Abstract
The very first issue of the journal of Translational Behavioral Medicine (TBM) was dedicated, in part, to the theme of Health Information Technology as a platform for evidence implementation. The topic was timely: legislation in the USA was passed with the intent of stimulating the adoption of electronic health records; mobile smartphones, tablets, and other devices were gaining traction in the consumer market, while members within the Society of Behavioral Medicine were gaining scientific understanding on how to use these tools to effect healthy behavior change. For the anniversary issue of TBM, we evaluated the progress and problems associated with deploying digital health technologies to support cancer treatment, prevention, and control over the last decade. We conducted a narrative review of published literature to identify the role that emerging digital technologies may take in achieving national and international objectives in the decade to come. We tracked our evaluation of the literature across three phases in the cancer control continuum: (a) prevention, (b) early detection/screening, and (c) treatment/survivorship. From our targeted review and analyses, we noted that significant progress had been made in the adoption of digital health technologies in the cancer space over the past decade but that significant work remains to be done to integrate these technologies effectively into the cancer control systems needed to improve outcomes equitably across populations. The challenge for the next 10 years is inherently translational.
Keywords: Behavioral informatics, Cancer control, Digital health, Technology, Prevention
Implications.
Practice: Emerging digital health technologies over the next decade, informed by scientific evidence and ethical standards, hold great promise and potential for engaging consumers and patients and supporting providers in delivery of evidence-based care across the continuum of cancer prevention, early detection, and treatment.
Policy: Future investments in digital health for cancer care need to incorporate behavioral, psychosocial, and environmental determinants of health and engage all relevant stakeholders in the design, development, and implementation of digital health solutions.
Research: The convergence of research in behavioral medicine and emerging digital technologies afford enormous opportunities to enhance screening, early detection, treatment, and survivorship, thereby reducing the burden of cancer. The future success of cancer care is predicated on drawing the best available scientific evidence from biomedicine and behavioral medicine as complementary resources.
Emerging Technologies in Cancer Prevention and Control
A platform for evidence implementation
The first issue of Translational Behavioral Medicine (TBM), published in March of 2011, contained a special section dedicated to the topic of “information technology as a platform for evidence implementation” [1]. The topic was especially timely for the newly launched, translationally focused journal. From the policy sphere, the U.S. Congress had just passed the “Health Information Technology for Economic and Clinical Health” (HITECH) Act in 2009 [2]. The HITECH Act provided a monetary incentive to qualified health care providers who attested to the “meaningful use” of health information technology (HIT) in their administrative and clinical operations [3]. Policy makers reasoned that broad adoption of HIT would be a necessary precondition for pivoting health care toward the prevention, personalization, and population-based strategies embraced by the Affordable Care Act under discussion in Congress [4].
From the commercial sector, Apple Computer had introduced the iPhone in 2007 and the iPad in 2010. Both products would be game changers in the consumer marketplace, with droves of consumers adopting mobile technologies for ubiquitous access to the Internet, personal navigation (through Global Positioning Systems; GPS), portable computing, digitally enhanced telecommunications, and (when paired with wearable sensors) to personal biometric data monitoring. Funding agencies, such as the National Institutes of Health (NIH) and the National Science Foundation, would soon begin working together in establishing the evidence base for utilizing signals from mobile and wireless devices to improve health [5]. Adoption of social media was also on the rise [6], and with it the ascendency of a business model that utilized increasingly sophisticated machine-learning algorithms to process behavioral signals as embedded within high-volume, high-velocity data streams [7].
Within this technological context, one of the principal themes for TBM’s first issue was that attention to the affordances and enhanced capacities of digital health infrastructures would offer translational specialists in behavioral medicine a platform upon which to expand the impact of their research [1,8]. As a case in point, an article by Spring et al. in the first issue illustrated how a Coordinated Anxiety and Learning Management (CALM) Health IT system could be utilized in a primary care environment to track anxiety-related symptoms, deliver cognitive behavioral therapy, and guide medication management [9]. Similarly, Hesse et al. presented a translational blueprint outlining how the meaningful use incentive program could be leveraged to create a health care system that would incorporate and be supportive of behavioral medicine principles and objectives [10]. Riley et al. published a paper in the first issue recognizing the potential of mobile technologies to provide just-in-time reinforcement for behavior change efforts as a complement to the episodic nature of in-person consultation [11]. The paper placed a high value on harnessing data flows to improve the responsiveness of just-in-time interventions to reinforce desired health behaviors in line with parallel applications derived from the engineering sciences. In a similar vein, Abernethy et al. predicted that the interoperability of data flows could help accelerate scientific discovery within a learning oncology system; could address health disparities through enhanced analysis of social determinants and identifying system flaws; and could provide input into predictive analytics as a way of promoting personalized and precision support for individuals [12].
How far have we come in the past decade?
When the U.S. Congress passed the HITECH Act in 2009, data suggested that only 12.2% of non-Federal acute care hospitals had adopted a basic electronic health record (EHR) system [13]. That number climbed to 83.8% by 2015 after the introduction of the HITECH-mandated meaningful use incentives [14]. The number has continued to increase, albeit at less precipitous pace, after the incentives had expired with 89% of all hospitals and 96% of critical care hospitals reporting the adoption of basic EHR functionality in 2020. Such a rapid expansion of digital capacity came at a cost though [15]. The American Medical Association funded research suggesting that the early accounting-based IT systems were difficult to use, were disruptive of workflows, posed undue demands on data entry, were not interoperable across systems, and were expensive to implement [16]. In response, the U.S. Congress included wording in the 2016, “21st Century Cures Act” [17] that would encourage data sharing between health systems through the provisions of a “trusted exchange framework” [18], and it included provisions to encourage the safety and usability of future Health IT initiatives through transparency and human factors research [19]. Standardized Application Programming Interfaces (APIs), most notably, the Fast Healthcare Interoperability Resources (FHIR) API, are facilitating data flows between technologies and health care systems to create an interconnected health care ecosystem that can be leveraged to support national [20–22] and international objectives [23]. Barriers to the adoption of these interfaces exist [24] and much work remains to be done, however, to realize the benefits of a trusted exchange network through changes in organizational behavior, institutional policy, and individual incentives.
