Abstract
Because of their similarities to infants in mucosal immune responses and their susceptibility to human rotavirus (HRV) diarrhea, gnotobiotic pigs provide a useful model for rotaviral disease. In this study, we performed quantitative enzyme-linked immunospot (ELISPOT) assays to measure local and systemic isotype-specific antibody-secreting cell (ASC) responses to individual structural (VP4, VP6, and VP7) and nonstructural (NSP3 and NSP4) proteins of Wa HRV. The Spodoptera frugiperda cells expressing each recombinant baculovirus HRV protein were formalin fixed and used as antigen for ELISPOT assays. Neonatal gnotobiotic pigs were orally inoculated once with virulent Wa (WaV) or three times with attenuated Wa (WaA) HRV or mock inoculated (Mock) and then were challenged with virulent Wa (WaV/PC) 28 days after the first inoculation. The ASCs from intestinal and systemic lymphoid tissues of pigs from each group were quantitated by ELISPOT assay at the day of challenge, at postinoculation day 28 (WaV, WaA, and Mock) or at postchallenge day (PCD) 7 (WaV+WaV/PC, WaA+WaV/PC, and Mock+WaV/PC). In all virus-inoculated pigs, regardless of the inoculum, lymphoid tissue, or isotype, VP6 induced the highest numbers of ASCs, followed by VP4; ASCs specific for VP7, NSP3, and NSP4 were less numerous. At challenge, total HRV- and HRV protein-specific immunoglobulin A (IgA) and IgG ASCs in intestinal lymphoid tissues were significantly greater in WaV- than in WaA-inoculated pigs, and WaV pigs were fully protected against diarrhea postchallenge, whereas the WaA pigs were partially protected. At PCD 7, there were no significant differences in ASC numbers for any HRV proteins between the WaV+WaV/PC and WaA+WaV/PC groups.
Group A rotaviruses are the single most important cause of viral gastroenteritis in infants and young children in developing and developed countries worldwide (18). Annually, in the United States, 3 million infants develop rotavirus-induced diarrhea, 82,000 are hospitalized, and approximately 150 die (11, 17). It is estimated that in developing countries, 18 million infants develop rotaviral diarrhea and 870,000 deaths occur annually (11, 17). Improved hygienic conditions have led to a reduction or elimination of bacterial diarrhea but have had little or no effect on rotavirus gastroenteritis, suggesting that improvements in hygiene alone will not reduce rotavirus disease (35). Evidence that infants develop natural immunity to rotavirus after exposure and the worldwide impact of this disease have led to extensive efforts to develop vaccines against rotavirus (10, 12, 18). Because rotaviruses replicate in the small intestine, local mucosal immunity is an important factor in protection against rotavirus diarrhea; thus, efficacious vaccines must induce immune responses which protect in the intestinal enterocytes (5, 27, 28). In 1998, an oral attenuated vaccine for human rotavirus (HRV) was licensed in the United States, but a potential risk of intussusception in vaccinated infants prompted its withdrawal (3).
Gnotobiotic pigs serve as excellent models for the study of rotavirus disease pathogenesis and immunity. Unlike other laboratory animals, pigs are susceptible to clinical infection with HRVs up to at least 8 weeks of age (26, 28, 29). Another advantage of pigs is that they resemble human infants in their gastrointestinal physiology, milk diets, and development of mucosal immune responses (21, 23, 28).
We previously studied the pathogenesis of and immune responses to virulent Wa (WaV) and attenuated Wa (WaA) HRV infections in gnotobiotic pigs (28, 36, 38). In the present study, we performed quantitative ELISPOT assays to measure local and systemic antibody-secreting cell (ASC) responses to individual structural (VP4, VP6, and VP7) and nonstructural (NSP3 and NSP4) Wa HRV proteins in gnotobiotic pigs inoculated with WaV HRV or WaA HRV and challenged with WaV HRV. Our goal was to determine the isotype and tissue distribution of the ASC responses to selected HRV proteins and to assess if particular isotype ASC responses to particular HRV proteins were associated with protection.
MATERIALS AND METHODS
Cells and viruses.
Rhesus monkey kidney cells (MA104) were grown and maintained in minimal essential medium (Life Technologies, Rockville, Md.) in a humid CO2 incubator at 37°C. Spodoptera frugiperda (Sf9) insect cells were grown and maintained in Hinks TNM-FH (JRH Biosciences, Lenexa, Kans.) with 10% fetal bovine serum (FBS) at 27°C. The WaV HRV strain (P1A[8]G1) was maintained by serial passages of Wa HRV-infected stools from an infant in gnotobiotic pigs as described previously (37). A suspension of intestinal contents from the 16th pig passage was used as the WaV HRV inoculum (36, 38). WaA HRV was adapted to growth in cell culture (37) and serially passaged in MA104 cells 27 times (36, 38).
Wa HRV gene clones and recombinant proteins.
Recombinant baculoviruses expressing individual Wa HRV proteins VP6, VP7, NSP3, and NSP4 were constructed using the pBlueBac 4.5 system (Invitrogen, Carlsbad, Calif.). The full-length individual genes were amplified by reverse transcription-PCR and cloned into pCR2.1 (Invitrogen). Each gene was then subcloned into the pBlueBac 4.5 baculovirus transfer vector. The recombinant baculoviruses were generated by cotransfecting the linearized baculovirus DNA and the recombinant pBlueBac 4.5 followed by plaque purification. The identity of each Wa HRV protein was confirmed using an immunoblotting assay with guinea pig hyperimmune antiserum prepared against WA HRV-infected MA104 cell lysates. A recombinant baculovirus expressing Ku (P1A[8]) VP4 was obtained from H. B. Greenberg, Stanford University School of Medicine, Palo Alto, Calif.
Hybridoma cells.
