Visual Abstract
Abstract
Evaluation of minimal residual disease (MRD) during first-line treatment and after salvage therapy is part of the standard management of acute lymphoblastic leukemia (ALL). Persistent or recurrent MRD is one of the most relevant prognostic factors and identifies a group of patients with resistance to standard chemotherapy. These patients have a high risk of relapse despite continued first-line therapy. Although stem cell transplantation (SCT) is an appropriate strategy, patients with high MRD show an increased relapse rate even after SCT. Approximately one-quarter of adult ALL patients develop an MRD failure, defined as MRD above 0.01% after standard induction and consolidation. The best time point and level of MRD for treatment modification are matters of debate. In order to eradicate MRD and thereby improve chances for a cure, new targeted compounds with different mechanisms of action compared to chemotherapy are being utilized. These compounds include monoclonal antibodies, chimeric antigen receptor T cells, and molecular targeted compounds. Essential factors for decision-making, available compounds, and follow-up therapies are discussed.
Learning Objectives
Understand the relevant factors for treatment decisions based on the individual course of MRD
Assess the available approaches and their impact on response and overall outcome
CLINICAL CASE
A 47-year-old woman with Philadelphia chromosome (Ph)/ BCR-ABL-negative acute lymphoblastic leukemia (ALL) received induction phase 1 and 2 chemotherapy according to a pediatric-based regimen (GMALL 08/2013 trial). After induction 1 she achieved a hematologic complete remission (CR). Minimal residual disease (MRD) testing using quantitative polymerase chain reaction (PCR) of clonal immunoglobulin (Ig)/T-cell receptor (TCR) rearrangements (Ig/TCR-PCR) was conducted in a central reference laboratory and revealed a level of 0.1%. When MRD results became available, the first consolidation had already been completed. The options for treatment decisions in this situation are discussed.
Introduction
For more than 2 decades, MRD has been routinely measured in patients with ALL and is now considered the most important prognostic factor in the disease.1,2 MRD testing identifies patients with insufficient responses to standard chemotherapy due to underlying disease biology.3 Whereas the rapid achievement of MRD negativity is associated with an excellent prognosis, the persistence of MRD during standard therapy for ALL is strongly associated with relapse despite continued chemotherapy.4 MRD has been shown to be prognostic in pediatric and adult ALL, in all traditionally defined risk groups, including high-risk (HR) groups such as KMT2A-positive ALL, and in the context of different treatment protocols. Due to its prognostic importance, it is standard to measure MRD in all adults with ALL.
Methods for MRD detection
Four approaches are routinely available for MRD evaluation in ALL: quantitative Ig/TCR-PCR, multiparameter flow cytometry of leukemia-specific surface markers (at least 6 color), measurement of Ig/TCR rearrangements based on next-generation sequencing, and real-time quantitative PCR of BCR-ABL or other specific fusion genes. The techniques, advantages, and disadvantages of the different methods have been described elsewhere.5 If MRD measurement is used as the basis for treatment decisions, several prerequisites and standardization steps should be fulfilled (Table 1).
Table 1.
Prerequisites for MRD testing as a basis for treatment decisions
| Optimal material | • Material from primary diagnosis (aspirate, biopsy) • Sufficient material and blast content • Selection of correct material (PB/BM) |
| Skilled laboratory | • Experience (number of ALL cases) • Participation in quality-control rounds • International standards for methods |
| Reporting of results | • Timely and reliable reporting • Results for each time point should include ○ Level of MRD ○ Sensitivity in case of a negative result ○ Further specification in case of nonquantifiable MRD |
| Terminology for MRD response | • MRD response is evaluated in patients with hematologic CR • Clear definition of results similar to definition of hematologic CR6,7 MRD CR: ○ MRD-negative with a minimum sensitivity of 0.01% MRD failure: ○ MRD-positive ≥0.01% MRD intermediate: ○ All other types of results, eg, ○ MRD-positive below 0.01% ○ Nonquantifiable but detectable MRD below 0.01% (PNQ) ○ MRD-negative with insufficient sensitivity |
The selection of optimal material for MRD testing—ie, peripheral blood (PB) or bone marrow (BM)—depends on the applied methods and aims of MRD detection. Whereas in B-cell precursor ALL (BCP-ALL) the MRD level in the PB tends to be lower than in the BM when evaluated with Ig/TCR-PCR, for early detection of upcoming relapses the BM appears to be more suitable. In T-cell ALL (T-ALL) a strong correlation exists between MRD levels in the PB and BM, and therefore follow-up tests for relapse identification may rely on PB.8 The selection of materials may change if more sensitive methods for MRD testing become available.
The chances of identifying a suitable marker through Ig/TCR- PCR is around 95%, and about 90% of ALL patients display a leukemia-associated phenotype.5 If the preferred method does not identify a clone-specific MRD marker, alternative methods should be applied, such as flow cytometry if primarily Ig/TCR- PCR was performed or vice versa. This effort may include the quantification of specific chromosome aberrations, such as KMT2A rearrangements.
