PURPOSE
We aimed to improve efficacy and reduce toxicity of high-risk human epidermal growth factor receptor 2 (HER2)–positive early breast cancer (EBC) treatment by replacing taxanes and trastuzumab with trastuzumab emtansine (T-DM1).
METHODS
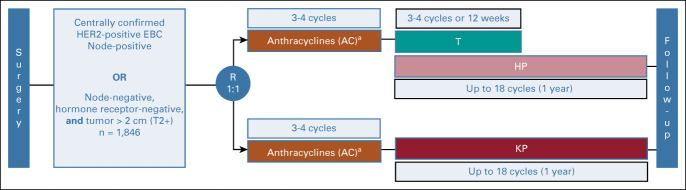
The phase III KAITLIN study (NCT01966471) included adults with excised HER2-positive EBC (node-positive or node-negative, hormone receptor–negative, and tumor > 2.0 cm). Postsurgery, patients were randomly assigned 1:1 to anthracycline-based chemotherapy (three-four cycles) and then 18 cycles of T-DM1 plus pertuzumab (AC-KP) or taxane (three-four cycles) plus trastuzumab plus pertuzumab (AC-THP). Adjuvant radiotherapy/endocrine therapy was permitted. Coprimary end points were invasive disease-free survival (IDFS) in the intention-to-treat node-positive and overall populations with hierarchical testing.
RESULTS
The median follow-up was 57.1 months (interquartile range, 52.1-60.1 months) for AC-THP (n = 918) and 57.0 months (interquartile range, 52.1-59.8 months) for AC-KP (n = 928). There was no significant IDFS difference between arms in the node-positive (n = 1,658; stratified hazard ratio [HR], 0.97; 95% CI, 0.71 to 1.32) or overall population (n = 1846; stratified HR, 0.98; 95% CI, 0.72 to 1.32). In the overall population, the three-year IDFS was 94.2% (95% CI, 92.7 to 95.8) for AC-THP and 93.1% (95% CI, 91.4 to 94.7) for AC-KP. Treatment completion rates (ie, 18 cycles) were 88.4% for AC-THP and 65.0% for AC-KP (difference driven by T-DM1 discontinuation because of laboratory abnormalities [12.5%]). Similar rates of grade ≥ 3 (55.4% v 51.8%) and serious adverse events (23.3% v 21.4%) occurred with AC-THP and AC-KP, respectively. KP decreased clinically meaningful deterioration in global health status versus THP (stratified HR, 0.71; 95% CI, 0.62 to 0.80).
CONCLUSION
The primary end point was not met. Both arms achieved favorable IDFS. Trastuzumab plus pertuzumab plus chemotherapy remains the standard of care for high-risk HER2-positive EBC.
INTRODUCTION
The standard of care for adjuvant treatment of human epidermal growth factor receptor 2 (HER2)–positive early breast cancer (EBC) is chemotherapy plus 1 year of HER2-directed therapy.1,2 However, high-risk populations (node-positive and/or tumors > 2 cm) receiving trastuzumab have 5-year disease-free survival rates of < 85%.3,4 In APHINITY,5 adjuvant chemotherapy and dual HER2 targeting with pertuzumab plus trastuzumab improved invasive disease-free survival (IDFS) compared with trastuzumab alone in the intention-to-treat (ITT) population and more markedly in patients with node-positive disease: the 6-year IDFS was 88% with dual targeting versus 83% with trastuzumab.6 Another challenge of current EBC treatment is systemic chemotherapy-associated toxicity. Taxanes are associated with well-characterized toxicities, including neutropenia, febrile neutropenia, alopecia, neuropathy, myalgia, and arthralgia.7-10
CONTEXT
Key Objective
Current treatments for high-risk human epidermal growth factor receptor 2 (HER2)–positive breast cancer have limitations in terms of efficacy and tolerability, the latter primarily because of toxicities associated with chemotherapy. Improved outcomes have been shown with the addition of pertuzumab to trastuzumab and chemotherapy in metastatic and neoadjuvant settings. Trastuzumab emtansine (T-DM1) is an antibody–drug conjugate composed of the cytotoxic agent DM1 linked to trastuzumab, which could provide an alternative to taxane-plus-trastuzumab to improve safety and efficacy, when combined with pertuzumab.
Knowledge Generated
In high-risk HER2-positive breast cancer, replacing the combination of taxane-plus-trastuzumab and pertuzumab with T-DM1 and pertuzumab (after surgery and anthracycline chemotherapy) did not yield a clinically meaningful improvement in efficacy and demonstrated a safety profile consistent with previous experience.
Relevance
The approach for management of high-risk HER2-positive breast cancer remains initial treatment with neoadjuvant trastuzumab/pertuzumab and chemotherapy and escalation to adjuvant T-DM1 for residual disease.
Trastuzumab emtansine (T-DM1) is an antibody–drug conjugate composed of the cytotoxic agent DM1 covalently linked to trastuzumab.11 T-DM1 retains the HER2-targeted effects of trastuzumab and provides targeted delivery of emtansine (MCC-DM1).12 In the metastatic setting, single-agent T-DM1 is effective, and is the standard of care, in patients with cancer resistant to trastuzumab plus a taxane.13-16
In KAITLIN, we aimed to improve efficacy and reduce toxicity of adjuvant chemotherapy in HER2-positive, high-risk EBC by replacing taxane-plus-trastuzumab with T-DM1 after anthracycline chemotherapy. On the basis of preclinical data showing synergistic antitumor activity with pertuzumab-plus-T-DM1,17 clinical data showing safety and activity of the combination,18 and improved outcomes with the addition of pertuzumab to trastuzumab in metastatic19 and neoadjuvant settings,20,21 KAITLIN was designed with each arm receiving pertuzumab. After KAITLIN was underway, data from the MARIANNE trial, testing the T-DM1-plus-pertuzumab combination in previously untreated metastatic disease, became available. T-DM1-plus-pertuzumab demonstrated similar efficacy, but not superiority, and better tolerability than trastuzumab-plus-taxane.22 Because the experimental arm in KAITLIN (anthracycline-plus-T-DM1-plus-pertuzumab) was expected to demonstrate a risk-benefit profile at least equivalent to the adjuvant standard of care at that time (anthracycline-plus-taxane-plus-trastuzumab) and because the question of whether it could be similar or superior to standard adjuvant regimens remained important, the trial continued. Here, we report the primary results of KAITLIN.
METHODS
Study Design and Patients
KAITLIN (NCT01966471) is a randomized, multinational, open-label study conducted in 288 centers in 36 countries (Data Supplement, online only). Eligible patients had newly diagnosed, HER2-positive, nonmetastatic, histologically confirmed, operable primary invasive breast carcinoma. Eligible patients had node-positive disease (pN ≥ 1), with any tumor size except T0 and any hormone receptor status, or node-negative disease (pN0) with the pathologic tumor size > 2.0 cm by local assessment and negative for estrogen receptor (ER) and progesterone receptor (PgR). Hormone receptor status and HER2 status were centrally determined. HER2 status was measured using the PATHWAY HER2 4B5 Immunohistochemistry Assay (Ventana Medical Systems, Inc, Oro Valley, AZ) and the INFORM HER2 Dual in Situ Hybridization Assay (Ventana Medical Systems, Inc, Oro Valley, AZ). HER2-positive status was defined as an immunohistochemistry (IHC) score of 3+ and/or an in situ hybridization HER2:chromosome 17 ratio of ≥ 2.0. Patients had to have a baseline left ventricular ejection fraction (LVEF) of ≥ 55% (by echocardiogram or multigated acquisition scans). Complete eligibility criteria are listed in the Data Supplement.
The Protocol (online only) was approved by the independent ethics committees and/or institutional review boards at each site. The trial was performed in accordance with the Declaration of Helsinki and guidelines for Good Clinical Practice. All participants provided written informed consent. The trial was overseen by a steering committee and an independent data monitoring committee. An independent clinical events committee adjudicated prespecified safety events of interest (cardiac and hepatic dysfunction events) in a blinded fashion.
Procedures
Patients were randomly assigned 1:1 within 9 weeks of definitive breast surgery to anthracycline chemotherapy followed by trastuzumab and a taxane plus pertuzumab (AC-THP) or anthracycline chemotherapy followed by T-DM1 plus pertuzumab (AC-KP; Appendix Fig A1, online only). Random assignment was performed according to a permuted block scheme, stratified by region (United States or Canada, Western Europe or Australia or New Zealand, Asia, and rest of world), nodal status (0, 1-3, or ≥ 4 positive nodes), hormone receptor status (ER- and/or PgR-positive or both ER- and PgR-negative), and anthracycline type (doxorubicin or epirubicin).
Anthracycline and taxane regimens were chosen by the investigator before random assignment and according to protocol-specified regimens (Data Supplement). T-DM1 was dosed at 3.6 mg/kg once every 3 weeks. Trastuzumab was dosed at 6 mg/kg once every 3 weeks after an 8-mg/kg loading dose and started concurrently with the taxane. Pertuzumab was dosed at 420 mg once every 3 weeks after an 840-mg loading dose and administered concurrently with T-DM1 or trastuzumab-plus-taxane. An interval of ≥ 3 weeks from the last dose of anthracycline to initiation of HER2-targeted therapy was required. After the taxane-concurrent phase in the trastuzumab-containing arm, trastuzumab-plus-pertuzumab was continued for ≥ 1 year. In the T-DM1–containing arm, T-DM1-plus-pertuzumab was continued for ≥ 1 year. For toxicities, dose delays and/or reductions were permitted for anthracyclines, taxanes, and T-DM1; only dose delays were permitted for trastuzumab and pertuzumab. For patients discontinuing T-DM1 because of toxicity, switching to trastuzumab was recommended to complete ≥ 1 year of HER2-targeted therapy. Adjuvant radiotherapy and/or hormonal therapy was given as clinically indicated at the end of taxane chemotherapy or after four cycles of T-DM1 during HER2-targeted therapy.