Hybridoma cells secreting immunoglobulin G (IgG) antibodies reactive with HRV proteins were used to standardize the expressed HRV proteins for ELISPOT assay. These included the following hybridoma cells: RG25A10, which is reactive with rotavirus VP6 (16); Common 60, which is reactive to rotavirus VP7 (13); and B42, which is reactive to rotavirus NSP4 (13). Common 60 and B42 were supplied by H. B. Greenberg. No Wa NSP3 and Ku VP4 hybridoma cells were available to standardize NSP3 and VP4 proteins, so the above antibodies to HRV were used for NSP3 and VP4.
Preparation of plates.
Separate cultures of Sf9 cells in Grace's medium (10% FBS) were infected with recombinant baculoviruses expressing individual rotavirus proteins, VP4, VP6, VP7, NSP3, and NSP4, at a multiplicity of infection of 5. The infected Sf9 cells were transferred to 96-well microtiter plates using 8.0 × 104 cells/well, and plates were incubated at 27°C for 40 h until cells expressed the corresponding individual rotavirus proteins. The cell culture medium was carefully removed so as not to disrupt the Sf9 cell monolayer, and the plates were air dried. The infected Sf9 cell monolayers were fixed by adding 4% formaldehyde at room temperature for 30 min as described previously (13). The fixed cells were permeabilized with 1% Triton X-100 in TNC buffer (10 mM Tris-HCl, 140 mM NaCl, 10 mM CaCl2 [pH 7.5]) at room temperature for 10 min. The plates were stored at −20°C up to 7 days before use. The MA104 cells in 96-well plates were infected with Wa HRV and fixed with 80% acetone as described previously (38) to examine total rotavirus-specific ASCs by ELISPOT assay.
Standardization of ELISPOT assay using hybridoma cells
The hybridomas used to standardize the protein-specific ELISPOT assay were RG25A10 against VP6, Common 60 against VP7, and B42 against NSP4. Hybridoma cells were centrifuged three times at 200 × g for 5 min, followed each time by resuspension in wash buffer (RPMI 1640; Life Technologies), and were resuspended with RPMI 1640 containing 2% FBS. Hybridoma cells were diluted in RPMI 1640 (2% FBS) and 3, 10, 30, 100, and 300 cells were added per well to 96-well plates. Hybridoma cells at these concentrations were transferred to triplicate wells of either the fixed MA104 cell plates infected with Wa HRV or the fixed Sf9 cell plates infected with the recombinant baculoviruses expressing the corresponding individual rotavirus proteins. To test the specificity of each assay, the hybridoma cells were also added to Sf9 cells infected with recombinant baculoviruses expressing the unrelated HRV proteins or wild-type baculoviruses. After addition of hybridoma cells, the plates were centrifuged for 5 min at 100 × g, incubated for 12 h at 37°C in a CO2 incubator, and then washed three times with phosphate-buffered saline (PBS) (pH 7.2). For the detection of IgG secreted by the hybridomas, the plates were incubated at 37°C for 1 h with horseradish peroxidase-labeled goat anti-mouse IgG (heavy and light chains) (Kirkegaard & Perry Laboratories, Gaithersburg, Md.) diluted 1:5,000 in PBS containing 1% nonfat dry milk. The plates were then washed three times with PBS. Bound enzyme conjugate was detected by adding TMB Membrane 3-component peroxidase substrate (Kirkegaard & Perry Laboratories) to the plates. The blue-purple spots were counted using a microscope with a 40× objective, and the mean numbers of spots at each concentration from at least three independent tests were obtained.
Animals.
Animal use protocols were approved by The Ohio State University Institutional Laboratory Animal Care and Use Committee. Near-term derived gnotobiotic pigs were assigned to one of the six groups shown in Table 1. All primary inoculations were performed at 3 to 5 days of age. The WaV HRV (n = 6) and WaV+WaV HRV, postchallenge (WaV/PC) (n = 6) pigs were inoculated once with WaV HRV (intestinal contents, ∼105 fluorescent focus units [FFU]). The WaA HRV (n = 6) and WaA+WaV/PC (n = 6) pigs were inoculated orally three times with WaA HRV (clarified rotavirus-infected MA104 cell lysates [∼2 × 107 FFU]) with intervals of 7 days between inoculations. Mock (n = 6) and Mock+WaV/PC (n = 6) pigs were inoculated three times with diluent as controls. The three WaV/PC groups were subsequently challenged with WaV HRV (intestinal contents, ∼106 FFU) at postinoculation day (PID) 28 (Table 1). WaV, WaA, and Mock pigs were euthanatized at PID 28 and postchallenge day (PCD) 0, and WaV+WaV/PC, WaA+WaV/PC, and Mock+WaV/PC pigs were euthanatized at PID 35 and PCD 7 (Table 1). After pigs were euthanatized, small intestine (duodenum and ileum), mesenteric lymph nodes (MLN), spleen, and blood were collected from each pig, and the mononuclear cells (MNC) were isolated and purified for ELISPOT assay as described previously (38).
TABLE 1.
Experimental design for virus inoculation of gnotobiotic pigs to monitor ASC responses, clinical signs, and fecal virus shedding
| Group designationa | Virus inoculumb | Virusc challenge WaV | Euthanatized at PID (PCD) |
|---|---|---|---|
| WaV | WaV (1X) | No | 28 (0) |
| WaV+WaV/PC | WaV (1X) | Yes | 35 (7) |
| WaA | WaA (3X) | No | 28 (0) |
| WaA+WaV/PC | WaA (3X) | Yes | 35 (7) |
| Mock | Mock | No | 28 (0) |
| Mock+WaV/PC | Mock | Yes | 35 (7) |
n = 6 pigs/group.
An intestinal content (WaV, ∼105 FFU) or clarified rotavirus-infected MA104 cell lysate (WaA, ∼2 × 107 FFU) was administered orally.
Virus challenge with an intestinal filtrate (WaV, ∼106 FFU).