Relevant factors for decision-making
Time point
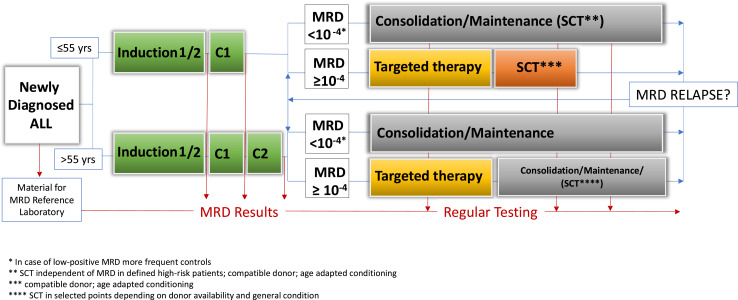
Making treatment decisions based on MRD failure at very early time points risks allocating patients slower to respond to the standard therapy to an HR group (with potential stem cell transplantation [SCT] indication). Using very late time points bears the risk that a significant proportion of patients with MRD failure will develop a hematologic relapse before any action can be taken. In the GMALL trials, the time point with the most prominent negative prognostic impact regarding MRD failure was after consolidation 1, approximately 3 to 4 months after diagnosis, when all relevant drugs had been administered at least once (Figure 1).4 In other treatment settings, postinduction and post-early consolidation time points have been identified as the most prognostic. It is important to recognize that the most prognostic time point may differ according to regimen and intended treatment decisions.
Figure 1.
Flow of MRD surveillance and treatment decisions (GMALL strategy).
Level of MRD
The level of blast cells during first-line treatment is a continuum. Conventional cytomorphology can identify persistent lymphoblasts present at a level of approximately 5% or higher. Below 5% the more sensitive MRD detection methods typically detect lymphoblasts to a level of 0.01%. Most reports on the prognostic impact of MRD on outcome refer to the persistence of MRD at levels of 0.01% to 5% simply due to the sensitivity of the available methods; the significance of persistent MRD at levels less than 0.01% is less clear. In addition to the clear correlation between MRD level and relapse risk is a correlation between MRD level and time to hematologic relapse.9,10 In the context of the GMALL trials, patients with MRD above 0.1% relapsed at a median of 4.9 months, while patients with MRD above 0.01% but less than 0.1% relapsed at a median of 7.6 months.4
Whereas MRD testing based on flow cytometry or next-generation sequencing usually does not provide sensitivity or a quantifiable range of results at each time point, this is routinely possible for Ig/TCR-PCR. Thus, it becomes evident that a significant proportion of patients, around 25% tested with Ig/TCR-PCR, do not meet the criteria of MRD CR or MRD failure (Table 1).11 The majority of patients in this group have detectable MRD below 0.01% or positive, nonquantifiable MRD (PNQ). Since the quantifiable range is usually above 0.01%, PNQ is most frequently MRD below 0.01%. The numbers in this “gray-zone” group might be even larger when methods with higher sensitivity are applied.
Patients with an indeterminate MRD response potentially have an intermediate prognosis compared to those with MRD CR and MRD failure. For instance, overall survival (OS) in the GMALL trials was 83% for patients with an MRD CR, 43% for patients with an MRD failure, and 68% for patients with intermediate MRD.11 In pediatric ALL a correlation between MRD level and survival was seen as well. The relapse risk ranged from 4% for negative MRD, 6% for MRD below 0.01%, and 8% for 0.01 to 0.1% to 16% for MRD between 0.1 and 1%.10
Thus, the level of MRD matters for MRD-based treatment decisions (Figure 1). Whereas patients with MRD failure may qualify for targeted therapies and/or SCT, patients with PNQ or low-positive MRD may be candidates for closer surveillance.
Why should MRD be eradicated?
MRD represents the persistence of malignant blasts resistant to standard chemotherapy. These cells are the source of future relapse and potential clonal evolution under the selection pressure of ongoing chemotherapy. With increasing numbers of residual malignant cells persisting in spite of standard chemotherapy comes an increasing statistical risk of acquiring potential deleterious additional biologic features. Persistent MRD is on the continuum of primary refractory hematologic disease but has a lower disease burden and attenuated disease growth dynamics. In addition, compared to patients with frankly refractory disease, patients in hematologic remission with persistent MRD are in better general condition and are less prone to the general risks associated with massive bone marrow infiltration and tumor load. Therefore, treating MRD before hematologic relapse is attractive because treatment may be both better tolerated and more effective.
Standard treatment options for MRD eradication
The best standard approach to eradicating measurable MRD in a large proportion of fitter and younger patients is with pediatric-based induction therapy. Approximately one-quarter of adult patients display MRD failure after the initial phase of therapy.
Continue or intensify standard chemotherapy
In patients with MRD failure, further chemotherapy adds little additional response. In the GMALL protocol, the rate of eventual achievement of MRD CR in patients with MRD failure before consolidation 2 continuing with chemotherapy was only 25%.11 Thus, continued chemotherapy in adult ALL bears the risk that patients will accrue additional toxic effects with limited benefits in terms of disease control.
SCT for eradication of MRD
Data indicate that SCT can improve outcomes of patients with MRD. The GRAALL group demonstrated the advantages of SCT specifically in MRD-positive patients.12 Similarly, the GMALL data indicate more favorable results for transplanted vs nontransplanted patients with MRD failure.4 However, transplanted patients represent a select group of patients with an available donor, with an acceptable general condition, and, most importantly, with a remission durable enough to undergo SCT. Furthermore, there is broad evidence that the MRD level before SCT influences the success of SCT. Patients with MRD above 0.01% before SCT have a poorer outcome after SCT compared to MRD-negative patients, mainly due to increased risk of relapse.13-15 Several factors may affect SCT outcomes in MRD-positive patients. The MRD level appears to have an impact on the risk of relapse. In an Italian study with an MRD-guided SCT indication, the outcome of SCT was poor in patients with MRD of 0.1% or higher before SCT, whereas patients with MRD between 0.01% and 0.1% and those with negative or lower MRD had similar more favorable outcomes.14 The direct impact of SCT on MRD level should be measured by comparing the results before and immediately after SCT. Although this comparison is extremely important in order to make decisions regarding posttransplant strategies, data are rare. One trial reported a 3-log reduction of MRD at day plus 100 compared to the level before SCT in a mixed cohort of adult BCR-ABL-negative and -positive patients. However, evidence of persistent or recurrent MRD was detected in 16 out of 36 (44%) samples. Patients with MRD detection after SCT had an extremely high relapse risk of 80%.16 These data indicate that any transplantation in MRD-positive patients should be closely followed by MRD testing, starting as early as 30 days after SCT in order to identify MRD relapses of persistence and to consider additional approaches for eradication.