Outcomes
The coprimary efficacy end points were IDFS in the node-positive subpopulation and IDFS in the overall population. IDFS was defined as the time from random assignment until the date of first occurrence of one of the following: ipsilateral invasive breast tumor recurrence, ipsilateral locoregional invasive breast cancer recurrence, contralateral or ipsilateral second primary invasive breast cancer, distant recurrence, or death because of any cause.
Secondary efficacy end points in the node-positive subpopulation and overall population were IDFS plus second primary nonbreast cancer, disease-free survival (time between random assignment and first occurrence of an IDFS event, second primary nonbreast cancer event, and contralateral or ipsilateral ductal carcinoma in situ), distant recurrence-free interval (time between random assignment and first occurrence of distant breast cancer recurrence), and overall survival (OS—time from random assignment to death because of any cause). Adverse events (AEs) were coded according to the Medical Dictionary for Regulatory Activities and graded according to the National Cancer Institute Common Terminology Criteria for AEs v4.0. The primary adjudicated cardiac safety end point comprised severe heart failure (New York Heart Association [NYHA] classification III or IV) and significant LVEF decline (decline of ≥ 10 percentage points to a value < 50%) or cardiac death. Secondary cardiac event end points were symptomatic left ventricular systolic dysfunction (LVSD; NYHA II) with significant LVEF decline and asymptomatic LVSD. Patient-reported outcomes (PROs) included the European Organisation for Research and Treatment of Cancer (EORTC) Quality of Life Questionnaire–Core 30 (QLQ-C30) and the modified EORTC Breast Cancer module (QLQ-BR23). Clinically meaningful deterioration in global health status/QOL was defined as a decrease in the baseline score of ≥ 10 points.23 Deterioration in function and symptoms was defined by minimally important differences.24
Statistical Analysis
To achieve 82.5% power in the overall population, approximately 171 IDFS events were required, and 160 events were needed to achieve 80% power in the node-positive subpopulation to detect a hazard ratio (HR) of 0.64, at a two-sided significance level of 5% (log-rank test). Approximately 1,850 and 1,665 patients were required for enrollment in the overall population and node-positive subpopulation, respectively. After the phase III MARIANNE trial showed noninferior (but not superior) progression-free survival with T-DM1, with or without pertuzumab, compared with trastuzumab-and-a-taxane in metastatic disease,22 it was assumed that the likelihood of the study meeting its primary efficacy end point for superiority had decreased, and hence, the sample size was reduced from 2,500 to approximately 1,850 to decrease the risk of exposing more patients to a potentially suboptimal treatment. Because this is a time-to-event end point and as such statistical power is dependent on the number of events, not the sample size, the reduction in sample size did not reduce statistical power, but potentially increased the time to attain the prespecified number of events for the primary end point analysis.
Efficacy was evaluated on an ITT basis. The primary end point, IDFS, was compared between treatment arms with the log-rank test, stratified by protocol-defined stratification factors, excluding region—because some strata could have few patients, limiting statistical power. Data from patients without an event at the time of data analysis were censored on the date they were last known to be alive and event-free. The Cox proportional hazards model was used to estimate HRs between treatment arms and corresponding 95% CIs for time-to-event end points. The Kaplan-Meier approach was used to estimate 3- and 5-year event rates and corresponding 95% CIs for time-to-event end points. A hierarchical testing procedure was used to control the overall study type I error rate at 5%: (1) IDFS in the node-positive subpopulation, (2) IDFS in the overall population, (3) OS in the node-positive subpopulation, and (4) OS in the overall population. An interim analysis of IDFS was performed after approximately 75% of IDFS events were observed in the overall protocol-defined population and node-positive subpopulation.
Patients with baseline and ≥ 1 post-treatment assessment were included in PRO analyses. Time to clinically meaningful deterioration in PRO scales, derived using cycle 1 day 1 of HER2-targeted treatment as the reference timepoint, was analyzed using the same time-to-event methods used to analyze IDFS.
RESULTS
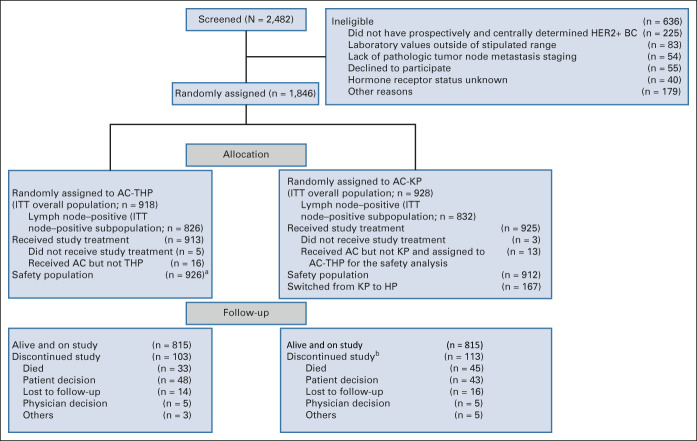
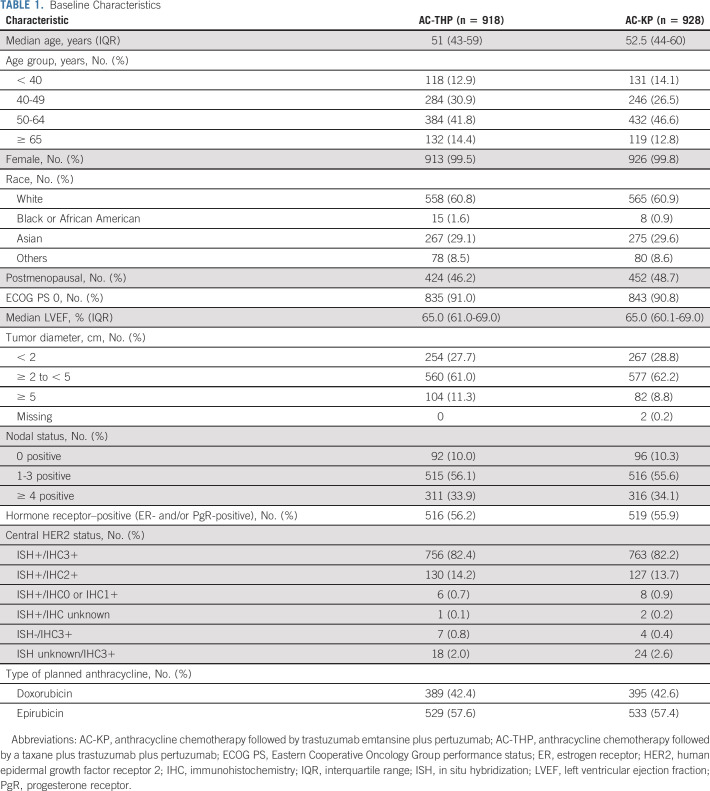
Between January 2014 and June 2015, 1,846 patients were enrolled (n = 918, AC-THP; n = 928, AC-KP [Fig 1]). Of these, 1,658 (89.8%) comprised the node-positive subpopulation (n = 826, AC-THP; n = 832, AC-KP). A total of 167 patients switched from T-DM1 to trastuzumab (Data Supplement). The median follow-up was 57.1 months (interquartile range, 52.1-60.1 months) in the AC-THP and 57.0 months (interquartile range, 52.1-59.8 months) in the AC-KP arm. Baseline characteristics were balanced between groups (Table 1).
FIG 1.
CONSORT diagram. aIncludes the 16 patients in the AC-THP arm who received AC but not THP and the 13 patients in the AC-KP arm who received AC but not KP. bOne patient in the AC-KP arm experienced an adverse event that first led to study discontinuation and eventually to death. This patient is counted both as discontinued study because of death and discontinued study because of other. AC, anthracycline chemotherapy; AC-KP, anthracycline chemotherapy followed by trastuzumab emtansine plus pertuzumab; AC-THP, anthracycline chemotherapy followed by a taxane plus trastuzumab plus pertuzumab; BC, breast cancer; HER2+, human epidermal growth factor receptor 2–positive; HP, trastuzumab plus pertuzumab; ITT, intention to treat; KP, trastuzumab emtansine plus pertuzumab.
TABLE 1.
