Abstract
Smoking is the leading preventable cause of death and disability in the U.S. Empirical evidence suggests that engaging in evidence-based self-regulatory strategies (e.g., behavioral substitution, mindful attention) can improve smokers’ ability to resist craving and build self-regulatory skills. However, poor engagement represents a major barrier to maximizing the impact of self-regulatory strategies. This paper describes the protocol for Mobile Assistance for Regulating Smoking (MARS) – a research study designed to inform the development of a mobile health (mHealth) intervention for promoting real-time, real-world engagement in evidence-based self-regulatory strategies. The study will employ a 10-day Micro-Randomized Trial (MRT) enrolling 112 smokers attempting to quit. Utilizing a mobile smoking cessation app, the MRT will randomize each individual multiple times per day to either: (a) no intervention prompt; (b) a prompt recommending brief (low effort) cognitive and/or behavioral self-regulatory strategies; or (c) a prompt recommending more effortful cognitive or mindfulness-based strategies. Prompts will be delivered via push notifications from the MARS mobile app. The goal is to investigate whether, what type of, and under what conditions prompting the individual to engage in self-regulatory strategies increases engagement. The results will build the empirical foundation necessary to develop a mHealth intervention that effectively utilizes intensive longitudinal self-report and sensor-based assessments of emotions, context and other factors to engage an individual in the type of self-regulatory activity that would be most beneficial given their real-time, real-world circumstances. This type of mHealth intervention holds enormous potential to expand the reach and impact of smoking cessation treatments.
Keywords: Smoking Cessation, Mobile Health (mHealth), Engagement, Self-Regulatory Strategies, Micro-Randomized Trial (MRT)
1. Introduction
Smoking is the leading preventable cause of death and disability in the U.S.[1–3] with about 30% of all cancer deaths directly attributable to smoking [4–6]. Although most smokers would like to quit, over 90% of quit attempts are unsuccessful [7, 8]. Empirical evidence suggests that engaging in evidence-based self-regulatory strategies (e.g., behavioral substitution, mindful attention) can improve smokers’ ability to resist craving and build self-regulatory skills [9–12]. As a result, self-regulatory strategies are key components in many smoking cessation interventions [13–15]. However, poor engagement represents a major barrier to maximizing the impact of self-regulatory strategies [9, 16]. Enhancing real-time, real-world engagement in evidence-based self-regulatory strategies has the potential to improve the efficacy of smoking cessation interventions.
Just-In-Time Adaptive Interventions (JITAIs) delivered via mobile devices have been developed for preventing and treating addictions [17–19]. JITAIs adapt over time to an individual’s changing status and are optimized to provide appropriate intervention strategies based on real time, real world context [20, 21]. Vulnerability (e.g., risk for lapse) and receptivity (i.e., ability and willingness to invest energy in a specific intervention) are two theoretical constructs that play a critical role in the formulation of effective JITAIs. Conceptual models of JITAIs emphasize the importance of minimizing disruptions to the daily lives of the individual by tailoring strategies not only to vulnerability, but also to receptivity [20, 22, 23]. Both vulnerability and receptivity are considered latent states that are dynamic and constantly changing based on the constellation and temporal dynamics of emotions, context, and other factors [20, 23] . However, limited research attention has been given to systematically investigating the nature of vulnerability and receptivity, as well as how knowledge of these states can be used to decide when and how to intervene during a smoking quit attempt. For example, although it is often assumed that the value of JITAIs lies in their ability to detect high vulnerability for lapse and intervene “just in time,” it may be equally or even more important to intervene in periods of low vulnerability but high receptivity to facilitate skill-learning and prevent future risk elevations. Further, different contextual and intrapersonal factors that increase vulnerability to lapse (e.g., the presence of other smokers [24, 25]) might decrease receptivity to certain self-regulatory strategies (e.g., complex and effortful exercises), but not to others (e.g., less effortful escape and active coping strategies).
The Mobile Assistance for Regulating Smoking (MARS) study will employ a 10-day Micro-Randomized Trial (MRT) [26] enrolling 112 smokers attempting to quit. Using a mobile smoking cessation app, the MRT will randomize each individual multiple times per day to either: (a) no intervention prompt; (b) a prompt recommending brief (low effort) cognitive and/or behavioral self-regulatory strategies; or (c) a prompt recommending more effortful cognitive or mindfulness-based strategies. The goal is to investigate whether, what type of, and under what conditions (e.g., current state of vulnerability and/or receptivity) prompting the individual via a mobile device to engage in self-regulatory strategies increases proximal engagement in self-regulatory strategies, which in turn reduces vulnerability (see conceptual model in Figure 1).
Figure 1:
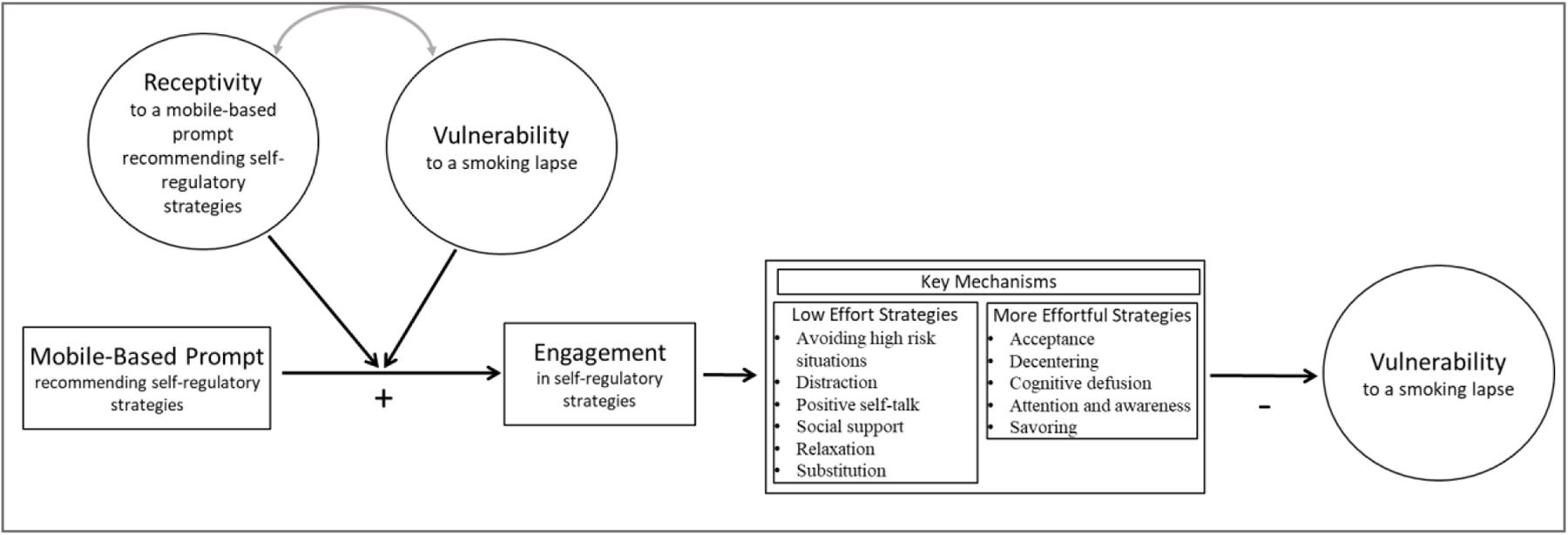
Conceptual Model for MARS
2. Materials and methods
The MRT study protocol and consent procedures were approved by the University of Utah Institutional Review Board before enrolling participants. The trial was registered on clinicaltrials.gov (NCT04376489) and the full study protocol and results will be published on clinicaltrials.gov upon completion of the trial.
2.1. Research Design
This study employs a MRT – an experimental design for optimizing mHealth interventions [26]. MRTs enable investigators to construct an empirically-informed JITAI prior to conducting a randomized control trial (RCT) to confirm its effectiveness [27]. Specifically, MRTs are suitable for addressing questions about whether and under what conditions it is beneficial to deliver mobile-based intervention prompts. Leveraging the rapidly growing and dynamic digital intervention environment, MRTs provide data that informs the development of an effective JITAI. The effectiveness of this empirically informed JITAI can then be evaluated via a RCT comparing the JITAI to a suitable control (e.g., standard of care).
In the MARS MRT, participants will be assessed from 3 days prior to their quit date through 6 days post-quit using Ecological Momentary Assessments (EMA), a suite of wireless sensors (MotionSense HRV [28]), and Global Positioning System (GPS). This 10 day period was selected in order to allow participants to learn and practice the use of self-regulatory strategies during the 3 days prior to their quit day, and (b) capture the period of time when first lapses are most likely to occur [29–31].
Participants will be asked 6 times per day via a smartphone to respond to a brief 2-question survey about availability of cigarettes and negative affect. Immediately following survey completion (or 5 minutes following survey initiation if the survey was not completed), the individual will be micro-randomized (2:1:1) to either: (a) no prompt; (b) a prompt recommending brief (low effort) cognitive and/or behavioral strategies (tailored to the participant’s response to the 2-questions survey); or (c) a prompt recommending relatively more effortful (5–10 minutes) self-regulatory strategies (cognitive or mindfulness-based exercises on the mobile device).
Approximately one hour following each micro-randomization, participants will be asked to complete an EMA (i.e., six EMAs per day). These EMAs are relatively short (less than 5 minutes to complete) and designed to assess engagement with self-regulatory strategies (primary proximal outcome), as well as gather information about affect, behaviors, cognitions, and context.
Participants will also attend three in-person or virtual visits, which will occur on Day 1, Day 4 (quit day) and Day 10. At each visit, smoking status will be assessed, participants will fill out standard questionnaires, receive brief tobacco cessation counseling, and be compensated for their time. The study design is summarized in Figure 2.
