Abstract
Objective
To identify the parameters associated with self-reported diagnostic delay (DD) in axial spondyloarthritis (axSpA) patients across Europe.
Methods
Data from 2652 patients from 13 countries who participated in the European Map of Axial Spondyloarthritis (EMAS) were collected through an online survey (2017–2018). DD was calculated as the difference between age at diagnosis and age at symptom onset reported by participants. Associations between DD and sociodemographic characteristics, as well as disease-related factors were explored through univariable and multivariable linear regression analysis.
Results
Average DD was 7.4 (8.4) years with a variation between countries. The variables associated with longer DD in the final multivariable regression model were: younger age at symptom onset (b = −0.26; 95% CI: −0.28, −0.23), female gender (b = 1.34; 95% CI: 0.73, 1.96) and higher number of health-care professionals (HCPs) seen before diagnosis (b = 1.19; 95% CI: 0.95, 1.43). There was a significant interaction between the female gender and the number of HCPs seen before diagnosis. A substantial variation of the DD across European countries was observed.
Conclusion
In this sample of axSpA patients, average DD was greater than 7 years. Younger age at symptom onset, female gender, higher number of HCPs seen before diagnosis, and being diagnosed by rheumatologist were the parameters associated with a longer DD in axSpA. These findings indicate a need for continuing efforts dedicated to recognition of patients with a high probability of axSpA on the level of non-rheumatology specialists and facilitating referral to a rheumatologist for timely diagnosis.
Keywords: axial spondyloarthritis, diagnostic delay, patient-reported outcomes, patient journey, Europe
Rheumatology key messages
Diagnostic delay in axial spondyloarthritis patients is on average greater than 7 years.
Compared with men, women with axial spondyloarthritis are diagnosed 2 years later.
Younger age at symptom onset, female gender, visit to HCPs, and being diagnosed by a rheumatologist are strongly associated with axSpA delayed diagnosis.
Introduction
Early diagnosis of axial spondyloarthritis (axSpA), an inflammatory disease characterized by an insidious progression that can cause irreversible structural damage to the spine, is crucial for initiation of optimal treatments that may result in a more favourable prognosis for patients [1, 2]. However, despite extensive research on the illness, diagnostic delay (DD) in axSpA remains high and in fact, is one of the longest among rheumatic diseases [3].
During the period in which axSpA is not accurately identified, patients are forced to cope not only with the insidious nature of axSpA onset [4], but also with the anxiety of uncertainty about their diagnosis [5]. Many patients may undergo unnecessary and/or invasive testing and receive inadequate treatment for their condition [6], which may ultimately lead to poorer prognostic outcomes [7].
In order to establish accurate clinical guidelines that enable early diagnosis, identification of the factors associated with its delay is key. Various studies have found different factors associated with increased DD, including female gender [8–10], younger age at symptom onset [9], HLA-B27 negativity [7, 9, 11], presence of enthesitis or entheseal pain [7, 12], no family history of SpA [10], older age at diagnosis [12], absence of peripheral arthritis or dactylitis [12], and presence of psoriasis [9]. However, to date, these findings have not been replicated in an international setting.
The objective of the present study was to evaluate DD and identify its associated parameters in a large and multinational real-world sample of unselected axSpA patients across Europe.
Method
Study design
EMAS was a cross-sectional online survey of patients with a self-reported diagnosis of axSpA encompassing 13 European countries: Austria, Belgium, France, Germany, Italy, the Netherlands, Norway, Russia, Slovenia, Spain, Sweden, Switzerland and the UK. The survey was adapted from the Spanish Atlas of Axial Spondyloarthritis 2017 [13], the results of which were added retrospectively to the EMAS database. The methodology of the EMAS study is described in detail elsewhere [14].
Working group
The EMAS project is a collaboration led by the Health & Territory Research group of the University of Seville, Axial Spondyloarthritis International Federation (ASIF), Novartis Pharma AG and a steering committee composed of patient research partners and internationally recognized rheumatologists, psychologists and researchers specialized in axSpA.