Targeted therapies for MRD eradication
Since persistent MRD is characterized by resistance to chemotherapy, its successful eradication requires treatment with compounds having different mechanisms of action. A variety of potential targeted drugs are theoretically applicable to ALL.17 With the exception of Ph-positive ALL, promising molecular targets are limited in BCP-ALL, whereas surface markers such as CD20 (around 40%), CD19 (>90%), and CD22 (>90%) are present in most cases. In T-ALL, immunotherapies currently have no role, and no potential molecular targets or therapeutic compounds have been identified.
Targeted immunotherapies of MRD in BCP-ALL
Rituximab
A randomized trial has demonstrated that the addition of rituximab to standard chemotherapy in CD20+ ALL can contribute to an improved outcome.18 Although results on MRD response are not conclusive, adding rituximab to chemotherapy in patients with MRD persistence may be an option when newer immunotherapies are not available or affordable.
Blinatumomab
Blinatumomab is a bispecific CD19/CD3-directed antibody that promotes the serial killing of CD19+ blast cells via the activation of native T cells. The first trial of blinatumomab for MRD in ALL was a small pilot trial with 20 patients displaying persistent or recurrent MRD above 0.01%.19 A molecular response was documented in 80% of the patients, and 45% received SCT. Relapse-free survival was 78%. Notably, 5 patients remained in long-term remission without receiving SCT, indicating a potential complete eradication of the disease reservoir.20 Based on these results, the international phase 2 BLAST trial was designed for patients with persistent MRD above 0.1%. Patients with MRD that persisted after salvage therapy for a prior hematologic relapse (CR2+) accounted for one-third of those enrolled. Overall, 113 patients were included. Importantly, blinatumomab in the MRD setting is administered without dose stepping since the risk of a cytokine release syndrome is low due to the limited leukemia burden. It is essential to give intrathecal prophylaxis before starting and between cycles and to regularly check for signs of potential extramedullary relapse. In addition to the loss of the target CD19, extramedullary manifestations have been shown to be one cause of failure after blinatumomab therapy. This is potentially due to the limited activity of the compound in sanctuary spaces.21
The MRD response rate in the BLAST trial was 88% (80% complete MRD response) after 1 cycle. Few additional responses were achieved after a second cycle, but the responses were potentially deepened. No differences were seen in terms of response with regard to MRD level, age, status of first or later remission, or other clinical factors.22 After long-term follow-up, the median OS was 36 months, which compares favorably to a median survival of 7.7 months for blinatumomab in hematologic relapse.23 The 5-year OS was 43%. Survival was significantly better in patients with an MRD response compared to those without (median not reached vs 14 months).24
Importantly, 67% of the patients in the BLAST trial received SCT in ongoing remission after blinatumomab. The median age of transplanted patients was 42 years; 34% had a mismatch donor, and 74% received myeloablative conditioning. Although the overall outcome appeared similar for transplanted and nontransplanted patients with a complete MRD response (median not reached vs 56 months), there were significant differences. Among transplanted patients, 40% remained alive in CR, 23% relapsed, and 36% died in CR. Among nontransplanted patients, 19% remained alive in CR (N = 7), 8% died in CR, and 72% relapsed.24 These data suggest two approaches for treatment optimization. While SCT appears to be the preferred option to achieve long-term remission in patients responding to blinatumomab, it should be restricted to patients anticipated to tolerate the procedure well based on age and/or comorbidities. Patients unable, unwilling, or not advised to pursue SCT should be offered consolidation and/or maintenance treatment. The best approach (and when to transition from blinatumomab back to standard approaches) is not certain but may include reinstitution of standard chemotherapy, standard low-dose maintenance, and/or “refresher” cycles with blinatumomab. This approach requires close MRD monitoring.
The toxicity of blinatumomab in the MRD setting appears to be less pronounced than in the relapsed/refractory (R/R) setting. Overall, 60% of the patients in the MRD trial developed grade 3 or 4 adverse events compared to 86% in the Tower trial for R/R ALL.22,23 The incidence of cytokine release syndrome was 1.7% compared to 4.9%. Lower incidences were reported for grade 3 and 4 elevations of liver enzymes (4%-5% vs 13%) or neutropenias (16% vs 38%). Consequently, the rate of grade 3 and 4 infections was 34% in the R/R setting but appeared to be less than 3% in the MRD setting.22,23
The overall incidence of neurological events (grade 3-4), however, was comparable, at 13% and 9.4% in the MRD setting and R/R setting, respectively.22,23 The terminology of the investigators' neurological event reporting was not standardized in both trials, and many neurological events are part of syndromes accumulating in individual patients. Therefore, it is difficult to compare both studies in detail. The fact that the infusion was started without dose stepping in MRD-positive patients may contribute to the equal levels of neurological events in these settings. Notably, most adverse events occur during the first cycle and can be well handled with increasing experience with the compound. Nevertheless, in order for practitioners to detect and mitigate neurological events early, patients should begin treatment as inpatients, and both patients and caregivers should receive strict instructions about potential neurological symptoms when they leave the ward.