Baseline Characteristics
Efficacy
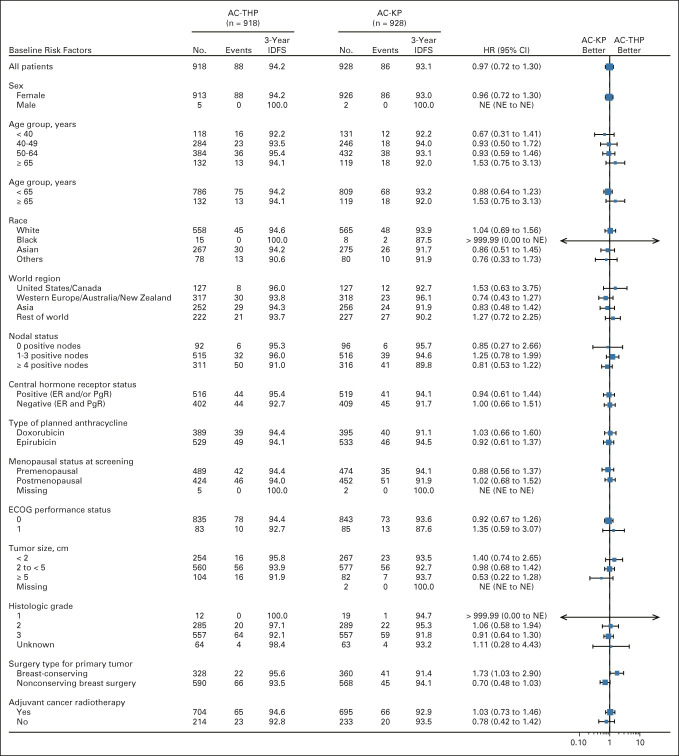
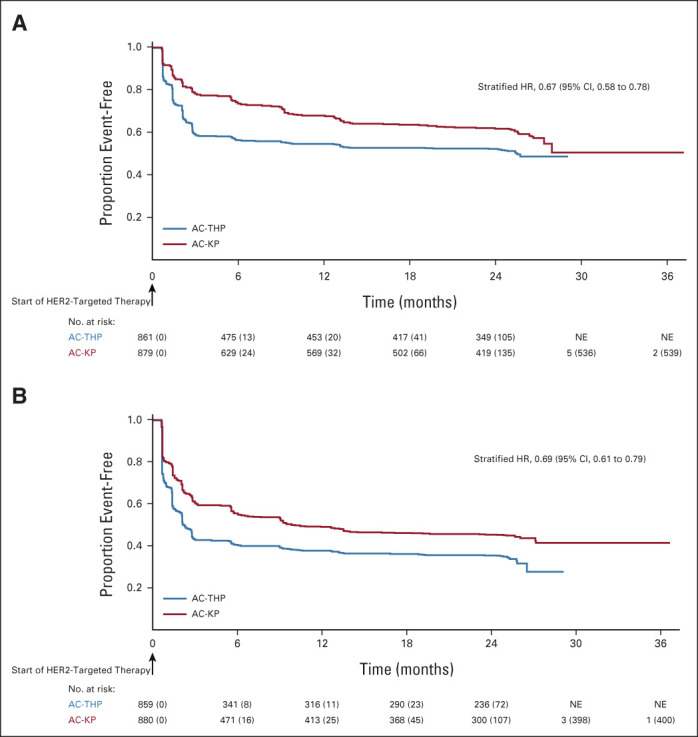
As of November 2019, among the ITT lymph node–positive subpopulation, 82 (9.9%) IDFS events had occurred in the AC-THP arm and 80 (9.6%) had occurred in the AC-KP arm. Treatment with AC-KP did not reduce the risk of an IDFS event compared with AC-THP in the node-positive subpopulation (stratified HR, 0.97; 95% CI, 0.71 to 1.32; P = .83; Fig 2A), so hierarchical testing was stopped. In this subpopulation, 3-year IDFS rates were 94.1% (95% CI, 92.5 to 95.7) with AC-THP and 92.8% (95% CI, 91.0 to 94.5) with AC-KP. Sites of recurrence were similar in each arm (Data Supplement). Among the ITT overall population, 88 (9.6%) IDFS events occurred in the AC-THP arm and 86 (9.3%) occurred in the AC-KP arm. AC-KP did not reduce the risk of an IDFS event compared with AC-THP (stratified HR, 0.98; 95% CI, 0.72 to 1.32; Fig 2B). Three-year IDFS rates were 94.2% (95% CI, 92.7 to 95.8) with AC-THP and 93.1% (95% CI, 91.4 to 94.7) with AC-KP. Sites of recurrence were similar in each arm (Data Supplement). In prespecified exploratory subgroup analysis, IDFS was consistently similar between arms across subgroups, except for a modest advantage with AC-THP versus AC-KP in the subgroup undergoing breast-conserving surgery (Fig 3). Rates of secondary nonprimary breast cancer, disease-free survival, and distant recurrence-free interval were similar between treatment arms (Data Supplement). OS data were immature with event rates of 3.6% and 4.7% in patients receiving AC-THP and AC-KP, respectively.
FIG 2.
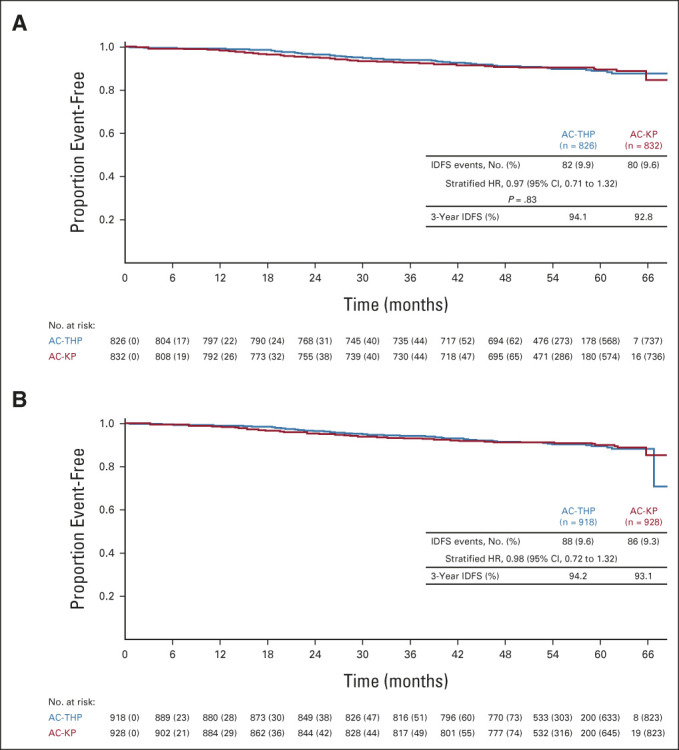
Kaplan-Meier plot of IDFS in the intention-to-treat lymph node–positive (A) subpopulation and (B) overall population. Stratified Cox regression and stratified log-rank test were used to assess HR (along with corresponding 95% CI) and P value. Stratification was based on the random assignment stratification factors: nodal status (0 positive nodes; 1-3 positive nodes; ≥ 4 positive nodes), centrally assessed hormone receptor status (ER- and/or PgR-positive or both ER- and PgR-negative), and type of planned anthracycline (doxorubicin or epirubicin). AC-KP, anthracycline chemotherapy followed by trastuzumab emtansine plus pertuzumab; AC-THP, anthracycline chemotherapy followed by a taxane plus trastuzumab plus pertuzumab; ER, estrogen receptor; HR, hazard ratio; IDFS, invasive disease-free survival; PgR, progesterone receptor.
FIG 3.
Subgroup analysis of IDFS. Forest plot showing comparison of AC-KP versus AC-THP in subgroups of the overall intention-to-treat population. Unstratified Cox proportional hazard regression was used to estimate HR and CI in each covariate subgroup. AC-KP, anthracycline chemotherapy followed by trastuzumab emtansine plus pertuzumab; AC-THP, anthracycline chemotherapy followed by a taxane plus trastuzumab plus pertuzumab; ECOG, Eastern Cooperative Oncology Group; ER, estrogen receptor; HER2, human epidermal growth factor receptor 2; HR, hazard ratio; IDFS, invasive disease-free survival; NE, not estimable; PgR, progesterone receptor.
Safety
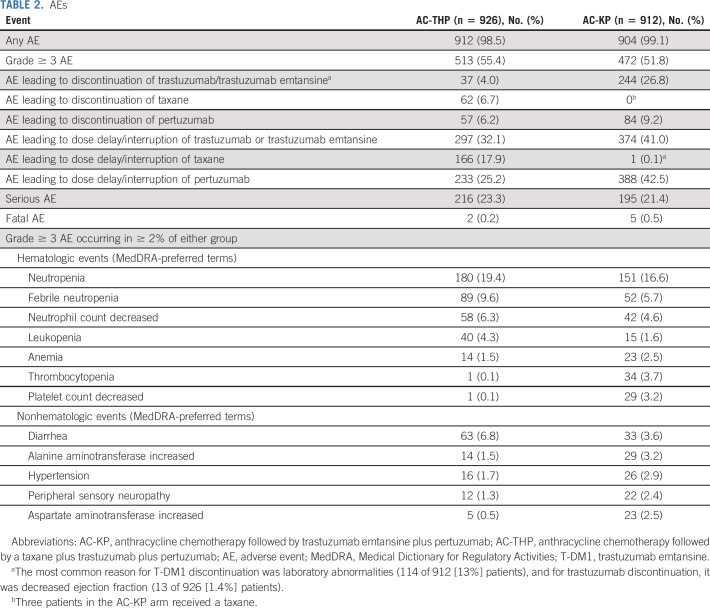
The safety population comprised 1838 patients who received ≥ 1 dose of study treatment (Table 2). All 18 cycles of trastuzumab or T-DM1 were completed by 819 (88.4%) patients receiving AC-THP and 593 (65.0%) receiving AC-KP (Data Supplement). Including patients who switched from T-DM1 to trastuzumab, 743 (81.5%) in the AC-KP arm completed HER2-targeted therapy. Of patients in the AC-THP arm, 167 (18.0%) had a taxane dose reduction. A total of 281 (30.8%) patients receiving AC-KP had a T-DM1 dose reduction (one-level, 172 [18.9%]; two-level, 109 [12.0%]; Data Supplement). A similar proportion of patients in each arm completed all 18 cycles of pertuzumab (Data Supplement).