Patients
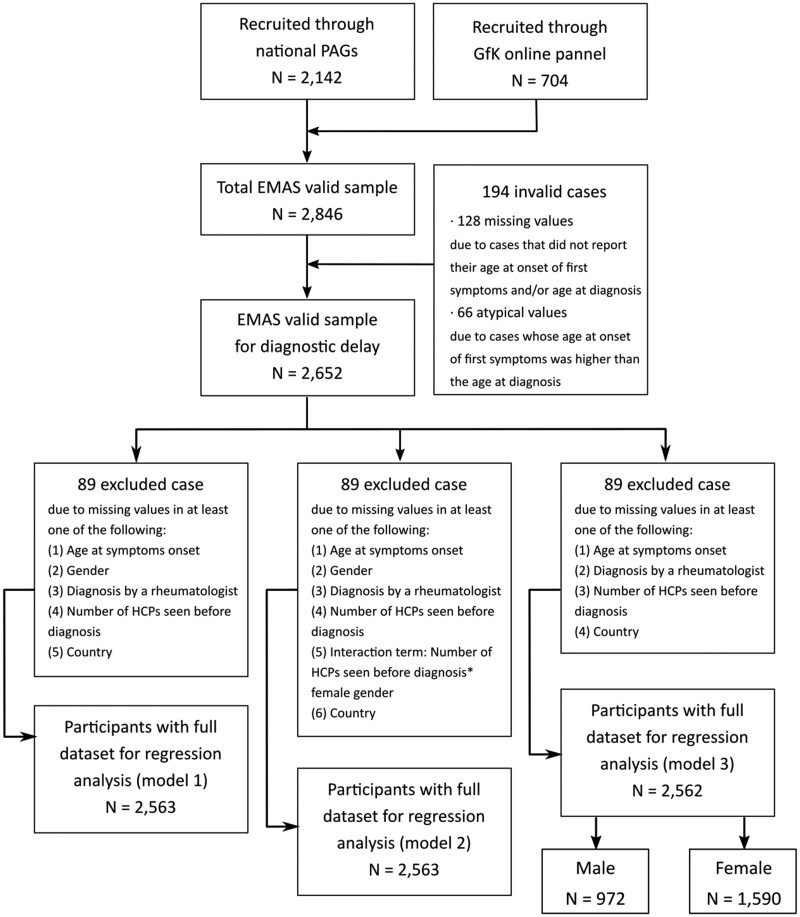
Participants were recruited between July 2017 and March 2018 by Ipsos SA (formerly GfK), a market research agency, through their existing database of respondents. Some national patient advocacy groups (PAGs) also supported recruitment by distributing the survey to their members in the following countries: Austria, Italy, the Netherlands, Norway, Russia, Slovenia, Spain, and Sweden (Fig. 1).
Fig. 1.
Sample selection flow chart
PAG: Patient Advisory Group; GfK: market research agency currently known as Ipsos Group S.A.; EMAS: European Map of Axial Spondyloarthritis.
The inclusion criteria were: aged ≥18 years, residents of an EMAS country, a self-reported clinician-provided diagnosis of axSpA, including ankylosing spondylitis or non-radiographic axSpA, and visit to a health-care professional for axSpA in the 12 months prior to inclusion.
Instruments
The EMAS survey consisted of a patient questionnaire administered online. The questionnaire included 108 items related to 12 different areas: sociodemographic and anthropometric characteristics, disability assessment, working life, daily life, lifestyle habits, diagnostic journey, health-care utilization, treatment, comorbidities (including extra-articular manifestations), psychological distress/mental health, axSpA-specific outcomes, and patient disease-related attitudes and treatment goals. All variables collected for the EMAS survey were patient-reported (see Supplementary Table S1, available at Rheumatology online).
Diagnostic delay assessment
DD was calculated with self-reported data from the following two items of the EMAS survey: ‘Age at first symptom onset (pain, inflammation, stiffness) associated with Spondylitis/Spondyloarthritis’ and ‘Age at which you were diagnosed with Spondylitis/Spondyloarthritis’. The year of diagnosis was calculated by subtracting the age of the patients at the time of the survey from the age at the time of diagnosis.
