Abstract
A total of 121 reference and clinical strains of both slowly and rapidly growing mycobacteria belonging to 54 species were studied for restriction fragment length polymorphism of a PCR-amplified 439-bp segment of the gene encoding the 65-kDa heat shock protein. Restriction digests were separated by 10% polyacrylamide gel electrophoresis (PAGE). By including a size standard in each sample, the restriction fragment profile was calculated using a computer-aided comparison program. An algorithm describing these 54 species (including 22 species not previously described) is proposed. We found that this assay based on 10% PAGE provided a more precise estimate than that based on agarose gel electrophoresis of the real size of restriction fragments as deduced from the sequence analysis and allowed identification of mycobacteria whose PCR-restriction fragment length polymorphism analysis patterns were unequivocally identified by fragments shorter than 60 bp.
Mycobacteria other than Mycobacterium tuberculosis (MOTT) are increasingly recognized as causing human infections (31). Conventional biochemical methods and phenotypic tests for species differentiation are laborious and time-consuming and frequently require specialized testing that is beyond the capacity of clinical laboratories. Genotypic methods for the identification of mycobacteria have been developed in recent years (3, 10, 23, 29). These molecular methods are gaining increasing importance because they yield rapid and, in most cases, unequivocal results.
In 1992 Plikaytis et al. (15) developed a method for differentiating among slowly growing Mycobacterium species by PCR and restriction fragment length polymorphism analysis (PRA). A similar approach was used by Telenti et al. (27) for rapid identification of mycobacteria to species level based on evaluation of the gene coding for the 65-kDa heat shock protein (22) by PCR and restriction enzyme analysis. Subsequently, this approach was used for the taxonomic separation of rapidly growing mycobacteria (18, 24), for routine identification of mycobacteria (1, 4, 7, 9, 11, 12, 23, 26), and for identifying Mycobacterium leprae (16, 25) and Mycobacterium kansasii subspecies (17).
In all these studies the algorithm describing the mycobacteria species is based on the use of two restriction enzymes (BstEII and HaeIII) and separation of the restriction fragments on an agarose gel. PRA patterns are then interpreted by converting the running distance in electrophoresis to apparent molecular size (in base pairs). Difficulties in PRA interpretation may stem from similarities in a number of band sizes that are critical for discrimination of species and are not sufficiently resolved by agarose-based gel electrophoresis.
In view of the application of PRA-based identification of mycobacteria in our diagnostic laboratory and its application to an increasing number of different species, we conducted the present study in order to propose an algorithm based on 10% polyacrylamide gel electrophoresis (PAGE) of restriction digests to improve the resolution of low-molecular-weight fragments and to extend the identification capacity of the method.
MATERIALS AND METHODS
Bacterial strains.
Sixty-eight clinical isolates were collected at the Clinical Mycobacteriology Laboratory of the Ospedale Civile Maggiore in Verona, Italy. The isolates were grown in a liquid medium (MB/BacT system; Organon Teknika Corp., Durham, N.C.) (6) or on Lowenstein-Jensen medium (Biotest, Heidelberg, Germany) and examined for growth rate, gross and microscopic colony morphology, and pigmentation. Identification at the species level was done by classical biochemical identification tests and AccuProbe tests (Gen-Probe Incorporated, San Diego, Calif.) for M. avium complex, M. tuberculosis complex, M. gordonae, and M. kansasii. Forty-four strains were from the collection of the Italian Reference Laboratory for mycobacteria. Nine strains were from the American Type Culture Collection (Rockville, Md.): M. bovis BCG ATCC 27291, M. intracellulare ATCC 35763, M. avium ATCC 15769, M. terrae ATCC 15755, M. fortuitum ATCC 19542, M. malmoense ATCC 29571, M. marinum ATCC 927, M. smegmatis ATCC 19420, and M. haemophilum ATCC 29548. The strains used in this study are listed in Table 1.
TABLE 1.
Mycobacterium strains studied for the hsp65 gene and development of the diagnostic algorithm
| Organism | No. of strains | Strain(s)a |
|---|---|---|
| M. bovis BCGb | 1 | ATCC 27291 |
| M. intracellulareb serotype 17 | 1 | ATCC 35763 |
| M. aviumb serotype 1 | 1 | ATCC 15769 |
| M. terraeb | 1 | ATCC 15755 |
| M. fortuitumb | 1 | ATCC 19542 |
| M. malmoenseb | 1 | ATCC 29571 |
| M. marinumb | 1 | ATCC 927 |
| M. smegmatis | 1 | ATCC 19420 |
| M. haemophilum | 1 | ATCC 29548 |
| M. tuberculosis complexb | 3 | OCM1, OCM2, OCM3 |
