked sera.
The NASBA assay has several advantages over the PCR technique (14). The amplification procedure of the NASBA assay is entirely isothermal and is conducted at 41°C; it does not require thermal cycling instrumentation. The NASBA product is single stranded and can therefore be readily detected by hybridization analysis. Furthermore, the final product is RNA, which is far less stable than DNA, minimizing the risk of contamination. Therefore, the NASBA method would be suitable for field epidemiologic studies in areas where dengue virus is endemic.
The current format of the NASBA-based dengue assay is semiquantitative. However, it is being configured for dengue viral RNA quantitation using previously described strategies (14). The NASBA assay is also being developed into a real-time detection assay through the inclusion of molecular beacon probes in the amplification reaction. Preliminary results indicate that the single-stranded NASBA reaction product works very well with molecular beacons (E. M. Lee, personal communication). Given the fact that the NASBA assay requires a constant single temperature, real-time beacon detection of NASBA products can be achieved with a simple fluorometric instrument (e.g., the Cytofluor 4000 [Perkin-Elmer Applied Biosystems, Foster City, Calif.]), and RT-PCR thermal cyclers with fluorescence detection capability are not required. Thus, the field applications of the NASBA assay may be more readily achieved compared to PCR.
In conclusion, a rapid NASBA assay was optimized for the detection of dengue viral RNA in clinical samples. The NASBA assay provided high sensitivity and specificity compared to the standard C6/36 viral isolation method. The total assay allows for the complete analysis of 20 samples in approximately 5 h, much faster than the tissue culture method, which requires 7 to 10 days. Because a thermal cycling instrument is not required, the cost of the assay relative to other amplification-based methods is immediately reduced. This study suggests that NASBA would be useful for diagnosing dengue infection and that results could be obtained in less than 1 day. This diagnostic assay may therefore be used to guide clinical care during the acute phase of illness. We are now planning to further evaluate the NASBA assay in a hospital setting using acute sera from clinically suspected dengue patients.
ACKNOWLEDGMENTS
This research was funded by U.S. Naval Medical Research Center Work Unit 62787A.870.L.1441 and by the U.S. Army Medical Research and Materiel Command.
REFERENCES
- 1.Blackburn G F, Shah H P, Kenten J H, Leland J, Kamin R A, Link J, Peterman J, Powell M J, Shah A, Talley D B, Tyagi S K, Wilkens E, Wu T G, Massey R J. Electrochemiluminescence detection for development of immunoassays and DNA probe assays for clinical diagnostics. Clin Chem. 1991;37:1534–1539. [PubMed] [Google Scholar]
- 2.Boom R, Sol C J, Salimans M M, Jansen C L, Wertheim-van Dillen P M, van der Noorda J. Rapid and simple method for purification of nucleic acids. J Clin Microbiol. 1990;28:495–503. doi: 10.1128/jcm.28.3.495-503.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Eckels K H, Brandt W E, Harrison V R, McCown J M, Russell P K. Isolation of temperature-sensitive dengue-2 virus under conditions suitable for vaccine development. Infect Immun. 1976;14:1221–1227. doi: 10.1128/iai.14.5.1221-1227.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Halstead S B. Pathogenesis of dengue: challenges to molecular biology. Science. 1988;239:476–481. doi: 10.1126/science.3277268. [DOI] [PubMed] [Google Scholar]