TABLE 2.
AEs
The rates of grade ≥ 3 AEs and serious AEs were similar between treatment arms (Table 2). AEs leading to discontinuation of trastuzumab or T-DM1 occurred in 4.0% of patients receiving AC-THP and 26.8% receiving AC-KP. AEs leading to discontinuation of taxane occurred in 6.7% of AC-THP patients. The most common AE leading to discontinuation of trastuzumab was decreased ejection fraction (13 [1.4%] patients). AEs leading to discontinuation of T-DM1 were largely laboratory abnormalities (114 of 244 [47%]), most commonly increased bilirubin (49 [5.4%] patients) and decreased platelet count (30 [3.3%]). Deaths related to an AE occurred in two (0.2%) patients receiving AC-THP (pneumonia and hydrothorax [n = 1] and uterine leiomyosarcoma [n = 1]) and in five (0.5%) patients receiving AC-KP (pneumonia [n = 2], colon neoplasm [n = 1], depression leading to suicide [n = 1], and metabolic acidosis [n = 1]). None was deemed related to study treatment by the investigator.
Adjudicated primary cardiac events occurred in 1.1% of patients receiving AC-THP and 0.4% receiving AC-KP; no cardiac deaths occurred (Data Supplement). A secondary cardiac event was observed in 5.7% of patients receiving AC-THP and 2.6% receiving AC-KP. Nodular regenerative hyperplasia occurred in four (0.4%) patients receiving AC-KP—two grade 2, one grade 3, and one not reported as an AE (so severity not assessed). Of the three cases reported as AEs, two resolved. Of AEs known to occur with either treatment regimen, the most common AEs occurring in more patients receiving AC-KP versus AC-THP were any-grade hepatotoxicity (47.6% v 15.6%), any-grade thrombocytopenia (32.3% v 4.0%), and any-grade hemorrhage (45.9% v 25.9%). Any-grade pulmonary toxicity occurred in 2.2% of patients receiving AC-THP and 3.3% receiving AC-KP, with grade ≥ 3 events occurring in one patient in each arm. Radiation-related pneumonitis occurred in 1.4% of patients receiving AC-THP and 2.3% receiving AC-KP.
PROs
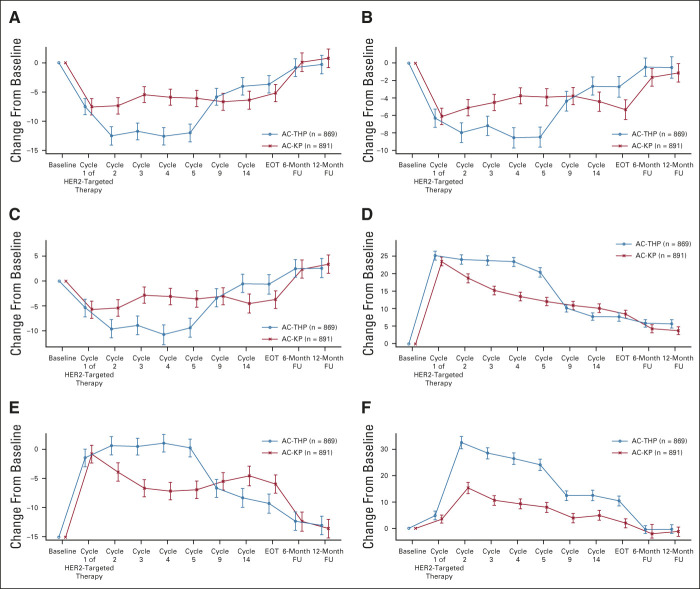
PRO completion rates were similar between arms (> 85% of evaluable patients across all assessment timepoints; Data Supplement). Baseline scores on the QLQ-C30 and QLQ-BR23 reflected moderate-to-high levels of functioning and overall QOL and low levels of disease- and treatment-related symptoms (Appendix Fig A2, online only). During the anthracycline treatment period, there was a consistent pattern of deterioration across subscales and treatment arms (Appendix Fig A3, online only). As expected, the adjusted mean scores for the QLQ-C30 and QLQ-BR23 function scales (Data Supplement) and the QLQ-C30 and QLQ-BR23 symptom scales were similar between arms at cycle 1 of the HER2-targeted treatment period, with the exception of arm symptoms (adjusted mean AC-THP, 18.0; AC-KP, 15.9; difference in adjusted means, –2.1 [95% CI, –3.9 to –0.3]; data not shown), a difference that was not clinically meaningful. From the start of HER2-targeted therapy, deterioration of functioning stopped in the AC-KP arm, whereas it continued during the taxane phase in the AC-THP arm. When taxane was stopped, functioning improved in the AC-THP arm and became similar to the AC-KP arm thereafter. The risk of experiencing a clinically meaningful deterioration in global health status from the start of HER2-targeted therapy was reduced by 29% in the AC-KP versus AC-THP arm (stratified HR, 0.71; 95% CI, 0.62 to 0.80), with the majority of the deterioration occurring during the first five cycles of HER2-targeted therapy (Fig 4 and Data Supplement). Similar results were observed in physical and role functioning (Appendix Fig A4, online only). Mean change from baseline and clinically meaningful deterioration in individual symptoms of the QLQ-C30 and QLQ-BR23 are shown in the Data Supplement.
FIG 4.
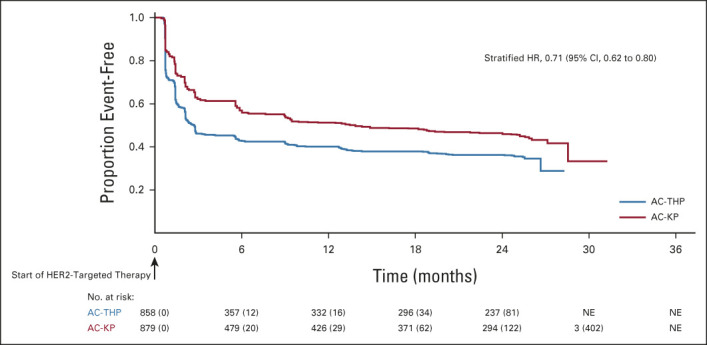
Time to deterioration in the European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire–Core 30 Global Health/Quality of Life Scale. Baseline defined as day 1 of HER2-targeted therapy. Stratified Cox regression was used to assess HR (along with corresponding 95% CI). Stratification was based on the random assignment stratification factors: nodal status (0 positive nodes; 1-3 positive nodes; ≥ 4 positive nodes), centrally assessed hormone receptor status (ER- and/or PgR-positive or both ER- and PgR-negative), and type of planned anthracycline (doxorubicin or epirubicin). AC-KP, anthracycline chemotherapy followed by trastuzumab emtansine plus pertuzumab; AC-THP, anthracycline chemotherapy followed by a taxane plus trastuzumab plus pertuzumab; ER, estrogen receptor; HER2, human epidermal growth factor receptor 2; HR, hazard ratio; NE, not estimable; PgR, progesterone receptor.
DISCUSSION
Adjuvant AC-KP did not result in statistically significant or clinically meaningful improvement in IDFS compared with AC-THP in the node-positive subpopulation or overall population of patients with high-risk EBC. AC-KP and AC-THP safety profiles were consistent with previous experience. There was a lower risk of deterioration in QOL with AC-KP, likely accounted for by taxanes in the AC-THP arm, since after completion of taxane therapy, QOL returned to levels comparable with the AC-KP arm.
The 3-year IDFS rates of 94.2% (AC-THP) and 93.1% (AC-KP) are notable in this high-risk population (89.8% having node-positive disease, with 34.0% having ≥ 4 positive nodes) and similar to those in patients with node-positive disease (25% with ≥ 4 positive nodes) in APHINITY.6 These data suggest an improvement in standard of care and/or more accurate initial staging of patients since the first adjuvant trials of trastuzumab, where the 3-year IDFS was approximately 82%-88% in populations in which 68%-94% had node-positive disease, with 28%-41% having ≥ 4 positive nodes.3,4,25
Of note, the treatment approach in EBC has changed since KAITLIN was initiated. A neoadjuvant strategy is currently recommended for high-risk HER2-positive disease, and patients with residual disease after surgery now receive T-DM1 on the basis of the KATHERINE trial results.1,2,26 In the KATHERINE study, adjuvant T-DM1 was superior to trastuzumab in patients with residual invasive disease after a taxane–trastuzumab-containing regimen.27 The KATHERINE data imply that there is a subset of cancers that has at least partial resistance to taxane-trastuzumab, but retains sensitivity to T-DM1. However, the observation that substituting T-DM1 for taxane-trastuzumab in KAITLIN does not improve outcomes suggests that there is also a subset of cancers resistant to T-DM1-plus-pertuzumab, for which taxane-trastuzumab-plus-pertuzumab therapy remains important. Ongoing research in samples from KAITLIN seeks to elucidate biomarkers to identify patients with cancers selectively sensitive to T-DM1 or taxanes.