| M. gordonae type Ib | 5 | MGI1, MGI2, MGI3, MGI4, MGI5 |
| M. gordonae type II | 9 | MGII1, MGII2, MGII3, MGII4, MGII5, MGII6, MGII7, MGII8, MGII9 |
| M. gordonae type IIIb | 2 | MGIII1, MGIII2 |
| M. gordonae type IVb | 2 | MGIV1, MGIV2 |
| M. chelonae subsp. chelonaeb | 8 | MCH1, MCH2, MCH3, MCH4, MCH5, MCH6, MCH7, MCH8 |
| M. chelonae subsp. abscessusb | 14 | MAB1, MAB2, MAB3, MAB4, MAB5, MAB6, MAB7, MAB8, MAB9, MAB10, MAB11, MAB12, MAB13, MAB14 |
| M. fortuitum subsp. fortuitumb | 4 | MFO1, MFO2, MFO3, MFO4 |
| M. marinumb | 2 | MMA1, MMA2 |
| M. avium-M. paratuberculosisb | 9 | MA1, MA2, MA3, MA4, MA5, MA6, MA7, MA8, MA9 |
| M. intracellulareb | 1 | MAC1 |
| M. kansasiib | 3 | MKA1, MKA2, MKA3 |
| M. xenopib | 4 | MXE1, MXE2, MXE3, MXE4 |
| M. terraeb | 1 | MTE1 |
| M. simiaeb | 1 | MSI1 |
| M. agri | 1 | OCF902 |
| M. aichiense | 1 | OCF896 |
| M. alvei | 1 | OCF886 |
| M. asiaticum | 1 | OCF877 |
| M. austroafricanum | 1 | OCF909 |
| M. branderi | 1 | OCF874 |
| M. brumaeb | 1 | OCF893 |
| M. chitaeb | 1 | OCF876 |
| M. confluentisb | 1 | OCF916 |
| M. duvalii | 1 | OCF894 |
| M. farcinogenes | 1 | OCF882 |
| M. gadium | 1 | OCF915 |
| M. gastrib | 1 | OCF873 |
| M. genavenseb | 1 | OCF869 |
| M. gilvum | 1 | OCF904 |
| M. haemophilum | 1 | OCF919 |
| M. hiberniae | 1 | OCF900 |
| M. interjectum | 1 | OCF910 |
| M. kansasii | 1 | OCF921 |
| M. komossense | 1 | OCF870 |
| M. malmoenseb | 1 | OCF880 |
| M. mucogenicumb | 1 | OCF906 |
| M. neoaurumb | 1 | OCF883 |
| M. nonchromogenicumb | 1 | OCF889 |
| M. obuense | 1 | OCF901 |
| M. parafortuitum | 1 | OCF898 |
| M. phleib | 1 | OCF878 |
| M. porcinum | 1 | OCF905 |
| M. poriferae | 1 | OCF892 |
| M. pulversb | 1 | OCF881 |
| M. rhodesiaeb | 1 | OCF908 |
| M. scrofulaceumb | 1 | OCF899 |
| M. senegalenseb | 1 | OCF903 |
| M. diernhoferi | 1 | OCF911 |
| M. simiaeb | 1 | OCF912 |
| M. sphagni | 1 | OCF885 |
| M. szulgai | 1 | OCF891 |
| M. terraeb | 1 | OCF895 |
| M. thermoresistible | 1 | OCF872 |
| M. tokaiense | 1 | OCF913 |
| M. triviale | 1 | OCF888 |
| M. xenopi | 1 | OCF897 |
ATCC, American Type Culture Collection; OCF, Italian Reference Laboratory for Mycobacteria; all the other strains were clinical isolates from the Mycobacteriology Laboratory of Verona Hospital.
The hsp65 gene sequence is available from The National Center for Biotechnology Information GenBank database.
Chromosomal DNA isolation.
Bacterial DNA was prepared as follows. The sediment from a 0.5-ml liquid culture or one loopful of bacteria cultured on Lowenstein-Jensen medium was suspended in 500 μl of TE buffer (0.01 M Tris-HCl, 0.01 M EDTA [pH 8.0]). Lysozyme (Sigma Chemical Co., St. Louis, Mo.) was added to a final concentration of 2 mg/ml, and the tube was incubated for 20 min at 37°C. Bacterial DNA was prepared as described by van Soolingen et al. (30). Briefly, 70 μl of 10% sodium dodecyl sulfate and 5 μl of proteinase K (at 10 mg/ml) were added, and the mixture was incubated for 10 min a 65°C. One hundred microliters of 5 M NaCl and 100 μl of N-cetyl-N,N,N,-trimethylammonium bromide were added. The tubes were incubated for 10 min a 65°C. An equal volume of chloroform was added, and the mixture was centrifuged for 5 min. Five hundred microliters of isopropanol was added to the supernatant to precipitate the DNA. After 30 min at −20°C and centrifuging for 30 min at 14,000 × g at 4°C, the pellet was washed once with 70% ethanol and the air-dried pellet was dissolved in 50 μl of 1× TE buffer.
PCR for PRA.
A segment of the 65-kDa heat shock protein gene (hsp65) was amplified by two specific primers (Tb11 [5′-ACCAACGATGGTGTGTCCAT] and Tb12 [5′-CTTGTCGAACCGCATACCCT]) as described by Telenti et al. (27). The presence of amplified products was confirmed by agarose gel electrophoresis.
Restriction digestion and analysis of restriction patterns.
BstEII and HaeIII enzyme digestion of the amplification product was performed essentially as described by Telenti et al. (27), with the following modification. Briefly, 5 μl of the amplified reaction solution was added to a mixture containing 2 μl (1 U/μl) of enzyme, 2 μl of appropriate restriction buffer (10×), and 15 μl of autoclaved distilled water. The mixtures were incubated for 60 min at 60°C for BstEII digestion and at 37°C for HaeIII digestion. Two protocols were used for electrophoresis: in one, restriction fragments were electrophoresed on a 3% agarose gel at 120 V for 2 h, and in the other, restriction fragments were electrophoresed on a 10% polyacrylamide gel in a Mini-Sub-Cell electrophoresis system (Bio-Rad, Richmond, Calif.) at 120 V until the dye front migrated to approximately 1 cm from the end of the gel. Fragment band sizes were estimated on a computerized Image Master VDS-Pharmacia Biotech system, using a HaeIII-digested φX174 DNA and a 100-bp ladder as the molecular size standard.
