Abstract
Objectives
The aim of this study was to identify sex-specific prevalence and strength of risk factors for the incidence of radiographic knee OA (incRKOA).
Methods
Our study population consisted of 10 958 Rotterdam Study participants free of knee OA in one or both knees at baseline. One thousand and sixty-four participants developed RKOA after a median follow-up time of 9.6 years. We estimated the association between each available risk factor and incRKOA using sex stratified multivariate regression models with generalized estimating equations. Subsequently, we statistically tested sex differences between risk estimates and calculated the population attributable fractions (PAFs) for modifiable risk factors.
Results
The prevalence of the investigated risk factors was, in general, higher in women compared with men, except that alcohol intake and smoking were higher in men and high BMI showed equal prevalence. We found significantly different risk estimates between men and women: high level of physical activity [relative risk (RR) 1.76 (95% CI: 1.29–2.40)] or a Kellgren and Lawrence score 1 at baseline [RR 5.48 (95% CI: 4.51–6.65)] was higher in men. Among borderline significantly different risk estimates was BMI ≥27, associated with higher risk for incRKOA in women [RR 2.00 (95% CI: 1.74–2.31)]. The PAF for higher BMI was 25.6% in women and 19.3% in men.
Conclusion
We found sex-specific differences in both presence and relative risk of several risk factors for incRKOA. Especially BMI, a modifiable risk factor, impacts women more strongly than men. These risk factors can be used in the development of personalized prevention strategies and in building sex-specific prediction tools to identify high risk profile patients.
Keywords: sex difference, risk factors, knee osteoarthritis, epidemiology, GEE
Rheumatology key messages
Studies on risk factors and designing prediction models have largely ignored sex differences in OA.
Physical activity and KL1 showed sex differences in their relationship with incidence of knee OA.
In women 1/4 and in men 1/5 new knee cases is attributable to a BMI ≥27.
Introduction
Men and women are similar in many ways, but when it comes to health the differences matter. Sex and gender influence our risk of developing certain diseases [1], their clinical manifestation, how well we respond to treatment [2] and how often we seek health care [3]. Knee OA (KOA) is one of the diseases that affect females and males differently. After the age of 50, there is a steep increase in incidence of KOA in women compared with men [4], leading to a higher prevalence in women [5]. Also, KOA in women is more often accompanied by pain and disability compared with men [6, 7]. Sex is a strong risk factor for KOA [8] and it is thought that different aetiology can underlie the disease in the different sexes [9]. Despite this knowledge, studies on risk factors and the design of prediction models have largely ignored sex differences.
There are several reasons why certain risk factors might display a different risk in men and women. First, a large number of factors have a different distribution depending on gender and socio-cultural influence (ethnicity, socio-economic status) visible in the study characteristics of various population-based studies. Moreover, behaviour connected to lifestyle factors might be different depending on gender. For instance physical activity (PA) in women, while similar in amount, might still be composed of different types of activity than in men, leading to differences in strength of association.
Second, several studies have indicated that certain aetiological pathways might be more predominant in one of the sexes [9]. For example, the metabolic OA-type is thought to be female-specific. Not only the prevalence of metabolic syndrome (MetS) differs between the sexes, but also its clinical representation with sex differences in fat distribution and adipocyte function [10]. The association of OA with MetS is not completely explained by an obesity-induced increased biomechanical load and obese people have also an increased risk for OA of the hand, a non-weight-bearing joint [11]. This suggests that the relationship between MetS and OA is through altered metabolism and inflammation. Due to its modifiable nature, obesity has already been the target in a randomized controlled trial to prevent KOA [12].
Third, women not only have different morphology of the knee joint [13] but also differ in neuromuscular control and gait kinematics [14]. In addition, the relation between hip-shape differences (dysplasia and CAM impingement) and OA has been shown to be sex-specific [15]. This evidence suggests that other joint- and OA-related risk factors constitute a valuable set to further investigate for sex differences.
In order to provide better healthcare for both men and women, the differences and similarities in the manifestation, aetiology and pathophysiology of KOA should be considered in health research and subsequently translated to clinical practice. The more we understand how sex and gender affect health, the more we can improve health and quality of life for everyone. With the study of sex differences we get closer to obtaining a more efficient and personalized health care, as sex- and gender-based prevention strategies or therapies are probably more effective than the usual ‘one size fits all’ approach and would benefit patients of both sexes.
It is high time to overcome this lack of knowledge and shed more light on the role sex differences play in OA. Therefore, the aim of the present study was to identify sex-specific prevalence and strength first of the usual person-related risk factors and then of OA-related risk factors for the development of radiographic KOA (RKOA) in a large population-based prospective study.
Methods
Participants
We selected our study population from the Rotterdam Study cohort, a population-based prospective study ongoing since 1990 in the city of Rotterdam in the Netherlands. Baseline measurements were collected from 1990 to 1993 for 7983 participants (RS-I). Two additional sub-cohorts were recruited in 2000–2001 including 3011 participants (RS-II) and in 2006–2008 comprising 3932 participants (RS-III). In this study we used visits 1, 3 and 4 of RS-I (1990–2004), visits 1, 2 and 3 of RS-II (2000–2012) and visits 1 and 2 of RS-III (2006–2014) where the radiographic measurements of the knees were available. As of 2008, 14 926 participants aged 45 years and over comprise the Rotterdam Study. The participants are followed for a variety of diseases that are frequent in the elderly with the aim to investigate determinants of disease occurrence and progression. The Rotterdam Study is approved by the medical ethics committee of the Erasmus University Medical Center and the review board of the Ministry of Health, Welfare and Sports of the Netherlands. Written informed consent was obtained from all participants in the study [16].