The lack of improved efficacy with AC-KP versus AC-THP in KAITLIN is consistent with results from KRISTINE28,29 and MARIANNE,22,30 two further studies evaluating KP as a chemotherapy-sparing regimen. Both failed to demonstrate that KP was superior to a taxane-plus-trastuzumab-(pertuzumab)–based regimen, particularly in patients with lower HER2 expression (HER2 IHC 2+; HER2 mRNA low) and higher HER2 heterogeneity. The KAITLIN results are consistent with other studies suggesting that longer duration of a cytotoxic agent is not beneficial in EBC.31
Together, data from KAITLIN and other recent studies of HER2-directed therapy support the current tailored approach for the management of high-risk HER2-positive breast cancer: initial treatment with neoadjuvant chemotherapy and trastuzumab plus pertuzumab and escalation to adjuvant T-DM1 in those patients with residual disease. Given the favorable outcomes seen in contemporary studies of HER2-positive EBC, ongoing research is now focused on strategies to de-escalate chemotherapy while maintaining optimal HER2-targeted therapy with trastuzumab plus pertuzumab in the majority of patients (eg, CompassHER2-pCR [NCT04266249] and Decrescendo [NCT04675827]) and to escalate therapy only for the minority of patients who are at risk for recurrence despite maximal current management (eg, CompassHER2 RD [NCT04457596] and DESTINY-Breast05 [NCT04622319]).
ACKNOWLEDGMENT
We gratefully acknowledge the contributions of the late Professor Mikhail Lichinitser, Blokhin Russian Oncology Research Center of the Russian Academy of Medical Sciences, Moscow, Russia, to the KAITLIN study. F. Hoffmann-La Roche/Genentech funded the study. The sponsor designed the trial in conjunction with the steering committee, provided the study drugs (T-DM1, pertuzumab, trastuzumab, docetaxel, and paclitaxel), except in certain countries in which trastuzumab, docetaxel, and paclitaxel were sourced locally, and was involved in data collection, data interpretation, and manuscript writing. Support for third-party writing assistance was provided by Laura Evans, PharmD, and Holly Strausbaugh, PhD (Twist Medical, Burlingame, CA), funded by F. Hoffmann-La Roche.
APPENDIX
FIG A1.
Study design. aAnthracyclines (AC) = investigator's choice of FEC, AC, or EC. A, doxorubicin; C, cyclophosphamide; E, epirubicin; F, fluorouracil. EBC, early breast cancer; HER2, human epidermal growth factor receptor 2; HP, trastuzumab plus pertuzumab; KP, T-DM1 plus pertuzumab; R, randomized; T, taxanes.
FIG A2.
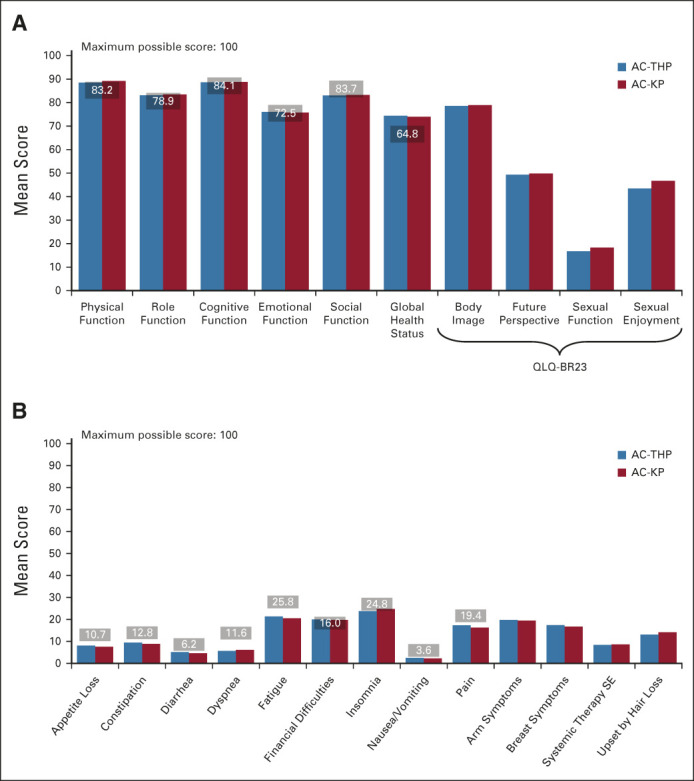
Baseline QLQ-C30 and QLQ-BR23: (A) function scale and (B) symptom scale scoresa with baseline defined as cycle 1, day 1 of anthracycline treatment. Horizontal lines over columns and associated numbers represent normative QLQ-C30 scores for patients with stage I-II breast cancer (Scott NW, et al: European Organisation for Research and Treatment of Cancer, 2008; www.eortc.org/app/uploads/sites/2/2018/02/reference_values_manual2008.pdf). aIn (A), higher scores denote better quality of life or functioning. In (B), higher scores denote greater symptoms. AC-KP, anthracycline chemotherapy followed by trastuzumab emtansine plus pertuzumab; AC-THP, anthracycline chemotherapy followed by a taxane plus trastuzumab plus pertuzumab; QLQ-BR23, Quality of Life Questionnaire–Breast Cancer 23; QLQ-C30, Quality of Life Questionnaire–Core 30; SE, side effects.
FIG A3.
Mean change from baseline in QOL: (A) EORTC QLQ-C30 Global Health/Quality of Life Scale, (B) EORTC QLQ-C30 Physical Functioning Scale, (C) EORTC QLQ-C30 Role Functioning Scale, (D) EORTC QLQ-BR23 Systemic Therapy Side Effects Scale, (E) EORTC QLQ-C30 Fatigue Scale, and (F) EORTC QLQ-C30 Diarrhea Scale. Baseline is defined as cycle 1, day 1 of anthracycline treatment. Cycles depicted in the graph indicate the cycle of HER2-targeted therapy. Error bars indicate 95% CIs. Higher scores on function scales (A-C) indicate better functioning. Higher scores on symptom scales (D-F) indicate a higher level of symptoms. AC-KP, anthracycline chemotherapy followed by trastuzumab emtansine plus pertuzumab; AC-THP, anthracycline chemotherapy followed by a taxane plus trastuzumab plus pertuzumab; EORTC, European Organisation for the Treatment of Cancer; EOT, end of treatment; FU, follow-up; HER2, human epidermal growth factor receptor 2; QLQ-BR23, Quality of Life Questionnaire–Breast Cancer 23; QLQ-C30, Quality of Life Questionnaire–Core 30; QOL, quality of life.
FIG A4.
Time to deterioration in (A) physical functioning and (B) role functioning from start of HER2-targeted therapy. Baseline is defined as cycle 1, day 1 of treatment with HER2-targeted therapy. Hazard ratios along with corresponding CIs were estimated by stratified Cox regression. Stratification was based on the random assignment stratification variables: nodal status (0 positive nodes; 1-3 positive nodes; ≥ 4 positive nodes), centrally assessed hormone receptor status (ER- and/or PgR-positive or both ER- and PgR-negative), and type of planned anthracycline (doxorubicin or epirubicin). AC-KP, anthracycline chemotherapy followed by trastuzumab emtansine plus pertuzumab; AC-THP, anthracycline chemotherapy followed by a taxane plus trastuzumab plus pertuzumab; ER, estrogen receptor; HER2, human epidermal growth factor receptor 2; HR, hazard ratio; PgR, progesterone receptor.