PCR for reverse cross-blot hybridization assay.
PCRs were performed under the conditions described by Kox et al. (13) with the primers PMyc14bio [5′-GRGRTACTCGAGTGGCGAAC] and PMyc7 [5′-GGCCGGCTACCCGTCGTC], derived from the sequence of the 16S rRNA gene common to all Mycobacterium species. The presence of amplified DNA was visualized by agarose gel electrophoresis (2% agarose in TE buffer) and staining with ethidium bromide.
Reverse cross-blot hybridization assay.
The amplicons were analyzed by hybridization assay as described by Kox et al. (13) with specific oligonucleotide probes (18). The oligonucleotide probes were subjected to the tailing reaction with dTTP to permit efficient capture of PCR products. The dTTP-tailed oligonucleotide probes were fixed to the nylon membrane. Two panels were prepared: the first included probes for Mycobacterium spp., M. tuberculosis complex, M. avium, M. intracellulare, M. fortuitum, M. xenopi, M. kansasii, and M. gordonae, and the second included probes specific for Mycobacterium spp., M. chelonae, M. genavense, M. malmoense-M. szulgai, M. marinum-M. ulcerans, M. smegmatis, M. terrae, and Nocardia asteroides. The PCR products were denatured by heating and were added to the membrane in the hybridization solution; the hybridized PCR products were detected by incubation with streptavidin-alkaline phosphatase and a color substrate.
Sequencing of PCR product hsp65 gene.
The amplified PCR products were captured and purified with silica gel columns (Qiagen; M-Medical-Genenco, Florence, Italy) as described in the manufacturer's instructions. Sequencing reactions were done by a standard sequencing method with a DNA sequencing kit (ABI Prism 6700 sequence detection system; Applied Biosystems); the primers Tb11 and Tb12 were used for sequencing. The sequence were analyzed by using the OMIGA 1.1.3 (Oxford Molecular) program to determine the locations of restriction sites.
Nucleotide sequence accession number.
The hsp65 gene sequences of the Mycobacterium spp. described in this study are available upon request and have been deposited in the National Center for Biotechnology Information GenBank database under accession numbers AJ310215 through AJ310239 and AJ307630 through AJ307654.
RESULTS
Comparison of 3% agarose gel electrophoresis and 10% PAGE.
The method originally developed by Telenti et al. (27) and adopted by others (8, 14, 17, 24, 26) evaluates restriction patterns by separating the fragments generated by BstEII and HaeIII digestion on an agarose gel. We initially conducted studies to compare the PRA patterns of several reference strains using 3% agarose gel electrophoresis and 5% and 10% PAGE to separate the restriction fragments with the aim of evaluating which procedure yielded more precise estimates of fragment sizes and resolution of bands of ≤100 bp.
Table 2 shows the comparison between sizes deduced from the position of the restriction sites in the sequence (real sizes), the sizes obtained in the present study by the use of 3% agarose gel electrophoresis and 10% PAGE, and the sizes published by Telenti et al. (27). Since values nearer to real sizes were obtained with 10% than with 3% PAGE, only results obtained with the former procedure are reported. When the fragment separation was performed on a 10% polyacrylamide gel, 16 of the total of 23 fragments generated by digestion with BstEII of the amplicons of the nine reference strains showed a size differing from the real size by less than ±5 bp and 7 fragments had a size differing by less than ±10 bp, whereas with agarose gel electrophoresis eight fragments differed by less than ±5 bp, 10 differed by ±10 bp and five differed by more than ±10 bp from the real size. Similarly, 23 of the total of 26 fragments generated by digestion with HaeIII of the amplicons of the nine reference strains differed by less than ±5 bp from the real size and three differed by less than ±10 bp. Of the total of 19 fragments separated by 3% agarose gel electrophoresis gel, 10 differed by less than ±5 bp, 6 differed by less than ±10 bp, and 3 differed by more than ±10 bp from the real size. The sizes of the corresponding BstEII and HaeIII fragments in the published algorithms based on agarose gel electrophoresis differed from the real sizes to a greater extent than those found in our study.
TABLE 2.
Fragment lengths of the hps65 PCR products after restriction with BstEII and HaeIII deduced from sequence analysis and comparison with the present study and previously published data
| Species |
BstEII fragment lengths (bp)
|
HaeIII fragment lengths (bp)
|
||||||
|---|---|---|---|---|---|---|---|---|
| Sequence | This study
|
Published dataa | Sequence | This study
|
Published dataa | |||
| Agarose gel electrophoresis | PAGE | Agarose gel electrophoresis | PAGE | |||||
| M. bovis ATCC 27291 | 231, 116, 79 | 238, 118, 84 | 236, 117, 82 | 245, 125, 80 | 152, 127, 69 | 153, 129, 77 | 152, 131, 71 | 160, 140, 70 |
| M. avium ATCC 15769 | 231, 212 | 238, 218 | 234, 209 | 245, 220 | 127, 103, 59 | 141, 65 | 127, 104, 55 | 140, 105 |
| M. intracellulare ATCC 15763 | 231, 116, 94 | 244, 123, 106 | 237, 120, 104 | 245, 125, 100 | 145, 127, 57, 45, 36, 34 | 152, 135, 60 | 152, 131, 63, 44, 39 | 155, 140, 60 |
| M. terrae ATCC 15755 | 325, 116 | 322, 118 | 323, 118 | 325, 125 | 181, 126, 38, 34, 24, 21 | 184, 131 | 187, 132, 42, 36 | 190, 140 |
| M. fortuitum ATCC 19542 | 231, 130, 79 | 241, 123, 90 | 229, 129, 82 | 245, 125, 80 | 139, 123, 69, 54 | 151, 128 | 137, 127, 67, 56 | 155, 135 |
| M. malmoense ATCC 29571 | 231, 116, 94 | 240, 120, 101 | 234, 116, 101 | 245, 125, 100 | 145, 105, 78, 40, 34, 21, 17 | 152, 116, 84 | 147, 109, 81 | 155, 110, 70 |
| M. marinum ATCC 927 | 230, 210 | 237, 212 | 232, 209 | 245, 220 | 145, 106, 78 | 149, 114, 83 | 146, 102, 81 | 160, 115, 80 |
| M. smegmatis ATCC 19420 | 231, 131, 79 | 246, 137, 90 | 239, 138, 87 | 245, 140, 85 | 154, 123, 58, 40, 36, 23 | 152, 129, | 154, 129, 63, 45, 41 | 160, 130 |
| M. haemophilum ATCC 29548 | 310, 130 | 312, 127 | 313, 133 | 325, 140 | 160, 112, 111, 39, 17 | 158, 109 | 163, 108 | 175, 125 |
Reference 27.