Meanwhile, supply and demand for consumer-facing HIT seem to have grown exponentially since the inaugural issue of TBM with more than 318,000 mHealth apps available in major app stores [25]. As might be expected, the quality and reliability of these health-related apps vary widely [26,27]. Efforts have been underway to provide consumers and health care providers with the tools they need to make better selections of tools based on scientific efficacy [28–30], interoperability [31], and conformity to ethical standards [30]. Success of wearable devices (e.g., Fitbit and the Apple Watch) opened the market to whole new groups of consumers interested in losing weight, making healthier dietary choices, or modifying their own habits through biometric and behavioral monitoring and feedback [32]. With the evolution of smart devices—such as WiFi-enabled glucometers, scales, and the like—clinical researchers have begun to explore ways of extending the monitoring capacity of the hospital into the home [33].
Early results in the cancer field have revealed promising increases in quality of life [34,35], preemptive interventions to curtail adverse events and symptoms [34–36], and decreases in mortality [35–38]. Easy-to-use voice recognition systems, such as Amazon’s Alexa, Apple’s Siri, and Microsoft’s Cortana, offer the ability to make telephone calls, query the web, or control these same smart devices with a simple voice command [39]. Evolution of tele-video capacity through online communication tools, such as Skype, FaceTime, and Zoom, has finally made telemedical consults a reality, while the SARS-CoV-2 pandemic accelerated that trend and prompted changes in the legal and business frameworks needed to support telemedicine [40–42]. Advances in machine learning/artificial intelligence (ML/AI) began showing returns on investment in cancer detection and diagnosis [43], with deep learning approaches outperforming human capacities in reading digital X-ray images [44], arriving more quickly at accurate diagnoses of underlying pathology [45], and in translating cancer genomics into precision medicine approaches [46].
What are the opportunities in cancer control and prevention?
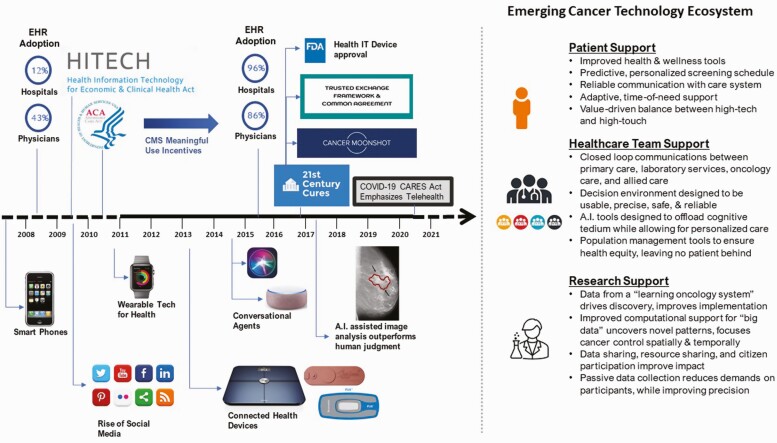
In Fig. 1, we recapitulate some of the crucial trends in digital health emerging over the past decade that have collectively contributed to a wholly new, emerging cancer technology ecosystem both in the USA and globally. The focus of translational research in the next decade, we contend, should not be as much on specific technologies per se but on the clinical integration of these technologies into the reliable workflows that will optimize patient support, reduce burden, and improve quality among health care teams and that will accelerate research in an interconnected oncology data system. Emerging technologies in this case should be considered to be a means to an end, where the overriding objective is to achieve better health outcomes. Such endeavors will offer synergy to national goals as expressed within the Department of Health and Human Services’ (DHHS’s) 2020–2025 Federal Health IT Strategic Plan. The overarching goals set by the U.S. national plan revolve around: (a) promoting health and wellness, (b) enhancing delivery and the experience of care, (c) building a secure, data-driven ecosystem to accelerate research and innovation, and (d) connecting health care with health data [20]. In the following sections, we highlight opportunities for leveraging the emerging digital technology ecosystem to improve outcomes in cancer prevention, screening, treatment and survivorship.
Fig 1.
Timeline of events over the previous decade that helped forge the new technological ecosystem for cancer treatment, prevention, and control.
Emerging Digital Technologies in Prevention
Digital health technologies are widely used in health promotion and cancer prevention. Many efforts that support cancer prevention overlap with overall efforts to improve population health. A multitude of digital health solutions have been developed and implemented to promote health behavior change addressing healthy eating, physical activity, sun protective behaviors, avoiding harmful substances (mainly targeting problematic drinking and smoking), and addressing mental health issues [47]. In most countries, including the USA, digital health resources are available to provide information, support self-monitoring, action planning, and maintaining health behaviors long-term [48,49]. Thousands of health-oriented websites and apps have been developed by governments, not-for-profit organizations, and commercial entities. Advantages of using these digital health technologies for cancer prevention include low cost, high scalability, and providing tailored and person-specific automated feedback [50]. Other important features include the use of engaging images, voice, infographics, and videos for enhanced health literacy, and data sharing for large-scale data analytics. Although development efforts in digital cancer prevention in the last decade were numerous and unprecedented [29], they also have not incorporated evidence-based components and came at the expense of initially, and notably, lacking robust regulations for data security, privacy, and digital ethics.