- 5.Harris E, Roberts T G, Smith L, Selle J, Kramer L D, Valle S, Sandoval E, Balmaseda A. Typing of dengue viruses in clinical specimens and mosquitoes by single-tube multiplex reverse transcriptase PCR. J Clin Microbiol. 1998;36:2634–2639. doi: 10.1128/jcm.36.9.2634-2639.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Henchal E A, Gentry M K, McCown J M, Brandt W E. Dengue virus-specific and flavivirus group determinants identified with monoclonal antibodies by indirect immunofluorescence. Am J Trop Med Hyg. 1982;31:830–836. doi: 10.4269/ajtmh.1982.31.830. [DOI] [PubMed] [Google Scholar]
- 7.Henchal E A, Putnak J R. The dengue viruses. Clin Microbiol Rev. 1990;3:376–396. doi: 10.1128/cmr.3.4.376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Houng H-S H, Hritz D, Kanesa-thasan N. Quantitative detection of dengue 2 virus using fluorogenic RT-PCR based on 3′-noncoding sequence. J Virol Methods. 2000;86:1–11. doi: 10.1016/s0166-0934(99)00166-4. [DOI] [PubMed] [Google Scholar]
- 9.Kievits T, van Gemen B, van Strijp D, Schukkink R, Dircks M, Adriaanse H, Malek L, Sooknanan R, Lens P. NASBA isothermal enzymatic in vitro nucleic acid amplification optimized for the diagnosis of HIV-1 infection. J Virol Methods. 1991;35:273–286. doi: 10.1016/0166-0934(91)90069-c. [DOI] [PubMed] [Google Scholar]
- 10.Lanciotti R S, Calisher C H, Gubler D J, Chang G J, Vorndam A V. Rapid detection and typing of dengue viruses from clinical samples by using reverse transcriptase-polymerase chain reaction. J Clin Microbiol. 1992;30:545–551. doi: 10.1128/jcm.30.3.545-551.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Laue T, Emmerich P, Schmitz H. Detection of dengue virus RNA in patients after primary or secondary dengue infection by using the TaqMan automated amplification system. J Clin Microbiol. 1999;37:2543–2547. doi: 10.1128/jcm.37.8.2543-2547.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Morre S A, Sillekens P, Jacobs M V, van Aarle P, de Blok S, van Gemen B, Walboomers J M M, Meijer C J L M, van den Brule A J C. RNA amplification by nucleic acid sequence-based amplification with an internal standard enables reliable detection of Chlamydia trachomatis in cervical scrapings and urine samples. J Clin Microbiol. 1996;34:3108–3114. doi: 10.1128/jcm.34.12.3108-3114.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rigau-Perez J G, Clark G G, Gubler D J, Reiter P, Sanders E J, Vorndam A V. Dengue and dengue haemorrhagic fever. Lancet. 1998;352:971–977. doi: 10.1016/s0140-6736(97)12483-7. [DOI] [PubMed] [Google Scholar]
- 14.Romano J W, Shurtliff R N, Dobratz E, Gibson A, Hickman K, Markham P, Pal R. Quantitative evaluation of simian immunodeficiency virus infection using NASBA technology. J Virol Methods. 2000;86:61–70. doi: 10.1016/s0166-0934(99)00184-6. [DOI] [PubMed] [Google Scholar]
- 15.Sudiro T M, Ishiko H, Green S, Vaughn D W, Nisalak A, Kalayanarooj S, Rothman A L, Raengsakulrach B, Janus J, Kurane I, Ennis F. Rapid diagnosis of dengue viremia by reverse transcriptase-polymerase chain reaction using 3′-noncoding region universal primers. Am J Trop Med Hyg. 1997;56:424–429. doi: 10.4269/ajtmh.1997.56.424. [DOI] [PubMed] [Google Scholar]
- 16.Tesh R B. A method for the isolation and identification of dengue viruses, using mosquito cell cultures. Am J Trop Med Hyg. 1979;28:1053–1059. doi: 10.4269/ajtmh.1979.28.1053. [DOI] [PubMed] [Google Scholar]
- 17.World Health Organization. Strengthening the implementation of the global strategy for dengue fever/dengue hemorrhagic fever prevention and control. Geneva, Switzerland: World Health Organization; 2000. [Google Scholar]
- 18.Wu S L, Grouard-Vogel G, Sun W, Mascola J R, Brachtel E, Putvatana R, Louder M, Filgueira L, Marovich M, Wong H K, Blauvelt A, Murphy G S, Robb M L, Innes B L, Birx D L, Hayes C G, Frankel S S. Human skin Langerhans cells are targets of dengue virus infection. Nat Med. 2000;6:816–820. doi: 10.1038/77553. [DOI] [PubMed] [Google Scholar]