Figure 2:
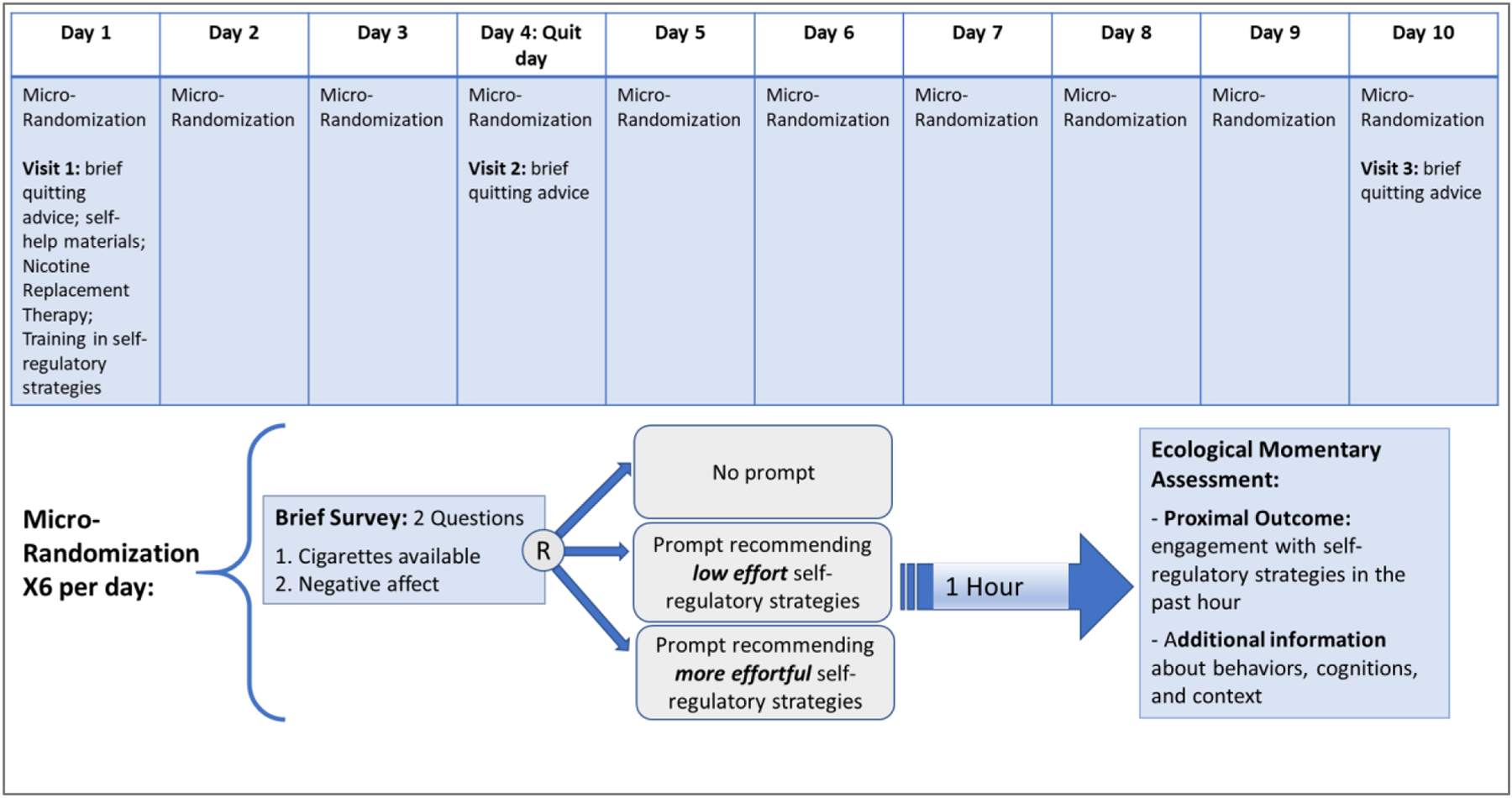
Study Design
2.2. Aims
The goal of the current study is to investigate whether, what type of, and under what conditions (e.g., current state of vulnerability and/or receptivity) a self-regulatory strategy recommendation increases proximal engagement in those strategies (primary outcome), which in turn reduces vulnerability.
The primary aim is to investigate whether prompting with a recommendation to engage in self-regulatory strategies (vs. no prompting) generally leads to more engagement in self-regulatory strategies. We hypothesize (H1) that prompting (vs. not prompting) increases proximal engagement in self-regulatory strategies.
The secondary aim is to investigate the difference between prompting to engage in brief (low effort) self-regulatory strategies vs. prompting to engage in more effortful self-regulatory strategies in promoting proximal engagement. Given the multiple demands competing for people’s time, effort, and attention in daily life [20, 23, 32], we anticipate that on average individuals are more inclined to engage in less effortful recommendations. Hence, we hypothesize (H2) that prompting with a recommendation to engage in brief (low effort) self-regulatory strategies generally leads to greater proximal engagement than prompting to engage in more effortful strategies.
Exploratory aims investigate whether the effect of prompting on proximal engagement is moderated by indicators of vulnerability and receptivity. Indicators of vulnerability include lower positive emotions such as pride, gratitude, and happiness; greater negative emotions such as anger, sadness, and restlessness; lower self-regulatory capacity; risky context, such as tobacco outlet proximity and specific locations [e.g., in a bar]; adverse intrapersonal factors, such as urge and low abstinence self-efficacy; as well as more stable risk factors, such as neighborhood disadvantage, financial stress, poor mental health, heaviness of smoking and other substance use. Indicators of receptivity include greater positive activating emotions, such as joy, happiness, and gratitude; lower negative deactivating emotions, such as boredom and hopelessness; higher self-regulatory capacity; contextual factors, such as distant tobacco outlet proximity and specific locations (e.g., home); beneficial intrapersonal factors, such as low urge and high abstinence self-efficacy; as well as more stable protective factors, such as agency, health literacy, social support and personal resources. We hypothesize (H3) that real-time mobile-based prompts increase proximal engagement under conditions that represent vulnerability to smoking lapse (H3a) and receptivity to intervention prompts (H3b). We will also investigate the association between engagement and subsequent vulnerability, hypothesizing that (H4) in general, engagement in self-regulatory strategies is associated with reductions in subsequent vulnerability (H4a), with greater reductions facilitated by engagement in more (vs. less) effortful strategies (H4b) as they are more likely to support skill-learning and resiliency. Given that the effect of prompting may dissipate over time due to accumulated exposure to the prompts which may lead to burden or habituation (i.e., diminishing tendency to respond to a frequently repeated stimulus [33]), exploratory analyses will also investigate whether the proximal effect of prompting (vs. no prompting) varies by time.
2.3. Counseling/Pharmacotherapy Interventions
All participants will receive 6 weeks of nicotine replacement therapy (combination of nicotine patch and nicotine gum), self-help materials, and counseling. Counseling is based on the Treating Tobacco Use and Dependence Clinical Practice Guideline [34]. Sessions will provide psychoeducation about nicotine dependence and quitting smoking, encourage participants to identify and plan for high-risk situations, and teach cognitive and behavioral techniques (e.g., distraction, modifying routines) for avoiding and coping with high-risk situations. Nicotine patch/gum dispensation and counseling will occur during the in-person/virtual visits.
2.4. mHealth Intervention Components
The MARS app was designed to deliver a suite of brief cognitive and/or behavioral strategies, as well as more effortful cognitive or mindfulness-based exercises. During the first in-person/virtual visit, participants will receive brief training in the self-regulatory strategies listed below. Participants will also receive a smartphone and training in accessing and using the MARS app. The MARS app is implemented in the mCerebrum smartphone framework [35] that includes apps to collect sensor data from wearable devices and sensors in the smartphone, deliver EMAs, and deliver mHealth interventions.
2.4.1. Brief Cognitive and/or Behavioral Strategies
The MARS app will deliver prompts (via push notifications) recommending relatively low effort, brief cognitive and behavioral strategies based on the current state of the science recommendations [7, 9]. A library of more than 180 prompts recommending low effort strategies that are tailored to cigarette availability and negative affect was created. Table 1 includes example prompts for different scenarios. In general, the prompts were designed to provide simple, easily implementable strategies, such as avoiding high risk situations (e.g., move away from places where you used to smoke), and using coping strategies like distraction (e.g., go for a walk, listen to a favorite song), positive self-talk (e.g., cigarettes will not change situations or problems, they only provide a brief escape), support systems (e.g., talk to your family and friends and ask them for support), relaxation (e.g., use deep breathing), substitution (e.g., fidget with a rubber band to keep your hands busy), and remind and reward (e.g., remind yourself of your reasons for quitting).
Table 1.
Example Prompts Recommending Low Effort Self-Regulatory Strategies
| Cigarettes are available | Cigarettes not available | |
|---|---|---|
| Negative affect present | - Leave the situation until the temptation to smoke has passed or call a supportive friend. Talking about what is stressful can help you feel better. - Believe in yourself – you can do it! If you are feeling stressed or down, be sure to avoid being around cigarettes right now. - When you are feeling stressed, leave if cigarettes are available or drink a tall glass of water. You’re in control! |
- Quitting can be tough, so try to relax. Listen to calming music or call or text a family member or friend to lean on. - By quitting you may feel you’ve lost part of yourself – use this time to start a new healthy habit like walking 10 minutes a day. You deserve success! - Occupy your mind by going out for fresh air, or remind yourself that you are capable of getting through the ups and downs that come with quitting. |
|
| ||
| Negative affect not present | - Instead of picking up a cigarette, do something new with your hands. An idea is to play a game on your phone! - If there are cigarettes nearby then leave, even if for a minute, or remind yourself that you stopped smoking for your health. Stay strong, even if it’s tough! - You got this! If cigarettes are around remind yourself that smoking is not an option and you chose not to smoke! |
- Be proud of the work you’re doing to change your life! Create a list of rewards you can give yourself for staying quit. - Surround yourself with people who give you positive support for the work you’re doing, or think of how much money you will save by not buying cigarettes. - Ask a friend to help you start healthy routines that don’t include smoking. Believe in yourself - you can do it! |
Note: This table contains 12 examples of prompts from a library of more than 180 possible prompts developed by our team. The prompts are tailored based on the participant’s response to the brief 2-questions survey delivered prior to each micro-randomization. If the participant does not complete the brief 2-question survey, the prompt will recommend more general strategies such as those described in the lower-right quadrant of the table.