The GMALL now includes blinatumomab treatment for all B-cell ALL patients with persistent MRD above 0.01% after consolidation 1 in the ongoing first-line trial 08/2013 (NCT02881086; Figure 1). The GMALL is also conducting a trial with blinatumomab for patients with MRD that includes those with MRD less than 0.01%, the level required for enrollment in the BLAST trial (NCT03109093). The overall MRD response rate in this trial was 82%, with 67% achieving complete MRD responses. The 2-year OS was 64%, with 67% of patients transplanted in CR. SCT-related mortality was 12% with only 3 relapses, whereas 13 relapses occurred in nontransplanted patients. The trial indicates a potentially poorer survival rate in patients achieving an incomplete response after 1 cycle of blinatumomab. This result suggests that patients with an incomplete MRD response to blinatumomab may benefit from additional, alternative MRD-directed therapy, as evident from the GMALL Molact1 trial.25 Further results with blinatumomab in MRD-positive ALL are summarized in Table 2.
Table 2.
Results with blinatumomab in MRD-positive ALL
| Author | Year | N | Population | MRD response | SCT rate (%) | Overall survival |
|---|---|---|---|---|---|---|
| Topp et al19 Gökbuget et al20 |
2011 | 10 | MRD > 0.01% Adult: 47 (20-77) y PCR |
80% | 45 | Nr |
| Gökbuget et al24,26 | 2018 2020 |
110 | MRD > 0.1% First/later CR Adult: 45 (18-76) y PCR |
88% 80% CR 83% CR1 73% CR2 |
67 | Median 36 mo 43% at 5 y |
| Gökbuget et al25 | 2020 | 64 | MRD > 0.01% First CR Adult: 44 (18-83) y PCR |
82% 67% CR |
67 | Median nr 64% at 2 y |
| Haddad et al27 | 2021 | 31 | MRD > 0.01% First/later CR Adult: 42 (22-84) y Ph-pos/neg Flow |
74% CR 84% Ph-neg 62% Ph-pos |
35 | Median nr 63% at 3 y |
| Keating et al28 | 2019 | 15 | MRD > 0.01% Pediatric: 0-21 y Flow |
14/15 MRD-neg | 100* | Median nr 93% 1 y |
| Locatelli et al29 | 2020 | 12 | MRD > 0.1% Pediatric PCR |
92% | Nr | Nr |
| Locatelli et al30 | 2021 | 29 | MRD > 0.01% Ped R/R: 1-18 y Mainly PCR |
93% | Nr | Nr |
| Bassan et al31 | 2021 | 23 | MRD > 0.01% Adult: 18-65 y PCR |
87% | Nr | Nr |
Selected for SCT. Neg, negative; nr, not reported; pos, positive.
Inotuzumab ozogamicin
Inotuzumab is a CD22-directed calicheamicin-conjugated antibody that induces high rates of hematologic and MRD response in R/R ALL.32 It is being tested now in first-line protocols as part of induction or consolidation therapy. While results from ongoing trials in the MRD setting (NCT03913559, NCT03441061, NCT03610438) are not yet available, it can be extrapolated that inotuzumab should be active in the MRD setting as well. The lower tumor burden offers the potential to reduce dosages and thereby the risk of inotuzumab-associated toxicities, such as prolonged cytopenia or veno-occlusive disease.
CAR T cells
CD19-directed chimeric antigen receptor (CAR) T cells have mainly been explored in R/R ALL. However, by bridging therapies after study inclusion, CAR T cells have been infused in a considerable proportion of patients in hematologic CR with persistent MRD. OS was significantly better if CAR T cells were used in patients with a lower disease burden—ie, in an MRD setting compared to persistent R/R disease. In one trial, the median OS was 20 months in patients with low disease burden compared to 12 months in patients with higher-burden disease.33 An analysis of the real-world application of CAR T cells in pediatric patients revealed that 22% were infused in the MRD setting and 25% without detectable disease. The OS was 85% and 95% at 1 year, respectively, in these populations compared to an OS of 58% in patients infused during frank cytologic relapse.34 Since the toxicities of CAR T cells are also less pronounced with lower disease burden, it will be of great interest to design future trials with CAR T cells in MRD-positive ALL.
Molecular targeted therapies of BCP-ALL
MRD responses are lower in HR molecular subtypes of ALL. For instance, patients with Ph-like ALL show a high rate of MRD failure and would potentially benefit from the addition of targeted therapies to standard chemotherapy to improve the chance of achieving an MRD response.35,36 However, the proportion of patients with druggable lesions such as ABL-class fusions is rather low in adults (<5%). Tyrosine kinase inhibitors and JAK inhibitors are being tested in clinical trials and are usually administered after standard induction—ie, in the MRD setting (NCT02723994). Single-case series indicate efficacy.37 One case collection reports on 12 patients with ABL-class fusions treated with a tyrosine kinase inhibitor for persistent MRD. MRD negativity was achieved in 4 of 12 patients at the first follow-up and in 10 of 12 patients as best response.38 Trials adding ruxolitinib to standard chemotherapy in newly diagnosed Ph-like ALL with CRLF2-R or other JAK pathway alterations are ongoing.39
Targeted therapies in T-ALL
Nelarabine is a compound with specific activity in R/R T-ALL. Based on these results, it has been successfully integrated into the first-line therapy of pediatric ALL as a consolidation treatment.40 As for all monotherapies in ALL, it can be assumed to be more efficacious when used in patients with a lower leukemia burden. The GMALL study group has therefore integrated nelarabine into first-line therapy for T-ALL patients with MRD failure, similar to the approach with blinatumomab for B-ALL. There are also case reports detailing the successful use of CD38 antibodies in the MRD setting.41,42 It may be preferable to test other compounds of interest in T-ALL, such as NOTCH inhibitors or venetoclax, in the MRD setting since the impact of a single drug in full hematologic relapse of T-ALL is certainly limited.