Electrophoresis was also performed both with undigested amplicons and with digested amplicons of species known to produce fragments of ≤100 bp and with primers submitted to the PCR protocol in the absence of the target DNA to rule out any possibility that artifacts may confound the interpretation of the PRA patterns obtained with 10% PAGE (data not shown). In no case were bands in the range of primers or primer-dimers observed.
Development of the algorithm.
PRA using 10% PAGE was then performed on strains belonging to 54 Mycobacterium species. Mycobacterium strains were reference strains from the American Type Culture Collection or from the collection of the Italian Reference Laboratory for Mycobacteria and clinical isolates from the Mycobacteriology Laboratory of our hospital. An algorithm was derived which included 32 species already described by others (8, 26, 27) and 22 additional species (Table 3).
TABLE 3.
Algorithm for differentiation of Mycobacterium isolates to species level
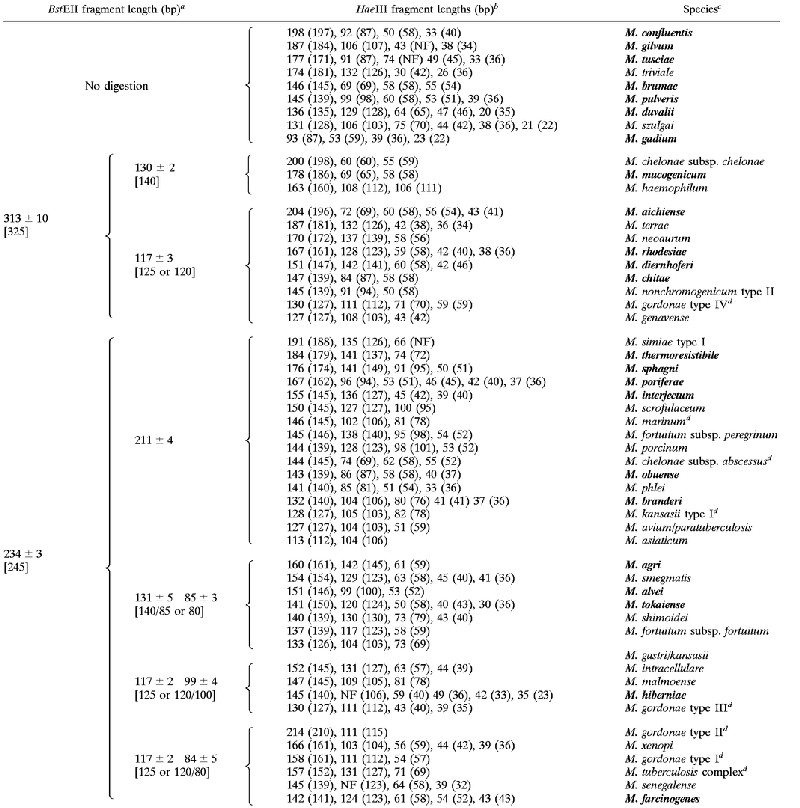 |
Fragment sizes in brackets are those observed for these fragments in previously reported patterns (8, 14, 17, 24, 26).
Fragment sizes in parentheses are those derived from sequence analysis. (NF), restriction site not found in the sequence; NF, fragment not found in the PRA pattern.
Boldface indicates PRA patterns that did not figure in the algorithms of Telenti et al. (27), Taylor et al. (26), and Devallois et al. (8).
Species for which more than two strains were examined; in this case the averaged size was reported (see also Table 6).
In the case of the previously described species, the algorithm based on 10% PAGE generally agreed with those previously published (8, 26, 27). The PRA patterns of the 22 additional species were introduced into the algorithm and a good measure of agreement was established between the size derived from restriction digest electrophoresis and the real length of the fragments derived from the position of the restriction sites in the hsp65 gene sequence of each species. If the sequence was not available in the data bank, we performed DNA sequencing of both hsp65 amplicons and amplicons of 16S rRNA to confirm species identification.
Distinct PRA patterns were obtained for most of the additional species by considering only two or three HaeIII fragments in the size range of 200 to 80 bp. However, in the case of some species, the size of the bands in this range differed by only ±5 bp from the pattern of other species, and bands smaller than 60 bp had to be considered for definitive identification (M. branderi versus M. kansasii; M. abscessus versus M. phlei, M. interjectum versus M. smegmatis, and M. farcinogenes versus M. intracellulare). In addition, some species had only one discriminant band longer than 80 bp, and interpretation of the patterns had to be based on shorter bands than this (M. brumae, M. gadium, M. aichiense, M. chelonae subsp. chelonae, M. chelonae subsp. abscessus, M. hiberniae, and M. senegalense).