However, in recent years, we also witnessed a rapid progress in terms of improving and setting standards in relation to digital health tools for prevention [51]. These included the development of mechanisms for obtaining consent, processing and granting rights to data access, data encryption, pseudonymization, and rights management. Processing of any health data generally entails high risk, and unauthorized access or manipulation can cause harms and expose users’ information; this progress in regulatory efforts has been supported by governments and international organizations [23]. In terms of digital ethics, we have also seen a considerable progress in the last 10 years, and initiatives such as ReCODE emerged to support technology developers, researchers, regulators, and organizations in ethically researching and applying digital health technologies (www.recode.health). Such organizations aim to increase awareness of ethical principles and practices from the initial stages of technology design to implementation.
As a result of the proliferation of consumer-facing digital health technologies, users are often overwhelmed with available digital health offerings. Recent efforts, such as the Open Digital Health initiative, have been launched to support navigation through the vast array of offerings while aiming to support researchers in systematizing evidence-based digital health tools (www.opendigitalhealth.org). Recommendations have also been made to improve training and employ a specialized workforce of “digital health navigators” to help administrators, clinical staff, and patients steer through the digital health ecosystem [52]. The goal is to create an active, open ecosystem [53] of digital health technologies, including specification and classification of active intervention components [31], transparent sharing of usability data [54], and rigorous testing of intervention effects on behavior change and health outcomes, all of which have been historically lacking for preventive digital health solutions. Moreover, technology designers often develop digital health solutions that are short lived and that do not reflect what the consumers needed, wanted, or were willing to pay for. Targeted development of digital technologies is also needed for culturally diverse groups [55]. In digital health promotion, still, a lot needs to be done to: (a) engage user-centered design approaches, (b) to assess available technologies in terms of user- and population-level outcomes; (c) to improve the selection and navigation to the most suitable resources (ensuring that clinicians and users are supported); (d) to adhere to best practices for data security, privacy, and ethical use; and (e) to design health promotion tools that are inclusive, effective, and cost-effective.
Emerging Digital Technologies in Screening
In cancer prevention and control, the term “screening” is typically evoked when considering the use of evidence-based tests administered to asymptomatic patients with the purpose of identifying an early manifestation of neoplastic processes before they become life threatening. Tests with sufficient scientific evidence to warrant an “A” (strong evidence) or “B” (fair evidence) recommendation by the U.S. Preventive Services Task Force—such as those for cervical cancer (e.g., Pap Smear) or colon cancer (e.g., colonoscopy or FIT tests) screening—are generally made available through a physician’s office, a community health care facility, or health maintenance organization. In a reactive and fragmented health care system, the public health response to promote adherence to screening guidelines has been one of increased outreach to patients, education to physicians, and negotiations for cost coverage with insurance companies. Public health campaigns have been launched to improve awareness of cancer screening options, while encouraging physicians to recommend screening as an option to their patients who fit the appropriate age and risk profiles [56]. As public health campaigns go online, cancer prevention specialists have become adept at using data-driven personalization techniques available through search engines and social media sites to deliver motivationally tailored educational opportunities to prospective test recipients. Those efforts will undoubtedly mature as ML and AI algorithms do a better job of tailoring motivational content to the right people at the right time [57].
Not just a test but a process
In considering the ways in which emerging digital technologies can bolster early detection efforts in the next decade, it is useful to consider screening as a process and not just as a singular test [58]. First, there needs to be significant evidence to suggest that a person with a given risk profile would benefit from undergoing a screening procedure. Next, there needs to be an opportunity to recommend and discuss the pros and cons of the procedure, after which appointments must be made and kept, results must be interpreted, a follow-up consultation must be scheduled, and an action plan for next steps if the test is positively agreed upon. Each link in the chain represents a point of vulnerability in the process [59,60]. If an asymptomatic patient is never made aware of the screening option, then the test may never be ordered; if there is a loss to follow-up after the test is ordered, then the results may never influence preemptive treatment plans; and so on. From a health care safety perspective, a breakdown at any point in the chain should be considered on par with a potentially lethal medical error; in this case, an error of omission [61,62]. Improving the quality of the screening process means fortifying the system to reduce the probability of such errors. Emerging digital technologies, if deployed properly, can support each step in the screening process, making it more intuitive and less effortful for the person.
This shift in perspective, from testing simple interventions around single tests to engineering health care systems to fortify the broader screening process, has been slow to evolve in medicine but with the integration of informatics systems into medical care is beginning to take hold. One stellar example is the system that the Oregon Community Health Information Network (OCHIN) established to support population-wide screening goals through its “Stop Colorectal Cancer” program instituted through Federally Qualified Health Centers (FHQC’s). The system took full advantage of EHR technology to systematize three core functions: (a) it used EHR data to identify patients who had become eligible for colorectal cancer screening; (b) it enabled the clinical workflow needed to support mailing a self-administered, fecal immunochemical test (FIT) screening packet to eligible patients; and (c) it provided tools for tracking compliance with the test and then for scheduling follow-up appointments. The emerging technologies deployed in the project included the development of: (a) interoperable data tools to transfer crucial information between EHRs across the FHQC system, (b) electronic ordering and tracking tools between the health care system and the contracted laboratories, (c) office management tools to guide clinical outreach when needed, and (d) the population management tools needed to promote equitable compliance across populations [63]. More importantly, the OCHIN design team followed a rigorous user-centered design protocol to optimize fit between the social and technological components of the system, reducing the threat of “reminder fatigue” [64] and bolstering uptake of the emerging technologies [58].
The OCHIN informatics model reflects views expressed in the inaugural issue of TBM that to be effective as an architecture for evidence implementation, the components of new emerging technological systems must work together in unison to nudge best practice [10]. Following human factors principles [65], such systems should be intuitive and easy to use; they should (a) work with or leverage existing mental models; (b) provide for default actions congruent with system objectives; (c) offer feedback to calibrate managerial actions to measures of success or deficit in the population; and (d) be resilient in the face of error [10,66]. Similar projects with equal promise have been instantiated within the Kaiser system in Southern California, in New York, and in the Parkland-UT Southwestern PROSPR Center [58]. Early reviews suggest up to a 10-fold increase in effective colorectal cancer screening rates using the integrated system approach and have demonstrated substantial progress in promoting equitable protections across populations [67].