MRD eradication in specific situations
Older patients
Older patients are treated with dose-reduced regimens and are therefore at higher risk of experiencing MRD failure. Therefore, it is of utmost importance to measure MRD and to act on MRD in a manner similar to younger patients. This approach has already been tested in first-line trials. The GMALL BOLD trial applies a dose-reduced chemotherapy induction with a hematologic response rate of around 70%, whereas nearly all patients remain MRD-positive.43 This is followed by blinatumomab cycles alternating with standard chemotherapy cycles. The first results in a limited number of patients show a promising rate of MRD negativity. Outside of clinical trial, we use a dose-reduced pediatric-based chemotherapy (GMALL-Elderly) and initiate targeted therapies in case of MRD failure after consolidation 2 (Figure 1).
Molecular relapse
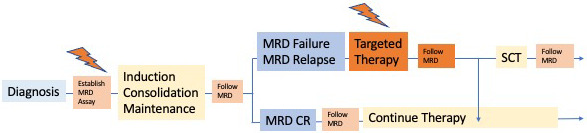
Although MRD evaluation is part of many protocols in the initial treatment phase, the follow-up tests to be used during therapy are less well defined. The balance between the cost and burden of multiple bone marrow evaluations and the chance of identifying a molecular relapse is debated. Clearly, molecular relapses have similar poor prognoses as molecular failure, and the same treatment principles apply.4 In the GMALL group, MRD follow-up investigations therefore take place every 2 to 3 months during consolidation and maintenance and approximately 1 year after the end of treatment (Figure 2).
Figure 2.
How I manage ALL based on MRD.
MRD and other prognostic factors
The definition of prognostic factors is not standardized in adult ALL. Whether the prognostic impact of MRD is similar in all potential risk groups, including distinct genetic settings, has not been established. Thus, the French GRAALL group reported that MRD level discriminated HR patients independent of the presence of HR genetics (MLL, IKZF-1 gene deletion) in BCP-ALL. In T-ALL, however, an HR genetic profile (the absence of NOTCH1/FBXW mutation and/or N/K-RAS mutation and/or PTEN gene alteration) discriminated HR patients independently of MRD level.44 In pediatric ALL, the genetic subtype correlated not only to the time and extent of the MRD response but also to the prognostic impact. Although the level of MRD correlated to relapse risk in all subtypes, there was a lower relapse risk in low-risk compared to HR genetics patients with similar MRD levels.10 A larger European group reported a new integrated risk model covering genetic markers and MRD as a continuous marker.45
Specific HR subgroups of pediatric and adult ALL, such as Ph-negative BCR-ABL (Ph)-like ALL or early T-precursor ALL, are known to present with higher rates of MRD failure. The optimal approaches remain to be defined. As discussed, few targeted approaches are available in T-ALL. An MRD-based SCT indication was reported as beneficial in one trial for early T-precursor ALL.46 In Ph-like ALL, there is increasing evidence of the efficacy of immunotherapies such as blinatumomab, which has been demonstrated for CRLF2-rearranged Ph-like ALL.47 According to a recent abstract report, all patients with Ph-like ALL and MRD failure (N = 10) converted to an MRD CR after 1 cycle.31 Therefore, a pragmatic approach for all patients might be to identify Ph-like ALL through MRD testing instead of up-front because, most probably, such patients will be allocated to the group of MRD failure.
Overall, the impact of MRD on outcome and the therapeutic consequences will have to be defined based on distinct treatment protocols.
MRD-based clinical trials and standard of care
Generally, a toolkit is required to make proper treatment decisions with the goal of eradicating MRD. For each treatment protocol, the correct time point, level of MRD, material, terminology, and treatment consequences should be defined, and all related issues, such as donor search and even decision-making for subsequent SCT and SCT procedures, should be as well standardized as possible. Consequently, for trials in MRD-positive patients with hematologic CR, the response criteria have to be adapted (Table 1). This applies to the MRD level and to the exact time point. Referring to MRD positivity without reporting the level or to a cumulative MRD response without a distinct time point will make conclusions difficult. Furthermore, it is essential to consider the fact that MRD mainly affects relapse risk. Therefore, remission duration or the cumulative incidence of relapse or both are appropriate parameters for long-term outcomes associated with MRD-directed approaches. OS is important but less conclusive since it is influenced not only by subsequent salvage approaches, which can lead to prolonged survival, but also by subsequent SCT mortality. It is important to decipher these consequences.
Figure 1 graphically illustrates the current strategy of the GMALL Study Group, and Figure 2 summarizes the most important steps. MRD-based treatment modifications are now the standard of care.48 However, a number of questions remain a matter of debate and are subjects of ongoing or future clinical trials (Figure 3).
Figure 3.
Open questions in MRD-based treatment.
CLINICAL CASE (continued)
An MRD level of 0.05% was confirmed in the patient after consolidation 1. The patient was treated with blinatumomab for 1 cycle, which was well tolerated with the exception of an interruption of a few days due to symptoms of an encephalopathy. A donor search had already taken place during induction therapy, and a matched unrelated donor had been identified. The transplant center was contacted at the start of blinatumomab in order to prepare for transplantation. The second cycle of blinatumomab was started. The result of the first cycle arrived and showed a complete molecular response at a sensitivity of 0.01%. One week before the planned transplant date, the second cycle was stopped, and the patient was transferred to the transplant unit. Conditioning was performed with 8-Gy total body irradiation and fludarabine since the patient was older than 45 in order to account for the increased transplant-related mortality in this age group. After transplant, MRD follow-up was started at day 60 and continued every 2 to 3 months thereafter in addition to chimerism analysis and confirmed a negative result.