2.4.2. Effortful Self-Regulatory Strategies
The MARS app includes five relatively more effortful self-regulatory strategies that (a) draw on empirically-supported self-regulation approaches, such as Acceptance and Commitment Therapy [10], and Mindfulness-Based Stress Reduction [13]; and (b) were pilot-tested for feasibility and acceptability in the mobile setting, showing promising results [14–16, 36, 37]. Table 2 provides a brief description of each of the five strategies, and the key mechanisms each is intended to target.
Table 2:
Effortful Self-regulatory Strategies
| Activity | Description | Example Key Mechanisms |
|---|---|---|
| Mood surf | Learning and practicing how to manage stressful thoughts and emotions (e.g., urges) by “riding the waves” as they fluctuate in intensity. | Acceptance: The non-judgmental embracing of cognitions, emotions, and sensations as they occur without efforts to control them [38, 39]. |
| Meditate | Learning and practicing how to cultivate mindfulness by directing attention to experiences that are occurring in the moment without judgment. | Decentering (mindful awareness): the capacity to observe thoughts, feelings, and memories with healthy psychological distance, greater self-awareness and perspective-taking [40, 41] |
| Imagine | Learning and practicing how to create distance between themselves and an unwanted thought or feeling. | Cognitive defusion: The separation of thoughts from the self and from what they refer to, without a direct attempt to modify their content [38, 39]. |
| Notice | Learning and practicing how to become more aware of and accept uncomfortable thoughts, feelings, and physical sensations. | Attention and awareness: Increasing focused attention and conscious awareness to current thoughts, feelings, and sensations and allowing them to enter the mind with acceptance [39, 42]. |
| Joy | Learning and practicing relaxation via deep breathing and directing attention to content (photos) that brings joy and gratitude. | Savoring: The capacity to attend to and appreciate positive experiences and thoughts in order to savor or prolong enjoyment (i.e., to up-regulate positive emotions)[43, 44]. |
Note: The mechanisms listed above are example key mechanisms targeted by each activity. However, there may be substantial overlap among these (e.g., acceptance may be a mechanism targeted by Mood surf, Meditate and Notice).
2.4.3. Randomization to Engagement Prompts
Prompts recommending engagement in self-regulatory strategies will be provided via push notifications. Each participant will be randomized 6 times per day with equal probability (0.5) to either: (1) an engagement prompt; or (2) no prompt. Engagement prompts are further randomized with equal probability (0.5) to either (a) low effort strategy prompt; or (b) effortful strategy prompt. The content of the low effort strategy prompt will be randomly selected from the library of brief, tailored cognitive/behavioral messages (see examples in Table 1). The strategy recommended in the effortful strategy prompt will be randomly selected from the strategies described in Table 2.
The decision to randomize participants 6 times per day, spreading them throughout the day with approximately 2 hours between each randomization was based on a range of considerations, including: (a) the motivation to investigate how to best adapt the delivery of engagement prompts across time in response to conditions (e.g., affect, behaviors, cognitions and context) that are highly dynamic [29, 45–51]; (b) the effort associated with the self-regulatory strategies; (c) the risk of satiation or burden leading to dropout; and (d) concerns that individuals would begin to ignore self-regulatory suggestions through habituation. Additionally, due to practical and ethical considerations, randomizations will not occur if driving is detected or if the participant activates the sleep mode on the smartphone.
3. Participants and Procedures
3.1. Eligibility
Participants will be at least 18 years of age, a current smoker who smokes an average of at least 3 cigarettes per day, motivated to quit smoking within the next 30 days, reside in Salt Lake County, Utah, have a valid home address and functioning telephone number, can speak, read, and write in English, and have at least marginal health literacy. Those participating via virtual visits must also self-report access to reliable Wi-fi. Exclusion criteria include: contraindication for nicotine patch or gum use; active substance misuse or dependence; current use of tobacco cessation medications; pregnancy or lactation; or, another household member enrolled in the study.
3.2. Recruitment
Participants will be recruited using a comprehensive, multi-pronged recruitment strategy that focuses on media and community outreach. Our primary recruitment efforts will focus on radio, websites (e.g., Google, Craigslist), social media outlets (e.g., Facebook, Instagram), and other online recruitment venues (e.g., Trialfacts). Recruitment flyers will be posted in public areas such as community clinics, local restaurants, and bars.
3.3. Initial Screening and Orientation Procedures
Recruitment materials will instruct interested participants to contact study staff by email or phone to learn more about the study. Participants will initially be screened for eligibility over the phone. Those deemed eligible will be scheduled for an information session, during which eligibility will be confirmed, participants will be given more information about the study procedures, and study staff will review the Informed Consent Document with the participants and answer any questions they may have regarding the study and their participation. These procedures will be followed exactly for participants who attend the information session virtually, except that the visit will take place via Zoom. Documents explaining the study will be projected on the screen so the participant can follow along with staff as they go through each detail of the study. At the information session, participants will be scheduled for study visits 1–3, which will occur on Day 1, Day 4 (quit date), and Day 10.
3.4. Assessments
3.4.1. EMA Protocol
Six EMAs will be triggered per day during the 10-day MRT. An EMA will be triggered ~1 hour following each intervention prompt randomization; hence, there will be ~2 hours between each EMA and the next. If driving is detected, EMAs will be delayed up to 15 minutes. Because alerts to complete EMAs may occur at inconvenient times, participants will be able to delay an EMA for up to 20 minutes, or put the smartphone into “sleep” mode for up to 2 hours when it is impossible or inconvenient to respond (e.g., attending church). This strategy improves compliance and does not bias the data [52]. Total time devoted to EMA is not excessively burdensome (19–34 minutes/day on average), with expected compliance rates >75% [53–56]. Our previous research has shown that >90% of completed assessments include no delays [57], and EMA reactivity is nonexistent or small [58].
Primary Proximal Outcome:
EMAs will be used to measure the primary proximal outcome -- engagement in self-regulatory activities in the past hour following randomization to engagement prompts. In particular, the EMAs will ask participants to think about the most recent tip (i.e., brief cognitive and/or behavioral strategy) or activity recommended by the MARS app and indicate whether (Yes/No) in the last hour they used this tip or activity; any other tip or activity recommended by MARS; or any other tip or activity not included in the MARS app.
Other EMA Items:
In addition to the three questions assessing engagement in self-regulatory activities, 44 additional items will be used to assess emotions, substance use, intrapersonal, and contextual information. Specific emotion items were derived from previous EMA studies, recent empirical data, conceptual models in affective and addiction science, and preliminary data [25, 51, 59–68]. Negative emotion items include: angry, ashamed, guilty, irritable, lonely, anxious, sad, restless, bored, and hopeless. Positive emotion items include: grateful, happy, proud, relaxed, and enthusiastic. Other items will assess substance use (e.g., smoking cigarettes, vaping, marijuana use, and alcohol consumption) intrapersonal (e.g. motivation, urge, smoking outcome expectancies, self-efficacy, and self-regulatory capacity) and contextual factors (e.g., cigarette availability, smoking restriction, being around other smokers, and social support), recent stressors, and patch use.
3.4.2. Visits
Participants will be scheduled for 3 visits that occur on Day 1, Day 4 (quit date) and Day 10. At each visit, participants will fill out a battery of questionnaires about demographic information as well as relatively stable risk and protective factors (see Table 3). Note that Visit 3 also includes usability and acceptability measures, asking participants how easy or difficult they found using the study smartphone and the wrist sensors (Likert scales: 1=Verry difficult to 5=Very easy), how often they had technical problems with the MARS app, and how much they liked various features of MARS (Likert scales: 1=Strongly dislike to 5=Strongly like), including the prompts and each of the self-regulatory strategies.
Table 3.
Study Visits Measures
| Demographic information, socioeconomic status [69–71] |
| Tobacco use, dependence, withdrawal, and other substance use [72–82] |
| Predispositions, mental health, stress, affect [83–89] |
| Agency, Self-regulation [90–92] |
| Social support, personal resources [93–95] |
| Neighborhood disadvantage [96] |
| Health behavior, health literacy, self-rated health, and medical conditions [78, 97–100] |
Information Session:
Study staff will confirm eligibility and will subsequently give participants more information about the study. Staff will review the Informed Consent Document with the participant, answer any questions participants may have concerning the study and their participation, and schedule study visits 1–3.
Visit 1 (Day 1):
Study staff will confirm that all inclusion criteria are met. Following informed consent, participants will be asked to complete a battery of questionnaires and then receive tobacco treatment including brief quitting advice, self-help materials, and Nicotine Replacement Therapy (NRT). Next, participants will be trained on the use of study equipment (i.e., study phone, wrist-worn sensors) and the self-regulatory strategies described in Tables 1 and 2. Finally, the next visit date will be confirmed, and participants will be compensated for their time. These procedures will be followed exactly for participants who attend Visit 1 virtually, except that the visit will take place via Zoom. Study staff will ship all necessary supplies (NRT and NRT guidelines, brief quitting advice booklet, study equipment, equipment instructions, equipment return instructions and return shipping label, and Adobe Sign instructions for consent) to the participant 5–7 days prior to the scheduled visit. During the virtual visit, study documents will be projected on the screen and consent will be obtained via Adobe Sign. Participants will also be trained on the use of study equipment over Zoom.