Incidence of RKOA
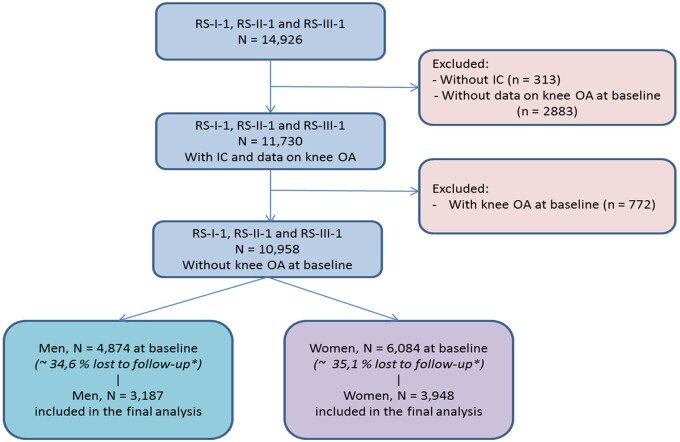
Knee radiographs were taken with the knee and patella in an extended and central position respectively [17]. Knee radiographs were available for 4874 men and 6084 women at baseline, and for 3187 men and 3948 women (Fig. 1) after a median follow-up time of 9.56 years [interquartile range (IQR): 5.65–10.60 years]. We defined RKOA according to the original Kellgren and Lawrence (KL) scoring system [18, 19]. Incidence of RKOA (incRKOA) was defined for each knee as a KL score of 0 or 1 at baseline and a KL score of 2–4 or a total joint replacement at the second or third visit during the total follow-up time of 10 years.
Fig. 1.
Flow chart of study participants from the Rotterdam Study cohorts
*The lost to follow-up percentages are also due to missing data on time between radiographs and other covariates (age, BMI). IC: informed consent; RS-I-1: Rotterdam Study cohort 1 visit 1; RS-II-1: Rotterdam Study cohort 2 visit 1; RS-III-1: Rotterdam Study cohort 3 visit 1.
Person-related risk factors
We selected the studied risk factors for KOA available in the three cohorts of the Rotterdam Study based on the latest systematic review [8]. Baseline information on the following characteristics was collected from participants using questionnaires, structured home interviews and visits to the research centre: age, highest level of education according to the UNESCO classification (primary education, lower = lower/intermediate general education or lower vocational education; intermediate = intermediate vocational education or higher general education; higher = higher vocational education or university) [20], BMI (kg/m2), waist and hip circumference (cm) used to calculate waist-to-hip ratio (WHR), femoral neck (FN) BMD derived from DEXA scans, alcohol consumption (yes/no) and cigarette smoking status (never/ever smoker) recorded during home interviews and total PA assessed in RS-I and RS-II using a validated adapted version of the Zutphen Physical Activity Questionnaire [21] and in RS-III cohort using the LASA Physical Activity Questionnaire (LAPAQ) [22]. In both questionnaires, total PA was expressed as the metabolic equivalent of task (MET) in hours per week [23]. Total PA comprised all physical activity types, and thus different METs.
OA-related risk factors
Information on hip OA as well as on hand OA was assessed at baseline during the visits at the research centre, where X-ray photographs were made and graded by two independent researchers according to the KL grading system [18, 19]. OA was defined as having a KL grade of 2 or higher. Hand OA was divided into bilateral finger OA (KL 2 or higher in at least one DIP joint or PIP joint on both hands) and bilateral thumb OA (defined as OA in CMC joint or scaphotrapeziotrapezoidal joint; CMC/TS) [24].
The Stanford HAQ was used to assess disability [25]. A comprehensive description of the way the HAQ was assessed during the home interview carried out by one of nine extensively trained interviewers has been presented earlier [26]. Locomotor disability of the lower limb was defined as the mean of the scores (with 0 indicating no impairment and 3 indicating unable to perform) on the six questions related to lower limb functions. Disability was defined as a lower limb disability index of 0.5 or over. Upper limb disability and an overall disability index were computed in a similar way.
Baseline KL score of 1 in the knee was also included as a risk factor in the analysis as it is widely known that it increases the risk of developing RKOA in the future.