After inoculation, pigs were examined twice daily for clinical signs and development of diarrhea, and the fecal consistency was scored (0 = normal, 1 = pasty, 2 = semiliquid, 3 = liquid). Pigs with scores of 2 or more were considered diarrheic. Feces (rectal swabs) were collected daily and stored at −20°C until tested. Each rectal swab sample was tested for virus shedding using a cell culture immunofluorescence test and enzyme-linked immunosorbent assay as described previously (38).
ELISPOT of MNC for detection of protein-specific ASCs.
After the MNC were isolated, they were counted using a hemacytometer, and the number of MNC was adjusted to 5 × 105 and 5 × 104 cells/100 μl suspended in Enhanced-RPMI (Gibco Life Technologies). The fixed Sf9 cell plates and MA104 cell plates were rehydrated by washing once with distilled water. Then, 100 μl of each dilution of MNC suspension (5 × 105 and 5 × 104 cells/well) was added in duplicate to the 96-well plates. The plates were centrifuged for 5 min at 100 × g and incubated at 37°C in a CO2 incubator for approximately 12 h. The plates were washed three times with PBS (pH 7.2) containing 0.05% Tween 20 (PBS-Tw) to remove the MNC. Each isotype-specific horseradish peroxidase-labeled secondary antibody (anti-pig IgA [α chain specific], anti-pig IgM [μ chain specific], and anti-pig IgG [γ specific] [Bethyl Laboratories, Montgomery, Tex.]) was added to separate plates and incubated for 2 h at 37°C. The plates were then washed three times with PBS-Tw and treated for 20 min with tetramethylbenzidine membrane 3-component peroxidase substrate for the development of spots. The number of ASCs was counted using a microscope with a 40× objective, and wells with fewer than 40 spots were averaged from the duplicate wells at each dilution.
Statistical analyses.
Significant differences in the numbers of total rotavirus-specific and protein-specific ASCs among groups were determined by the Kruskal-Wallis test. The differences among groups in the protection rates of virus shedding and clinical signs after challenge were determined by Fisher's exact test and one-way analysis of variance, respectively. Statistical analyses were done using the Statistical Analysis Systems (SAS 6.12) program. Statistical significance was assessed at a P of <0.05.
RESULTS
Standardization of protein-specific ELISPOT assay with hybridoma cells.
The ELISPOT assay to detect ASCs against individual HRV proteins using Sf9 cells infected with recombinant baculoviruses was standardized with hybridoma cells secreting antibodies against the rotavirus proteins VP6, VP7, and NSP4, and the numbers of spots were compared with those on MA104 cells infected with Wa HRV (Table 2). Overall, the numbers of spots observed on MA104 cells and Sf9 cells closely corresponded to the numbers of hybridoma cells added per well except for the Common 60 hybridoma cells reactive with VP7 (Table 2). In the case of VP7, the number of spots on Sf9 cells infected with recombinant VP7 baculoviruses was lower than those on MA104 cells (12 versus 24 using 30 hybridoma cells, 2 versus 8 using 10 hybridoma cells, and 0 versus 1 using 3 hybridoma cells, respectively) (Table 2). When hybridoma cells were added to mock-infected Sf9 cells, Sf9 cells infected with HRV recombinant baculoviruses coding for other or with wild baculoviruses, no spots were observed (data not shown). The antigenicity of the recombinant proteins secreted by the recombinant VP4 and NSP3 baculoviruses was confirmed by immunoblotting with the guinea pig polyclonal antiserum prepared against MA104 cell lysates infected with Wa HRV (data not shown).
TABLE 2.
Standardization of ELISPOT assay using hybridomas secreting antibodies against individual rotavirus proteins
| No. of hybridoma cells added per well | No. of spots observeda (mean ± SD)
|
|||||
|---|---|---|---|---|---|---|
| MA104 cells infected with Wa HRV
|
Sf9 cells expressing the corresponding gene encoding each individual Wa HRV proteinb
|
|||||
| RG25A10c (VP6) | Common 60 (VP7) | B42 (NSP4) | RG25A10 (VP6) | Common 60 (VP7) | B42 (NSP4) | |
| 300d | >50 | >50 | >50 | >50 | >50 | >50 |
| 100 | >50 | >50 | >50 | >50 | >50 | >50 |
| 30 | 28 ± 1 | 24 ± 3 | 21 ± 1 | 23 ± 2 | 12 ± 3 | 19 ± 1 |
| 10 | 10 ± 3 | 8 ± 2 | 7 ± 2 | 7 ± 2 | 2 ± 1 | 8 ± 1 |
| 3 | 3 ± 1 | 1 ± 1 | 3 ± 1 | 2 ± 0 | 0 | 2 ± 1 |
Numbers are means ± standard deviations of at least three independent tests; spots were counted in wells containing 50 spots or less.
No spots were seen in wells with the unrelated HRV proteins.
Hybridoma cells added (specificity).
Wells containing 300 hybridomas had more spots than those containing 100 hybridomas.
ASC responses to individual HRV proteins after inoculation of gnotobiotic pigs with WaV or WaA or mock inoculation (prechallenge).
In all animals, regardless of the Wa HRV inoculum, tissue, or isotype, ASC responses to VP6 were the most numerous, followed by VP4; ASC responses to VP7, NSP3, and NSP4 were less numerous (Fig. 1). The numbers of ASCs for VP6 and VP4 were about 60 and 50%, respectively, and those for VP7, NSP3, and NSP4 were less than 10%, respectively, of the numbers of ASCs for total rotavirus (Fig. 1). In general, the numbers of IgG, IgA, and IgM ASCs against the five rotavirus proteins tested and against total rotavirus were greater in the intestinal lymphoid tissues (intestine and MLN) than in the systemic lymphoid tissues (spleen and peripheral blood lymphocytes [PBL]) (Fig. 1). No spots were observed in the mock-inoculated group.