Statistical analysis
In order to identify the associated parameters to DD, only variables that could be present at the time of disease onset/diagnosis were assessed. The Mann–Whitney test was used to evaluate the difference DD between variables with two categories: gender (male, female), occupation (manual worker, non-manual worker), diagnosed by rheumatologist (yes, no), HLA-B27 (positive, negative), presence of psoriasis (yes, no), presence of uveitis (yes, no), and presence of inflammatory bowel disease (yes, no). Although the presence of family history of axSpA was collected in the EMAS survey, this variable was not considered in the analysis because it did not differentiate between patients who knew about these cases before or after the time of their diagnosis. The Kruskal-Wallis test was used to evaluate the differences in DD between variables with more than two categories (age at symptom onset, educational status).
Univariable and multivariable linear regression analysis was used to evaluate the relationship between DD and candidate variables (age at symptom onset, gender, diagnosed by rheumatologist, number of HCPs seen before diagnosis, and country). In addition, we performed an assessment of the interaction between the candidate variables. Due to a significant interaction between the number of HCPs seen before diagnosis and female gender (see results below), we constructed an additional model stratified by gender that included the following independent variables: age at symptom onset, being diagnosed by rheumatologist, number of HCPs seen before diagnosis, and country. The factor country was introduced as a dummy variable taking France (country with the largest sample size and the DD close to the mean in the entire sample) as a reference.
The regression coefficients (b) and corresponding 95% CIs were reported. SPSS 26.0 version was used to carry out the analysis.
Results
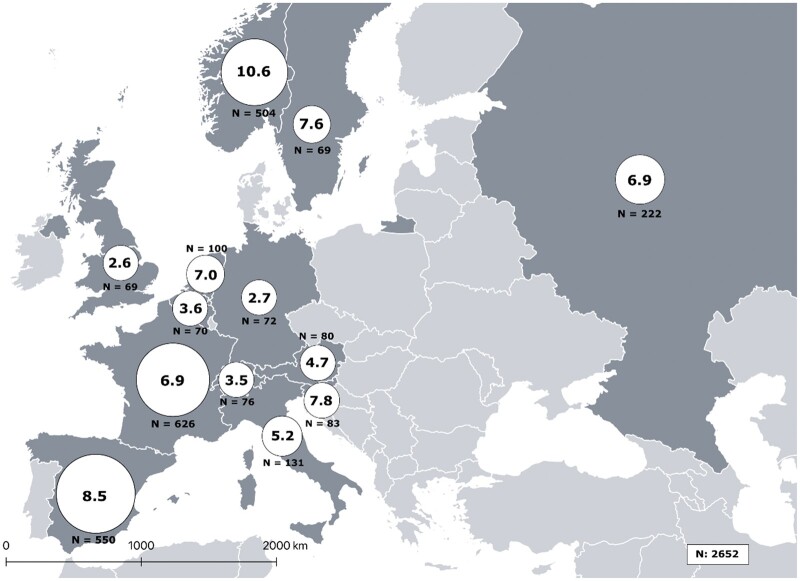
Of the 2846 EMAS participants, 2652 were included as they provided information on both age at symptom onset and age at diagnosis (Fig. 1). Mean age at symptom onset was 26.6 (11.0), mean age at diagnosis was 34.1 (11.1), and resulting mean DD was 7.4 (8.4) years (Table 1). DD was unevenly distributed among participating European countries, varying significantly from one to another. The highest average reported DD was that of Norway, followed by Spain, Slovenia and Sweden (from 10.6–7.6 years), while the countries with the lowest average were identified as the UK, Germany, Switzerland and Belgium (from 2.6–3.6 years) in that order (Fig. 2).
Table 1.