An evolving capacity for early detection
The goal of the NIH-funded “All of Us” initiative is to gather enough data from a million-person cohort recruited across a broad swath of volunteers to improve the predictability of disease models, moving beyond a one-size-fits-all approach in medical care to an age of precision medicine [68]. With respect to screening, precision medicine promises to improve triage efficiencies by moving away from broad bands of screening eligibility (e.g., colorectal cancer screening for those over 50 or sooner if the patient has a family history) to a more precise continuum of risk informed by the data collected longitudinally in an individual’s EHR over time. Needless to say, emerging technologies will be needed to maintain coherence among the myriad facts projected to steer decision-making under a move toward a more predictive, preemptive, and precise medicine. New data-intensive technologies must evolve, much of it informed by the rapid advances of “big data” [69] technology and ML/AI [43,45,46] to distill increasingly more precise recommendations for early detection into actionable patient care plans [70].
As the era of precision medicine continues to unfold, emerging digital technologies can be utilized to offer early warning signals for other aspects of the oncology care trajectory. For example, teledermatological approaches have been used in remote settings to provide a point-of-care analysis of potentially malignant carcinomas [71]. Under stay-at-home orders during the COVID-19 pandemic, these types of telemedical consults have been extended into the home as a stop-gap measure for patients seeking to avoid a trip to the clinic [72]. Calls have been given to expand the legislative and licensing frameworks to encourage continued use of telemedical services after the pandemic is over [42]. Likewise, an emerging technique referred to as “digital phenotyping”—using data GPS, social behavior, and other behavioral patterns to construct predictive models for identifying maladaptive behavior patterns—has shown promise in scaling up attention to individuals at risk for addictive behaviors while staying at home during the COVID-19 pandemic [73]. Continued evolution of this remote monitoring approach could offer hope to psycho-oncologists seeking to minimize mental and physical health risks before and during treatment. In all of these cases, work will need to be conducted at the systems level to balance workloads, to generate timely and responsive action when a screening anomaly is detected, and to create a closed-loop clinical system that is reliable and responsive to patient need [74].
Emerging Digital Technologies in Treatment and Survivorship
Reviews conducted during the initial years following passage of the HITECH Act revealed evidence of promising increases to health care quality and timeliness as automated mechanisms worked to prevent adverse pharmaceutical reactions, to align practice with evidence-based guidelines, to improve information flow between clinical team members, and to engage patients more proactively in their own care [75]. Ample work remains to be done, however, in distributing the benefits of these improvements across the full spectrum of cancer care. The work must begin with an emphasis on the whole patient [76], recognizing that oncology patients are often forced to navigate the gamut of services from primary care, oncology care, radiology, surgery, infusion, psychosocial counseling, and other allied services on their own. Communication technologies and system-wide monitoring systems will need to be employed to ensure that needed services are not delayed or sacrificed [77], a lesson learned during the SARS-CoV-2 pandemic [72,78]. Population health management tools will need to be deployed to ensure that all patients have equitable access to services and to guarantee that no patient is left behind [79].
This latter point, regarding population health management tools, merits particular emphasis. When the HITECH Act was passed, one of the less-popularized but more substantive changes proposed by the meaningful use incentives was an accountability requirement to track services across patients to ensure that no ball was dropped in meeting the medical needs equitably for all patients in an accountable system of care [80]. In cancer, where health disparities have historically created deficits in access across the continuum of care [81], an effective instantiation of population management strategies at the system level is precisely what cancer control will need to achieve equity in oncology care [22]. It is a self-correcting mechanism, enabled by digital health infrastructure that will be needed paradoxically to counter the projected deficits of a digital divide; that is, to help oncology practices balance communications equitably across all patients irrespective of channel preference or digital literacy [55].
Behavioral medicine and the new ecosystems of care
One way to foster the inclusion of behaviorally oriented elements into the health care marketplace is to take full advantage of a movement away from monolithic EHR systems to the ecosystem approach advocated by the DHHS to achieve interoperability through a trusted exchange framework [20]. Although the movement may be in its early stages, regulatory incentives specified through the 21st Century Cures Act will likely nudge progress down this path. Like the “app stores” of mobile telephones and tablets [82], emerging applications in digital health will be able to exchange data with legacy EHR systems in ways that improve their usability and functionality [83]. The shift is helping health systems grow more nimbly to meet the pace of a patient-driven economy [84], an area in which behavioral medicine has much to contribute. Work on Just in Time Adaptive Interventions, tailored decision-making, medication adherence, behavioral phenotyping, and many other facets of behavioral informatics should find new currency in an ecosystem driven by patient engagement. Modules that provide self-paced support for cognitive behavioral therapy techniques [85], for pain management [86], for smoking cessation [87], for stress reduction [88], and for other types of behaviorally oriented therapies [89–91] can be woven into the new ecosystem as a complement to in-person care. Understanding how best to harmonize automated, virtual, and in-person visits within the new ecosystem will require its focus in research.
Following the ecosystem approach, sensors, patient-facing treatment apps, and medical devices can all be engineered to support better exchange of data between patients and their care teams during the time of treatment and, if engineered properly, can serve to overcome the “lost in transition” [92] phenomenon often experienced by cancer survivors [77]. Such systems can and should begin to use the powerful analytic capabilities made possible through just-in-time data analytic systems to intervene early when adverse events are detected [34] and to help hospital administrators allocate scarce resources proactively to those at highest risk for worse outcomes and in greatest need [93]. Ultimately, the goal of these additions will be to provide greater support to patients who will use these tools to remain actively engaged in their own care [94] and to their care teams who can relinquish the tedium of cumbersome data exchange tasks in favor of caring directly for their patients [70]. As with the prevention tools described earlier, policymakers and health care system administrators will need to perform due diligence in selecting devices and apps for use in treatment that follow Hippocratic principles of nonmaleficence (first, do no harm) and beneficence (evidence of contributed value) [30,95].