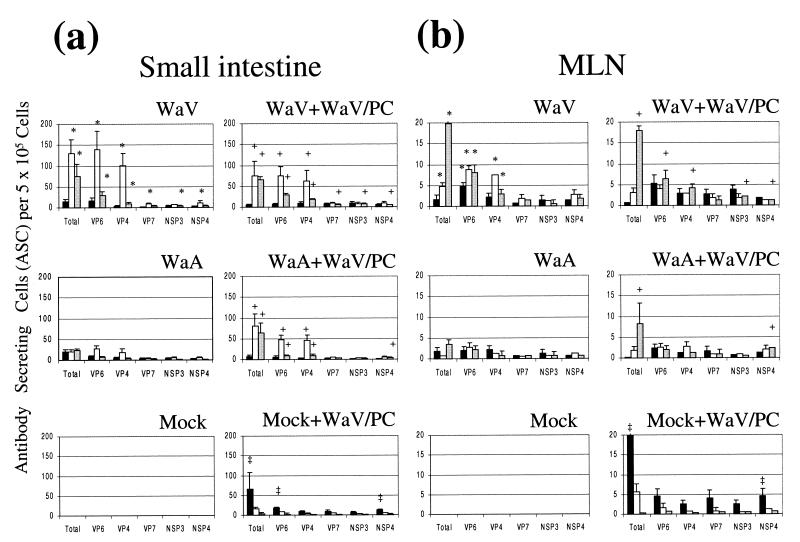
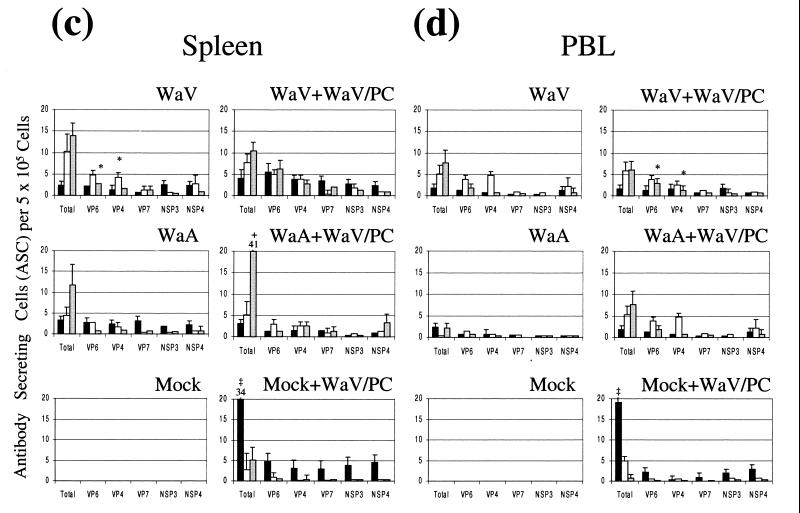
FIG. 1.
Isotype-specific ASCs to total Wa HRV (Wa HRV-infected MA104 cells) and Wa HRV proteins (recombinant baculovirus-infected Sf9 cells) in the small intestine (average of duodenum and ileum) (a), MLN (b), spleen (c), and PBL (d) of gnotobiotic pigs following oral inoculation and challenge with Wa HRV. Data are the mean numbers of total or protein-specific ASCs per 5 × 105 MNC for six pigs in each group. An asterisk denotes significant differences (P < 0.05) in numbers of ASCs between WaV (one dose of WaV HRV, before challenge) and WaA (three doses of WaA HRV, before challenge) pigs. The plus mark denotes significant differences (P < 0.05) in numbers of ASCs between WaV+WaV/PC or WaA+WaV/PC and Mock+WaV/PC pigs. The double dagger denotes significant differences (P < 0.05) in numbers of ASCs between Mock+WaV/PC and WaV+WaV/PC and WaV/PC pigs. Solid bars, IgM; open bars, IgA; shaded bars, IgG.
Overall, one dose of WaV HRV induced greater ASC responses than three doses (7 days apart) of WaA HRV in gnotobiotic pigs at PID 28 (Fig. 1). The numbers of total rotavirus-, VP6-, and VP4-specific IgG and IgA ASCs in intestinal lymphoid tissues (intestine and MLN) were significantly greater in WaV (one dose of WaV HRV) pigs than in WaA (three doses of WaV HRV) pigs (P < 0.05). For VP7, NSP3, and NSP4, the numbers of specific IgA (VP7 and NSP4) or IgG (NSP3) ASCs in the intestine were significantly greater in WaV pigs than in WaA pigs (P < 0.05), but no significant differences were observed in the MLN. In systemic tissues, overall there were few significant differences between WaV and WaA pigs (VP6-specific IgG in spleen and VP4-specific IgA in spleen were significantly higher in the WaV pigs) (Fig. 1). No ASC responses were observed in the mock-inoculated pigs.
ASC responses postchallenge (WaV+WaV/PC, WaA+WaV/PC, and Mock+WaV/PC).
Although overall numbers of ASCs in WaV+WaV/PC (one dose of WaV) pigs were greater than those in the WaA+WaV/PC (three doses of WaA) pigs, no statistically significant differences were seen in the numbers of ASCs between WaV+WaV/PC and WaA+WaV/PC pigs after challenge with WaV HRV (PID 35 and PCD 7) (Fig. 1). Comparing pre- and postchallenge ASC numbers (WaV versus WaV+WaV/PC and WaA versus WaA+WaV/PC), mean numbers of total and protein-specific ASCs in intestinal and systemic tissues were either increased (WaA versus WaA+ WaV/PC) or decreased (WaV versus WaV+WaV/PC) after challenge, but differences were not significant (Fig. 1).