Visit 2 (Day 4: quit day):
Participants will fill out questionnaires and receive tobacco treatment (brief quitting advice). Staff will confirm study equipment is working properly. Finally, the next visit date will be confirmed, and participants will be compensated for their time. Similar procedures will be followed for participants who attend Visit 2 virtually, such that participants will receive brief quitting advice and staff will confirm study equipment is working properly during the Zoom session. Study staff will send participants a link to their questionnaires and will instruct them to complete the questionnaires before midnight of the following day (i.e., 48 hours from the start of the scheduled quit date).
Visit 3 (Day 10):
Participants will fill out questionnaires and receive tobacco treatment (brief quitting advice). Staff will collect the study equipment and participants will be compensated for their time. Similar procedures will be followed for participants who attend Visit 3 virtually, such that they will receive brief quitting advice during the zoom session. Study staff will send participants a link to their questionnaires and will instruct the participants to complete the questionnaires within 4 days from the start of the Visit 3 scheduled date. Participants will also be reminded to return all study equipment using the shipping label provided in the materials they received before Visit 1.
3.4.3. Sensor-Based Assessments
The MotionSense HRV wrist-worn sensor suite includes a pulse plethysmography (PPG), three-axis accelerometer, and three-axis gyroscope. Sensor data collected from MotionSense HRV sensors are streamed to the MARS app. Apps within the mCerebrum [35] will apply machine learning (ML) algorithms to detect smoking and index of self-regulatory capacity [101, 102].
Smoking:
We will employ puffMarker (97% sensitivity; 1.1% false positive rate) -- a smoking detection method that uses MotionSense HRV to track hand-to-mouth gestures to detect smoking puffs [103, 104]. These puffs are then composed into smoking episodes if the puffs have plausible inter-puff duration and frequency. When compared with daily CO-monitoring in an independent smoking cessation study data, puffMarker detected 28 out of 32 first lapses (all cases when the sensor suite was worn and collected data at the time of lapse). When tested on abstainers, the false positive rate was once every 6 days [104].
Self-Regulatory Capacity (HRV):
Variability in heart rate attributable to respiration is directly mediated by the vagus nerve and serves as a marker for parasympathetic activity (i.e., high frequency heart rate variability or HRV) [105]. HRV is a likely index of self-regulatory capacity because brain structures involved in self-regulation and in the autonomic nervous system have considerable overlap—especially in the prefrontal cortex [106, 107]. Although not without debate, heightened HRV may provide an index of active regulatory effort and/or strength [108, 109]. HRV has been assessed in the natural environment [110, 111] by examining root mean square of successive differences (RMSSD). RMSSD is calculated by a time series method, which appears to be the most reliable and cost efficient way to calculate HRV using ambulatory heart rate data [112, 113]. HRV will be continuously monitored using multispectral PPG data (using green and infrared LEDs). As the PPG signal is noisy and prone to motion artifacts, a probabilistic algorithm [114] will be used to generate likely sequences of interbeat intervals. Then, HRV features such as RMSSD will be calculated as averages over the inferred intervals. All procedures will follow the recommendations of the Task Force of the European Society of Cardiology and the North American Society of Pacing Electrophysiology [115, 116].
GPS/GIS:
We will utilize: (1) assisted GPS technology to track participants, and (2) high-resolution Geographic Information System (GIS) datasets (e.g., tobacco retailers; stores, bars and other entities serving/selling alcohol) to assess the likelihood of each participant being exposed to key environmental factors within their own mobility patterns. GIS data will be collected from multiple sources such as the Utah State Tax Commission and the Utah State Geographic Information Database [117]. We will first use proven geo-computational methods [118–120] to calculate participants’ minute-by-minute mobility pattern (e.g., driving, staying, walking). Then we will overlay these patterns with GIS layer to estimate exposures using either density (e.g., number of tobacco stores within 0.25 miles) or proximity (e.g., distance from the closest tobacco store) based measures.
3.4.4. Compensation
Participants will be compensated up to $400 for participating in the study. They will receive $20 each for visits 1–3, $40 for visit 4, and $60 for returning all the study equipment. Participants will also receive up to $240 for completing EMAs and wearing study equipment (i.e., wrist-worn sensors). In particular, they will receive $4 for each completed EMA if they wear the study equipment for at least 60% of the time since the prior EMA. If they wear the study equipment for less than 60% of the time since the prior EMA, they will receive $2 for each completed survey (6 EMAs per day x 10 days at $4 per survey = $240). Note that participants will not be compensated for completing the 2-question survey since these questions are considered part of the just-in-time intervention. Participants will be compensated for study visits at the end of each visit and compensated for EMA completion at Visit 3 when they return study equipment. Participants who attend Visit 1-Visit 3 virtually will be compensated via mail once study staff confirms they have completed their questionnaires. Upon receipt of study equipment via mail, staff will mail the compensation for returning equipment and will send a gift card for EMA completion.
3.5. Treatment Fidelity
All participants will receive 6 weeks of nicotine replacement therapy, self-help materials, and tobacco cessation counseling to help them quit. To measure treatment receipt and compliance, study personnel will record participants’ visit attendance, whether participants receive counseling during a visit, and the number of nicotine patches/gums dispensed. Furthermore, participants will be asked to report patch/gum compliance via EMA (i.e., whether they use a patch/gum during the first 7 days of quitting).
To ensure proper delivery of mHealth intervention components via the MARS app, 15% of participants’ smartphone data will be monitored through the custom dashboard every 2–3 days and the data will be used to evaluate the functioning and integrity of the micro-randomization procedures (e.g., by summarizing the rate at which each prompt option was delivered across participants). Paradata on app usage and participants’ feedback regarding the usefulness of each strategy included in the MARS app will be summarized at the end of the study.
3.6. Privacy and Confidentiality
Study participation will be confidential and protected by identifying all subjects by ID numbers only, with their names and other identifying information (e.g., address) kept in a firewall and password protected file on the institutional server, separate from other individual level data files. Data are collected during study visits or in the field. Data collected during visits will directly enter via workstations into secure server. The majority of the data collected in the field (e.g., GPS and accelerometer data) will be stored in encrypted format on a microSD card, which will be uploaded onto a secure server at the University of Utah during study visits. This server is in a protected facility monitored physically as well as virtually via secure alphanumeric login passwords. Data collected in the field can potentially be compromised. For example, participants could lose the smartphone before returning it to study staff. However, data on the smartphone is not visible to anyone from the device itself and requires specialized software for download and interpretation and will be in encrypted format. These data cannot be manipulated by anyone simply by fidgeting with the device.
3.7. Statistical Analysis
3.7.1. Primary Aim Analysis
The primary aim is to investigate whether prompting with a recommendation to engage in self-regulatory strategies (vs. no prompting) generally leads to more proximal engagement in self-regulatory strategies. To analyze the data, we will use a generalization of regression analysis that ensures unbiased estimation of causal effects of time-varying intervention prompts in mobile health settings [26, 121]. These analyses pool time-varying, longitudinal data across all study participants. Specifically, let t1 denote time points at which an engagement prompt may or may not be delivered; t1 ranges from 1 to 60 (10 days*6 times per day). The proximal outcome is binary (engaged or did not in the next hour following t1). We will test the prompt effects on a binary outcome using a log-linear type model [122, 123]. The causal effect of a prompt recommending self-regulatory strategies will be expressed on the (log) “risk-ratio” scale, namely on a scale that measures the probability (“risk”) of engagement in the next hour, when a prompt was delivered at t1, divided by the probability of engagement in the next hour, when a prompt was not delivered at t1. The risk-ratio will be greater than 1 if delivering (vs. not delivering) a prompt at t1 has a causal effect on the probability of engagement in self-regulatory strategies in the next hour. As noted in section 2.4.3., given practical and ethical considerations, randomization will not occur at t1 if the participant is driving a vehicle or if they activated the “sleep mode” on their smartphone. Hence, the causal effect the prompt will be estimated only among those time points t1 at which driving is not detected and the smartphone is not on a “sleep mode”. The model will adjust for gender, age, race/ethnicity, response to the 2-questions survey at t1, engagement prior to t1, time of day, and day in the study.
3.7.2. Secondary Aim Analyses
The secondary aim is to investigate the difference between a prompt recommending brief (low effort) self-regulatory strategies and a prompt recommending more effortful self-regulatory strategies in terms of promoting proximal engagement. The secondary aim will focus on the marginal effect of delivering a prompt to engage in low effort (vs. more effortful) strategies at t1 on engagement in self-regulatory strategies in the next hour following t1 (binary proximal outcome). We will test this effect using a log-linear type model [122, 124] that includes indicators for whether at t1 (i) a prompt recommending low effort strategies was delivered (vs. otherwise); and (ii) a prompt recommending more effortful strategies was delivered (vs. otherwise). The causal effect will be expressed on the (log) “risk-ratio” scale, measuring the probability (“risk”) of engagement in the next hour, when a low effort prompt was delivered at t1, divided by the probability of engagement in the next hour, when a more effortful prompt was delivered at t1. The risk-ratio will be greater than 1 if delivering a prompt recommanding low effort (vs. more effortful) strategies has a causal effect on the probability of engagement in self-regulatory strategies in the next hour. Similar to the primary aim, the analysis will be restricted to those time points t1 in which driving is not detected and the sleep-mode is not activated. The model will adjust for gender, age, race/ethnicity, engagement prior to t1, time of day, and day in the study.