Ian E. Krop
Employment: Freeline Therapeutics (I), PureTech (I)
Leadership: AMAG Pharmaceuticals (I), Freeline Therapeutics (I), PureTech (I)
Stock and Other Ownership Interests: AMAG Pharmaceuticals (I), Freeline Therapeutics (I), PureTech (I)
Honoraria: Genentech/Roche, AstraZeneca¸ Celltrion
Consulting or Advisory Role: Genentech/Roche, Seattle Genetics, Daiichi Sankyo, MacroGenics, Novartis, Merck, Bristol Myers Squibb, AstraZeneca
Research Funding: Genentech (Inst), Pfizer (Inst)
Seock-Ah Im
Consulting or Advisory Role: AstraZeneca, Novartis, Roche/Genentech, Eisai, Pfizer, Amgen, Hanmi, Lilly, GlaxoSmithKline, MSD, Daiichi Sankyo
Research Funding: AstraZeneca (Inst), Pfizer (Inst), Roche/Genentech (Inst), Daewoong Pharmaceutical (Inst), Eisai (Inst)
Other Relationship: Roche
Carlos Barrios
Stock and Other Ownership Interests: Biomarker, MedSIR, Tummi
Honoraria: Novartis, Roche/Genentech, Pfizer, GlaxoSmithKline, Sanofi, Boehringer Ingelheim, Eisai¸ MSD, Bayer, Lilly, AstraZeneca, Zodiac Pharma
Consulting or Advisory Role: Boehringer Ingelheim, Roche/Genentech, Novartis, GlaxoSmithKline, Eisai, Pfizer, AstraZeneca, Libbs, MSD Oncology, United Medical, Lilly
Research Funding: Pfizer (Inst), Novartis (Inst), Amgen (Inst), AstraZeneca (Inst), Boehringer Ingelheim (Inst), GlaxoSmithKline (Inst), Roche/Genentech (Inst), Lilly (Inst), Sanofi (Inst), Taiho Pharmaceutical (Inst), Mylan (Inst), Merrimack (Inst), Merck (Inst), AbbVie (Inst), Astellas Pharma (Inst), BioMarin (Inst), Bristol Myers Squibb (Inst), Daiichi Sankyo (Inst), Abraxis BioScience (Inst), AB Science (Inst), Asana Biosciences (Inst), Medivation (Inst), Exelixis (Inst), ImClone Systems (Inst), LEO Pharma (Inst), Millennium (Inst), Janssen (Inst), Clinica Atlantis (Inst), INC Research (Inst), Halozyme (Inst), Covance (Inst), Celgene (Inst), inVentiv Health (Inst), Merck KGaA (Inst), Shanghai Henlius Biotech (Inst), Polyphor (Inst), PharmaMar (Inst)
Travel, Accommodations, Expenses: Roche/Genentech, Novartis, Pfizer, BMS Brazil, AstraZeneca, MSD Oncology, Lilly
Hervé Bonnefoi
Travel, Accommodations, Expenses: Roche, Pfizer
Julie Gralow
Consulting or Advisory Role: Genentech, AstraZeneca, Hexal, Puma Biotechnology, Roche, Novartis, Seattle Genetics, Genomic Health
Masakazu Toi
Honoraria: Novartis, Takeda, Eisai, AstraZeneca, Chugai Pharma, Taiho Pharmaceutical, Yakult Pharmaceutical, Shimadzu, Pfizer, Lilly, Kyowa Kirin, Devicor Medical Products, Exact Sciences, Nippon Kayaku
Consulting or Advisory Role: Daiichi Sankyo, Kyowa Kirin, Bertis, Athenex, BMS, Kansai Medical, Terumo, Lux Biosciences, AstraZeneca¸ Lilly
Speakers' Bureau: Pfizer, AstraZeneca, Lilly, Daiichi Sankyo
Research Funding: Taiho Pharmaceutical, Chugai Pharma, Shimadzu, Astellas Pharma, AFI technology, Japan Breast Cancer Research Group, Pfizer, Eisai, Daiichi Sankyo, AstraZeneca, Nippon Kayaku, Kyoto Breast Cancer Research Network, Shionogi, Lilly, Yakult Pharmaceutical, Lux Biosciences (Inst), GL Science (Inst)
Patents, Royalties, Other Intellectual Property: JP 2017-143763 WO2017/131162A1, PCT/JP2016/004374
Travel, Accommodations, Expenses: Eisai
Other Relationship: Japan Breast Cancer Research Group, Kyoto Breast Cancer Research Network, Organization for Oncology and Translational Research, Breast Cancer research and Treatment, Frontier in Women's Cancer, Scientific Reports, International Journal of Clinical Oncology, Cancer Science, Asian Journal of Surgery, Asian Journal of Breast Surgery, British Journal of Cancer
Luca Gianni
Consulting or Advisory Role: Roche, Pfizer, AstraZeneca, Genomic Health, Merck Sharp & Dohme, Synaffix, Celgene, Lilly, ADC Therapeutics, Odonate Therapeutics, Oncolytics, Genenta Science, Sandoz, METIS Precision Medicine, Novartis, Revolution Medicines, Zymeworks, G1 Therapeutics, Genentech, Onkaido Therapeutics, Taiho Pharmaceutical, Seattle Genetics, Synthon, Forty Seven, Sanofi, Amgen, Menarini, BioMedical Insights, Artemida Pharma Ltd
Speakers' Bureau: Roche Product, Zymeworks, Revolution Medicines
Patents, Royalties, Other Intellectual Property: Roche
Travel, Accommodations, Expenses: Celgene, Roche, Chugai Pharma, Pfizer
Sandra M. Swain
Consulting or Advisory Role: Inivata, Genentech/Roche, Daiichi Sankyo, Molecular Templates, Athenex, Silverback Therapeutics, AstraZeneca, Exact Sciences, Natera, Beijing Medical Award Foundation, Lilly, Merck
Research Funding: Genentech (Inst), Kailos Genetics (Inst)
Travel, Accommodations, Expenses: Caris Life Sciences, Daiichi Sankyo, Genentech/Roche
Other Relationship: AstraZeneca, Roche
Open Payments Link: https://openpaymentsdata.cms.gov/physician/801195/associated-research-funding
Michelino De Laurentiis
Honoraria: Roche, Novartis, Pfizer, Lilly, Amgen, Pierre Fabre, AstraZeneca, MSD, Seattle Genetics, Gilead Sciences, Takeda, Ipsen
Consulting or Advisory Role: Roche, Novartis, Pfizer, Lilly, Amgen, AstraZeneca, MSD, Pierre Fabre, Seattle Genetics, Gilead Sciences, Ipsen, Takeda
Speakers' Bureau: Novartis
Research Funding: Novartis, Roche, Lilly, Puma Biotechnology, Pfizer, Daiichi Sankyo, MSD, MacroGenics, Bristol Myers Squibb
Zbigniew Nowecki
Travel, Accommodations, Expenses: Roche/Genentech
Chiun-Sheng Huang
Honoraria: Roche, Novartis, Pfizer, AstraZeneca
Consulting or Advisory Role: Amgen, Pfizer, Roche, Lilly, AstraZeneca, Novartis
Speakers' Bureau: Amgen, Pfizer, Roche, Novartis, AstraZeneca
Research Funding: AstraZeneca (Inst), Lilly (Inst), MSD (Inst), Novartis (Inst)¸ Pfizer (Inst), Roche (Inst), OBI Pharma (Inst), EirGenix (Inst), Daiichi Sankyo (Inst)
Travel, Accommodations, Expenses: Pfizer, Roche, AstraZeneca
Yoshinori Ito
Research Funding: Daiichi Sankyo (Inst), Chugai Pharma (Inst), Novartis (Inst), Parexel (Inst), EPS Associates Co, Ltd (Inst), MSD (Inst), AstraZeneca (Inst), Lilly (Inst)
Jigna Shah
Employment: Genentech
Stock and Other Ownership Interests: Genentech
Travel, Accommodations, Expenses: Genentech
Other Relationship: Genentech
Thomas Boulet
Employment: Roche/Genentech
Haiying Liu
Employment: Genentech
Stock and Other Ownership Interests: Roche/Genentech
Travel, Accommodations, Expenses: Roche/Genentech
Harrison Macharia
Employment: Roche/Genentech
Peter Trask
Employment: Roche/Genentech
Stock and Other Ownership Interests: Genentech, Pfizer
Chunyan Song
Employment: Roche/Genentech
Stock and Other Ownership Interests: Roche/Genentech
Eric P. Winer
Honoraria: Genentech/Roche, Genomic Health
Consulting or Advisory Role: Leap Therapeutics, Seattle Genetics, Jounce Therapeutics, GlaxoSmithKline, Carrick Therapeutics, Lilly, G1 Therapeutics, Syros Pharmaceuticals, Genentech/Roche, Gilead Sciences, Zymeworks, Athenex
Research Funding: Genentech (Inst)
Other Relationship: InfiniteMD
Nadia Harbeck
Stock and Other Ownership Interests: West German Study Group
Honoraria: Roche, Novartis, Amgen, Pfizer, AstraZeneca¸ Pierre Fabre, Daiichi-Sankyo, Exact Sciences, MSD, Seattle Genetics
Consulting or Advisory Role: Roche/Genentech, Novartis, Pfizer, Lilly, Sandoz, Daiichi Sankyo, AstraZeneca, West German Study Group (Inst), Pierre Fabre, Seattle Genetics, MSD
Research Funding: Roche/Genentech, Lilly, MSD¸ AstraZeneca
No other potential conflicts of interest were reported.
PRIOR PRESENTATION
Presented in part at the American Society of Clinical Oncology Annual (virtual) Meeting, May 29-June 2, 2020.
SUPPORT
Supported by F. Hoffmann-La Roche Ltd.
CLINICAL TRIAL INFORMATION
DATA SHARING STATEMENT
Qualified researchers may request access to individual patient-level data through the clinical study data request platform (https://vivli.org/). Further details on Roche's criteria for eligible studies are available at https://vivli.org/members/ourmembers/. For further details on Roche's Global Policy on the Sharing of Clinical Information and how to request access to related clinical study documents, see https://www.roche.com/research_and_development/who_we_are_how_we_work/clinical_trials/our_commitment_to_data_sharing.htm.