WaV+WaV/PC and WaA+WaV/PC pigs had significantly greater numbers of total, VP6-, and VP4-specific IgA and IgG ASCs in the small intestine than did the Mock+WaV/PC pigs (Fig. 1a and b). In the MLN, the numbers of total and NSP4-specific IgG ASCs in WaV+WaV/PC and WaA+WaV/PC pigs and VP6-, VP4-, and NSP3-specific IgG ASCs in WaV+WaV/PC pigs were significantly greater than those in Mock+WaV/PC pigs (Fig. 1b). In intestine and MLN, numbers of total and NSP4 (and VP6- in intestine)-specific IgM ASCs in Mock+WaV/PC pigs were significantly greater than those in WaV+WaV/PC and WaA+WaV/PC pigs (Fig. 1a and b). In systemic tissues, no significant differences between vaccinated (WaV+WaV/PC or WaA+WaV/PC) and Mock+WaV/PC pigs were observed (except total specific IgM and IgG in spleen and IgM in PBL) (Fig. 1c and d).
Clinical signs and virus shedding.
The temporal appearance of clinical signs, virus shedding, and pathological changes in virulent or attenuated Wa HRV-inoculated gnotobiotic pigs was described in detail elsewhere (36, 38). As summarized in Table 3, 100% of pigs inoculated with one dose of virulent Wa HRV were protected from virus shedding and clinical diarrhea, whereas pigs inoculated with three doses of attenuated Wa HRV were partially protected from virus shedding (33% protection rate) and diarrhea (50% protection rate) after challenge with WaV HRV. Pigs mock inoculated and challenged with WaV HRV at PID 28 developed diarrhea (100%) and virus shedding (100%), and the pigs mock inoculated but not challenged showed neither diarrhea nor virus shedding (Table 3).
TABLE 3.
Summary of virus shedding and diarrhea in pigs inoculated or mock inoculated and challenged with WaV HRV at PID 28
| Exptl groupa | Virus shedding
|
Diarrhea
|
||
|---|---|---|---|---|
| % of pigsb | Mean duration (days)c | % of pigsb | Mean duration (days)c | |
| WaA+WaV/PC | 33 AB | 3.0 A | 50 AB | 1.5 A |
| WaV+WaV/PC | 0 B | NAd | 0 B | NA |
| Mock | 0 B | NA | 0 B | NA |
| Mock+WaV/PC | 100 A | 2.5 A | 100 A | 3.0 A |
n = 6 pigs/group.
Percentages in the same column with different letters beside them differ significantly (P < 0.05 [Fisher's exact test]).
Means in the same column with different letters beside them differ significantly (P < 0.05 [one-way analysis of variance]).
NA, not applicable.
DISCUSSION
When the specificity and sensitivity of the HRV protein-specific ELISPOT assay were standardized using hybridoma cell lines, the numbers of spots on the Wa HRV-infected MA104 cells agreed with the numbers of spots on the recombinant baculovirus-infected Sf9 cells except for the numbers for the hybridoma Common 60 secreting antibody to VP7 (Table 2). The Common 60 hybridoma secretes broadly G-serotype-reactive, but nonneutralizing, monoclonal antibodies (30). The numbers of spots on the Sf9 cells using Common 60 were less than half those on MA104 cells, which suggests that although the baculovirus-expressed VP7 was reactive with the monoclonal antibody, its antigenic authenticity may differ from that of the native VP7 on the virion. This might account for the unexpectedly (since VP7 is the major outer capsid protein) low numbers of VP7-specific ASCs detected in gnotobiotic pigs after inoculation with Wa HRV in this study. When another independent recombinant baculovirus expressing the VP7 gene was generated and the same procedure was applied to examine whether our results were related to the individual VP7 gene clone used, we observed similar results (data not shown). In previous studies, it was suggested that expressed rotavirus VP7 may not maintain its antigenicity (7, 8). Dormitzer and Greenberg (7) observed that VP7 expressed using a herpes simplex virus type 1 system showed conformational changes and lacked several neutralizing epitopes when assessed by enzyme-linked immunosorbent assay.
Ishida et al. (13, 14) studied the immune responses to individual baculovirus-expressed rotavirus proteins following murine rotavirus infection of adult mice. When they detected rotavirus antibodies in serum (IgG) and feces (IgA), VP6 and VP4 were the most immunogenic; VP2 was less immunogenic than VP6 or VP4, and titers of antibody to VP7, NSP2, and NSP4 were very low in serum and undetectable in feces. In the present study, among all groups, for both the systemic and mucosal lymphoid tissues, the most immunogenic protein was VP6, followed by VP4. VP7, NSP3, and NSP4 were less immunogenic, in agreement with the findings of Ishida et al. (13). Previous investigators have also reported that VP6 was the most immunogenic rotavirus protein (13, 14, 24, 25, 33, 34). The greatest mass of the virion is composed of VP6 (about 50% of virion), which could explain why VP6 is immunodominant among the rotavirus proteins (22).
Another structural protein, VP4 (the outer capsid hemagglutinin), also induced high ASC responses in gnotobiotic pigs, which is also similar to the findings of Ishida et al. (13) for mice, but contrary to the results of Shaw et al. (32). The latter authors reported that the response to VP4 was only a small portion (1.5%) of the overall total rotavirus ASC response in the intestinal tract in neonatal mice infected with a heterologous rhesus rotavirus. In the present study, the number of VP4-specific ASCs was about 50% of the total rotavirus-specific ASCs, and the discrepancy with the study of Shaw et al. (32) may be due to different hosts, viruses, and VP4 ELISPOT systems (they used baculovirus-expressed VP4 to coat the ELISPOT plates).
Although VP7 is the major outer capsid protein, comprising about 30% of the virion mass (22), the numbers of VP7-specific ASCs were only about 10% of the total rotavirus-specific ASC numbers. The reason for the low ASC responses against VP7 detected in this study and a previous study by Ishida et al. (13) is unclear, but the altered antigenicity of baculovirus-expressed VP7 may be one explanation.