The importance of human-centered design
Another area of contribution from behavioral medicine is to offer a human-centered perspective on the ways in which constituent elements of the sociotechnical ecosystem come together to achieve system-wide goals [96]. For example, many members of the advocacy community have lobbied for the inclusion of survivorship care plans as patients transition from curative treatment to a prolonged phase of routine vigilance for recurrence, new cancers, or late-stage side effects from treatment. Just as with the topic of screening, however, it is myopic to consider a singly produced static document as sufficient in guiding and protecting cancer survivors over the course of their lives [97]. Rather, it would be more accurate to replace the concept of a singular cancer control plan with the broader concept of survivorship care planning, where survivorship care planning is a process and broader conversation evoked repeatedly as individuals interact with their primary care physicians, oncology specialists, other care specialists, and even clinical pharmacists [98].
A human-centered, systems engineering approach could then be deployed following best translational practices to account for the roles that Health IT should play to inform future planning, monitoring, and testing activities across the patient’s life span [99]. Following a careful human factors analysis, components of the planning document would likely reside within the patient’s interoperable EHR where future tests and checkups can be scheduled following evidentiary guidelines. The routinicity of those checkups may undoubtedly change as new guidelines are set, and new types of patient generated health data become available. For example, researchers at Stanford University published data in the New England Journal of Medicine compiled over a large virtual cohort of 400,000 patients demonstrating how wearable technologies (e.g., a smart watch) could be used to detect atrial fibrillation safely and without overdiagnosis in a nonclinical population [100]. Signals such as these could be incorporated into future versions of a cancer survivor’s interoperable EHR to indicate potential late effects from cardiotoxic treatments.
Other human-centered research efforts are currently investigating the use of patient-facing sensor technologies to help detect cancer fatigue, low white cell counts (neutropenia), sepsis, cortisol levels, cognitive decline, depression, and other symptoms while patients are at home away from the clinic [95,101]. Signals from devices such as these will undoubtedly be useful for helping to enable patients to self-monitor and manage their conditions as well as support data sharing as they recover from treatment but would also open up capacity for survivorship planning as patients and their care teams navigate the complexities of comorbid conditions. The human factors question is how to balance the sociotechnical components of the emerging health care ecosystem in a way that will continuously evolve toward greater usability, increases in effectiveness and efficiency, and reductions in both provider and patient burnout [102].
A Final Caveat
In reviewing the technological advances of the last 10 years and considering the promise and potential of emerging digital technologies for the next 10, we have become acutely aware of the harsh realities associated with unanticipated effects. We no longer have the luxury of imagining what the world would be like if social media became the dominant mode of social information sharing; it has. Rather, we must now come to grips with the stunning reality of a world in which medical misinformation has been weaponized by hostile agents and used in targeted disinformation campaigns [103]; a world in which snake oil and conspiracy mingle with medical facts [104]; and a world in which attentional addiction and “doom-scrolling” can stifle mental health [105]. Similarly, we no longer need wait for EHR adoption, thanks to the fact that HITECH Act adoption rates have gone up dramatically. Rather, we now have to come to grips with a digital health environment for which proprietary algorithms and “information blocking” (i.e., refusing to share patients’ data between health care systems and services) have been the norm [24]; wherein a sudden infusion of ill-fitting technologies causes burnout among the physicians they are intended to serve [15,106]; in which fee-for-service reimbursement policies have weighed against predictive, preemptive strategies in oncology [107]; and for which efficient organizational design results by accident rather than planning [15,74]. On top of that, we must also be prepared to operate in an oncology system that has been unexpectedly derailed by the SARS-CoV-2 pandemic, a derailment that experts project will cost tens of thousands of lives from missed screening and delayed treatment [108].
As we rebuild, we will need the agenda and funding to “build back better.” We must look to technology and digital health not as a panacea but as the enhanced capacity needed to fortify the behavioral aspects of our cancer control system [95] against future shocks. In some cases, this means that we can use the new technologies to bridge barriers in access for patients who are too wrapped up in the struggles of day-to-day life, or who live too far away in rurally remote areas, to drop everything for a visit to the clinic [109]. For some patients, this may mean empowering them with direct access to the data, educational tools, and decision support technologies they need to remain active in their own personal pursuit of health and wellness. For others, it may mean expanding access to family members, community health centers, pharmacists, or urgent care facilities. In all of these endeavors, we must be sure that no one is left behind, especially attentive to the risk of perpetuating long-standing structural inequities in the existing health care system. Indeed, technology can often leave behind the most vulnerable people who have limited access to resources and who are unable to navigate the complexities of a digital health ecosystem. Leaving behind underserviced groups and underprivileged populations within a digital health economy will increase the well-established social inequalities [110]. Ensuring equitable access and helping vulnerable individuals navigate the system will be a necessary first principle in creating an oncology care system that is truly equitable and of high quality. This is a time when translational behavioral research will not just be desirable; it will be essential.
Acknowledgments:
The commentary represents volunteer work for the Society of Behavioral Medicine.
Contributor Information
Bradford W Hesse, Retired, Behavioral Research Program, National Cancer Institute, Bethesda, MD 20850, USA.
Dominika Kwasnicka, NHMRC CRE in Digital Technology to Transform Chronic Disease Outcomes, Melbourne School of Population and Global Health, University of Melbourne, Melbourne, Australia and Faculty of Psychology, SWPS University of Social Sciences and Humanities, Wrocław, Poland.
David K Ahern, Digital Behavioral Health and Informatics Research Program, Department of Psychiatry, Brigham and Women’s Hospital, Boston, MA 02215, USA.