Conflict-of-interest disclosure
Nicola Gökbuget: speaking honoraria, travel support, advisory board membership: Amgen, AstraZeneca, Celgene, Gilead, Novartis, Pfizer, Jazz Pharmaceuticals, Incyte, Cellestia, Erytech, Morphosys, Servier; research funding: Amgen, Pfizer, Novartis, Servier, Jazz Pharmaceuticals, Incyte, Abbvie.
Off-label drug use
Nicola Gökbuget: Most targeted compounds have no label for treatment of MRD in ALL; this includes inotuzumab, rituximab, CD38 antibodies, venetoclax, tyrosine kinase inhibitors, JAK inhibitors, and CAR T cells.
References
- 1.Bassan R, Brüggemann M, Radcliffe HS, Hartfield E, Kreuzbauer G, Wetten S. A systematic literature review and meta-analysis of minimal residual disease as a prognostic indicator in adult B-cell acute lymphoblastic leukemia. Haematologica. 2019;104(10):2028-2039. doi: 10.3324/haematol.2018.201053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Berry DA, Zhou S, Higley H, et al.. Association of minimal residual disease with clinical outcome in pediatric and adult acute lymphoblastic leukemia: a meta-analysis. JAMA Oncol. 2017;3(7):e170580. doi: 10.1001/jamaoncol.2017.0580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Luskin MR, Murakami MA, Manalis SR, Weinstock DM. Targeting minimal residual disease: a path to cure? Nat Rev Cancer. 2018;18(4):255-263. doi: 10.1038/nrc.2017.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gökbuget N, Kneba M, Raff T, et al; German Multicenter Study Group for Adult Acute Lymphoblastic Leukemia. Adult patients with acute lymphoblastic leukemia and molecular failure display a poor prognosis and are candidates for stem cell transplantation and targeted therapies. Blood. 2012;120(9):1868-1876. doi: 10.1182/blood-2011-09-377713. [DOI] [PubMed] [Google Scholar]
- 5.Brüggemann M, Kotrova M. Minimal residual disease in adult ALL: technical aspects and implications for correct clinical interpretation. Blood Adv. 2017;1(25):2456-2466. doi: 10.1182/bloodadvances.2017009845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brüggemann M, Schrauder A, Raff T, et al.. Standardized MRD quantification in European ALL trials: proceedings of the Second International Symposium on MRD Assessment in Kiel, Germany, 18–20 September 2008. Leukemia. 2010;24(3):521-535. doi: 10.1038/leu.2009.268. [DOI] [PubMed] [Google Scholar]
- 7.Hoelzer D, Bassan R, Dombret H, et al; European Society for Medical Oncology Guidelines Committee. Acute lymphoblastic leukaemia in adult patients: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2016;27(suppl 5):v69-v82. doi: 10.1093/annonc/mdw025. [DOI] [PubMed] [Google Scholar]
- 8.Kotrova M, Volland A, Kehden B, et al.. Comparison of minimal residual disease levels in bone marrow and peripheral blood in adult acute lymphoblastic leukemia. Leukemia. 2020;34(4):1154-1157. doi: 10.1038/s41375-019-0599-1. [DOI] [PubMed] [Google Scholar]
- 9.Gökbuget N, Dombret H, Giebel S, et al.. Minimal residual disease level predicts outcome in adults with Ph-negative B-precursor acute lymphoblastic leukemia. Hematology. 2019;24(1):337-348. doi: 10.1080/16078454.2019.1567654. [DOI] [PubMed] [Google Scholar]
- 10.O‘Connor D, Enshaei A, Bartram J, et al.. Genotype-specific minimal residual disease interpretation improves stratification in pediatric acute lymphoblastic leukemia. J Clin Oncol. 2018;36(1):34-43. doi: 10.1200/JCO.2017.74.0449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gökbuget N, Brüggemann M, Beck J, et al.. Evaluation of minimal residual disease (MRD) and MRD-based treatment decisions in Ph/BCR-ABL negative adult acute lymphoblastic leukemia (ALL): experience from the German Multicenter Study Group for Adult ALL (GMALL). Blood. 2017;130(suppl 1):138. doi: 10.1182/blood.V130.Suppl_1.139.139. [DOI] [Google Scholar]
- 12.Dhédin N, Huynh A, Maury S, et al; GRAALL investigators. Role of allogeneic stem cell transplantation in adult patients with Ph-negative acute lymphoblastic leukemia. Blood. 2015;125(16):2486-2496, quiz 2586. doi: 10.1182/blood-2014-09-599894. [DOI] [PubMed] [Google Scholar]
- 13.Bar M, Wood BL, Radich JP, et al.. Impact of minimal residual disease, detected by flow cytometry, on outcome of myeloablative hematopoietic cell transplantation for acute lymphoblastic leukemia. Leuk Res Treatment. 2014;2014(23 March):421723. doi: 10.1155/2014/421723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bassan R, Spinelli O, Oldani E, et al.. Different molecular levels of post-induction minimal residual disease may predict hematopoietic stem cell transplantation outcome in adult Philadelphia-negative acute lymphoblastic leukemia. Blood Cancer J. 2014;4(11 July):e225. doi: 10.1038/bcj.2014.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pavlů J, Labopin M, Niittyvuopio R, et al.. Measurable residual disease at myeloablative allogeneic transplantation in adults with acute lymphoblastic leukemia: a retrospective registry study on 2780 patients from the acute leukemia working party of the EBMT. J Hematol Oncol. 2019;12(1):108. doi: 10.1186/s13045-019-0790-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Spinelli O, Peruta B, Tosi M, et al.. Clearance of minimal residual disease after allogeneic stem cell transplantation and the prediction of the clinical outcome of adult patients with high-risk acute lymphoblastic leukemia. Haematologica. 2007;92(5):612-618. doi: 10.3324/haematol.10965. [DOI] [PubMed] [Google Scholar]