There are only limited studies of the immune responses to the nonstructural proteins of rotavirus after immunization or infection with rotavirus. Studies of immune responses to NSP4 of rotavirus are especially important for vaccine development and understanding if and how protection is induced by this protein, because it has been shown to be a viral enterotoxin in the mouse model (2). Furthermore, antibodies to NSP4 partially protected infant mice from diarrhea after challenge with virulent rotavirus (2). Previous investigators reported that humoral immune responses to NSP4 after rotavirus infection in mice and humans were either undetectable or modest compared to those to VP6 (5, 13, 25). It has been suggested that because of possible genetic and antigenic variation of NSP4 among different rotavirus strains (20), a detection system homologous to the target strain would be important in detecting immune responses to NSP4 after vaccination or natural infection (15). Recently, Johansen et al. (15) reported humoral and cell-mediated immune responses to NSP4 of HRV in children. However, both natural infection and immunization using a tetravalent live rhesus-human reassortant rotavirus vaccine containing rhesus rotavirus NSP4 induced only low humoral and cell-mediated immune responses to NSP4 in children. In the present study, both one dose of WaV and three doses of WaA HRV induced low numbers of NSP3- and NSP4-specific ASCs. This may be due to expression of the nonstructural proteins inside the infected intestinal epithelial cells, with low quantities released from the cells.
Previous descriptions of immune responses of gnotobiotic pigs to WaV and WaA HRV have been reported from our laboratory (36, 38, 39). We found that a single dose of WaV HRV induced a high protection rate, whereas two doses of WaA HRV induced only partial protection in the gnotobiotic pigs (38). In the present study, similar experiments (but using three instead of two doses of WaA to mimic the three-dose HRV vaccines used in infants) were performed to measure ASC responses to individual rotavirus proteins. Similar to previous studies, one dose of WaV induced significantly greater ASC responses than three doses of WaA in intestinal lymphoid tissues at PID 28 (Fig. 1a and b). However, in systemic tissues, no significant differences in the numbers of total virus-specific and each individual protein-specific ASCs were observed between the WaV and WaA pigs (Fig. 1c and d). As previously reported (38), high ASC responses in intestinal tissues induced by WaV corresponded to the high protection rates in these pigs, and the lower ASC responses induced by the WaA were associated with the lower protection rates observed (Table 3). The low ASC responses to multiple doses of attenuated rotavirus compared to the response to a single dose of virulent rotavirus in pigs may be due to the limited replication of the WaA HRV in the intestinal epithelial cells (36). Because of this reduced level of replication of the WaA in the intestinal epithelial cells compared to the more extensive replication of WaV, we expected to see greater ASC responses to the nonstructural HRV proteins NSP3 and NSP4 in the latter group. Although the ASC responses to NSP3 and NSP4 were consistently lower both pre- and postchallenge in intestinal tissues from the WaA-inoculated pigs compared to the WaV-inoculated pigs, these differences were not significant because of the low magnitude of these ASC responses in both groups. Thus, greater numbers of MNC should be tested to discern these differences in future studies.
After challenge at PCD 7, no significant differences were seen in the number of total rotavirus-specific- and protein-specific ASCs between WaV+WaV/PC and WaA+WaV/PC pigs. One dose of WaV conferred full protection, whereas three doses of WaA induced partial protection, which corresponded to the significantly lower numbers of ASCs in intestinal lymphoid tissues before challenge in the WaA pigs. However, pigs given three doses of WaA had less diarrhea and virus shedding after challenge than pigs given two doses of WaA in the previous study (38). Thus, the greater protection rate in the three-dose WaA pigs likely contributed to the lack of significantly increased intestinal ASC responses we observed after challenge.
Mock+WaV/PC pigs were used as mock controls to compare the magnitude of the primary ASC response at PCD 7 with the ASC responses of the WaV- and WaA-inoculated and WaV-challenged pigs. The Mock+WaV/PC pigs had lower IgG and IgA and higher IgM ASC responses due to a primary antibody response to rotavirus, whereas the WaV+WaV/PC and WaA+WaV/PC pigs exhibited typical secondary immune responses characterized by IgG and IgA ASC (Fig. 1). No significant differences were observed between the numbers of ASCs at PID 28 and PCD 0 (prechallenge) and those at PID 35 and PCD 7 (postchallenge) (WaV versus WaV+WaV/PC pigs and WaA versus WaA+WaV/PC pigs). Yuan et al. (38) suggested that the absence of significant increases in ASC numbers upon challenge with virulent rotavirus correlated with the presence of protective immunity. Besides differences in the number of doses of WaA in the present study, ASCs were enumerated at PCD 7 in the challenged pigs, whereas Yuan et al. (38) enumerated ASCs at PCD 4 and PCD 7 and observed that the numbers of ASCs were greatest at PCD 4. This may further explain why no significant differences were seen in the numbers of ASCs in pigs prechallenge (PID 28 and PCD 0) and postchallenge (PID 35 and PCD 7) in our present study in comparison to the previous findings (38).
Because intact live rotaviruses were used for immunization, each protein-specific ASC response was stimulated (VP6, VP4, VP7, NSP3, and NSP4) regardless of the inoculum. The specific immune responses to VP6 (common inner capsid antigen), VP4 and VP7 (neutralization antigens), and NSP4 (potential enterotoxin) need to be clarified in future studies using each protein to assess its role in induction of immune responses and protection against diarrhea upon challenge of gnotobiotic pigs.
In conclusion, a protein-specific ELISPOT assay was developed and was utilized to enumerate ASCs against individual HRV proteins in gnotobiotic pigs. Results of this assay have provided a better understanding of ASC responses to structural and nonstructural rotavirus proteins in pigs inoculated with WaA or WaV HRV. We conclude that vaccination of gnotobiotic pigs with three doses of WaA HRV induced significantly lower ASC responses to total rotavirus and almost all individual rotavirus proteins compared to one dose of WaV HRV, and the former provided only partial protection against virulent Wa HRV.