Baseline characteristics of participants included in the diagnostic delay analysis (N = 2652, unless otherwise specified)
| Sociodemographic characteristics | Mean (s.d.)/Median or n (%) | |
|---|---|---|
| Age (years) | 43.9 (12.2)/43.0 | |
| Female gender | 1644 (62.0) | |
| Educational level n = 2846 | No school completed | 28 (1.1) |
| Primary school | 226 (8.5) | |
| High school | 1092 (41.2) | |
| University | 1306 (49.2) | |
| Currently employed n = 2532 | 1369 (54.1) | |
| Occupation n = 1174 | Manual worker | 175 (14.9) |
| Non-manual worker | 999 (85.1) | |
| Lifestyle habits | ||
| Current smoker n = 2651 | 858 (32.4) | |
| BMI (kg/m2) | 26.1 (5.4)/25.3 | |
| Patient organization member | 1032 (38.9) | |
| Clinical characteristics | ||
| Age at onset of first symptoms (years) | 26.6 (11.0)/25.0 | |
| Age at diagnosis (years) | 34.1 (11.1)/33.0 | |
| Diagnostic delay (years) | 7.4 (8.4)/4.0 | |
| Disease duration since symptom onset (years) n = 2649 | 17.2 (12.3)/15.0 | |
| Positive family history of SpA n = 2186 | 851 (38.9) | |
| Diagnosed by rheumatologist n = 2564 | 2023 (78.9) | |
| Number of HCPs seen before diagnosis | 2.7 (1.3)/3.0 | |
| HLA-B27 positivity n = 1744 | 1258 (72.1) | |
| BASDAI (0–10) n = 2512 | 5.5 (2.0)/5.7 | |
| BASDAI ≥4 (0–10) n = 2512 | 1961 (78.1) | |
| Presence of inflammatory bowel disease (ever) n = 1871 | 272 (14.5) | |
| Presence of acute anterior uveitis (ever) n = 2402 | 454 (18.9) | |
| GHQ-12 (0–12) n = 2565 | 4.9 (4.1)/4.0 | |
| GHQ-12 ≥ 3 (0–12) n = 2565 | 1576 (61.4) | |
| Global Limitation Index (0–54) n = 2652 | 20.2 (16.1)/17.0 | |
| Global Stiffness Index (3–12) n = 2585 | 7.7 (2.5)/8.0 | |
| NSAIDs intake (ever) n = 2091 | 1318 (63.0) | |
| bDMARDs intake (ever) n = 2091 | 773 (37.0) | |
HLA-B27: human leucocyte antigen B27; BASDAI: Bath Ankylosing Spondylitis Disease Activity Index; GHQ-12: 12-item General Health Questionnaire; bDMARDs: biologic disease-modifying anti-rheumatic drugs.
Fig. 2.
Distribution of diagnostic delay of axSpA across EMAS countries (N = 2652)
Circle area represents the relative sample size of their respective countries.
In the bivariate analysis, a longer DD was associated with younger age at symptom onset, female gender, and diagnosis made by a rheumatologist (Table 2), which were also evident in the univariable regression analysis (Table 3). In addition, a higher number of HCPs visited before diagnosis showed an association with a longer DD (Table 2).
Table 2.
Associations between sociodemographic and disease-related variables and diagnostic delay
| Variable | Diagnostic delay Mean (s.d.) | P-value | |
|---|---|---|---|
| Age at symptom onset | <18 | 12.7 (10.7) | <0.001 |
| 19–34 | 6.8 (7.4) | ||
| 35–51 | 3.7 (4.5) | ||
| 52–70 | 1.9 (2.4) | ||
| Gender | Male | 6.1 (7.4) | <0.001 |
| Female | 8.2 (8.9) | ||
| Education level | No schooling completed | 8.0 (10.7) | 0.397 |
| Primary school | 7.6 (8.9) | ||
| High school | 7.6 (8.4) | ||
| University | 7.3 (8.3) | ||
| Occupation | Manual worker | 6.7 (8.3) | 0.163 |
| Non-manual worker | 7.3 (8.4) | ||
| Diagnosed by rheumatologist | Yes | 7.9 (8.7) | <0.001 |
| No | 5.7 (7.3) | ||
| Number of HCPs seen before diagnosis | 0 | 6.5 (7.0) | <0.001 |
| 1–2 | 5.2 (7.2) | ||
| 3 or more | 9.4 (9.0) | ||
| HLA-B27 | Positive | 8.3 (8.3) | 0.775 |
| Negative | 8.7 (9.0) | ||
| Psoriasis (ever) | Yes | 8.2 (10.0) | 0.516 |
| No | 7.4 (8.1) | ||
| Uveitis (ever) | Yes | 8.0 (8.3) | 0.098 |
| No | 7.6 (8.4) | ||
| Inflammatory bowel disease (ever) | Yes | 7.7 (8.7) | 0.944 |
| No | 7.5 (8.5) | ||
Table 3.