3.7.3. Exploratory Analyses
Exploratory aims will investigate whether the effects of prompting are moderated by indicators of vulnerability and receptivity. This will be done by extending the models described in 3.7.1. and 3.7.2 to include interactions between the engagement prompt indicators and covariates that represent vulnerability and/or receptivity. Covariates will include both dynamic (measured via EMAs and sensor-based assessments) and more stable (measured during visits 1–3) constructs. We will also investigate whether the proximal effects of prompting, described in the primary and secondary aim analyses, vary by time. This will be done by extending the models described in sections 3.7.1. and 3.7.2 to include interactions between the prompt indicators and time in the study. Estimates of the risk ratios for comparing the prompt options at different levels of each candidate moderator and their 95% confidence intervals will be reported. Finally, we will investigate the effect of engagement in the next hour following t1 on subsequent indicators of vulnerability (observational comparison), conditional on indicators of receptivity, vulnerability, and the engagement prompt option at t1.
3.7.4. Missing Data
A thorough investigation of mechanisms for missing data will be carried out. Missing data will be dealt with explicitly using multiple imputation (MI) procedures [125]. In sensitivity analyses, data for all aims will be analyzed with and without MI. Since non-completion of EMAs may be associated with intervention disengagement, the data will also be analyzed with EMA non-completion considered as non-engagement and any discrepancies will be reported.
3.8. Sample Size and Power
Sample size (N=112) was planned based on the primary aim comparison between delivering vs. not delivering a prompt, assuming 10% dropout (N=100 after attrition), two-sided 5% Type-I error, within person correlations ranging between 0.65 – 0.67 and 0.15 probability of engagement in the next hour if a prompt was not delivered at t1. Power calculators do not exist for MRTs with a binary proximal outcome, hence we approximated power using simulations (code and documentation are available online [126]). Given that micro-randomization at a given time point t1 will occur only if the individual is not driving and the sleep-mode is not activated on the smartphone, power is reported (see Table 4) for varying average rates of micro-randomized time points throughout the study (i.e., 80%, 85%, and 90%). As can be seen in Table 4, even if on average micro-randomizations occur on only 80% of the 60 possible time points during the study, a sample size of N=100 will allow us to address the primary aim with adequate power. Specifically, sample size of N=100 will enable us to reject the null hypothesis of no difference between prompting and no prompting (primary aim) with 84% power when the risk ratio for the effect is ≥ 23% greater engagement in favor of the engagement prompt, which is considered small [127]. This sample size will also allow us to address the secondary aim with adequate power (see Table 4). Specifically, we will reject the null hypothesis of no difference between the two types of engagement prompts with 82% power when the RR for the effect is ≥31% greater engagement in favor of a prompt recommending a low effort strategy (vs. a prompt recommending a more effortful strategy), which is considered between small to moderate [127]. Note that Type 1 error rates for the primary aim and the secondary aim are each set to 0.05.
Table 4.
Estimated Power for N=100
| Average rate of no driving and no sleep-mode at t1 | Primary Aim: Power when RR=1.23 | Secondary Aim: Power when RR=1.31 |
|---|---|---|
| 80% | 0.84 | 0.82 |
| 85% | 0.85 | 0.85 |
| 90% | 0.86 | 0.87 |
4. Summary
4.1. Overview of Study
The MARS study will conduct a micro-randomized trial (MRT) to investigate whether, what type, and under what conditions prompting smokers attempting to quit to engage in self-regulatory strategies promotes proximal engagement in these strategies. JITAIs hold tremendous potential for promoting smoking cessation [36] and there is increased interest in empirically developing JITAIs that deliver real-time support to smokers attempting to quit [128–131]. However, there is scant evidence to guide the development of a JITAI that effectively engages smokers attempting to quit in self-regulatory strategies in real-time.
4.2. Importance of Optimizing a JITAI to Support Smoking Cessation
To the best of our knowledge, MARS is the first full-scale MRT designed to inform the construction of a JITAI that promotes real-time, real-world engagement in self-regulatory strategies among smokers attempting to quit. To our knowledge, existing studies focusing on the development of JITAIs for smoking cessation primarily concern feasibility and acceptability, providing initial evidence of efficacy or proof of concept [128–130]. While these studies represent an important first step in laying the groundwork for building effective JITAIs, the Multiphase Optimization Strategy [MOST; 132] highlights the importance of conducting full-scale trials to inform the optimization of JITAIs. Here, optimization refers to gathering information to decide when and how to intervene in order to comprise an effective and practical (i.e., effective given real-world constraints) mHealth intervention, prior to evaluating its effectiveness relative to control [132]. To optimize a JITAI, it is critical to systematically answer questions about when and how to intervene given constraints that are imposed by the person’s natural environment, where multiple demands compete for their time and attention. Hence, the current study will employ a full-scale MRT to answer scientific questions about whether and how to prompt engagement in self-regulatory strategies. We will also investigate whether prompting engagement in self-regulatory strategies is more beneficial under conditions that represent vulnerability to a lapse and receptivity to mobile-based prompting. Answering these questions will inform the development of JITAIs that effectively address real-time vulnerability for a lapse, while minimizing participant burden. Given that mobile technology can overcome many of the barriers that have hampered the use of other evidence-based smoking cessation treatments (e.g., the lack of time, transportation issues, and cost) [129] an effective and practical JITAI has enormous potential to expand the reach and impact of smoking cessation interventions.
4.3. Novel Aspects of Design
This trial is uniquely designed to address open scientific questions concerning the construction of a JITAI to support smoking cessation. The MRT is a novel trial design developed explicitly for use in optimizing JITAIs. MRTs are intended to address research questions about whether and under what conditions just-in-time intervention options are effective. The current study employs a MRT whereby smokers attempting to quit are randomized 6 times per day to just-in-time prompts intended to engage them in self-regulatory strategies. These data can be used not only to inform the development of a JITAI, but also to develop more dynamic health behavior theories that explain how dynamically changing mechanisms, such as self-regulatory capacity and emotions, interact to shape an individual’s responsiveness to real-time engagement prompts.
Another novel aspect of the design is the integration of EMAs with objective, real time, real world assessments of lapse, self-regulatory capacity and context. Although participants generally show reasonable compliance with triggered EMAs, they are particularly likely to fail to initiate or respond during critical moments (e.g., lapse) [133]. Because MotionSense HRV indexes real time lapse and self-regulatory capacity without requiring any volitional action, a fundamental problem plaguing previous momentary assessment approaches is minimized. Further, the current study will investigate specific locations as indicators of vulnerability and/or receptivity. Research examining place has suffered from an almost exclusive focus on residential address and use of static census block data, which ignores the fact that individuals spend much of their day outside of this sphere of influence, hindering accurate assessment of exposure to environmental factors (e.g. tobacco outlets, bars). Here, we overcome these barriers by leveraging assisted GPS technology and high-resolution GIS datasets to objectively ascertain key environmental factors within the person’s own mobility patterns. In addition, detection of exposures to personal and public smoking spots [134] will provide potent exposure measures not utilized in prior studies.
Finally, the current study is designed to examine differential effects of distinct emotions, and self-regulatory capacity. Building on modern affective science, the proposed research will be among the first to investigate how dynamic fluctuations in distinct emotions represent vulnerability and receptivity. Moreover, although self-regulatory capacity is hypothesized to be perhaps the most proximal indicator of lapse risk, and to dynamically fluctuate over time, the proposed study will be the first to investigate how patterns over time in self-regulatory capacity relate to vulnerability and receptivity to engagement prompts. The hypothesized effects are consistent with theory, but have been impossible to accurately assess to date in the absence of real-time, real-world data [135, 136].
4.4. Limitations
The study design described in this paper may have several limitations. First, caution should be used in generalizing the results of the MRT to individuals who use exclusively e-cigarettes or marijuana. Although electronic cigarette/vaping and marijuana use are prevalent (about 3% and 7% of adults, respectively; [137, 138]), the Surgeon General notes the importance of continued efforts to reduce the use of all tobacco products. Additionally, even though we include participants who may use multiple nicotine/tobacco products, cigarettes remain the most commonly used product by far among adults. As such, studies of cigarette smoking remain extremely relevant [137–139]. Given that co-use of tobacco and cannabis and tobacco and e-cigarettes is prevalent, the EMA protocol in the current study includes items that ask about the use and timing of use of both e-cigarettes and marijuana at every assessment. Further, EMAs ask about motivation to be abstinent from e-cigarettes, urge to use e-cigarettes, e-cigarette related expectancies (e.g., whether e-cigarettes will improve mood), and self-efficacy to be abstinent from e-cigarettes. These data will allow future exploration of co-use of combustible cigarettes, e-cigarettes, and marijuana, and the association between engagement in self-regulatory strategies and abstinence from these products. Second, while the focus of the current study is on promoting real-time, real-world engagement in self-regulatory strategies, promoting engagement with other treatment components such NRT and brief counseling is also of practical and scientific importance. Future studies can be designed to investigate how mobile technology can be leveraged to prompt engagement in multiple smoking cessation intervention components.
5. Conclusions
This paper describes the protocol of a research project designed to inform the development of a JITAI to support smokers attempting to quit. An MRT will be utilized to micro-randomize each individual 6 times per day to either (a) a prompt recommending brief (low effort) self-regulatory strategies; (b) a prompt recommending relatively more effortful self-regulatory strategies; or (c) no prompt. The primary proximal outcome is engagement in self-regulatory strategies, self-reported via an EMA approximately 1 hours following micro-randomization. The results will build the empirical foundation necessary to develop JITAIs that effectively utilize intensive longitudinal self-report and sensor-based assessments of emotions, self-regulatory capacity, context and other factors to engage an individual in the type of self-regulatory activity that would be most beneficial given their real-time, real-world level of risk (vulnerability) and ability/willingness to engage (receptivity).
Acknowledgments
This project is funded by NIH grants U01 CA229437, U01 CA229437–03S1, U54 EB020404, P41 EB028242, R01 AA023187, P50 DA039838, P30 CA042014, UL1 TR002538, TL1 TR002540; and the Huntsman Cancer Foundation.