ACKNOWLEDGMENTS
We thank Peggy Lewis and Paul Nielsen for technical assistance.
This work was supported by grants from the National Institutes of Health (NIH) (RO1AI33561 and RO1AI37111). Salaries and research support were provided by state and federal funds appropriated to the Ohio Agricultural Research and Development Center, The Ohio State University.
REFERENCES
- 1.Arvilommi H. ELISPOT for detecting antibody-secreting cells in response to infections and vaccinations. APMIS. 1996;104:401–410. doi: 10.1111/j.1699-0463.1996.tb00734.x. [DOI] [PubMed] [Google Scholar]
- 2.Ball J M, Tian P, Zeng C Q, Morris A P, Estes M K. Age dependent diarrhea induced by a rotaviral nonstructural glycoprotein. Science. 1996;272:101–104. doi: 10.1126/science.272.5258.101. [DOI] [PubMed] [Google Scholar]
- 3.Barnes G. Intussusception and rotavirus vaccine. J Pediatr Gastroenterol Nutr. 1999;29:375. doi: 10.1097/00005176-199910000-00001. [DOI] [PubMed] [Google Scholar]
- 4.Conner M E, Matson D O, Estes M K. Rotavirus vaccines and vaccination potential. In: Kapikian A J, editor. Rotaviruses. Berlin, Germany: Springer-Verlag; 1994. pp. 285–338. [DOI] [PubMed] [Google Scholar]
- 5.Conner M E, Estes M K, Graham D Y. Rabbit model of rotavirus infection. J Virol. 1988;62:1625–1633. doi: 10.1128/jvi.62.5.1625-1633.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.De Zoysa I, Feachem R G. Interventions for the control of diarrhoeal diseases among young children: rotavirus and cholera immunization. Bull W H O. 1985;63:569–583. [PMC free article] [PubMed] [Google Scholar]
- 7.Dormitzer P R, Greenberg H B. Calcium chelation induces a conformational change in recombinant herpes simplex virus-1-expressed rotavirus VP7. Virology. 1992;189:828–832. doi: 10.1016/0042-6822(92)90616-w. [DOI] [PubMed] [Google Scholar]
- 8.Dormitzer P R, Ho D Y, Mackow E R, Mocarski E S, Greenberg H B. Neutralizing epitopes on herpes simplex virus-1-expressed rotavirus VP7 are dependent on coexpression of other rotavirus proteins. Virology. 1992;187:18–32. doi: 10.1016/0042-6822(92)90291-v. [DOI] [PubMed] [Google Scholar]
- 9.Estes M K. Rotaviruses and their replication. In: Fields B N, Knipe D M, Howley P M, editors. Fields virology. 3rd ed. Philadelphia, Pa: Lippincott-Raven; 1990. pp. 1329–1352. [Google Scholar]
- 10.Glass R I, Lang D R, Ivanoff B N, Compans R W. Introduction: rotavirus—from basic research to a vaccine. J Infect Dis. 1996;174(Suppl. 1):S1–2. doi: 10.1093/infdis/174.supplement_1.s1. [DOI] [PubMed] [Google Scholar]
- 11.Glass R I, Kilgore P E, Holman R C, Jin S, Smith J C, Woods P A, Clarke M J, Ho M S, Gentsch J R. The epidemiology of rotavirus diarrhea in the United States: surveillance and estimates of disease burden. J Infect Dis. 1996;174(Suppl. 1):S5–11. doi: 10.1093/infdis/174.supplement_1.s5. [DOI] [PubMed] [Google Scholar]
- 12.Hoshino Y, Kapikian A Z. Rotavirus vaccine development for the prevention of severe diarrhea in infants and young children. Trends Microbiol. 1994;2:242–249. doi: 10.1016/0966-842x(94)90629-7. [DOI] [PubMed] [Google Scholar]
- 13.Ishida S, Feng N, Tang B, Gilbert J M, Greenberg H B. Quantification of systemic and local immune responses to individual rotavirus proteins during rotavirus infection in mice. J Clin Microbiol. 1996;34:1694–1700. doi: 10.1128/jcm.34.7.1694-1700.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ishida S, Feng N, Joanna J M, Tang B, Greenberg H B. Immune responses to individual rotavirus proteins following heterologous and homologous rotavirus infection in mice. J Infect Dis. 1997;175:317–323. doi: 10.1086/516462. [DOI] [PubMed] [Google Scholar]
- 15.Johansen K, Hinkula J, Espinoza F, Levi M, Zeng C, Ruden U, Vesikari T, Estes M, Svensson L. Humoral and cell-mediated immune responses in humans to the NSP4 enterotoxin of rotavirus. J Med Virol. 1999;59:369–377. [PubMed] [Google Scholar]
- 16.Kang S Y, Benfield D A, Gorziglia M, Saif L J. Characterization of the neutralizing epitopes of VP7 of the Gottfried strain of porcine rotavirus. J Clin Microbiol. 1993;31:2291–2297. doi: 10.1128/jcm.31.9.2291-2297.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kapikian A Z. Viral gastroenteritis. JAMA. 1993;269:627–630. [PubMed] [Google Scholar]
- 18.Kapikian A Z, Chanock R M. Rotaviruses. In: Fields B N, Knipe D M, Howley P M, editors. Fields virology. 3rd ed. Philadelphia, Pa: Lippincott-Raven; 1996. pp. 1657–1708. [Google Scholar]
- 19.Kapikian A Z, Hoshino Y, Chanock R, Perez-Schael L. Efficacy of a quadrivalent rhesus rotavirus-based human rotavirus vaccine aimed at preventing severe rotavirus induced diarrhea in infants and young children. J Infect Dis. 1996;174(Suppl. 1):S65–72. doi: 10.1093/infdis/174.supplement_1.s65. [DOI] [PubMed] [Google Scholar]
- 20.Kim Y B. Developmental immunity in the piglet. Birth Defects. 1975;11:549–557. [PubMed] [Google Scholar]