Univariable and multivariable linear regression analysis of the association between diagnostic delay and candidate variables in patients with axial spondyloarthritis
| Variables | Ref. | Univariable analysis |
Multivariable analysis |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Model 1 (n = 2563) |
Model 2 |
||||||||
| Male (n = 972) |
Female (n = 1590) |
||||||||
| B | 95% CI | B | 95% CI | B | 95% CI | B | 95% CI | ||
| Age at symptom onset, years | – | −0.29 | −0.32, −0.26 | −0.26 | −0.28, −0.23 | −0.18 | −0.21, −0.14 | −0.32 | −0.35, −0.28 |
| Female gender | Male | 2.10 | 1.44, 2.76 | 1.34 | 0.73, 1.96 | – | – | – | – |
| Diagnosed by rheumatologist, yes | No | 2.12 | 1.32, 2.91 | 0.41 | −0.33, 1.15 | 0.34 | −0.66, 1.35 | 0.37 | −0.68, 1.41 |
| Number of HCPs seen before diagnosis | – | 1.72 | 1.49, 1.96 | 1.19 | 0.95, 1.43 | 0.92 | 0.56, 1.29 | 1.31 | 1.00, 1.62 |
| Country, Austria | France | −2.22 | −4.09, −0.35 | 0.44 | −1.37, 2.24 | −0.29 | −2.85, 2.28 | 1.23 | −1.27, 3.74 |
| Country, Belgium | −3.39 | −5.36, −1.41 | −1.74 | −3.61, 0.12 | −0.81 | −3.39, 1.76 | −2.63 | −5.28, 0.03 | |
| Country, Germany | −4.28 | −6.22, −2.34 | −1.68 | −3.53, 0.17 | −1.78 | −4.20, 0.65 | −1.54 | −4.37, 1.29 | |
| Country, Italy | −1.71 | −3.25, −0.17 | 0.03 | −1.39, 1.45 | 0.23 | −1.92, 2.38 | −0.10 | −1.99, 1.79 | |
| Country, Netherlands | 0.08 | −1.69, 1.86 | 1.07 | −0.54, 2.68 | 2.78 | 0.40, 5.17 | −0.21 | −2.37, 1.95 | |
| Country, Norway | 3.68 | 2.63, 4.73 | 2.78 | 1.88, 3.68 | 1.67 | 0.05, 3.29 | 3.24 | 2.15, 4.32 | |
| Country, Russia | −0.06 | −1.27, 1.15 | 0.48 | −0.69, 1.66 | 1.70 | −0.09, 3.49 | −0.38 | −1.97, 1.21 | |
| Country, Slovenia | 0.84 | −1.05, 2.72 | 1.27 | −0.45, 2.98 | 0.91 | −1.48, 3.30 | 1.87 | −0.57, 4.30 | |
| Country, Spain | 1.58 | 0.67, 2.49 | 1.17 | 0.28, 2.07 | 1.64 | 0.19, 3.10 | 1.09 | −0.09, 2.27 | |
| Country, Sweden | 0.61 | −1.46, 2.67 | 1.92 | 0.05, 3.80 | 2.43 | −0.54, 5.40 | 1.73 | −0.66, 4.13 | |
| Country, Switzerland | −3.42 | −5.34, −1.50 | −0.72 | −2.52, 1.09 | −0.06 | −2.49, 2.36 | −1.67 | −4.34, 1.00 | |
| Country, United Kingdom | −4.38 | − 6.35, −2.41 | −1.71 | −3.62, 0.19 | −1.98 | −4.78, 0.83 | −1.19 | −3.74, 1.37 | |
Dependent variable in all models: diagnostic delay in years. CI: confidence interval; HCP: health-care professional.
In the multivariable model 1, a longer DD was associated with younger age at symptom onset (b = −0.26; 95% CI: −0.28, −0.23), with female gender (b = 1.34; 95% CI: 0.73, 1.96) and with the number of HCPs seen before diagnosis (b = 1.19; 95% CI: 0.95, 1.43). Furthermore, in model 1 Norway, Spain and Sweden were associated with longer DD using France as a reference (Table 3).
There was a significant interaction between gender and the number of HCPs seen before diagnosis (b = 0.56; 95% CI: 0.06, 1.05 for the interaction term), therefore, we performed an analysis stratified by gender (model 2). In this model, younger age at symptom onset showed a stronger association with longer DD in females (b= −0.32; 95% CI: −0.35, −0.28) than males (b= −0.18; 95% CI: −0.21, −0.14). Furthermore, DD was more strongly associated with a higher number of HCPs seen before diagnosis in females (b = 1.31; 95% CI 1.00, 1.62) than males (b = 0.92; 95% CI: 0.56, 1.29) (Table 3).