The authors would like to thank Timothy Hnat, Shahin Samiei, and Syed Monowar Hossain at the MD2K Center of Excellence in University of Memphis; and Stephanie Carpenter, Blake Wagner and Daniel Almirall at the d3lab, University of Michigan.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
CRediT statement:
Inbal Nahum-Shani: Conceptualization, Supervision, Writing—Original Draft; Lindsey N. Potter: Writing—Original Draft; Cho Y. Lam: Writing—Original Draft; Jamie Yap: Formal Analysis; Supervision; Moreno Alex: Methodology; Rebecca Stoffel: Project Administration; Zhenke Wu: Methodology; Neng Wan: Methodology; Walter Dempsey: Methodology; Santosh Kumar: Methodology, Software; Emre Ertin: Methodology, Sensors; Susan, A. Murphy: Methodology; James M. Rehg: Methodology; David W. Wetter: Conceptualization, Supervision, Writing—Original Draft
Contributor Information
Inbal Nahum-Shani, Institute for Social Research; University of Michigan;.
Lindsey N. Potter, Huntsman Cancer Institute; University of Utah
Cho Y. Lam, Huntsman Cancer Institute; University of Utah
Jamie R. Yap, Institute for Social Research; University of Michigan
Alexander Moreno, College of Computing; Georgia Institute of Technology.
Rebecca Stoffel, Huntsman Cancer Institute; University of Utah.
Zhenke Wu, School of Public Health; University of Michigan.
Neng Wan, Department of Geography; University of Utah.
Walter Dempsey, School of Public Health; University of Michigan.
Santosh Kumar, Department of Computer Science; University of Memphis.
Emre Ertin, Department of Electrical and Computer Engineering; The Ohio State University.
Susan A. Murphy, Departments of Statistics & Computer Science; Harvard University
James M. Rehg, College of Computing; Georgia Institute of Technology
David W. Wetter, Huntsman Cancer Institute; University of Utah
References
- [1].Ries LG, et al. , eds. SEER Cancer Statistics Review, 1975–2001, National Cancer Institute, Bethesda, MD, 2004. [Google Scholar]
- [2].Mokdad AH, et al. , Actual causes of death in the United States, 2000, J. Am. Med. Assoc 291 (10) (2004) 1238–1245. [DOI] [PubMed] [Google Scholar]
- [3].National Center for Chronic Disease Prevention and Health Promotion Office on Smoking and, H, Reports of the surgeon general, in: The Health Consequences of Smoking—50 Years of Progress: A Report of the Surgeon General, Centers for Disease Control and Prevention (US), Atlanta (GA), 2014. [Google Scholar]
- [4].Islami F, et al. , Proportion and number of Cancer cases and deaths attributable to potentially modifiable risk factors in the United States, CA-A Cancer.J. Clin 68 (1) (2018) 31–54. [DOI] [PubMed] [Google Scholar]
- [5].Lortet-Tieulent J, et al. , State-level Cancer mortality attributable to cigarette smoking in the United States, JAMA Intern. Med 176 (12) (2016) 1792–1798. [DOI] [PubMed] [Google Scholar]
- [6].Jacobs EJ, et al. , What proportion of cancer deaths in the contemporary United States is attributable to cigarette smoking? Ann. Epidemiol 25 (3) (2015) 179–182. [DOI] [PubMed] [Google Scholar]
- [7].Fiore M, Jaen C, Baker T, Treating Tobacco Use and Dependence: 2008 Update. Clinical Practice Guidelines, U.S. Department of Health and Human Services Public Health Service, Rockville, MD, 2008. [Google Scholar]
- [8].Babb S, et al. , Quitting smoking among adults - United States, 2000–2015, MMWR Morbid. Mortal. Weekly Rep 65 (52) (2017) 1457–1464. [DOI] [PubMed] [Google Scholar]
- [9].Vettese LC, et al. , Do mindfulness meditation participants do their homework? And does it make a difference? A review of the empirical evidence, J. Cogn. Psychother 23 (3) (2009) 198–225. [Google Scholar]
- [10].Elwafi HM, et al. , Mindfulness training for smoking cessation: moderation of the relationship between craving and cigarette use, Drug Alcohol Depend 130 (1) (2013) 222–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Davis JM, et al. , A pilot study on mindfulness based stress reduction for smokers, BMC Complement. Altern. Med 7 (1) (2007) 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Goldberg SB, et al. , The secret ingredient in mindfulness interventions? A case for practice quality over quantity, J. Couns. Psychol 61 (3) (2014) 491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Michie S, Free C, West R, Characterising the ‘Txt2Stop’ smoking cessation text messaging intervention in terms of behaviour change techniques, J. Smok. Cessat 7 (1) (2012) 55–60. [Google Scholar]
- [14].Lorencatto F, et al. , Developing a method for specifying the components of behavior change interventions in practice: the example of smoking cessation, J. Consult. Clin. Psychol 81 (3) (2013) 528. [DOI] [PubMed] [Google Scholar]
- [15].Westbrook C, et al. , Mindful attention reduces neural and self-reported cue-induced craving in smokers, Soc. Con. Affect. Neurosci 8 (1) (2011) 73–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Cavanagh K, et al. , Can mindfulness and acceptance be learnt by self-help?: a systematic review and meta-analysis of mindfulness and acceptance-based self-help interventions, Clin. Psychol. Rev 34 (2) (2014) 118–129. [DOI] [PubMed] [Google Scholar]
- [17].Gustafson DH, et al. , A smartphone application to support recovery from alcoholism: a randomized clinical trial, JAMA Psychiatry 71 (5) (2014) 566–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Shaw BR, et al. , Testing the feasibility of Mobile audio-based recovery material as an adjunct to intensive outpatient treatment for veterans with substance abuse disorders, J. Technol. Hum. Serv 31 (4) (2013) 321–336. [Google Scholar]
- [19].Muench F, et al. , Developing a theory driven text messaging intervention for addiction care with user driven content, Psychol. Addict. Behav 27 (1) (2013) 315–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Nahum-Shani I, Hekler EB, Spruijt-Metz D, Building health behavior models to guide the development of just-in-time adaptive interventions: a pragmatic framework, Health Psychol 34 (S) (2015) 1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Spruijt-Metz D, Nilsen W, Dynamic models of behavior for just-in-time adaptive interventions, Perv. Comp. IEEE 13 (3) (2014) 13–17. [Google Scholar]
- [22].Czerwinski M, et al. , Challenges for designing notifications for affective computing systems, in: Proceedings of the 2016 ACM International Joint Conference on Pervasive and Ubiquitous Computing: Adjunct, ACM, 2016. [Google Scholar]
- [23].Nahum-Shani I, et al. , Just-in-time adaptive interventions (JITAIs) in Mobile health: key components and design principles for ongoing health behavior support, Ann. Behav. Med 52 (2018) 446–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Cambron C, et al. , Socioeconomic status, mindfulness, and momentary associations between stress and smoking lapse during a quit attempt, Drug Alcohol Depend 209 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Shiffman S, et al. , First lapses to smoking: within-subjects analysis of real-time reports, J. Consult. Clin. Psychol 64 (2) (1996) 366–379. [DOI] [PubMed] [Google Scholar]
- [26].Liao P, et al. , Sample size calculations for micro-randomized trials in mHealth, Stat. Med 35 (12) (2016) 1944–1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Qian T, et al. , The micro-randomized trial for developing digital interventions: experimental design and data analysis considerations, Under Rev (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Holtyn AF, et al. , Towards detecting cocaine use using smartwatches in the NIDA clinical trials network: design, rationale, and methodology, Contemp. Clin. Trials Commun 15 (2019) 100392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Hughes JR, Keely J, Naud S, Shape of the relapse curve and long-term abstinence among untreated smokers, Addiction 99 (1) (2004) 29–38. [DOI] [PubMed] [Google Scholar]
- [30].Shiffman S, et al. , First lapses to smoking: within-subjects analysis of real-time reports, J. Consult. Clin. Psychol 64 (2) (1996) 366. [DOI] [PubMed] [Google Scholar]
- [31].Shiffman S, Reflections on smoking relapse research, Drug Alcohol Rev 25 (1) (2006) 15–20. [DOI] [PubMed] [Google Scholar]
- [32].Klingberg T, The Overflowing Brain: Information Overload and the Limits of Working Memory, Oxford University Press, 2009. [Google Scholar]
- [33].Thompson RF, Spencer WA, Habituation: a model phenomenon for the study of neuronal substrates of behavior, Psychol. Rev 73 (1) (1966) 16. [DOI] [PubMed] [Google Scholar]
- [34].CDC, Smoking-attributable mortality, years of potential life lost, and productivity losses–United States, 2000–2004, MMWR Morbid. Mortal. Weekly Rep 57 (45) (2008) 1226. [PubMed] [Google Scholar]
- [35].Chatterjee S, et al. , mCrave: continuous estimation of craving during smoking cessation, in: Proceedings of the 2016 ACM International Joint Conference on Pervasive and Ubiquitous Computing, ACM, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Naughton F, Delivering “Just-in-time” smoking cessation support via mobile phones: current knowledge and future directions, Nicotine Tob. Res 19 (3) (2017) 379–383. [DOI] [PubMed] [Google Scholar]
- [37].McClure JB, et al. , The effect of program design on engagement with an internet-based smoking intervention: randomized factorial trial, J. Med. Internet Res 15 (3) (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Hofmann SG, Asmundson GJG, Acceptance and mindfulness-based therapy: new wave or old hat? Clin. Psychol. Rev 28 (1) (2008) 1–16. [DOI] [PubMed] [Google Scholar]
- [39].Baer RA, Krietemeyer J, Overview of mindfulness-and acceptance-based treatment approaches, in: Mindfulness-Based Treatment Approaches: Clinician’s Guide to Evidence Base and Applications, 2006, pp. 3–27. [Google Scholar]