Statistical analysis
We reported the prevalence (or mean or median) of each of the available risk factors. We estimated the association between each available risk factor measurement and the incRKOA using sex-stratified multivariate regression models with generalized estimating equations models that take into account the correlation between the two knees in an individual. We report a relative risk (RR) per 1 s.d. with 95% CI, where s.d. was based on the whole study population, for continuous variables and per presence of risk factors in cases of dichotomous variables. FN-BMD and total PA were analysed per sex-specific tertile and the lowest tertile used as reference. P-values <0.05 were considered to be statistically significant. In the basic model we adjusted for age, months between radiographs and sub-cohort. Further, in the second model we additionally adjusted for BMI to verify if the associations are independent of this well-known modifiable risk factor. We used the Z-test statistic to test if the RR estimates were statistically significantly different between men and women [27]. We decided to use the Z-test because the interpretation of the interaction term would only be valid when the other variables in the regression model are the same between the men and women. This is a method often used in similar settings, i.e. the work of Schiphof et al. [28]. For the calculation, we used d = E1 − E2, which is the difference of estimates from two groups and has standard error s.e.(d) = √[s.e.(E1)2 + s.e.(E2)2]. The ratio z = d/s.e.(d) gives a test of the null hypothesis that there is no difference between the two groups, by comparing the value of z to the standard normal distribution. A two-sided test with a significance level of 0.05 was used. There is a significant difference in the factor for the specific grade if z is less than −1.96 or if z is >1.96. The 95% CI for the difference is d − 1.96 × s.e.(d) to d + 1.96 × s.e.(d) [29]. We defined a risk factor as being sex-specific if the RR estimates were statistically significantly different between men and women according to the Z-test when the P-value was <0.05 and borderline significant when this was <0.1.
Finally, we built sex-specific multivariable models where we included all factors that showed a P-value ≤0.1 in the second, BMI adjusted model. This model, conditioning on the other risk factors, was run to get an estimate of the net effect of a risk factor on the outcome accounting for the effects of other risk factors. Subsequently, to illustrate the contribution of the risk factors to the incRKOA, we estimated the sex-specific population attributable fractions (PAFs) for the modifiable factors that showed a significant association in the multivariable model. PAF is an impact measure that allows estimation of the proportion of new cases of KOA in the population that could be avoided if the risk factor was removed. We used the following formula for calculating sex-specific PAFs in our study sample: (Pe × adjRR)/[(Pe × adjRR) + 1], where Pe is the proportion of exposed male/female knees, and adjRR is the adjusted RR from the sex-specific multivariable generalized estimating equations model. All statistical analyses were performed using R version 3.5.2 [30].
Results
The number of participants who underwent longitudinal radiographic measurements of the knee and baseline measurements of several person- and OA-related factors was 11 730. Our study population included 10 958 participants at baseline who did not have KOA in at least one knee at the first visit (Fig. 1). Subsequently, we excluded participants without follow-up data on radiographic measurements. The lost to follow-up group (35% of 10 958) was older on average, suffering from disabilities and other comorbidities, which are reasons why they were not able to participate in any of the follow-up measurements that took place at the research centre. In contrast, the participants included in our analysis were younger and healthier on average, with fewer cases of hypertension and diabetes, less OA in other joints and less disability (Supplementary Table S1b, available at Rheumatology online).
A total number of 1064 participants, 713 women and 351 men, developed uni- or bilateral RKOA during follow-up. The proportion of women developing RKOA during follow-up was 17.3% and of men was 10.7%. The median follow-up time was 9.7 years (IQR: 5.7–10.6 years). We included 13 586 knees with KL score <2 in our analysis. During the follow-up 1303 incident knee cases (9.6%) developed, 884 in women and 419 in men.
We compared the baseline characteristics between men and women in our study population and observed that they differed significantly in almost every aspect (Table 1) except age, BMI and hip OA status. Women had on average lower FN-BMD, consumed significantly less alcohol and a larger proportion had never smoked compared with men. Women were, on average, more physically active than the male population and were less educated, indicated by a higher proportion with primary and lower education (66%) compared with the men (37.4%) in our study population. Moreover, finger OA and thumb OA were more frequently present in women, with 26.2% and 16.1%, respectively, which is almost twice the proportion in men.