- 21.Kirkwood C D, Palombo E A. Genetic characterization of the rotavirus nonstructural protein, NSP4. Virology. 1997;236:258–265. doi: 10.1006/viro.1997.8727. [DOI] [PubMed] [Google Scholar]
- 22.Mattion N M, Cohen J, Estes M K. The rotavirus proteins. In: Kapikian A Z, editor. Viral infections of the gastrointestinal tract. New York, N.Y: Marcel Dekker Inc.; 1994. pp. 169–249. [Google Scholar]
- 23.Phillips R W, Tumbleson M E. Models. In: Tumbleson M E, editor. Swine in biomedical research. New York, N.Y: Plenum Press; 1986. pp. 437–440. [Google Scholar]
- 24.Richardson S C, Bishop R F. Homotypic serum antibody response to rotavirus proteins following primary infection of young children with serotype 1 rotavirus. J Clin Microbiol. 1990;28:1891–1897. doi: 10.1128/jcm.28.9.1891-1897.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Richardson S C, Grimwood K, Bishop R F. Analysis of homotypic and heterotypic serum immune responses to rotavirus proteins following primary rotavirus infection by using the radioimmunoprecipitation technique. J Clin Microbiol. 1993;31:377–385. doi: 10.1128/jcm.31.2.377-385.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Saif L J, Yuan L, Ward L A, To T. Comparative studies of the pathogenesis, antibody immune responses, and homologous protection to porcine and human rotaviruses in gnotobiotic piglets. Ad Exp Med Biol. 1997;412:397–403. doi: 10.1007/978-1-4899-1828-4_62. [DOI] [PubMed] [Google Scholar]
- 27.Saif L J, Jackwood D J. Enteric virus vaccines: theoretical considerations, current status and future approaches. In: Saif L J, Theil K W, editors. Viral diarrheas of man and animals. Boca Raton, Fla: CRC Press; 1990. pp. 313–329. [Google Scholar]
- 28.Saif L J, Yuan L, Ward L, Rosen B L, To T L. The gnotobiotic piglet as a model for studies of disease pathogenesis and immunity to human rotaviruses. Arch Virol. 1996;12(Suppl.):153–161. doi: 10.1007/978-3-7091-6553-9_17. [DOI] [PubMed] [Google Scholar]
- 29.Schaller J P, Saif L J, Cordle C T. Prevention of human rotavirus-induced diarrhea in gnotobiotic pigs using bovine antibody. J Infect Dis. 1992;165:623–630. doi: 10.1093/infdis/165.4.623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shaw R D, Vo P T, Offit P A, Coulson B S, Greenberg H B. Antigenic mapping of the surface proteins of rhesus rotavirus. Virology. 1986;155:434–451. doi: 10.1016/0042-6822(86)90205-9. [DOI] [PubMed] [Google Scholar]
- 31.Shaw R D, Groene W S, Mackow E R, Merchant A A, Cheng E H. Recombinant baculovirus-expressed rotavirus protein (VP4) in an ELISPOT assay of antibody secretion. Viral Immunol. 1992;5:51–59. doi: 10.1089/vim.1992.5.51. [DOI] [PubMed] [Google Scholar]
- 32.Shaw R D, Groene W S, Mackow E R, Merchant A A, Cheng E H. VP4-specific intestinal antibody response to rotavirus in a murine model of heterotypic infection. J Virol. 1991;65:3052–3059. doi: 10.1128/jvi.65.6.3052-3059.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Svensson L, Sheshberadaran H, Vene S, Norrby E, Grandien M, Wadell G. Serum antibody responses to individual viral polypeptides in human rotavirus infections. J Gen Virol. 1987;68:643–651. doi: 10.1099/0022-1317-68-3-643. [DOI] [PubMed] [Google Scholar]
- 34.Svensson L, Sheshberadaran H, Vesikari T, Norrby E, Wadell G. Immune response to rotavirus polypeptides after vaccination with heterologous rotavirus vaccines (RIT 4237, RRv-1) J Gen Virol. 1987;68:1993–1999. doi: 10.1099/0022-1317-68-7-1993. [DOI] [PubMed] [Google Scholar]
- 35.Vesikari T. Rotavirus vaccines against diarrhoeal disease. Lancet. 1997;350:1538–1541. doi: 10.1016/S0140-6736(97)03254-6. [DOI] [PubMed] [Google Scholar]
- 36.Ward L A, Rosen B L, Yuan L, Saif L J. Pathogenesis of an attenuated and a virulent strain of group A human rotavirus in neonatal gnotobiotic pigs. J Gen Virol. 1996;77:1431–1441. doi: 10.1099/0022-1317-77-7-1431. [DOI] [PubMed] [Google Scholar]
- 37.Wyatt R G, Mebus C A, Yolken R H, Kalica A R, James H D, Kapikian A Z, Chanock R M. Rotavirus immunity in gnotobiotic calves: heterologous resistance to human virus induced by bovine virus. Science. 1979;203:548–550. doi: 10.1126/science.216077. [DOI] [PubMed] [Google Scholar]
- 38.Yuan L, Ward L A, Rosen B L, To T L, Saif L J. Systemic and intestinal antibody-secreting cell responses and correlates of protective immunity to human rotavirus in a gnotobiotic pig model of disease. J Virol. 1996;70:3075–3083. doi: 10.1128/jvi.70.5.3075-3083.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yuan L, Kang S Y, Ward L A, To T L, Saif L J. Antibody-secreting cell responses and protective immunity assessed in gnotobiotic pigs inoculated orally or intramuscularly with inactivated human rotavirus. J Virol. 1998;72:330–338. doi: 10.1128/jvi.72.1.330-338.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]