Discussion
In this large sample of axSpA patients from 13 different European countries, the average DD was more than 7 years. This is compatible with the recent data obtained on a national level in the UK [15] and Germany [9]. For axSpA patients, whose symptoms typically start in their mid-twenties [16], these are 7 years of intermittent pain, inflammation, fatigue, numerous medical tests, unnecessary visits to specialists, uncertainty, anxiety, depression and impact on quality of life [2, 6]. Moreover, as axSpA appears in a life stage when patients are supposed to be entering the labour market, the negative consequences it can have over the course of a lifetime may interfere with an individual’s ability to reach their full potential. As such, early detection of axSpA is critical for mitigating the physical, psychological and social impact of the disease.
The pan-European figure for DD is comparable to that of other studies [10]. However, what this study adds is the variability of DD among European countries. Our understanding is that the DD reported by EMAS is representative of the real-world situation of axSpA patients in Europe, compared with the data collected by studies relying exclusively on clinical records. In the EMAS study, participants were recruited through patient panels and patient organizations and, therefore, the resulting sample included a variety of patients who do not necessarily regularly attend health-care centres. This length of DD of axSpA patients in Europe is unacceptable and undoubtedly impacts on many aspects of the health condition and life of the patient. Thus, solutions for its reduction must be found urgently.
Several factors associated with a longer DD could be identified. The association of the longer DD with young age of symptom onset might look paradoxical at first glance. However, the same factor was found recently to be associated with longer DD in a nationwide study from Germany [9]. Most likely, back pain in young adults is often neglected or is not taken seriously enough to initiate referral to a rheumatologist or a diagnostic process to confirm or exclude an inflammatory nature of back pain.
We also found that the number of HCPs seen before diagnosis and being diagnosed by a rheumatologist (as opposed to general practitioners and other specialists) are associated with a longer DD. Most probably, patients who are diagnosed by a rheumatologist may account for axSpA cases that are difficult to detect for non-rheumatologists, therefore patients visit many non-rheumatologist HCPs before presenting to a rheumatologist who finally makes or confirms the axSpA diagnosis.
Furthermore, we revealed that female axSpA patients suffer from longer DD as compared with males even when they present with similar symptoms—a fact that has been shown in previous studies [8, 9, 17]. Longer DD in females may be due to the fact that in women with back pain, axSpA is often considered an unlikely diagnosis that results in the referral to health-care professionals other than rheumatologists. This could be confirmed by a significant interaction between female gender and number of HCPs seen before diagnosis shown in the regression analysis. Furthermore, in our gender-stratified model, we could show that in female patients both the younger age at symptom onset and the number of HCPs seen before diagnosis were more strongly associated with higher DD than in male patients. Data from research carried out in Spain supports this hypothesis. In this study, male and female patients with similar symptoms of inflammatory back pain attended a general practitioner, but only men were referred to a specialized rheumatologist [5]. Reasons for this phenomenon may be related to women presenting a higher frequency of non-classical forms of axial spondyloarthritis, such as non-radiographic [18] or to their increased frequency of comorbidity with other conditions like fibromyalgia [19]. These issues may have led to consideration of axSpA as a ‘male disease’ further perpetuating the DD in women [8].
The country factor helps to control for the effect of the other independent variables on the association with DD, with Norway, Sweden and Spain being the countries associated with the highest DD. However, the country factor should be interpreted with caution because countries are compared with a reference country (France) that had the largest sample size and a mean DD similar to the overall mean of our study.
As the results of our study show, Sweden, Spain, and Norway present a greater DD. This could be due to the fact that in these three countries the symptoms onset appeared on average in the late 1990s (1998, 1996 and 1994). This is 10 years earlier than in other countries such as Italy, Germany or the UK where patients reported a more recent symptom onset. During this period, knowledge about the disease has improved. Furthermore, in Sweden, Spain and Norway the number of visits to HCPs (2.2, 2.9 and 3.2) and the number of tests performed (2.6, 2.8 and 2.4) before diagnosis were higher, which could be due to application of inappropriate diagnostic tests for axSpA.