Table 1.
Baseline characteristics of the study population
| Characteristic | Overall (n = 7135) |
Men (n = 3187, 44.7%) |
Women (n = 3948, 55.3%) |
P-value |
|---|---|---|---|---|
| Age, mean (s.d.), years | 62.18 (7.08) | 62.12 (6.96) | 62.22 (7.19) | 0.557 |
| BMI ≥ 27, n (%) | 3014 (42.2) | 1353 (42.5) | 1661 (42.1) | 0.764 |
| Height, mean (s.d.), cm | 169.10 (9.23) | 176.45 (6.74) | 163.17 (6.21) | <0.001 |
| Weight, mean (s.d.), kg | 76.66 (13.49) | 83.21 (12.23) | 71.38 (12.06) | <0.001 |
| Waist-to-hip ratio, mean (s.d.) | 0.89 (0.09) | 0.95 (0.07) | 0.85 (0.08) | <0.001 |
| Femoral neck bone mineral density, mean (s.d.), g/cm2 | 0.90 (0.14) | 0.94 (0.13) | 0.87 (0.14) | <0.001 |
| Femoral neck bone mineral density, sex-specific tertiles, n (%) | 0.897 | |||
| Low (tertile 1) | 1950 (30.1) | 884 (30.4) | 1066 (29.9) | |
| Moderate (tertile 2) | 2252 (34.8) | 1009 (34.7) | 1243 (34.9) | |
| High (tertile 3) | 2267 (35.0) | 1012 (34.8) | 1255 (35.2) | |
| Alcohol intake, median (IQR), g/day | 6.43 (0.54, 15.00) | 8.57 (1.85, 20.75) | 2.50 (0.25, 8.57) | <0.001 |
| Any alcohol consumption, n (%) | 4651 (84.0) | 2162 (90.5) | 2489 (79.1) | <0.001 |
| Ever smoker, n (%) | 5143 (72.6) | 2357 (74.4) | 2786 (71.2) | <0.001 |
| Total physical activitya, median (IQR), MET h/week | 73.10 (45.41, 106.32) | 61.33 (35.11, 93.10) | 81.62 (55.85, 115.88) | <0.001 |
| Total physical activitya, sex-specific tertiles, n (%) | 0.363 | |||
| Low (tertile 1) | — | 724 (30.3) | 876 (28.6) | |
| Moderate (tertile 2) | — | 817 (34.2) | 1069 (34.8) | |
| High (tertile 3) | — | 848 (35.5) | 1123 (36.6) | |
| Education level (UNESCO), n (%) | <0.001 | |||
| Primary | 802 (11.3) | 263 (8.3) | 539 (13.8) | |
| Lower | 2964 (41.9) | 920 (29.1) | 2044 (52.2) | |
| Intermediate | 2088 (29.5) | 1193 (37.7) | 895 (22.8) | |
| Higher | 1228 (17.3) | 789 (24.9) | 439 (11.2) | |
| Disability, n (%) | 786 (12.1) | 209 (7.2) | 577 (16.0) | <0.001 |
| Lower-limb disability, n (%) | 679 (10.4) | 196 (6.7) | 483 (13.4) | <0.001 |
| Upper-limb disability, n (%) | 147 (2.3) | 26 (0.9) | 121 (3.4) | <0.001 |
| Hip OA at baseline, n (%) | 364 (5.2) | 154 (4.9) | 210 (5.4) | 0.393 |
| Finger OA at baseline, n (%) | 1308 (21.9) | 447 (16.6) | 861 (26.2) | <0.001 |
| Thumb OA at baseline, n (%) | 761 (12.7) | 234 (8.6) | 527 (16.1) | <0.001 |
For total physical activity the measurements from the third visit (RS-I-3) were used, as the measurement was not available at the first visit (RS-I-1). All the other measurements were available at the first visit of RS-I cohort. Therefore: n = 6106 (Overall), n = 2714 (Men), n = 3392 (Women). IQR: interquartile range; MET: metabolic equivalent of task.
Person-related risk factors and KOA
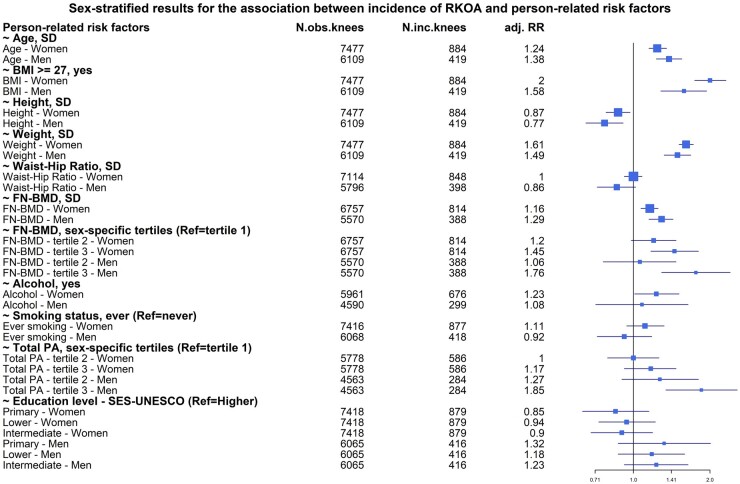
Figure 2 shows the results obtained for the person-related risk factors in the two models tested. Well-known risk factors such as age, BMI, weight and height were associated with the development of RKOA in both sexes. Additionally, we found that 1 s.d. increase in FN-BMD resulted in higher risk of developing KOA irrespective of sex [RR = 1.29 (95% CI: 1.15–1.43) in men, RR = 1.16 (95% CI: 1.07–1.25) in women] independently from BMI (Supplementary Table S2, available at Rheumatology online). Although associated in both sexes, the risk of both age and BMI was significantly different between the sexes, with age being stronger in men and BMI stronger in women (Fig. 2 and Supplementary Tables S2 and S4, available at Rheumatology online).
Fig. 2.
Forest plot of the associations between incident RKOA and person-related risk factors
FN: femoral neck; inc.: incident; obs.: observed; PA: physical activity; RKOA: radiographic knee OA; RR: relative risk; SES: socio-economic status.
Total PA, alcohol intake and ever smoker were significantly associated with the incRKOA in only one of the sexes. Using the same model adjusted for age, BMI and time of follow-up, we found that higher level of total PA [RR = 1.76 (95% CI: 1.29–2.40)] was associated with increased risk of developing KOA in our male population and this estimate was significantly higher compared with women (P = 0.01). In women, we found that any alcohol intake [RR = 1.23 (95% CI: 1.01–1.51)] was associated with a higher risk of RKOA at follow-up. In addition, female ever smokers showed a near significant association with developing RKOA [RR = 1.19 (95% CI: 0.99–1.43)] compared with female never smokers during follow-up independently from BMI (Supplementary Table S2, available at Rheumatology online). The RR of these latter two risk factors did not, however, differ significantly between the sexes.
Although having a higher WHR was associated with higher risk of RKOA in women, this association disappeared when we adjusted for BMI.