AUTHOR CONTRIBUTIONS
Conception and design: Ian E. Krop, Julie Gralow, Masakazu Toi, Paul A. Ellis, Luca Gianni, Sandra M. Swain, Haiying Liu, Harrison Macharia, Chunyan Song, Eric P. Winer, Nadia Harbeck
Provision of study materials or patients: Ian E. Krop, Seock-Ah Im, Hervé Bonnefoi, Masakazu Toi, Luca Gianni, Young-Hyuck Im, Michelino De Laurentiis, Zbigniew Nowecki, Chiun-Sheng Huang, Louis Fehrenbacher, Yoshinori Ito, Chunyan Song, Nadia Harbeck
Collection and assembly of data: Ian E. Krop, Seock-Ah Im, Hervé Bonnefoi, Masakazu Toi, Paul A. Ellis, Luca Gianni, Zbigniew Nowecki, Chiun-Sheng Huang, Louis Fehrenbacher, Yoshinori Ito, Jigna Shah, Haiying Liu, Harrison Macharia, Chunyan Song, Nadia Harbeck
Data analysis and interpretation: Ian E. Krop, Seock-Ah Im, Carlos Barrios, Hervé Bonnefoi, Julie Gralow, Paul A. Ellis, Luca Gianni, Sandra M. Swain, Young-Hyuck Im, Michelino De Laurentiis, Chiun-Sheng Huang, Louis Fehrenbacher, Yoshinori Ito, Jigna Shah, Thomas Boulet, Haiying Liu, Harrison Macharia, Peter Trask, Chunyan Song, Nadia Harbeck
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Trastuzumab Emtansine Plus Pertuzumab Versus Taxane Plus Trastuzumab Plus Pertuzumab After Anthracycline for High-Risk Human Epidermal Growth Factor Receptor 2–Positive Early Breast Cancer: The Phase III KAITLIN Study
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Ian E. Krop
Employment: Freeline Therapeutics (I), PureTech (I)
Leadership: AMAG Pharmaceuticals (I), Freeline Therapeutics (I), PureTech (I)
Stock and Other Ownership Interests: AMAG Pharmaceuticals (I), Freeline Therapeutics (I), PureTech (I)
Honoraria: Genentech/Roche, AstraZeneca¸ Celltrion
Consulting or Advisory Role: Genentech/Roche, Seattle Genetics, Daiichi Sankyo, MacroGenics, Novartis, Merck, Bristol Myers Squibb, AstraZeneca
Research Funding: Genentech (Inst), Pfizer (Inst)
Seock-Ah Im
Consulting or Advisory Role: AstraZeneca, Novartis, Roche/Genentech, Eisai, Pfizer, Amgen, Hanmi, Lilly, GlaxoSmithKline, MSD, Daiichi Sankyo
Research Funding: AstraZeneca (Inst), Pfizer (Inst), Roche/Genentech (Inst), Daewoong Pharmaceutical (Inst), Eisai (Inst)
Other Relationship: Roche
Carlos Barrios
Stock and Other Ownership Interests: Biomarker, MedSIR, Tummi
Honoraria: Novartis, Roche/Genentech, Pfizer, GlaxoSmithKline, Sanofi, Boehringer Ingelheim, Eisai¸ MSD, Bayer, Lilly, AstraZeneca, Zodiac Pharma
Consulting or Advisory Role: Boehringer Ingelheim, Roche/Genentech, Novartis, GlaxoSmithKline, Eisai, Pfizer, AstraZeneca, Libbs, MSD Oncology, United Medical, Lilly
Research Funding: Pfizer (Inst), Novartis (Inst), Amgen (Inst), AstraZeneca (Inst), Boehringer Ingelheim (Inst), GlaxoSmithKline (Inst), Roche/Genentech (Inst), Lilly (Inst), Sanofi (Inst), Taiho Pharmaceutical (Inst), Mylan (Inst), Merrimack (Inst), Merck (Inst), AbbVie (Inst), Astellas Pharma (Inst), BioMarin (Inst), Bristol Myers Squibb (Inst), Daiichi Sankyo (Inst), Abraxis BioScience (Inst), AB Science (Inst), Asana Biosciences (Inst), Medivation (Inst), Exelixis (Inst), ImClone Systems (Inst), LEO Pharma (Inst), Millennium (Inst), Janssen (Inst), Clinica Atlantis (Inst), INC Research (Inst), Halozyme (Inst), Covance (Inst), Celgene (Inst), inVentiv Health (Inst), Merck KGaA (Inst), Shanghai Henlius Biotech (Inst), Polyphor (Inst), PharmaMar (Inst)
Travel, Accommodations, Expenses: Roche/Genentech, Novartis, Pfizer, BMS Brazil, AstraZeneca, MSD Oncology, Lilly
Hervé Bonnefoi
Travel, Accommodations, Expenses: Roche, Pfizer
Julie Gralow
Consulting or Advisory Role: Genentech, AstraZeneca, Hexal, Puma Biotechnology, Roche, Novartis, Seattle Genetics, Genomic Health
Masakazu Toi
Honoraria: Novartis, Takeda, Eisai, AstraZeneca, Chugai Pharma, Taiho Pharmaceutical, Yakult Pharmaceutical, Shimadzu, Pfizer, Lilly, Kyowa Kirin, Devicor Medical Products, Exact Sciences, Nippon Kayaku
Consulting or Advisory Role: Daiichi Sankyo, Kyowa Kirin, Bertis, Athenex, BMS, Kansai Medical, Terumo, Lux Biosciences, AstraZeneca¸ Lilly
Speakers' Bureau: Pfizer, AstraZeneca, Lilly, Daiichi Sankyo
Research Funding: Taiho Pharmaceutical, Chugai Pharma, Shimadzu, Astellas Pharma, AFI technology, Japan Breast Cancer Research Group, Pfizer, Eisai, Daiichi Sankyo, AstraZeneca, Nippon Kayaku, Kyoto Breast Cancer Research Network, Shionogi, Lilly, Yakult Pharmaceutical, Lux Biosciences (Inst), GL Science (Inst)
Patents, Royalties, Other Intellectual Property: JP 2017-143763 WO2017/131162A1, PCT/JP2016/004374
Travel, Accommodations, Expenses: Eisai
Other Relationship: Japan Breast Cancer Research Group, Kyoto Breast Cancer Research Network, Organization for Oncology and Translational Research, Breast Cancer research and Treatment, Frontier in Women's Cancer, Scientific Reports, International Journal of Clinical Oncology, Cancer Science, Asian Journal of Surgery, Asian Journal of Breast Surgery, British Journal of Cancer
Luca Gianni
Consulting or Advisory Role: Roche, Pfizer, AstraZeneca, Genomic Health, Merck Sharp & Dohme, Synaffix, Celgene, Lilly, ADC Therapeutics, Odonate Therapeutics, Oncolytics, Genenta Science, Sandoz, METIS Precision Medicine, Novartis, Revolution Medicines, Zymeworks, G1 Therapeutics, Genentech, Onkaido Therapeutics, Taiho Pharmaceutical, Seattle Genetics, Synthon, Forty Seven, Sanofi, Amgen, Menarini, BioMedical Insights, Artemida Pharma Ltd
Speakers' Bureau: Roche Product, Zymeworks, Revolution Medicines
Patents, Royalties, Other Intellectual Property: Roche
Travel, Accommodations, Expenses: Celgene, Roche, Chugai Pharma, Pfizer
Sandra M. Swain
Consulting or Advisory Role: Inivata, Genentech/Roche, Daiichi Sankyo, Molecular Templates, Athenex, Silverback Therapeutics, AstraZeneca, Exact Sciences, Natera, Beijing Medical Award Foundation, Lilly, Merck
Research Funding: Genentech (Inst), Kailos Genetics (Inst)
Travel, Accommodations, Expenses: Caris Life Sciences, Daiichi Sankyo, Genentech/Roche
Other Relationship: AstraZeneca, Roche
Open Payments Link: https://openpaymentsdata.cms.gov/physician/801195/associated-research-funding
Michelino De Laurentiis
Honoraria: Roche, Novartis, Pfizer, Lilly, Amgen, Pierre Fabre, AstraZeneca, MSD, Seattle Genetics, Gilead Sciences, Takeda, Ipsen
Consulting or Advisory Role: Roche, Novartis, Pfizer, Lilly, Amgen, AstraZeneca, MSD, Pierre Fabre, Seattle Genetics, Gilead Sciences, Ipsen, Takeda
Speakers' Bureau: Novartis
Research Funding: Novartis, Roche, Lilly, Puma Biotechnology, Pfizer, Daiichi Sankyo, MSD, MacroGenics, Bristol Myers Squibb
Zbigniew Nowecki
Travel, Accommodations, Expenses: Roche/Genentech
Chiun-Sheng Huang
Honoraria: Roche, Novartis, Pfizer, AstraZeneca
Consulting or Advisory Role: Amgen, Pfizer, Roche, Lilly, AstraZeneca, Novartis
Speakers' Bureau: Amgen, Pfizer, Roche, Novartis, AstraZeneca
Research Funding: AstraZeneca (Inst), Lilly (Inst), MSD (Inst), Novartis (Inst)¸ Pfizer (Inst), Roche (Inst), OBI Pharma (Inst), EirGenix (Inst), Daiichi Sankyo (Inst)
Travel, Accommodations, Expenses: Pfizer, Roche, AstraZeneca
Yoshinori Ito
Research Funding: Daiichi Sankyo (Inst), Chugai Pharma (Inst), Novartis (Inst), Parexel (Inst), EPS Associates Co, Ltd (Inst), MSD (Inst), AstraZeneca (Inst), Lilly (Inst)
Jigna Shah
Employment: Genentech
Stock and Other Ownership Interests: Genentech
Travel, Accommodations, Expenses: Genentech
Other Relationship: Genentech
Thomas Boulet
Employment: Roche/Genentech
Haiying Liu
Employment: Genentech
Stock and Other Ownership Interests: Roche/Genentech
Travel, Accommodations, Expenses: Roche/Genentech
Harrison Macharia
Employment: Roche/Genentech
Peter Trask
Employment: Roche/Genentech
Stock and Other Ownership Interests: Genentech, Pfizer
Chunyan Song
Employment: Roche/Genentech
Stock and Other Ownership Interests: Roche/Genentech
Eric P. Winer
Honoraria: Genentech/Roche, Genomic Health
Consulting or Advisory Role: Leap Therapeutics, Seattle Genetics, Jounce Therapeutics, GlaxoSmithKline, Carrick Therapeutics, Lilly, G1 Therapeutics, Syros Pharmaceuticals, Genentech/Roche, Gilead Sciences, Zymeworks, Athenex
Research Funding: Genentech (Inst)
Other Relationship: InfiniteMD
Nadia Harbeck
Stock and Other Ownership Interests: West German Study Group
Honoraria: Roche, Novartis, Amgen, Pfizer, AstraZeneca¸ Pierre Fabre, Daiichi-Sankyo, Exact Sciences, MSD, Seattle Genetics
Consulting or Advisory Role: Roche/Genentech, Novartis, Pfizer, Lilly, Sandoz, Daiichi Sankyo, AstraZeneca, West German Study Group (Inst), Pierre Fabre, Seattle Genetics, MSD
Research Funding: Roche/Genentech, Lilly, MSD¸ AstraZeneca
No other potential conflicts of interest were reported.