In summary, as ASAS-EULAR management recommendations state, early detection and diagnosis of axSpA are paramount to its primary treatment goal, which is to maximize long-term health, quality of life, and prevent progressive structural damage [20]. Understanding that the number of HCPs visited before diagnosis is strongly associated with DD, suggests that both HCP education and effective referral practices are key to decreasing DD in axSpA.
EMAS is the largest survey carried out to date for axSpA patients, gathering 2846 respondents from 13 European countries. In this study, women and people with university studies may seem over-represented. Research methods for this study (online survey panel) may have biased sampling towards female and highly educated participants. However, EMAS sociodemographic characteristics (age and marital status) are in line with what is published in other axSpA-related studies. The inclusion of information from a sample of axSpA patients from 13 European countries has served to capture differences across the European continent, generally ignored in previous published studies. The novel focus in the EMAS study was to achieve a holistic approach using a questionnaire designed for patients, relying on data collected from the patient perspective. Unlike many other studies that tend to rely heavily on clinical records and patients presenting to rheumatologists, the EMAS survey allowed for the collection of real-world data. Data collected in this way has represented people in different geographical locations, not just urban, with different health lifestyles, and different relationships with their healthcare system.
The EMAS study is not without limitations. First, the survey relied on self-reported data, and did not attempt to confirm participant diagnosis nor support participant responses with clinician-reported assessments. As a result, lab markers of disease activity were not available, and HLA-B27 status was reported for a relatively small proportion of patients and could not be verified. The same is also true for the classification status (radiographic or non-radiographic), which cannot be verified; therefore, we focused on the analysis of the entire population.
In addition, information on the presence of extra-musculoskeletal manifestations was gathered at the time of the survey and was not restricted to the period before diagnosis. Finally, the possibility of recall bias cannot be ruled out for those variables referring to events that occurred decades back in the patients’ lives, such as the age at symptom onset—this is, however, true for any study collecting symptom duration since this information often cannot be verified.
Conclusion
In this large sample of axSpA patients from 13 different European countries, the average DD was more than 7 years. Younger age at symptom onset, female gender, higher number of HCPs seen before diagnosis, and being diagnosed by a rheumatologist were factors associated with a longer DD; there was a significant interaction between female gender and the number of HCPs seen before diagnosis. HCP education and effective referral practices to a rheumatologist are key to decreasing the DD in axSpA.
Ethics
All participants were asked to provide explicit opt-in consent prior to participating in the survey.
Funding: This study was supported by Novartis Pharma AG.
Disclosure statement: The authors have declared no conflicts of interest.
Data availability statement
The authors confirm that the data supporting the findings of this study are available within the article and its supplementary materials.
Supplementary data
Supplementary data are available at Rheumatology online.
Supplementary Material
Contributor Information
Marco Garrido-Cumbrera, Health & Territory Research (HTR), Universidad de Sevilla, Seville, Spain; Axial Spondyloarthritis International Federation (ASIF), London, UK.
Victoria Navarro-Compán, University Hospital La Paz, IdiPAZ, Madrid, Spain.
Christine Bundy, School of Healthcare Sciences, Cardiff University, Cardiff, United Kingdom.
Raj Mahapatra, Axial Spondyloarthritis International Federation (ASIF), London, UK.
Souzi Makri, Cyprus League Against Rheumatism, Nicosia, Cyprus.
José Correa-Fernández, Health & Territory Research (HTR), Universidad de Sevilla, Seville, Spain.
Laura Christen, Novartis Pharma AG, Basel, Switzerland.
Carlos Jesús Delgado-Domínguez, Health & Territory Research (HTR), Universidad de Sevilla, Seville, Spain.
Denis Poddubnyy, Charité-Universitätsmedizin Berlin; Rheumatology Department, German Rheumatism Research Centre, Berlin, Germany.