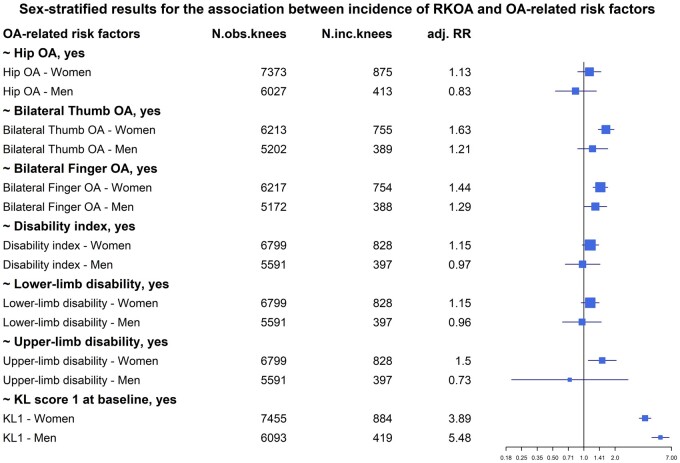
OA-related risk factors and KOA
Having OA at other joints showed some sex-specific associations with incRKOA as illustrated in Fig. 3. In both men and women, having bilateral finger OA increases the risks of developing RKOA [RR = 1.29 (95% CI: 1.00–1.66) in men, RR = 1.44 (95% CI: 1.22–1.70) in women]. Also, a KL score of 1, the strongest OA-related factor, has strong associations in both sexes, but with slightly higher risk in men [RR = 5.48 (95% CI: 4.51–6.65)] than in women [RR = 3.89 (95% CI: 3.41–4.43)] (Supplementary Table S3, available at Rheumatology online). This difference in risk estimates between men and women was significant (P = 0.002), but we did not include it in our multivariable model because it is arguably more likely a sign of early OA than a risk factor.
Fig. 3.
Forest plot of the associations between incident RKOA and OA-related risk factors
inc.: incident; KL: Kellgren–Lawrence; obs.: observed; RKOA: radiographic knee OA; RR: relative risk.
In women, we found a few additional risk factors. Having bilateral thumb OA increased the risk in women [RR = 1.63 (95% CI: 1.37–1.95)], which was borderline significantly different from the risk in men (P = 0.07, Supplementary Table S4, available at Rheumatology online). Having upper-limb disability increased the risk of developing KOA in 10 years only in women and the association remained after adjustment for BMI [RR = 1.50 (95% CI: 1.10–2.05)]. Similarly, lower-limb disability and general disability showed an association with KOA in women only. However, the risk estimates were reduced significantly and the associations attenuated after BMI adjustment. Also, the RRs of the disability-related risk factors did not differ significantly between the sexes. Having hip OA was not associated with the onset of KOA.
Population attributable fractions
For the modifiable factors BMI and total PA, we estimated the PAFs using the adjusted risk estimates from the multivariable sex-specific models (Supplementary Table S5 and S6, available at Rheumatology online).
In women, the estimated PAF for having a BMI of 27 or higher was 25.6% indicating that 25.6% of new knee cases are attributable to being overweight or obese in our female study population. In men, this proportion was significantly lower, with 19.3% of new knee cases attributable to a BMI ≥27.
The PAF for total PA was only estimated in men because in women it was not associated with the incRKOA. The estimated PAF in men for values of total PA in the highest male-specific tertile [median = 107 (range 78–1005) MET h/week] was 11.5%.
Discussion
In this population-based study of adults aged 45 years or older, we found sex differences in the relationship between risk factors and incKOA. We detected significantly different risk estimates between men and women according to the Z-test: high level of total PA or a KL score of 1 at baseline was associated with significantly higher risk in men. Other sex-related risk factors showed a borderline significant difference: higher age, a BMI of 27 or higher, lower WHR and lower education level suggest a tendency of higher risk in men, while having bilateral thumb OA suggests a significantly higher risk in women. The associations remained in the sex-specific conditional multivariable models. We further focus on discussing our main findings while also touching upon a much-debated risk factor—smoking.
Our findings show evidence that obesity has a higher impact on KOA in women compared with men. Although the association is present in both sexes, the risk for RKOA was borderline significantly different between the sexes (P = 0.056), stronger in women [RR = 2 (95% CI: 1.74–2.31)] compared with men [RR = 1.58 (95% CI: 1.28–1.94)]. Using BMI as the continuous variable provided similar results (data not shown). Despite the almost equal percentage of men and women with a BMI above 27 in our study population [31], a significantly higher proportion of new knee cases is attributable to a BMI of 27 or higher in women compared with men, with 25.6% and 19.3%, respectively. This suggests that obesity, indicated by higher BMI, acts in a different way in women compared with men in the context of RKOA. Obesity is one of the MetS components and is known to be connected to low-grade systemic inflammation [32]. In previous work from Visser and co-authors [33], it has been shown that the skeletal muscle mass to fat mass ratio is important in knee OA and that the underlying mechanisms differ between men and women. More specifically, they found that fat mass was most strongly associated in women, whereas in men skeletal muscle mass was the most important factor. Future research is needed to disentangle this difference. Thus, our results support the hypothesis of the female-specific metabolic OA type.