- 17.Bassan R, Bourquin JP, DeAngelo DJ, Chiaretti S. New approaches to the management of adult acute lymphoblastic leukemia. J Clin Oncol. 2018;36(35):3504-3519. doi: 10.1200/jco.2017.77.3648. [DOI] [PubMed] [Google Scholar]
- 18.Maury S, Chevret S, Thomas X, et al.. Addition of rituximab improves the outcome of adult patients with CD20-positive, Ph-negative, B-cell precursor acute lymphoblastic leukemia (BCP-ALL): results of the randomized GRAALL-R 2005 study. Blood. 2015;126(23):1. doi: 10.1182/blood.V126.23.1.1.26138534 [DOI] [Google Scholar]
- 19.Topp MS, Kufer P, Gökbuget N, et al.. Targeted therapy with the T-cell-engaging antibody blinatumomab of chemotherapy-refractory minimal residual disease in B-lineage acute lymphoblastic leukemia patients results in high response rate and prolonged leukemia-free survival. J Clin Oncol. 2011;29(18):2493-2498. doi: 10.1200/JCO.2010.32.7270. [DOI] [PubMed] [Google Scholar]
- 20.Gökbuget N, Zugmaier G, Klinger M, et al.. Long-term relapse-free survival in a phase 2 study of blinatumomab for the treatment of patients with minimal residual disease in B-lineage acute lymphoblastic leukemia. Haematologica. 2017;102(4):e132-e135. doi: 10.3324/haematol.2016.153957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Aldoss I, Song J, Stiller T, et al.. Correlates of resistance and relapse during blinatumomab therapy for relapsed/refractory acute lymphoblastic leukemia. Am J Hematol. 2017;92(9):858-865. doi: 10.1002/ajh.24783. [DOI] [PubMed] [Google Scholar]
- 22.Gökbuget N, Dombret H, Zugmaier G, et al.. Blinatumomab for minimal residual disease (MRD) in adults with B-cell precursor acute lymphoblastic leukemia (BCP-ALL): median overall survival (OS) is not reached in complete MRD responders at a median follow-up of 53.1 months. Blood. 2018;132(suppl 1):554. doi: 10.1182/blood-2017-08-798322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kantarjian H, Stein A, Gökbuget N, et al.. Blinatumomab versus chemotherapy for advanced acute lymphoblastic leukemia. N Engl J Med. 2017;376(9):836-847. doi: 10.1056/NEJMoa1609783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gökbuget N, Zugmaier G, Dombret H, et al.. Curative outcomes following blinatumomab in adults with minimal residual disease B-cell precursor acute lymphoblastic leukemia. Leuk Lymphoma. 2020;61(11):2665-2673. doi: 10.1080/10428194.2020.1780583. [DOI] [PubMed] [Google Scholar]
- 25.Gökbuget N, Werman WK, Schwartz S, et al.. Interim results of a multicenter, single-arm study to assess blinatumomab in adult patients (pts) with minimal residual disease (MRD) of B-precursor (BCP) acute lymphoblastic leukemia (GMALLMOLACT1-BLINA). Blood. 2020;136(suppl 1):39-40. doi: 10.1182/blood-2020-137350. [DOI] [Google Scholar]
- 26.Gökbuget N, Dombret H, Bonifacio M, et al.. Blinatumomab for minimal residual disease in adults with B-cell precursor acute lymphoblastic leukemia. Blood. 2018;131(14):1522-1531. doi: 10.1182/blood-2017-08-798322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Haddad F, Kantarjian H, Short NJ, et al.. A phase II study of blinatumomab for the treatment of measurable residual disease-positive B-cell acute lymphoblastic leukemia. Blood. 2021;138(suppl 1):4398. doi: 10.1182/blood-2021-149261. [DOI] [Google Scholar]
- 28.Keating AK, Gossai N, Phillips CL, et al.. Reducing minimal residual disease with blinatumomab prior to HCT for pediatric patients with acute lymphoblastic leukemia. Blood Adv. 2019;3(13):1926-1929. doi: 10.1182/bloodadvances.2018025726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Locatelli F, Zugmaier G, Mergen N, et al.. Blinatumomab in pediatric patients with relapsed/refractory acute lymphoblastic leukemia: results of the RIALTO trial, an expanded access study. Blood Cancer J. 2020;10(7):77. doi: 10.1038/s41408-020-00342-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Locatelli F, Zugmaier G, Rizzari C, et al.. Effect of blinatumomab vs chemotherapy on event-free survival among children with high-risk first-relapse B-cell acute lymphoblastic leukemia: a randomized clinical trial. JAMA. 2021;325(9):843-854. doi: 10.1001/jama.2021.0987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bassan R, Chiaretti S, Della Starza I, et al.. Preliminary results of the GIMEMA LAL2317 sequential chemotherapy-blinatumomab frontline trial for newly diagnosed adult Ph-negative B-lineage ALL patients. EHA Library. 9 June 2021. Abstract S114. [Google Scholar]