As regards the clinical isolates, we studied 65 MOTT and three M. tuberculosis complex strains isolated consecutively in our Clinical Mycobacteriology Laboratory. For rapid identification of the most frequently isolated species, we had already developed a method based on amplification of a sequence of 16S rRNA (13, 21) (see Materials and Methods) and reverse cross-blot hybridization assay (13, 21). This test together with biochemical identification was used for comparison with the PRA results (Table 4). PRA allowed identification of M. simiae and subspecies identification of M. gordonae, M. chelonae, M. avium, and M. fortuitum (Table 5).
TABLE 4.
Summary of results of identification of clinical isolates by different methods
| Organism | No. of isolates tested | No. of isolates identified bya:
|
|||
|---|---|---|---|---|---|
| PRA | PHA | Biochemical testing | AccuProbe | ||
| M. tuberculosis complex | 3 | 3 | 3 | NT | 3 |
| M. gordonae group | 18 | 18 | 18 | 18 | 18 |
| M. chelonae group | 22 | 22 | 22 | 22 | NT |
| M. avium complex | 10 | 10 | 10 | NT | 7 |
| M. xenopi | 4 | 4 | 4 | 4 | NT |
| M. kansasii | 3 | 3 | 3 | NT | 3 |
| M. marinum | 2 | 2 | 2 | 2 | NT |
| M. fortuitum group | 4 | 4 | 4 | 4 | NT |
| M. terrae | 1 | 1 | 1 | 1 | NT |
| M. simiae | 1 | 1 | NT | NT | NT |
PHA, PCR–reverse cross-blot hybridization assay; NT, not tested.
TABLE 5.
Subspecies of mycobacteria identified by PRA
| Species | Subspecies | No. of strains |
|---|---|---|
| M. gordonae | Type I | 5 |
| Type II | 9 | |
| Type III | 2 | |
| Type IV | 2 | |
| M. chelonae | chelonae | 8 |
| abscessus | 14 | |
| M. avium | avium | 9 |
| intracellulare | 1 | |
| M. fortuitum | fortuitum | 4 |
Finally, standard deviations were calculated for both 10% PAGE- and agarose gel electrophoresis-PRA patterns of species for which at least two strains were examined (Table 6). Both 10% PAGE-PRA and agarose gel electrophoresis-PRA yielded reproducible patterns, as the range of variation never exceeded the ±5-bp interval. With 10% PAGE, the average length of BstEII fragments differed from the real size by more than ±5 bp but less than ±10 bp for M. tuberculosis (the 79-bp HaeIII band), M. chelonae subsp. chelonae (the 311-bp band), M. fortuitum subsp. fortuitum (the 79-bp band), and M. marinum (the 230-bp band). In 3% agarose, nearly all species showed at least one fragment whose length differed from the real size by more than ±5 bp. With 10% PAGE, none of the HaeIII fragments differed from the real size by more than ±5 bp, but in 3% agarose nearly all species showed at least one fragment whose length differed from the real size by more than ±5 bp.
TABLE 6.
Standard deviation of molecular sizes of restriction fragments detected after 10% PAGE or 3% agarose gel electrophoresis
| Species (no. of strains) |
BstEII fragment length(s) (bp)
|
HaeIII fragment length(s) (bp)
|
||||
|---|---|---|---|---|---|---|
| Sequence | PAGE | Agarose gel electrophoresis | Sequence | PAGE | Agarose gel electrophoresis | |
| M. tuberculosis complex (3) | 231 | 236 ± 2 | 238 ± 3 | 152 | 156 ± 2 | 153 ± 4 |
| 116 | 116 ± 1 | 118 ± 4 | 127 | 131 ± 2 | 129 ± 5 | |
| 79 | 87 ± 1 | 84 ± 3 | 69 | 70 ± 2 | 77 ± 3 | |
| M. gordonae type I (5) | 231 | 234 ± 5 | 240 ± 4 | 161 | 158 ± 4 | 155 ± 5 |
| 116 | 117 ± 4 | 117 ± 3 | 112 | 111 ± 5 | 120 ± 3 | |
| 79 | 84 ± 5 | 86 ± 5 | 57 | 54 ± 5 | 59 ± 4 | |
| 40, 36, 33 | ||||||
| M. gordonae type II (9) | 231 | 234 ± 5 | 236 ± 2 | 210 | 214 ± 5 | 218 ± 4 |
| 116 | 117 ± 4 | 115 ± 2 | 115 | 111 ± 5 | 110 ± 3 | |
| 79 | 84 ± 5 | 86 ± 2 | ||||
| M. gordonae type III (2) | 231 | 233 ± 3 | 237 ± 5 | 127 | 130 ± 5 | 127 ± 4 |
| 115 | 117 ± 2 | 114 ± 3 | 112 | 111 ± 5 | 114 ± 4 | |
| 94 | 99 ± 4 | 99 ± 2 | 39, 36, 36, 36, 34, 23, 23, 23 | 43 ± 5 | ||
| M. gordonae type IV (2) | 325 | 322 ± 5 | 330 ± 5 | 127 | 130 ± 5 | 135 ± 5 |
| 116 | 118 ± 5 | 126 ± 5 | 112 | 111 ± 4 | 122 ± 5 | |
| 70 | 71 ± 5 | 76 ± 4 | ||||
| 59 | 59 ± 5 | |||||
| 40, 33 | ||||||
| M. chelonae subsp. chelonae (8) | 311 | 303 ± 5 | 303 ± 5 | 198 | 200 ± 5 | 179 ± 5 |
| 131 | 130 ± 5 | 119 ± 4 | 60 | 60 ± 4 | 46 ± 2 | |
| 59 | 55 ± 3 | |||||
| 54 | ||||||
| 23, 9 | ||||||
| M. chelonae subsp. abscessus (14) | 231 | 236 ± 5 | 237 ± 2 | 145 | 144 ± 2 | 145 ± 3 |