The sex-specific effect of smoking on the risk of KOA suggests the influence of gender roles in this case. We observed a higher proportion of male ever smokers at baseline, which may reflect the fact that three decades ago it was more acceptable for a man to smoke compared with women. Still, in our study, ever smoker showed a nearly significant positive association in women [RR = 1.19 (95% CI: 0.99–1.43)] while there was no effect in men [RR = 0.92 (95% CI: 0.72–1.18)]. The association increased in the female-specific multivariable model conditional on other risk factors [adjRR = 1.29 (95% CI: 1.1–1.49); Supplementary Table S6, available at Rheumatology online]. Smoking is a known risk factor for hypertension [34], and we therefore performed a sensitivity analysis where we also adjusted for systolic blood pressure, a reported causal factor for clinical KOA [35], which did not change the association (Supplementary Table S7, available at Rheumatology online). In previous studies, contrary to our findings, a protective effect of smoking on KOA was detected in diverse cohorts worldwide [36–38]. Still, the overall conclusion is that it has no effect [8]. Different study populations, different study designs and different categorization of exposure levels are the main reasons for the inconsistent and sometimes intriguing results. Smoking remains a controversial topic in KOA and given the contradictory results of previous research, further large prospective studies should employ sex-stratified analysis in order to replicate and contribute to this evidence.
In the case of PA, we observe a sex difference in the total amount of PA in baseline characteristics with higher values in women. This difference may partly be due to differences in the intensity and types of PA in which men and women engage [23]. Women more often performed low or moderate intensity PA comprising walking or domestic work, while men performed on average more high intensity activities, like sports. It is important to note that these characteristics are from a previous generation and may not reflect the current trend. We show that higher total PA is associated with higher risk of RKOA only in men, which might indicate that men were indeed engaged in different activities from women. Mainly high intensity PA has been shown to be detrimental to the knee joints and has been linked to the development or progression of OA in the future [39, 40]. The association remained but reduced in effect size in the multivariable model conditional on other risk factors in men [RR = 1.38 (95% CI: 1.14–1.61)]. History of knee injury is a major risk factor for the development of KOA [41, 42], and may be responsible for a part of the association we found with higher levels of total PA.
Strengths and limitations
We acknowledge that our study has limitations. First, due to the long follow-up period and the requirement for the participants to visit the research centre, there is a high percentage of participants lost to follow-up. As a consequence, the participants included in the analysis were, on average, healthier than the participants lost to follow-up. This could have led to underestimation of the risk of KOA in this population [43]. A second limitation, the lack of data on injury history and surgery in the whole study population, hindered us in investigating its sex-specific impact for KOA [41] and disentangling the attribution of PA alone to the risk of KOA. Furthermore, the PAF calculation was based on the knee cases of incKOA, meaning that for some participants we included two knees, and therefore the PAF estimates reported may have been overestimated due to the correlation between the knees. Finally, the observed sex-specific effects are the result of both biological sex effects (i.e. BMI) and sociological gender effects (i.e. smoking, PA) and it is difficult to disentangle the two. The first measurements in the Rotterdam Study took place more than three decades ago. Thus, distributions of the aforementioned risk factors might have changed among the two sexes and might not be representative for the present generation at risk for KOA. This study focuses on radiographic knee OA and did not consider symptomatic knee OA. Sex-specific risk factors may be very different for symptomatic knee OA. But this was outside the scope of this paper and is subject to future research.
Conclusion
In conclusion, our findings provide evidence for sex-specific differences in both presence and relative risks of several risk factors for incRKOA. BMI remains, however, the most important modifiable risk factor that impacts women to a larger extent than men. The new evidence we present should raise awareness of the sex differences that exist in the relationship between risk factors and KOA. The identified sex-specific risk factors can be used in the development of prevention strategies and in building sex-specific prediction tools to identify high-risk profile patients.
Supplementary Material
Acknowledgements
The Rotterdam Study is funded by Erasmus Medical Center and Erasmus University, Rotterdam, Netherlands Organization for the Health Research and Development (ZonMw), the Research Institute for Diseases in the Elderly (RIDE), the Ministry of Education, Culture and Science, the Ministry for Health, Welfare and Sports, the European Commission (DG XII) and the Municipality of Rotterdam. The authors are grateful to the study participants, the staff from the Rotterdam Study and the participating general practitioners and pharmacists. All authors contributed substantially to the conception and design of the article. I.S. performed the analysis and drafted the initial manuscript. All authors critically revised it for interpretation of results and important intellectual content. All authors approved the final version of the manuscript.
Funding: This research was funded by The Netherlands Organization for Health Research and Development (ZonMw), project number 849200003.
Disclosure statement: S.M.A.B.-Z. declares doing consultancy for Pfizer (tanezumab) and reports grants from The Netherlands Organization for Health Research and Development (ZonMw), Dutch Arthritis Association, Foreum. The remaining authors declare no competing financial interests. All authors declare no non-financial conflicts of interest.
Data availability statement
Rotterdam Study data can be made available to interested researchers upon request. Requests can be directed to data manager, Frank J. A. van Rooij (f.vanrooij@erasmusmc.nl), or visit the following website for more information: http://www.ergo-onderzoek.nl/wp/contact. We are unable to place data in a public repository due to legal and ethical restraints. Sharing of individual participant data was not included in the informed consent of the study, and there is potential risk of revealing participants’ identities as it is not possible to completely anonymize the data.
Supplementary data
Supplementary data are available at Rheumatology online.