Conflicts of Interest: D.K.A. has equity in Abacus Health Solutions.
References
- 1. Abernethy AP, Hesse BW. Guest editors’ introduction to the special section on information technology and evidence implementation. Transl Behav Med. 2011;1(1):11–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. U.S. House of Representatives, ed. 111th Congress of U.S. HITECH Act, 42 USC 139w-4(0)(2). Washington, DC: U.S. House of Representatives; 2009. [Google Scholar]
- 3. Blumenthal D, Tavenner M. The “meaningful use” regulation for electronic health records. N Engl J Med. 2010;363(6):501–504. [DOI] [PubMed] [Google Scholar]
- 4. Buntin MB, Jain SH, Blumenthal D. Health information technology: Laying the infrastructure for national health reform. Health Aff (Millwood). 2010;29(6):1214–1219. [DOI] [PubMed] [Google Scholar]
- 5. Wolff-Hughes DL, Conroy R, McClain JJ, Nilsen WJ, Riley WT. Building the infrastructure to accelerate evidence-generating mobile and wireless health research: National Institutes of Health and National Science Foundation perspectives. Transl Behav Med. 2018;8(2):295–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chou WY, Hunt YM, Beckjord EB, Moser RP, Hesse BW. Social media use in the United States: Implications for health communication. J Med Internet Res. 2009;11(4):e48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zuboff S. The Age of Surveillance Capitalism: The Fight for A Human Future at the New Frontier of Power. 1st ed. New York: Public Affairs; 2019. [Google Scholar]
- 8. Spring B. Translational behavioral medicine: a pathway to better health. Transl Behav Med. 2011;1(1):1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Spring B, Ferguson MJ. CALM technology-supported intervention: Synopsis of evidence for an emerging class of practice tool. Transl Behav Med. 2011;1(1):8–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hesse BW, Ahern DK, Woods SS. Nudging best practice: The HITECH act and behavioral medicine. Transl Behav Med. 2011;1(1):175–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Riley WT, Rivera DE, Atienza AA, Nilsen W, Allison SM, Mermelstein R. Health behavior models in the age of mobile interventions: Are our theories up to the task? Transl Behav Med. 2011;1(1):53–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Abernethy AP, Wheeler JL, Courtney PK, Keefe FJ. Supporting implementation of evidence-based behavioral interventions: The role of data liquidity in facilitating translational behavioral medicine. Transl Behav Med. 2011;1(1):45–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Jha AK, Ferris TG, Donelan K, et al. How common are electronic health records in the United States? A summary of the evidence. Health Aff (Millwood). 2006;25(6):w496–w507. [DOI] [PubMed] [Google Scholar]
- 14. Henry J, Pylypchuk Y, Searcy T, Patel V. Adoption of Electronic Health Record Systems Among U.S. Non-Federal Acute Care Hospitals: 2008–2015. Washington, DC: The Office of the National Coordinator for Health Information Technology; 2016. [Google Scholar]
- 15. Wachter RM. The Digital Doctor: Hope, Hype, and Harm at the Dawn of Medicine’s Computer Age. New York: McGraw-Hill Education; 2017. [Google Scholar]
- 16. Ratwani RM, Savage E, Will A, et al. A usability and safety analysis of electronic health records: A multi-center study. J Am Med Inform Assoc. 2018;25(9):1197–1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. U.S. Congress, ed. 21st Century Cures Act. (Vol. Pub.L. 114 - 255 114 – 255). Washington, DC:U.S. Congress; 2016. [Google Scholar]
- 18. Majumder MA, McGuire AL. Data sharing in the context of health-related citizen science. J Law Med Ethics. 2020;48(1_suppl):167–177. [DOI] [PubMed] [Google Scholar]
- 19. Ratwani RM, Hodgkins M, Bates DW. Improving electronic health record usability and safety requires transparency. JAMA. 2018;320(24):2533–2534. [DOI] [PubMed] [Google Scholar]
- 20. The Office of the National Coordinator for Health Information Technology. 2020–2025 Federal Health IT Strategic Plan. Washington, DC: Department of Health and Human Services; 2020. [Google Scholar]
- 21. Commission on Cancer. Optimal Resources for Cancer Care: 2020 Standards. Chicago, IL: American College of Surgeons; 2019. [Google Scholar]
- 22. National Academies of Sciences Engineering and Medicine (U.S.). Committee on a National Strategy for Cancer Control in the United States. In: Johns MME, Madhavan G, Amankwah FK, et al. , eds. Guiding Cancer Control: A Path to Transformation. Washington, DC: The National Academies Press; 2019. [PubMed] [Google Scholar]
- 23. World Health Organization. WHO Guideline: Recommendations on Digital Interventions for Health System Strengthening. Geneva, Switzerland: World Health Organization; 2019. [PubMed] [Google Scholar]
- 24. Everson J, Patel V, Adler-Milstein J. Information blocking remains prevalent at the start of 21 Century cures: Results from a survey of Health Information Exchange Organizations. J Am Med Inform Assoc. 2021;28(4):727–732. doi: 10.1093/jamia/ocaa323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Franklin R. 11 Surprising Mobile Health Statistics. Mobius MD (Vol. 2020). San Antonio, TX: Mobius MD; 2019. [Google Scholar]
- 26. Ana FA, Loreto MS, José LM, Pablo SM, María Pilar MJ, Myriam SA. Mobile applications in oncology: A systematic review of health science databases. Int J Med Inform. 2020;133(January):104001. [DOI] [PubMed] [Google Scholar]
- 27. McKay FH, Cheng C, Wright A, Shill J, Stephens H, Uccellini M. Evaluating mobile phone applications for health behaviour change: A systematic review. J Telemed Telecare. 2018;24(1):22–30. [DOI] [PubMed] [Google Scholar]