| 210 | 211 ± 4 | 211 ± 2 | 69 | 74 ± 0 | 66 ± 4 | |
| 58 | 62 ± 2 | 52 ± 3 | ||||
| 52 | 55 ± 1 | |||||
| 48, 40, 23 | ||||||
| M. fortuitum subsp. fortuitum (4) | 231 | 234 ± 4 | 240 ± 1 | 139 | 137 ± 5 | 149 ± 2 |
| 131 | 131 ± 3 | 119 ± 2 | 123 | 118 ± 3 | 124 ± 3 | |
| 79 | 85 ± 5 | 88 ± 1 | 63 | 59 ± 4 | 85 ± 5 | |
| 54 | ||||||
| M. marinum (2) | 230 | 236 ± 4 | 241 ± 3 | 145 | 146 ± 3 | 155 ± 1 |
| 210 | 211 ± 3 | 211 ± 4 | 106 | 102 ± 4 | 108 ± 2 | |
| 78 | 81 ± 4 | 78 ± 4 | ||||
| 40, 22, 21, 17, 12 | ||||||
| M. avium-M. paratuberculosis (9) | 231 | 236 ± 5 | 233 ± 5 | 127 | 127 ± 3 | 121 ± 5 |
| 212 | 211 ± 3 | 205 ± 5 | 103 | 104 ± 2 | 98 ± 5 | |
| 59 | 51 ± 4 | |||||
| 42, 40, 36, 34 | ||||||
| M. kansasii type I (3) | 231 | 236 ± 5 | 240 ± 5 | 127 | 128 ± 5 | 135 ± 3 |
| 208 | 211 ± 5 | 218 ± 5 | 103 | 105 ± 5 | 115 ± 2 | |
| 78 | 82 ± 5 | 89 ± 5 | ||||
| 42, 42, 38, 36, 34, 17 | ||||||
| M. xenopi (4) | 231 | 234 ± 4 | 241 ± 2 | 161 | 166 ± 3 | 166 ± 1 |
| 119 | 117 ± 2 | 119 ± 1 | 104 | 103 ± 5 | 104 ± 2 | |
| 79 (15) | 84 ± 4 | 87 ± 2 | 59 | 56 ± 5 | 52 ± 5 | |
| 42 | 44 ± 1 | |||||
| 36 | ||||||
DISCUSSION
Identification of mycobacteria by PRA of the hsp65 gene was developed by Telenti et al. and has been established practice since 1993 (27). Thirty-three PRA patterns were originally identified, of which 19 corresponded to single species and 14 were associated with five species (M. flavescens, two types; M. chelonae, two subspecies; M. kansasii, two types; M. gordonae, five types; M. fortuitum, three subspecies). Taylor et al. (26) introduced five additional PRA patterns into Telenti's algorithm (one additional species and four new subtypes of species already described); Devallois et al. (8) introduced 11 additional PRA patterns (five additional species and six subtypes of species already described).
In the present study, we also performed PRA of the hsp65 gene for identification of Mycobacterium species using 10% PAGE for detection of restriction fragments. We evaluated 32 species already described and 22 additional species. For the latter species only single isolates were analyzed, and the RLFP pattern described cannot be considered the discriminant one, since genetic heterogeneity leading to more than one RLFP pattern in a species cannot be excluded.
The PAGE-based method provided more precise estimates than those based on agarose gel electrophoresis of the real sizes of restriction fragments as deduced by sequence analysis and allowed identification of mycobacteria whose PRA patterns were unequivocally identified by fragments shorter than 60 bp. In our study, the size ranges for each data point obtained with the PAGE-based PRA for species already studied could be adjusted within the ranges described in the agarose gel electrophoresis-based algorithm. However, within these ranges, the PAGE-based PRA was better than agarose gel electrophoresis in resolving species with similar HaeIII patterns made by bands differing by ±10 bp. We found that the agarose gel electrophoresis-based PRA yielded fragment lengths which often differed from the real sizes by more than ±10 bp. Most of the additional species we introduced are not frequently isolated from humans, but their identification could improve our understanding of their clinical significance.
Recently, a novel diagnostic algorithm was proposed based on PRA of the 16S-23S DNA spacer sequence (19, 20). Eighty-three patterns which identified 48 species, 40 subspecies, and 4 subtypes were described. The method was proposed as an alternative to PRA of the hsp65 gene. With both methods, most species yielded unique patterns, but some were more variable. For instance, using the 16S-23S DNA spacer-based method, 10 different patterns were observed for M. fortuitum and 1 was observed fro M. gordonae, whereas with the hsp65 gene-based method, 2 and 6 patterns were found, respectively, in the same species. However, intraspecies variability should not be considered a drawback of the PRA method if the patterns are distinct. This can help us to trace the epidemiology of MOTT (31), in terms of both geographical distribution and pathogenicity (24, 26), as is clearly revealed by studies on M. kansasii demonstrating that only some subtypes are associated with human infections (2, 5, 28).