Contributor Information
Ingrid A Szilagyi, Department of General Practice.
Jan H Waarsing, Department of Orthopedics.
Dieuwke Schiphof, Department of General Practice.
Joyce B J van Meurs, Department of Internal Medicine and Department of Epidemiology, Erasmus MC, University Medical Center, Rotterdam, The Netherlands.
Sita M A Bierma-Zeinstra, Department of General Practice; Department of Orthopedics.
References
- 1. Berkley KJ. Sex differences in pain. Behav Brain Sci 1997;20:371–80. Discussion 435–513. [DOI] [PubMed] [Google Scholar]
- 2. Nechas E, Foley D. Unequal treatment: what you don't know about how women are mistreated by the medical community. New York: Simon & Schuster, 1994. [Google Scholar]
- 3. Courtenay WH. Constructions of masculinity and their influence on men's well-being: a theory of gender and health. Soc Sci Med 2000;50:1385–401. [DOI] [PubMed] [Google Scholar]
- 4. Prieto-Alhambra D, Judge A, Javaid MK et al. Incidence and risk factors for clinically diagnosed knee, hip and hand osteoarthritis: influences of age, gender and osteoarthritis affecting other joints. Ann Rheum Dis 2014;73:1659–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Srikanth VK, Fryer JL, Zhai G et al. A meta-analysis of sex differences prevalence, incidence and severity of osteoarthritis. Osteoarthritis Cartilage 2005;13:769–81. [DOI] [PubMed] [Google Scholar]
- 6. de Kruijf M, Stolk L, Zillikens MC et al. Lower sex hormone levels are associated with more chronic musculoskeletal pain in community-dwelling elderly women. Pain 2016;157:1425–31. [DOI] [PubMed] [Google Scholar]
- 7. Odding E, Valkenburg HA, Algra D et al. Associations of radiological osteoarthritis of the hip and knee with locomotor disability in the Rotterdam Study. Ann Rheum Dis 1998;57:203–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Silverwood V, Blagojevic-Bucknall M, Jinks C et al. Current evidence on risk factors for knee osteoarthritis in older adults: a systematic review and meta-analysis. Osteoarthritis Cartilage 2015;23:507–15. [DOI] [PubMed] [Google Scholar]
- 9. Huffman KM, Kraus WE. Osteoarthritis and the metabolic syndrome: more evidence that the etiology of OA is different in men and women. Osteoarthritis Cartilage 2012;20:603–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Pradhan AD. Sex differences in the metabolic syndrome: implications for cardiovascular health in women. Clin Chem 2014;60:44–52. [DOI] [PubMed] [Google Scholar]
- 11. Strand MP, Neogi T, Niu J, Felson DT, Haugen IK. Association between metabolic syndrome and radiographic hand osteoarthritis: data from a community-based longitudinal cohort study. Arthritis Care Res (Hoboken) 2018;70:469–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Runhaar J, van Middelkoop M, Reijman M et al. Prevention of knee osteoarthritis in overweight females: the first preventive randomized controlled trial in osteoarthritis. Am J Med 2015;128:888–95.e4. [DOI] [PubMed] [Google Scholar]
- 13. Mahfouz MR, Merkl BC, Fatah EE, Booth R Jr, Argenson JN. Automatic methods for characterization of sexual dimorphism of adult femora: distal femur. Comput Methods Biomech Biomed Engin 2007;10:447–56. [DOI] [PubMed] [Google Scholar]
- 14. McKean KA, Landry SC, Hubley-Kozey CL et al. Gender differences exist in osteoarthritic gait. Clin Biomech (Bristol) 2007;22:400–9. [DOI] [PubMed] [Google Scholar]
- 15. Saberi Hosnijeh F, Zuiderwijk ME, Versteeg M et al. Cam deformity and acetabular dysplasia as risk factors for hip osteoarthritis. Arthritis Rheumatol 2017;69:86–93. [DOI] [PubMed] [Google Scholar]
- 16. Ikram MA, Brusselle G, Ghanbari M et al. Objectives, design and main findings until 2020 from the Rotterdam Study. Eur J Epidemiol 2020;35:483–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hoeven TA, Kavousi M, Clockaerts S et al. Association of atherosclerosis with presence and progression of osteoarthritis: the Rotterdam Study. Ann Rheum Dis 2013;72:646–51. [DOI] [PubMed] [Google Scholar]
- 18. Kellgren JH, Lawrence JS. Radiological assessment of osteo-arthrosis. Ann Rheum Dis 1957;16:494–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Schiphof D, Boers M, Bierma-Zeinstra SM. Differences in descriptions of Kellgren and Lawrence grades of knee osteoarthritis. Ann Rheum Dis 2008;67:1034–6. [DOI] [PubMed] [Google Scholar]
- 20.UNESCO Institute for Statistics, International Standard Classification of Education (ISCED 1997), Montreal, 2006. https://unesdoc.unesco.org/ark:/48223/pf0000146967?posInSet=1&queryId=a8d996ff-eddd-4153-9468-5d7f9e8f7199.