References
- 1. Furst DE, Louie JS. Targeting inflammatory pathways in axial spondyloarthritis. Arthritis Res Ther 2019;21:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Yi E, Ahuja A, Rajput T, George AT, Park Y. Clinical, economic, and humanistic burden associated with delayed diagnosis of axial spondyloarthritis: a systematic review. Rheumatol Ther 2020;7:65–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Poddubnyy D, Sieper J. Current unmet needs in spondyloarthritis. Curr Rheumatol Rep 2019;21:43. [DOI] [PubMed] [Google Scholar]
- 4. Khan MA. Ankylosing spondylitis: introductory comments on its diagnosis and treatment. Ann Rheum Dis 2002;61(Suppl 3):iii3–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Jovani V, Blasco-Blasco M, Pascual E, Ruiz-Cantero MT. Challenges to conquer from the gender perspective in medicine: the case of spondyloarthritis. PLoS One 2018;13:e0205751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ozgocmen S, Khan MA. Current concept of spondyloarthritis: special emphasis on early referral and diagnosis. Curr Rheumatol Rep 2012;14:409–14. [DOI] [PubMed] [Google Scholar]
- 7. Fallahi S, Jamshidi AR. Diagnostic delay in ankylosing spondylitis: related factors and prognostic outcomes. Arch Rheumatol 2016;31:24–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Jovaní V, Blasco-Blasco M, Ruiz-Cantero MT, Pascual E. Understanding how the diagnostic delay of spondyloarthritis differs between women and men: a systematic review and metaanalysis. J Rheumatol 2017;44:174–83. [DOI] [PubMed] [Google Scholar]
- 9. Redeker I, Callhoff J, Hoffmann F et al. Determinants of diagnostic delay in axial spondyloarthritis: an analysis based on linked claims and patient-reported survey data. Rheumatol 2019;58:1634–8. [DOI] [PubMed] [Google Scholar]
- 10. Dincer U, Cakar E, Kiralp MZ, Dursun H. Diagnosis delay in patients with ankylosing spondylitis: possible reasons and proposals for new diagnostic criteria. Clin Rheumatol 2008;27:457–62. [DOI] [PubMed] [Google Scholar]
- 11. Feldtkeller E, Khan MA, van der Heijde D, van der Linden S, Braun J. Age at disease onset and diagnosis delay in HLA-B27 negative vs. positive patients with ankylosing spondylitis. Rheumatol Int 2003; 23:61–6. [DOI] [PubMed] [Google Scholar]
- 12. Masson Behar V, Dougados M, Etcheto A et al. Diagnostic delay in axial spondyloarthritis: a cross-sectional study of 432 patients. Joint Bone Spine 2017;84:467–71. [DOI] [PubMed] [Google Scholar]
- 13. Garrido-Cumbrera M, Gálvez-Ruiz D, Chacón García J, et al. Atlas of axial spondyloarthritis in Spain 2017. Profile of the disease. Madrid: Max Weber Institute; 2017.
- 14. Garrido-Cumbrera M, Poddubnyy D, Gossec L et al. ; EMAS Working Group. The European map of axial spondyloarthritis: capturing the patient perspective—an analysis of 2846 patients across 13 countries. Curr Rheumatol Rep 2019;21:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sykes MP, Doll H, Sengupta R, Gaffney K. Delay to diagnosis in axial spondyloarthritis: are we improving in the UK? Rheumatol 2015;54:2283–4. [DOI] [PubMed] [Google Scholar]
- 16. Sieper J, Poddubnyy D. Axial spondyloarthritis. Lancet 2017;390:73–84. [DOI] [PubMed] [Google Scholar]
- 17. Rusman T, van Vollenhoven RF, van der Horst-Bruinsma IE. Gender Differences in Axial Spondyloarthritis: Women Are Not So Lucky. Curr Rheumatol Rep 2018;20:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kiltz U, Baraliakos X, Karakostas P et al. Do patients with non-radiographic axial spondylarthritis differ from patients with ankylosing spondylitis? Arthritis Care Res 2012;64:1415–22. [DOI] [PubMed] [Google Scholar]
- 19. Salaffi F, De Angelis R, Carotti M et al. Fibromyalgia in patients with axial spondyloarthritis: epidemiological profile and effect on measures of disease activity. Rheumatol Int 2014;34:1103–10. [DOI] [PubMed] [Google Scholar]
- 20. van der Heijde D, Ramiro S, Landewé R et al. 2016 update of the ASAS-EULAR management recommendations for axial spondyloarthritis. Ann Rheum Dis 2017;76:978–91. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The authors confirm that the data supporting the findings of this study are available within the article and its supplementary materials.