REFERENCES
- 1.National Comprehensive Cancer Network : NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines). Breast Cancer. Version 3, March 24, 2020
- 2.Cardoso F, Kyriakides S, Ohno S, et al. : Early breast cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 30:1194-1220, 2019 [DOI] [PubMed] [Google Scholar]
- 3.Romond EH, Perez EA, Bryant J, et al. : Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N Engl J Med 353:1673-1684, 2005 [DOI] [PubMed] [Google Scholar]
- 4.Slamon D, Eiermann W, Robert N, et al. : Adjuvant trastuzumab in HER2-positive breast cancer. N Engl J Med 365:1273-1283, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.von Minckwitz G, Procter M, de Azambuja E, et al. : Adjuvant pertuzumab and trastuzumab in early HER2-positive breast cancer. N Engl J Med 377:122-131, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Piccart M, Procter M, Fumagalli D, et al. : Interim overall survival analysis of APHINITY: A randomized multicenter, double-blind, placebo-controlled trial comparing chemotherapy plus trastuzumab plus pertuzumab versus chemotherapy plus trastuzumab plus placebo as adjuvant therapy in patients with operable HER2-positive early breast cancer. Cancer Res 80, 2020. (4 suppl; abstr GS1-04) [Google Scholar]
- 7.von Minckwitz G, Raab G, Caputo A, et al. : Doxorubicin with cyclophosphamide followed by docetaxel every 21 days compared with doxorubicin and docetaxel every 14 days as preoperative treatment in operable breast cancer: The GEPARDUO study of the German Breast Group. J Clin Oncol 23:2676-2685, 2005 [DOI] [PubMed] [Google Scholar]
- 8.Sparano JA, Wang M, Martino S, et al. : Weekly paclitaxel in the adjuvant treatment of breast cancer. N Engl J Med 358:1663-1671, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tolaney SM, Barry WT, Dang CT, et al. : Adjuvant paclitaxel and trastuzumab for node-negative, HER2-positive breast cancer. N Engl J Med 372:134-141, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Willson ML, Burke L, Ferguson T, et al. : Taxanes for adjuvant treatment of early breast cancer. Cochrane Database Syst Rev 9:CD004421, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lewis Phillips GD, Li G, Dugger DL, et al. : Targeting HER2-positive breast cancer with trastuzumab-DM1, an antibody–cytotoxic drug conjugate. Cancer Res 68:9280-9290, 2008 [DOI] [PubMed] [Google Scholar]
- 12.Junttila TT, Li G, Parsons K, et al. : Trastuzumab-DM1 (T-DM1) retains all the mechanisms of action of trastuzumab and efficiently inhibits growth of lapatinib insensitive breast cancer. Breast Cancer Res Treat 128:347-356, 2011 [DOI] [PubMed] [Google Scholar]
- 13.Verma S, Miles D, Gianni L, et al. : Trastuzumab emtansine for HER2-positive advanced breast cancer. N Engl J Med 367:1783-1791, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Diéras V, Miles D, Verma S, et al. : Trastuzumab emtansine versus capecitabine plus lapatinib in patients with previously treated HER2-positive advanced breast cancer (EMILIA): A descriptive analysis of final overall survival results from a randomised, open-label, phase 3 trial. Lancet Oncol 18:732-742, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Krop IE, Kim SB, González-Martín A, et al. : Trastuzumab emtansine versus treatment of physician’s choice for pretreated HER2-positive advanced breast cancer (TH3RESA): A randomised, open-label, phase 3 trial. Lancet Oncol 15:689-699, 2014 [DOI] [PubMed] [Google Scholar]
- 16.Krop IE, Kim S-B, González-Martín A, et al. : Trastuzumab emtansine versus treatment of physician's choice in patients with previously treated HER2-positive metastatic breast cancer (TH3RESA): Final overall survival results from a randomised open-label phase 3 trial. Lancet Oncol 18:743-754, 2017 [DOI] [PubMed] [Google Scholar]
- 17.Lewis Phillips GD, Fields CT, Li G, et al. : Dual targeting of HER2-positive cancer with trastuzumab emtansine and pertuzumab: Critical role for neuregulin blockade in antitumor response to combination therapy. Clin Cancer Res 20:456-468, 2014 [DOI] [PubMed] [Google Scholar]
- 18.Miller KD, Diéras V, Harbeck N, et al. : Phase IIa trial of trastuzumab emtansine with pertuzumab for patients with human epidermal growth factor receptor 2-positive, locally advanced, or metastatic breast cancer. J Clin Oncol 32:1437-1444, 2014 [DOI] [PubMed] [Google Scholar]
- 19.Baselga J, Cortes J, Kim SB, et al. : Pertuzumab plus trastuzumab plus docetaxel for metastatic breast cancer. N Engl J Med 366:109-119, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gianni L, Pienkowski T, Im YH, et al. : Efficacy and safety of neoadjuvant pertuzumab and trastuzumab in women with locally advanced, inflammatory, or early HER2-positive breast cancer (NeoSphere): A randomised multicentre, open-label, phase 2 trial. Lancet Oncol 13:25-32, 2012 [DOI] [PubMed] [Google Scholar]
- 21.Schneeweiss A, Chia S, Hickish T, et al. : Pertuzumab plus trastuzumab in combination with standard neoadjuvant anthracycline-containing and anthracycline-free chemotherapy regimens in patients with HER2-positive early breast cancer: A randomized phase II cardiac safety study (TRYPHAENA). Ann Oncol 24:2278-2284, 2013 [DOI] [PubMed] [Google Scholar]
- 22.Perez EA, Barrios C, Eiermann W, et al. : Trastuzumab emtansine with or without pertuzumab versus trastuzumab plus taxane for human epidermal growth factor receptor 2-positive, advanced breast cancer: Primary results from the phase III MARIANNE study. J Clin Oncol 35:141-148, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Osoba D, Rodrigues G, Myles J, et al. : Interpreting the significance of changes in health-related quality-of-life scores. J Clin Oncol 16:139-144, 1998 [DOI] [PubMed] [Google Scholar]
- 24.Cocks K, King MT, Velikova G, et al. : Evidence-based guidelines for determination of sample size and interpretation of the European Organisation for the Research and Treatment of Cancer Quality of Life Questionnaire Core 30. J Clin Oncol 29:89-96, 2011 [DOI] [PubMed] [Google Scholar]
- 25.Gianni L, Dafni U, Gelber RD, et al. : Treatment with trastuzumab for 1 year after adjuvant chemotherapy in patients with HER2-positive early breast cancer: A 4-year follow-up of a randomised controlled trial. Lancet Oncol 12:236-244, 2011 [DOI] [PubMed] [Google Scholar]
- 26.Harbeck N, Wuerstlein R: Truly personalized therapy—An end to the era of one size fits all. Nat Rev Clin Oncol 16:77-78, 2019 [DOI] [PubMed] [Google Scholar]
- 27.von Minckwitz G, Huang CS, Mano MS, et al. : Trastuzumab emtansine for residual invasive HER2-positive breast cancer. N Engl J Med 380:617-628, 2019 [DOI] [PubMed] [Google Scholar]
- 28.Hurvitz SA, Martin M, Fraser Symmans W, et al. : Neoadjuvant trastuzumab, pertuzumab, and chemotherapy versus trastuzumab emtansine plus pertuzumab in patients with HER2-positive breast cancer (KRISTINE): A randomised, open-label, multicentre, phase 3 trial. Lancet Oncol 19:115-126, 2018 [DOI] [PubMed] [Google Scholar]
- 29.Hurvitz SA, Martin M, Jung KH, et al. : Neoadjuvant trastuzumab emtansine and pertuzumab in human epidermal growth factor receptor 2–positive breast cancer: Three-year outcomes from the phase III KRISTINE study. J Clin Oncol 37:2206-2216, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Perez EA, Barrios C, Eiermann W, et al. : Trastuzumab emtansine with or without pertuzumab versus trastuzumab with taxane for human epidermal growth factor receptor 2–positive advanced breast cancer: Final results from MARIANNE. Cancer 125:3974-3984, 2019 [DOI] [PubMed] [Google Scholar]
- 31.Shulman LN, Cirrincione CT, Berry DA, et al. : Six cycles of doxorubicin and cyclophosphamide or paclitaxel are not superior to four cycles as adjuvant chemotherapy for breast cancer in women with zero to three positive axillary nodes: Cancer and Leukemia Group B 40101. J Clin Oncol 30:4071-4076, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Qualified researchers may request access to individual patient-level data through the clinical study data request platform (https://vivli.org/). Further details on Roche's criteria for eligible studies are available at https://vivli.org/members/ourmembers/. For further details on Roche's Global Policy on the Sharing of Clinical Information and how to request access to related clinical study documents, see https://www.roche.com/research_and_development/who_we_are_how_we_work/clinical_trials/our_commitment_to_data_sharing.htm.