- 21. Caspersen CJ, Bloemberg BP, Saris WH, Merritt RK, Kromhout D. The prevalence of selected physical activities and their relation with coronary heart disease risk factors in elderly men: the Zutphen Study, 1985. Am J Epidemiol 1991;133:1078–92. [DOI] [PubMed] [Google Scholar]
- 22. Stel VS, Smit JH, Pluijm SM et al. Comparison of the LASA Physical Activity Questionnaire with a 7-day diary and pedometer. J Clin Epidemiol 2004;57:252–8. [DOI] [PubMed] [Google Scholar]
- 23. Koolhaas CM, Dhana K, Golubic R et al. Physical activity types and coronary heart disease risk in middle-aged and elderly persons: the Rotterdam Study. Am J Epidemiol 2016;183:729–38. [DOI] [PubMed] [Google Scholar]
- 24. Dahaghin S, Bierma-Zeinstra SM, Reijman M et al. Does hand osteoarthritis predict future hip or knee osteoarthritis? Arthritis Rheum 2005;52:3520–7. [DOI] [PubMed] [Google Scholar]
- 25. Fries JF, Spitz PW, Young DY. The dimensions of health outcomes: the health assessment questionnaire, disability and pain scales. J Rheumatol 1982;9:789–93. [PubMed] [Google Scholar]
- 26. Odding E, Valkenburg HA, Algra D et al. Association of locomotor complaints and disability in the Rotterdam study. Ann Rheum Dis 1995;54:721–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Altman DG, Bland JM. Interaction revisited: the difference between two estimates. BMJ 2003;326:219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Schiphof D, Kerkhof HJ, Damen J et al. Factors for pain in patients with different grades of knee osteoarthritis. Arthritis Care Res (Hoboken) 2013;65:695–702. [DOI] [PubMed] [Google Scholar]
- 29. Altman DG, Bland JM. Statistics Notes – Interaction revisited: the difference between two estimates. BMJ 2003;326:219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.R Core Team. R: a language and environment for statistical computing. Vienna: R Foundation for Statistical Computing, 2016. [Google Scholar]
- 31. Reijman M, Pols HA, Bergink AP et al. Body mass index associated with onset and progression of osteoarthritis of the knee but not of the hip: the Rotterdam Study. Ann Rheum Dis 2007;66:158–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Visser M, Bouter LM, McQuillan GM, Wener MH, Harris TB. Elevated C-reactive protein levels in overweight and obese adults. JAMA 1999;282:2131–5. [DOI] [PubMed] [Google Scholar]
- 33. Visser AW, de Mutsert R, Loef M, et al. ; NEO Study Group. The role of fat mass and skeletal muscle mass in knee osteoarthritis is different for men and women: the NEO study. Osteoarthr Cartilage 2014;22:197–202. [DOI] [PubMed] [Google Scholar]
- 34. Mills KT, Stefanescu A, He J. The global epidemiology of hypertension. Nat Rev Nephrol 2020;16:223–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Funck‐Brentano T, Nethander M, Movérare‐Skrtic S, Richette P, Ohlsson C. Causal factors for knee, hip and hand osteoarthritis: a Mendelian randomization study in the UK Biobank. Arthritis Rheumatol 2019;71:1634–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Felson DT, Zhang Y, Hannan MT et al. Risk factors for incident radiographic knee osteoarthritis in the elderly: the Framingham Study. Arthritis Rheum 1997;40:728–33. [DOI] [PubMed] [Google Scholar]
- 37. Felson DT, Anderson JJ, Naimark A et al. Does smoking protect against osteoarthritis? Arthritis Rheum 1989;32:166–72. [DOI] [PubMed] [Google Scholar]
- 38. Samanta A, Jones A, Regan M, Wilson S, Doherty M. Is osteoarthritis in women affected by hormonal changes or smoking? Br J Rheumatol 1993;32:366–70. [DOI] [PubMed] [Google Scholar]
- 39. D'Souza JC, Werner RA, Keyserling WM et al. Analysis of the Third National Health and Nutrition Examination Survey (NHANES III) using expert ratings of job categories. Am J Ind Med 2008;51:37–46. [DOI] [PubMed] [Google Scholar]
- 40. Driban JB, Hootman JM, Sitler MR, Harris KP, Cattano NM. Is participation in certain sports associated with knee osteoarthritis? A systematic review. J Athl Train 2017;52:497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Muthuri SG, McWilliams DF, Doherty M, Zhang W. History of knee injuries and knee osteoarthritis: a meta-analysis of observational studies. Osteoarthritis Cartilage 2011;19:1286–93. [DOI] [PubMed] [Google Scholar]
- 42. Richmond SA, Fukuchi RK, Ezzat A et al. Are joint injury, sport activity, physical activity, obesity, or occupational activities predictors for osteoarthritis? A systematic review. J Orthop Sports Phys Ther 2013;43:515–B19. [DOI] [PubMed] [Google Scholar]
- 43. Zhang Y, Niu J, Felson DT et al. Methodologic challenges in studying risk factors for progression of knee osteoarthritis. Arthritis Care Res (Hoboken) 2010;62:1527–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Rotterdam Study data can be made available to interested researchers upon request. Requests can be directed to data manager, Frank J. A. van Rooij (f.vanrooij@erasmusmc.nl), or visit the following website for more information: http://www.ergo-onderzoek.nl/wp/contact. We are unable to place data in a public repository due to legal and ethical restraints. Sharing of individual participant data was not included in the informed consent of the study, and there is potential risk of revealing participants’ identities as it is not possible to completely anonymize the data.