- 32.Kantarjian HM, DeAngelo DJ, Stelljes M, et al.. Inotuzumab ozogamicin versus standard of care in relapsed or refractory acute lymphoblastic leukemia: final report and long-term survival follow-up from the randomized, phase 3 INO-VATE study. Cancer. 2019;125(14):2474-2487. doi: 10.1002/cncr.32116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Park JH, Rivière I, Gonen M, et al.. Long-term follow-up of CD19 CAR therapy in acute lymphoblastic leukemia. N Engl J Med. 2018;378(5):449-459. doi: 10.1056/NEJMoa1709919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schultz LM, Baggott C, Prabhu S, et al.. Disease burden impacts outcomes in pediatric and young adult B-cell acute lymphoblastic leukemia after commercial tisagenlecleucel: results from the Pediatric Real World CAR Consortium (PRWCC). Blood. 2020;136(suppl 1):14-15. doi: 10.1182/blood-2020-134472. [DOI] [Google Scholar]
- 35.Bastian L, Schroeder MP, Eckert C, et al.. PAX5 biallelic genomic alterations define a novel subgroup of B-cell precursor acute lymphoblastic leukemia. Leukemia. 2019;33(8):1895-1909. doi: 10.1038/s41375-019-0430-z. [DOI] [PubMed] [Google Scholar]
- 36.Roberts KG, Reshmi SC, Harvey RC, et al.. Genomic and outcome analyses of Ph-like ALL in NCI standard-risk patients: a report from the Children's Oncology Group. Blood. 2018;132(8):815-824. doi: 10.1182/blood-2018-04-841676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Moorman AV, Schwab C, Winterman E, et al.. Adjuvant tyrosine kinase inhibitor therapy improves outcome for children and adolescents with acute lymphoblastic leukaemia who have an ABL-class fusion. Br J Haematol. 2020;191(5):844-851. doi: 10.1111/bjh.17093. [DOI] [PubMed] [Google Scholar]
- 38.Tanasi I, Ba I, Sirvent N, et al.. Efficacy of tyrosine kinase inhibitors in Ph-like acute lymphoblastic leukemia harboring ABL-class rearrangements. Blood. 2019;134(16):1351-1355. doi: 10.1182/blood.2019001244. [DOI] [PubMed] [Google Scholar]
- 39.Tasian SK, Assad A, Hunter DS, Du Y, Loh ML. A phase 2 study of ruxolitinib with chemotherapy in children with Philadelphia chromosome-like acute lymphoblastic leukemia (INCB18424-269/AALL1521): dose-finding results from the part 1 safety phase. Blood. 2018;132(suppl 1):555. doi: 10.1182/blood-2018-99-110221.30093383 [DOI] [Google Scholar]
- 40.Dunsmore KP, Winter SS, Devidas M, et al.. Children's oncology group AALL0434: a phase III randomized clinical trial testing nelarabine in newly diagnosed T-cell acute lymphoblastic leukemia. J Clin Oncol. 2020;38(28):3282-3293. doi: 10.1200/JCO.20.00256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cerrano M, Castella B, Lia G, et al.. Immunomodulatory and clinical effects of daratumumab in T-cell acute lymphoblastic leukaemia. Br J Haematol. 2020;191(1):e28-e32. doi: 10.1111/bjh.16960. [DOI] [PubMed] [Google Scholar]
- 42.Ofran Y, Ringelstein-Harlev S, Slouzkey I, et al.. Daratumumab for eradication of minimal residual disease in high-risk advanced relapse of T-cell/ CD19/CD22-negative acute lymphoblastic leukemia. Leukemia. 2020; 34(1):293-295. doi: 10.1038/s41375-019-0548-z. [DOI] [PubMed] [Google Scholar]
- 43.Gökbuget N, Stoltefuß A, Schwartz S, et al.. Dose reduced chemotherapy in combination with blinatumomab for newly diagnosed older patients with Ph-negative B-precursor ALL: first results of the BOLD Trial. EHA Library. 14 May 2020. Abstract EP414. [Google Scholar]
- 44.Beldjord K, Chevret S, Asnafi V, et al; Group for Research on Adult Acute Lymphoblastic Leukemia. Oncogenetics and minimal residual disease are independent outcome predictors in adult patients with acute lymphoblastic leukemia. Blood. 2014;123(24):3739-3749. doi: 10.1182/blood-2014-01-547695. [DOI] [PubMed] [Google Scholar]
- 45.Enshaei A, O‘Connor D, Bartram J, et al.. A validated novel continuous prognostic index to deliver stratified medicine in pediatric acute lymphoblastic leukemia. Blood. 2020;135(17):1438-1446. doi: 10.1182/blood.2019003191. [DOI] [PubMed] [Google Scholar]
- 46.Bond J, Graux C, Lhermitte L, et al.. Early response-based therapy stratification improves survival in adult early thymic precursor acute lymphoblastic leukemia: a Group for Research on Adult Acute Lymphoblastic Leukemia Study. J Clin Oncol. 2017;35(23):2683-2691. doi: 10.1200/JCO.2016.71.8585. [DOI] [PubMed] [Google Scholar]
- 47.Zhao Y, Aldoss I, Qu C, et al.. Tumor-intrinsic and -extrinsic determinants of response to blinatumomab in adults with B-ALL. Blood. 2021;137(4):471-484. doi: 10.1182/blood.2020006287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Short NJ, Jabbour E, Albitar M, et al.. Recommendations for the assessment and management of measurable residual disease in adults with acute lymphoblastic leukemia: a consensus of North American experts. Am J Hematol. 2019;94(2):257-265. doi: 10.1002/ajh.25338. [DOI] [PMC free article] [PubMed] [Google Scholar]