- 28. Sucala M, Ezeanochie NP, Cole-Lewis H, Turgiss J. An iterative, interdisciplinary, collaborative framework for developing and evaluating digital behavior change interventions. Transl Behav Med. 2020;10(6):1538–1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Arigo D, Jake-Schoffman DE, Wolin K, Beckjord E, Hekler EB, Pagoto SL. The history and future of digital health in the field of behavioral medicine. J Behav Med. 2019;42(1):67–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Boudreaux ED, Waring ME, Hayes RB, Sadasivam RS, Mullen S, Pagoto S. Evaluating and selecting mobile health apps: Strategies for healthcare providers and healthcare organizations. Transl Behav Med. 2014;4(4):363–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Larsen KR, Michie S, Hekler EB, et al. Behavior change interventions: The potential of ontologies for advancing science and practice. J Behav Med. 2017;40(1):6–22. [DOI] [PubMed] [Google Scholar]
- 32. Kvedar JC, Colman C, Cella G. The New Mobile Age: How Technology Will Extend the Healthspan and Optimize the Lifespan. Boston, MA: Partners Health; 2017. [Google Scholar]
- 33. Kvedar JC, Colman C, Cella G. The Internet of Healthy Things. Boston, MA: Partners HealthCare Connected Health; 2015. [Google Scholar]
- 34. Basch E, Deal AM, Kris MG, et al. Symptom monitoring with patient-reported outcomes during routine cancer treatment: A randomized controlled trial. J Clin Oncol. 2016;34(6):557–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Garg S, Williams NL, Ip A, Dicker AP. Clinical integration of digital solutions in health care: An overview of the current landscape of digital technologies in cancer care. JCO Clin Cancer Inform. 2018;2(December):1–9. [DOI] [PubMed] [Google Scholar]
- 36. Peterson SK, Shinn EH, Basen-Engquist K, et al. Identifying early dehydration risk with home-based sensors during radiation treatment: A feasibility study on patients with head and neck cancer. J Natl Cancer Inst Monogr. 2013;2013(47):162–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Chih MY, DuBenske LL, Hawkins RP, et al. Communicating advanced cancer patients’ symptoms via the Internet: A pooled analysis of two randomized trials examining caregiver preparedness, physical burden, and negative mood. Palliat Med. 2013;27(6):533–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Basch E, Deal AM, Dueck AC, et al. Overall survival results of a trial assessing patient-reported outcomes for symptom monitoring during routine cancer treatment. JAMA. 2017;318(2):197–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Bickmore TW, Trinh H, Olafsson S, et al. Patient and consumer safety risks when using conversational assistants for medical information: An observational study of Siri, Alexa, and Google Assistant. J Med Internet Res. 2018;20(9):e11510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Paterson C, Bacon R, Dwyer R, et al. The role of telehealth during the COVID-19 Pandemic across the interdisciplinary cancer team: Implications for practice. Semin Oncol Nurs. 2020;36(6):151090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Royce TJ, Sanoff HK, Rewari A. Telemedicine for cancer care in the time of COVID-19. JAMA Oncol. 2020;6(11):1698–1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Slomski A. Telehealth success spurs a call for greater post-COVID-19 license portability. JAMA. 2020;324(11):1021–1022. [DOI] [PubMed] [Google Scholar]
- 43. Ardila D, Kiraly AP, Bharadwaj S, et al. End-to-end lung cancer screening with three-dimensional deep learning on low-dose chest computed tomography. Nat Med. 2019;25(6):954–961. [DOI] [PubMed] [Google Scholar]
- 44. Coudray N, Ocampo PS, Sakellaropoulos T, et al. Classification and mutation prediction from non-small cell lung cancer histopathology images using deep learning. Nat Med. 2018;24(10):1559–1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Hu L, Bell D, Antani S, et al. An observational study of deep learning and automated evaluation of cervical images for cancer screening. J Natl Cancer Inst. 2019;111(9):923–932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Xu J, Yang P, Xue S, et al. Translating cancer genomics into precision medicine with artificial intelligence: Applications, challenges and future perspectives. Hum Genet. 2019;138(2):109–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Baumel A, Kane JM. Examining predictors of real-world user engagement with self-guided ehealth interventions: Analysis of mobile apps and websites using a novel dataset. J Med Internet Res. 2018;20(12):e11491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Labrique AB, Wadhwani C, Williams KA, et al. Best practices in scaling digital health in low and middle income countries. Global Health. 2018;14(1):103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Frost MJ, Tran JB, Khatun F, Friberg IK, Rodriquez DC. What does it take to be an effective national steward of digital health integration for health systems strengthening in low-and middle-income countries? Global Health Sci Pract. 2018;6(Suppl 1):S18–S28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Prochaska JJ, Coughlin SS, Lyons EJ. Social media and mobile technology for cancer prevention and treatment. Am Soc Clin Oncol Educ Book. 2017;37:128–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Kunz T, Lange B, Selzer A. [Digital public health: data protection and data security]. Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz. 2020;63(2):206–214. [DOI] [PubMed] [Google Scholar]
- 52. Wisniewski H, Gorrindo T, Rauseo-Ricupero N, Hilty D, Torous J. The role of digital navigators in promoting clinical care and technology integration into practice. Digit Biomark. 2020;4(suppl 1):119–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Hesse BW, Conroy DE, Kwasnicka D, et al. We’re all in this together: recommendations from the Society of Behavioral Medicine’s Open Science Working Group. Transl Behav Med. 2021. [DOI] [PubMed] [Google Scholar]
- 54. Ratwani RM, Reider J, Singh H. A decade of health information technology usability challenges and the path forward. JAMA. 2019;321(8):743–744. [DOI] [PubMed] [Google Scholar]