To achieve restriction fragment length polymorphism detection by automatic fluorescent fragment analysis, Hernandez et al. (11) combined PRA of the hsp65 gene and of the hypervariable region of the 16S rRNA gene with two enzymes. Height data points were obtained (two for each gene and each enzyme). Unique patterns were obtained for the 19 species analyzed. No subtypes of M. fortuitum and M. gordonae were detected, since the fragments identifying the types were not end fragments and were not labeled by the fluorescent dye (11). The method cannot be applied to identification of species where end fragments are not distinctive. In addition, it relies on very expensive instrumentation.
In conclusion, the many studies bearing witness to the specificity, rapidity, cost-effectiveness, and efficiency of PRA-based methods for identification of mycobacteria have now made the routine application of this technology possible. However, laboratories wishing to adopt them should produce their own algorithms with the species most frequently isolated. The technical improvement provided by 10% PAGE, as shown by the results of our study, could certainly enhance the performance of this assay method.
ACKNOWLEDGMENTS
This work was supported by a grant from the Italian Ministry of the University and Scientific Research (60%). F.B. was the recipient of a doctoral fellowship from the University of Verona.
REFERENCES
- 1.Alcaide A, Benitez M A, Escribà J M, Martin R. Evaluation of the BACTEC MGIT960 and MB/BacT system for recovery of mycobacteria from clinical specimens and for species identification by DNA AccuProbe. J Clin Microbiol. 2000;38:398–401. doi: 10.1128/jcm.38.1.398-401.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alcaide F, Richter I, Bernasconi C, Springer B, Hagenau C, Schulze-Robbecke R, Tortoli E, Martin R, Bottger E C, Telenti A. Heterogeneity and clonality among isolates of Mycobacterium kansasii: implications for epidemiological and pathogenicity studies. J Clin Microbiol. 1997;35:1959–1964. doi: 10.1128/jcm.35.8.1959-1964.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Avaniss-Aghajani E, Jones K, Holtzman A, Aronson T, Glover N, Boian M, Froman S, Brunk C F. Molecular technique for rapid identification of mycobacteria. J Clin Microbiol. 1996;34:98–102. doi: 10.1128/jcm.34.1.98-102.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bahrmand A R, Bakayeva T G, Bakayev V V. Use of restriction enzyme analysis of amplified DNA coding for the hsp65 gene and polymerase chain reaction with universal primer for rapid differentiation of mycobacterium species in the clinical laboratory. Scand J Infect Dis. 1998;30:477–480. doi: 10.1080/00365549850161476. [DOI] [PubMed] [Google Scholar]
- 5.Bloch K C, Zwerling L, Pletcher M J, Hahn J A, Geberding J L, Ostroff S M, Vugia D J, Reingold A L. Incidence and clinical implications of isolation of Mycobacterium kansasii: results of a 5-year, population-based study. Ann Intern Med. 1998;129:698–704. doi: 10.7326/0003-4819-129-9-199811010-00004. [DOI] [PubMed] [Google Scholar]
- 6.Brunello F, Favari F, Fontana R. Comparison of MB/BacT and Bactec 460 TB systems for recovery of mycobacteria from various clinical specimens. J Clin Microbiol. 1999;37:1206–1209. doi: 10.1128/jcm.37.4.1206-1209.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.da Silva Rocha A, da Costa Leite C, Torres H M, de Mirando A B, Pires Lopes M Q, Degrave W M, Suffys P N. Use of PCR-restriction fragment length polymorphism analysis of the hsp65 gene for rapid identification of mycobacteria in Brazil. J Microbiol Methods. 1999;37:223–229. doi: 10.1016/s0167-7012(99)00062-7. [DOI] [PubMed] [Google Scholar]
- 8.Devallois A, Goh K S, Rastogi N. Rapid identification of mycobacteria to species level by PCR-restriction fragment length polymorphism analysis of the hsp65 gene and proposition of an algorithm to differentiate 34 mycobacterial species. J Clin Microbiol. 1997;35:2969–2973. doi: 10.1128/jcm.35.11.2969-2973.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Devallois A, Picardeau M, Goh K S, Sola C, Vincent V, Rastogi N. Comparative evaluation of PCR and commercial DNA probes for detection and identification to species level of Mycobacterium avium and Mycobacterium intracellulare. J Clin Microbiol. 1996;34:2756–2759. doi: 10.1128/jcm.34.11.2756-2759.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hance A J, Grandchamp B, Levy-Frebault V, Lecossier D, Rauzier J, Bocart D, Giquel B. Detection and identification of mycobacteria by amplification of mycobacterial DNA. Mol Microbiol. 1989;3:843–849. doi: 10.1111/j.1365-2958.1989.tb00233.x. [DOI] [PubMed] [Google Scholar]