- [40].Fresco DM, et al. , Initial psychometric properties of the experiences questionnaire: validation of a self-report measure of decentering, Behay. Ther (3) (2007) 234–246. [DOI] [PubMed] [Google Scholar]
- [41].Bernstein A, et al. , Decentering and related constructs: a critical review and metacognitive processes model, Perspect. Psychol. Sci 10 (5) (2015) 599–617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Brown KW, Ryan RM, Perils and promise in defining and measuring mindfulness: observations from experience, Clin. Psychol. Sci. Pract 11 (3) (2004) 242–248. [Google Scholar]
- [43].Kiken LG, Lundberg KB, Fredrickson BL, Being present and enjoying it: dispositional mindfulness and savoring the moment are distinct, interactive predictors of positive emotions and psychological health, Mindfulness 8 (5) (2017) 1280–1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Bryant F, Savoring beliefs inventory (SBI): a scale for measuring beliefs about savouring, J. Ment. Health 12 (2) (2003) 175–196. [Google Scholar]
- [45].Businelle MS, et al. , Using intensive longitudinal data collected via mobile phone to detect imminent lapse in smokers undergoing a scheduled quit attempt, J. Med. Internet Res 18 (10) (2016), e275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Myers MG, et al. , Adolescent first lapse following smoking cessation: situation characteristics, precipitants and proximal influences, Addict. Behav 36 (12) (2011) 1253–1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Potter LN, et al. , A time-varying model of the dynamics of smoking lapse, Health Psychol 40 (1) (2021) 40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Piper ME, et al. , Tobacco withdrawal components and their relations with cessation success, Psychopharmacology 216 (4) (2011) 569–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Potter LN, et al. , A Time-Varying Effects Approach for Understanding the Dynamics of Smoking Lapse, Invited revision, Health Psychology, 2020. [Google Scholar]
- [50].Shiffman S, et al. , Natural history of nicotine withdrawal, Addiction 101 (12) (2006) 1822–1832. [DOI] [PubMed] [Google Scholar]
- [51].Shiyko MP, et al. , Using the time-varying effect model (TVEM) to examine dynamic associations between negative affect and self confidence on smoking urges: differences between successful quitters and Relapsers, Prey. Sci 13 (3) (2012) 288–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Businelle M, Lam C, Kendzor D, Cofta-Woerpel L, McClure J, Wetter DW, Alcohol Consumption and Urges to Smoke among Women during a Smoking Cessation Attempt, Experimental & Clinical Psychopharmacology 21 (2013) 29–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Lam CY, Businelle MS, Aigner CJ, et al. , Individual and combined effects of multiple high-risk triggers on postcessation smoking urge and lapse, Nicotine Tob Res 16 (2014) 569–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Lam CY, Businelle MS, Cofta-Woerpel L, McClure JB, Cinciripini PM, Wetter DW, Positive smoking outcome expectancies mediate the relation between alcohol consumption and smoking urge among women during a quit attempt, Psychol Addict Behav 28 (2014) 163–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Cofta-Woerpel LM, et al. , Early cessation success or failure among women attempting to quit smoking: trajectories and volatility of urge and negative mood during the first postcessation week, J. Abnorm. Psychol 120 (3) (2011) 596–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Rowan PJ, et al. , Evaluating reactivity to ecological momentary assessment during smoking cessation, Exp. Clin. Psychopharmacol 15 (4) (2007) 382–389. [DOI] [PubMed] [Google Scholar]
- [57].Deiches JF, et al. , Early lapses in a cessation attempt: lapse contexts, cessation success, and predictors of early lapse, Nicotine Tob. Res 15 (11) (2013) 1883–1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Shiffman S, et al. , Temptations to smoke after quitting: a comparison of lapsers and maintainers, Health Psychol 15 (6) (1996) 455–461. [DOI] [PubMed] [Google Scholar]
- [59].Shiffman S, et al. , Immediate antecedents of cigarette smoking: an analysis from ecological momentary assessment, J. Abnorm. Psychol 111 (4) (2002) 531–545. [DOI] [PubMed] [Google Scholar]
- [60].Shiffman S, Waters AJ, Hickcox M, The nicotine dependence syndrome scale: a multidimensional measure of nicotine dependence, Nicotine Tob. Res 6 (2) (2004) 327–348. [DOI] [PubMed] [Google Scholar]
- [61].Almeida DM, Kessler RC, Everyday stressors and gender differences in daily distress, J. Pers. Soc. Psychol 75 (3) (1998) 670–680. [DOI] [PubMed] [Google Scholar]
- [62].Barrett LF, Emotions are real, Emotion 12 (3) (2012) 413–429. [DOI] [PubMed] [Google Scholar]
- [63].Barrett LF, Bliss-Moreau E, Affect as a psychological primitive, Adv. Exp. Soc. Psychol 41 (2009) 167–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Barrett LF, Russell JA, Independence and bipolarity in the structure of current affect, J. Pers. Soc. Psychol 74 (4) (1998) 967–984. [Google Scholar]
- [65].Feldman LA, Valence focus and arousal focus - individual-differences in the structure of affective experience, J. Pers. Soc. Psychol 69 (1) (1995) 153–166. [Google Scholar]
- [66].Scott SB, et al. , Age, stress, and emotional complexity: results from two studies of daily experiences, Psychol. Aging 29 (3) (2014) 577–587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Pearlin LI, et al. , The stress process, J. Health Soc. Behav 22 (4) (1981) 337–356. [PubMed] [Google Scholar]
- [68].Young J, et al. , A valid two-item food security questionnaire for screening HIV-1 infected patients in a clinical setting, Public Health Nutr 12 (11) (2009) 2129–2132. [DOI] [PubMed] [Google Scholar]
- [69].Adler NE, et al. , Relationship of subjective and objective social status with psychological and physiological functioning: preliminary data in healthy white women, Health Psychol 19 (6) (2000) 586–592. [DOI] [PubMed] [Google Scholar]
- [70].Heatherton TF, et al. , Measuring the heaviness of smoking: using self-reported time to the first cigarette of the day and number of cigarettes smoked per day, Br. J. Addict 84 (7) (1989) 791–799. [DOI] [PubMed] [Google Scholar]
- [71].Heppner WL, et al. , The role of Prepartum motivation in the maintenance of postpartum smoking abstinence, Health Psychol 30 (6) (2011) 736–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Castro YA, et al. , The Texas Smoking Abstinence Motivation Scale Validation, 2010. [Google Scholar]
- [73].Piper ME, et al. , A multiple motives approach to tobacco dependence: the Wisconsin inventory of smoking dependence motives (WISDM-68), J. Consult. Clin. Psychol 72 (2) (2004) 139–154. [DOI] [PubMed] [Google Scholar]
- [74].Welsch SK, et al. , Development and validation of the Wisconsin smoking withdrawal scale, Exp. Clin. Psychopharmacol 7 (4) (1999) 354–361. [DOI] [PubMed] [Google Scholar]
- [75].WHO, The alcohol, smoking and substance involvement screening test (ASSIST): development, reliability and feasibility, Addiction 97 (9) (2002) 1183–1194. [DOI] [PubMed] [Google Scholar]
- [76].Spitzer RL, et al. , Validation and utility of a self-report version of PRIME-MD -the PHQ primary care study, JAMA J. Am. Med. Assoc 282 (18) (1999) 1737–1744. [DOI] [PubMed] [Google Scholar]
- [77].Bose J, et al. , Key Substance Use and Mental Health Indicators in the United States: Results from the 2015 National Survey on Drug Use and Health, S.A.A.M. H.S. Administration, Editor, 2020. [Google Scholar]
- [78].Bold KW, et al. , Measuring E-cigarette dependence: initial guidance, Addict. Behav 79 (2018) 213–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Health, N.I.O, Population Assessment of Tobacco and Health (PATH) Study, 2014. [Google Scholar]
- [80].NIDA, Screening for Drug Use in General Medical Settings: Quick Reference Guide, 2011. [Google Scholar]
- [81].Taylor S, et al. , Robust dimensions of anxiety sensitivity: development and initial validation of the anxiety sensitivity index-3, Psychol. Assess 19 (2) (2007) 176–188. [DOI] [PubMed] [Google Scholar]
- [82].Simons JS, Gaher RM, The distress tolerance scale: development and validation of a self-report measure, Motiv. Emot 29 (2) (2005) 83–102. [Google Scholar]
- [83].Spitzer RL, et al. , A brief measure for assessing generalized anxiety disorder - the GAD-7, Arch. Intern. Med 166 (10) (2006) 1092–1097. [DOI] [PubMed] [Google Scholar]
- [84].Cacioppo JT, Petty RE, Kao CF, The efficient assessment of need for cognition, J. Pers. Assess 48 (3) (1984) 306–307. [DOI] [PubMed] [Google Scholar]
- [85].Pressler SJ, et al. , Measuring depressive symptoms in heart failure: validity and reliability of the patient health Questionnaire-8, Am. J. Crit Care 20 (2) (2011) 146–152. [DOI] [PubMed] [Google Scholar]
- [86].Cohen S, Kamarck T, R Mermelstein A global measure of perceived stress, J. Health Soc. Behav 24 (4) (1983) 385–396. [PubMed] [Google Scholar]
- [87].Gosling SD, Rentfrow PJ, Swann WB, A very brief measure of the big-five personality domains, J. Res. Pers 37 (6) (2003) 504–528. [Google Scholar]
- [88].Steinberg L, et al. , New tricks for an old measure: the development of the Barratt impulsiveness scale-brief (BIS-brief), Psychol. Assess 25 (1) (2013) 216–226. [DOI] [PubMed] [Google Scholar]
- [89].Baer RA, Carmody J, Hunsinger M, Weekly change in mindfulness and perceived stress in a mindfulness-based stress reduction program, J. Clin. Psychol 68 (7) (2012) 755–765. [DOI] [PubMed] [Google Scholar]