- 11.Hernandez S M, Morlock G P, Butler W R, Crawford J T, Cooksey R. Identification of Mycobacterium species by PCR-restriction fragment length polymorphism analyses using fluorescence capillary electrophoresis. J Clin Microbiol. 1999;37:3688–3692. doi: 10.1128/jcm.37.11.3688-3692.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kirschner P, Springer B, Vogel U, Meier A, Wrede A, Kiekenbeck M, Bange F C, Bottger E C. Genotyping identification of mycobacteria by nucleic acid sequence determination: report of a 2-year experience in a clinical laboratory. J Clin Microbiol. 1993;31:2882–2889. doi: 10.1128/jcm.31.11.2882-2889.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kox L F F, van Leeuwen J, Knijper S, Jansen H M, Kolk A H J. PCR assay based on DNA coding for 16S rRNA for detection and identification of mycobacteria in clinical samples. J Clin Microbiol. 1995;33:3225–3233. doi: 10.1128/jcm.33.12.3225-3233.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Picardeau M, Prod'Hom G, Raskine L, LePennec M P, Vincent V. Genotypic characterization of five subspecies of Mycobacterium kansasii. J Clin Microbiol. 1997;35:25–32. doi: 10.1128/jcm.35.1.25-32.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Plikaytis B B, Plikaytis B D, Yakrus M A, Butler R, Woodley C L, Silcox V A, Shinnick T M. Differentiation of slowly growing Mycobacterium species, including Mycobacterium tuberculosis, by gene amplification and restriction fragment length polymorphism analysis. J Clin Microbiol. 1992;30:1815–1822. doi: 10.1128/jcm.30.7.1815-1822.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rastogi N, Goh K S, Berchel M. Species-specific identification of Mycobacterium leprae by PCR-restriction fragment length polymorphism analysis of the hsp65 gene. J Clin Microbiol. 1999;37:2016–2019. doi: 10.1128/jcm.37.6.2016-2019.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Richter E, Niemann S, Rüsch-Gerdes S, Hoffner S. Identification of Mycobacterium kansasii by using a DNA probe (AccuProbe) and molecular techniques. J Clin Microbiol. 1999;37:964–970. doi: 10.1128/jcm.37.4.964-970.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ringuet H, Akoua-Koffi C, Honore S, Varnerot A, Vincent V, Berche P, Gaillard J L, Pierre-Audigier C. hsp65 sequencing for identification of rapidly growing mycobacteria. J Clin Microbiol. 1999;37:852–857. doi: 10.1128/jcm.37.3.852-857.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Roth A, Fischer M, Hamid M E, Michalke S, Ludwig W, Mauch H. Differentiation of phylogenetically related slowly growing mycobacteria based on 16S–23S rRNA gene internal transcribed spacer sequences. J Clin Microbiol. 1998;36:139–147. doi: 10.1128/jcm.36.1.139-147.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Roth A, Reischl U, Streubel A, Naumann L, Kroppenstedt R M, Habicht M, Fischer M, Mauch H. Novel diagnostic algorithm for identification of mycobacteria using genus-specific amplification of the 16S–23S rRNA gene spacer and restriction endonucleases. J Clin Microbiol. 2000;38:1094–1104. doi: 10.1128/jcm.38.3.1094-1104.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sanguinetti M, Posteraro B, Ardito F, Zanetti S, Cingolani A, Sechi L, De Luca A, Ortona L, Fadda G. Routine use of PCR-reverse cross-blot hybridization assay for rapid identification of Mycobacterium species growing in liquid media. J Clin Microbiol. 1998;36:1530–1533. doi: 10.1128/jcm.36.6.1530-1533.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shinnick T M. The 65-kilodalton antigen of Mycobacterium tuberculosis. J Bacteriol. 1987;169:1080–1088. doi: 10.1128/jb.169.3.1080-1088.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Springer B, Stockman L, Teschner K, Roberts G D, Bottger E C. Two-laboratory collaborative study on identification of mycobacteria: molecular versus phenotypic methods. J Clin Microbiol. 1996;34:296–303. doi: 10.1128/jcm.34.2.296-303.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Steingrube V A, Gibson J L, Brown B A, Zhang Y, Wilson R W, Rajagopalan M, Wallace R J., Jr PCR amplification and restriction endonuclease analysis of a 65-kilodalton heat shock protein gene sequence for taxonomic separation of rapidly growing mycobacteria. J Clin Microbiol. 1995;33:149–153. doi: 10.1128/jcm.33.1.149-153.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Talhari S. Diagnosis, classification and prognosis. Int J Lepr Other Mycobact Dis. 1996;64(Suppl.):13–S15. [PubMed] [Google Scholar]
- 26.Taylor T B, Patterson C, Hale Y, Safranek W W. Routine use of PCR-restriction fragment length polymorphism analysis for identification of mycobacteria growing in liquid media. J Clin Microbiol. 1997;35:79–85. doi: 10.1128/jcm.35.1.79-85.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Telenti A, Marchesi F, Balz M, Bally F, Bottger E C, Bodmer T. Rapid identification of mycobacteria to the species level by polymerase chain reaction and restriction enzyme analysis. J Clin Microbiol. 1993;31:175–178. doi: 10.1128/jcm.31.2.175-178.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tortoli E, Simonetti M T, Lacchini C, Urbano P. Tentative evidence of AIDS-associated biotype of Mycobacterium kansasii. J Clin Microbiol. 1994;32:1779–1782. doi: 10.1128/jcm.32.7.1779-1782.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vaneechoutte M, Beenhouwer H D, Claeys G, Verschraegen G, Rouk A D, Paepe N, Elaichouni A, Portaels F. Identification of Mycobacterium species by using amplified ribosomal DNA restriction analysis. J Clin Microbiol. 1993;31:2061–2065. doi: 10.1128/jcm.31.8.2061-2065.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.van Soolingen D, Hermans P W M, de Haas P E W, Soll D R, van Embden J D A. Occurrence and stability of insertion sequences in Mycobacterium tuberculosis complex strains: evaluation of an insertion sequence-dependent DNA polymorphism as a tool in the epidemiology of tuberculosis. J Clin Microbiol. 1991;29:2578–2586. doi: 10.1128/jcm.29.11.2578-2586.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wayne L G, Sramek H A. Agents of newly recognized or infrequently encountered mycobacterial disease. Clin Microbiol Rev. 1992;5:1–25. doi: 10.1128/cmr.5.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]