- [90].Carey KB, Neal DJ, Collins SE, A psychometric analysis of the self-regulation questionnaire, Addict. Behav 29 (2) (2004) 253–260. [DOI] [PubMed] [Google Scholar]
- [91].Cohen S, et al. , Measuring the functional components of social support, in: Social Support Theory, Research, and Applications, Springer, Dordrecht, 1985, pp. 73–94. [Google Scholar]
- [92].Neto F, Loneliness among Portuguese adolescents, Soc. Behav. Pers 20 (1) (1992) 15–21. [Google Scholar]
- [93].Seeman TE, et al. , Social network ties and mortality among the elderly in the Alameda County study, Am. J. Epidemiol 126 (4) (1987) 714–723. [DOI] [PubMed] [Google Scholar]
- [94].L Winstanley E, et al. , The association of self-reported neighborhood disorganization and social capital with adolescent alcohol and drug use, dependence, and access to treatment, Drug Alcohol Depend 92 (1–3) (2008) 173–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Group, W.A.W, The alcohol, smoking and substance involvement screening test (ASSIST): development, reliability and feasibility, Addiction 97 (9) (2002) 1183–1194. [DOI] [PubMed] [Google Scholar]
- [96].Nebeling LC, et al. , The FLASHE study: survey development, dyadic perspectives, and participant characteristics, Am. J. Prey. Med 52 (6) (2017) 839–848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Chew LD, Bradley KA, Boyko EJ, Brief questions to identify patients with inadequate health literacy, Fam. Med 36 (8) (2004) 588–594. [PubMed] [Google Scholar]
- [98].Spitzer RL, et al. , Health-related quality of life in primary-care patients with mental disorders - results from the PRIME-MD 1000 study, JAMA J. Am. Med. Assoc 274 (19) (1995) 1511–1517. [PubMed] [Google Scholar]
- [99].Ertin E, Stohs N, Kumar S, et al. , AutoSense: Unobtrusively wearable sensor suite for inferencing of onset, causality, and consequences of stress in the field, in: Proceedings of the 9th ACM Conference on Embedded Networked Sensor Systems (SenSys), 2011, pp. 274–287. [Google Scholar]
- [100].Ali AA, Hossain SM, Hovsepian K, Rahman MM, Plarre K, Kumar S, et al. , mPuff: automated detection of cigarette smoking puffs from respiration measurements, in: Proceedings of the 11th international conference on Information Processing in Sensor Networks, 2011. [Google Scholar]
- [101].Nakajima M, et al. , Using novel mobile sensors to assess stress and smoking lapse, Int. J. Psychophysiol 158 (2020) 411–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Saleheen N, et al. , puffMarker: a multi-sensor approach for pinpointing the timing of first lapse in smoking cessation, in: Proceedings of the 2015 ACM International Joint Conference on Pervasive and Ubiquitous Computing, 2015. [PMC free article] [PubMed] [Google Scholar]
- [103].Berntson GG, Cacioppo JT, Quigley KS, Respiratory sinus arrhythmia: autonomic origins, physiological mechanisms, mid psychophysiological implications, Psychophysiology 30 (2) (1993) 183–196. [DOI] [PubMed] [Google Scholar]
- [104].Hagemann D, Waldstein SR, Thayer JF, Central and autonomic nervous system integration in emotion, Brain Con 52 (1) (2003) 79–87. [DOI] [PubMed] [Google Scholar]
- [105].Thayer JF, Lane RD, A model of neurovisceral integration in emotion regulation and dysregulation, J. Affect. Disord 61 (3) (2000) 201–216. [DOI] [PubMed] [Google Scholar]
- [106].Beauchaine T, Vagal tone, development, and Gray’s motivational theory: toward an integrated model of autonomic nervous system functioning in psychopathology, Dev. Psychopathol 13 (2) (2001) 183–214. [DOI] [PubMed] [Google Scholar]
- [107].Segerstrom SC, Nes LS, Heart rate variability reflects self-regulatory strength, effort, and fatigue, Psychol. Sci 18 (3) (2007) 275–281. [DOI] [PubMed] [Google Scholar]
- [108].Schwerdtfeger AR, Friedrich-Mai P, Social interaction moderates the relationship between depressive mood and heart rate variability: evidence from an ambulatory monitoring study, Health Psychol 28 (4) (2009) 501–509. [DOI] [PubMed] [Google Scholar]
- [109].Schwerdtfeger AR, Gerteis AK, The manifold effects of positive affect on heart rate variability in everyday life: distinguishing within-person and between-person associations, Health Psychol 33 (9) (2014) 1065–1073. [DOI] [PubMed] [Google Scholar]
- [110].Berntson GG, Lozano DL, Chen YJ, Filter properties of root mean square successive difference (RMSSD) for heart rate, Psychophysiology 42 (2) (2005) 246–252. [DOI] [PubMed] [Google Scholar]
- [111].Shaffer F, Ginsberg J, An overview of heart rate variability metrics and norms, Front. Public Health 5 (2017) 258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Gao J, Teng D, Ertin E, A probabilistic approach for heart rate variability analysis using explicit duration hidden markov models, in: 2018 IEEE Statistical Signal Processing Workshop (SSP), IEEE, 2018. [Google Scholar]
- [113].Tarvainen MP, et al. , Kubios HRV - A software for advanced heart rate variability analysis, in: 4th European Conference of the International Federation for Medical and Biological Engineering 22(1–3), 2009, pp. 1022–1025. [Google Scholar]
- [114].Camm AJ, et al. , Heart rate variability. Standards of measurement, physiological interpretation, and clinical use, Eur. Heart J 17 (3) (1996) 354–381. [PubMed] [Google Scholar]
- [115].Utah Automated Geographic Reference Center, Data from: The Utah State Geographic Information Database (SGID), 2020. [Google Scholar]
- [116].Wan N, Lin Kan G, Wilson G, Addressing location uncertainties in GPS-based activity monitoring: a methodological framework, Trans. GIS 21 (4) (2017) 764–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Wan N, Lin G, Classifying human activity patterns from smartphone collected GPS data: a fuzzy classification and aggregation approach, Trans. GIS 20 (6) (2016) 869–886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Wan N, Lin G, Life-space characterization from cellular telephone collected GPS data, Comput. Environ. Urban. Syst 39 (2013) 63–70. [Google Scholar]
- [119].Boruvka A, et al. , Assessing time-varying causal effect moderation in mobile health, J. Am. Stat. Assoc 113 (523) (2018) 1112–1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Rabbi M, et al. , SARA - Substance Abuse Research Assistant 2017. [Google Scholar]
- [121].Qian T, et al. , Estimating time-varying causal excursion effects in mobile health with binary outcomes, Biometrika (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Schafer JL, Multiple imputation: a primer, Stat. Methods Med. Res 8 (1) (1999) 3–15. [DOI] [PubMed] [Google Scholar]
- [123].Power Calculation for the Mobile-Assistance for Regulating Smoking (MARS) Micro-Randomized Trial, Available from, https://d3lab.isr.umich.edu/software/. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Olivier J, L May W, Bell ML, Relative effect sizes for measures of risk, Commun. Stat. Theory Methods 46 (14) (2017) 6774–6781. [Google Scholar]
- [125].Hébert ET, et al. , An ecological momentary intervention for smoking cessation: the associations of just-in-time, tailored messages with lapse risk factors, Addict. Behav 78 (2018) 30–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Hébert ET, et al. , A Mobile just-in-time adaptive intervention for smoking cessation: pilot randomized controlled trial, J. Med. Internet Res 22 (3) (2020), e16907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].Huh J, et al. , Effect of a mobile just-in-time implementation intention intervention on momentary smoking lapses in smoking cessation attempts among Asian American young adults, Transl. Behav. Med 11 (1) (2021) 216–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Cerrada CJ, et al. , Development of a just-in-time adaptive intervention for smoking cessation among Korean American emerging adults, Int. J. Behav. Med 24 (5) (2017) 665–672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [129].Collins LM, Optimization of Behavioral, Biobehavioral, and Biomedical Interventions: The Multiphase Optimization Strategy (MOST), Springer, Cham, 2018. [Google Scholar]
- [130].Stone AA, Shiffman S, Capturing momentary, self-report data: a proposal for reporting guidelines, Ann. Behav. Med 24 (3) (2002) 236–243. [DOI] [PubMed] [Google Scholar]
- [131].Chatterjee S, et al. , SmokingOpp: detecting the Smoking’Opportunity’Context using Mobile sensors, Proc. ACM Interact. Mob. Wear. Ubiquit. Technol 4 (1) (2020) 1–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [132].G. Marlatt, D. Donovan (Eds.), Relapse Prevention: Maintenance Strategies in the Treatment of Addictive Behaviors, 2nd ed., Guilford Press, New York, 2005. [Google Scholar]
- [133].Witkiewitz K, Marlatt GA, Relapse prevention for alcohol and drug problems: that was Zen, this is Tao, Am. Psychol 59 (4) (2004) 224–235. [DOI] [PubMed] [Google Scholar]
- [134].Wang TW, et al. , Tobacco product use among adults - United States, 2017, Morb. Mortal. Wkly Rep 67 (44) (2018) 1225–1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [135].Akbar SA, et al. , Tobacco and cannabis co-use and interrelatedness among adults, Addict. Behav 90 (2019) 354–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [136].Cornelius ME, et al. , Tobacco product use among adults - United States, 2019, MMWR Morb. Mortal. Wkly Rep 69 (46) (2020) 1736–1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [137].Hayes Steven C., Strosahl Kirk D., Wilson Kelly G.. Acceptance and commitment therapy, American Psychological Association, Washington, DC, 2009. [Google Scholar]
- [138].Kabat-Zinn Jon, Mindfulness-based stress reduction (MBSR). Constructivism in the Human Sciences 8 (2) (2003) 73–107. [Google Scholar]