- 55. Smith B, Magnani JW. New technologies, new disparities: The intersection of electronic health and digital health literacy. Int J Cardiol. 2019;292(October):280–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Hannon P, Lloyd GP, Viswanath K, et al. Mass media and marketing communication promoting primary and secondary cancer prevention. J Health Commun. 2009;14(suppl 1):30–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Plackett R, Kaushal A, Kassianos AP, et al. Use of social media to promote cancer screening and early diagnosis: Scoping review. J Med Internet Res. 2020;22(11):e21582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Abdiwahab E, Taplin SH, Coronado G, et al. Early detection in the age of information technology. In Hesse BW, Ahern DK, Beckjord EB, eds. Oncology Informatics: Using Health Information Technology to Improve Processes and Outcomes in Cancer Care. Boston, MA: Elsevier; 2016:123–143. [Google Scholar]
- 59. Taplin SH, Rodgers AB. Toward improving the quality of cancer care: Addressing the interfaces of primary and oncology-related subspecialty care. J Natl Cancer Inst Monogr. 2010;2010(40):3–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Zapka JG, Puleo E, Taplin SH, et al. Processes of care in cervical and breast cancer screening and follow-up–the importance of communication. Prev Med. 2004;39(1):81–90. [DOI] [PubMed] [Google Scholar]
- 61. Institute of Medicine (U.S.). Committee on Quality of Health Care in America. Crossing the Quality Chasm: A New Health System for the 21st Century. Washington, DC: National Academy Press; 2001. [Google Scholar]
- 62. Kohn LT, Corrigan J, Donaldson MS. To Err is Human: Building A Safer Health System. Washington, DC: National Academy Press; 2000. [PubMed] [Google Scholar]
- 63. Coronado GD, Burdick T, Petrik A, et al. Using an automated data-driven, EHR-Embedded program for mailing FIT kits: Lessons from the STOP CRC Pilot Study. J Gen Pract (Los Angel). 2014;2(January):1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Backman R, Bayliss S, Moore D, Litchfield I. Clinical reminder alert fatigue in healthcare: A systematic literature review protocol using qualitative evidence. Syst Rev. 2017;6(1):255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Vicente KJ. The Human Factor: Revolutionizing the Way People Live with Technology. 1st ed. New York: Taylor and Francis Books; 2003. [Google Scholar]
- 66. Thaler RH, Sunstein CR. Nudge: Improving Decisions About Health, Wealth, and Happiness (Rev. and expanded Ed.). New York: Penguin Books; 2009. [Google Scholar]
- 67. President’s Cancer Panel. Improving Cancer-Related Outcomes with Connected Health. Washington, DC: The National Cancer Institute; 2016. [Google Scholar]
- 68. Collins FS, Varmus H. A new initiative on precision medicine. N Engl J Med. 2015;372(9):793–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Shaikh AR, Butte AJ, Schully SD, Dalton WS, Khoury MJ, Hesse BW. Collaborative biomedicine in the age of big data: The case of cancer. J Med Internet Res. 2014;16(4):e101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Topol EJ. Deep Medicine: How Artificial Intelligence Can Make Healthcare Human Again. 1st ed. New York: Basic Books; 2019. [Google Scholar]
- 71. Finnane A, Dallest K, Janda M, Soyer HP. Teledermatology for the diagnosis and management of skin cancer: A systematic review. JAMA Dermatol. 2017;153(3):319–327. [DOI] [PubMed] [Google Scholar]
- 72. Elkaddoum R, Haddad FG, Eid R, Kourie HR. Telemedicine for cancer patients during COVID-19 pandemic: Between threats and opportunities. Future Oncol. 2020;16(18):1225–1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Hsu M, Ahern DK, Suzuki J. Digital phenotyping to enhance substance use treatment during the COVID-19 Pandemic. JMIR Ment Health. 2020;7(10):e21814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Dorn SD. Backslide or forward progress? Virtual care at U.S. healthcare systems beyond the COVID-19 pandemic. NPJ Digit Med. 2021;4(1):6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Buntin MB, Burke MF, Hoaglin MC, Blumenthal D. The benefits of health information technology: A review of the recent literature shows predominantly positive results. Health Aff (Millwood). 2011;30(3):464–471. [DOI] [PubMed] [Google Scholar]
- 76. Adler NE, Page A. Cancer Care for the Whole Patient: Meeting Psychosocial Health Needs. Washington, DC: National Academies Press; 2008. [PubMed] [Google Scholar]
- 77. Alfano CM, Mayer DK, Beckjord E, et al. Mending disconnects in cancer care: setting an agenda for research, practice, and policy. JCO Clin Cancer Inform. 2020;4(June):539–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Richards M, Anderson M, Carter P, Ebert BL, Mossialos E. The impact of the COVID-19 pandemic on cancer care. Nat Cancer. 2020:1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Hesse BW. Role of the internet in solving the last mile problem in medicine. J Med Internet Res. 2019;21(10):e16385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Ahier B. Entering a New Era of Population Health: We Have Reached an Inflection Point in the History of Health It. Washington DC: HIMSS; 2015. [Google Scholar]
- 81. Mokdad AH, Dwyer-Lindgren L, Fitzmaurice C, et al. Trends and Patterns of disparities in cancer mortality among US Counties, 1980–2014. JAMA. 2017; 317(4):388–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Mandl KD, Kohane IS. No small change for the health information economy. N Engl J Med. 2009;360(13):1278–1281. [DOI] [PubMed] [Google Scholar]
- 83. Mandl KD, Kohane IS. Escaping the EHR trap—The future of health IT. N Engl J Med. 2012;366(24):2240–2242. [DOI] [PubMed] [Google Scholar]
- 84. Mandl KD, Kohane IS. Time for a patient-driven health information economy? N Engl J Med. 2016;374(3):205–208. [DOI] [PubMed] [Google Scholar]
- 85. Rathbone AL, Clarry L, Prescott J. Assessing the efficacy of mobile health apps using the basic principles of cognitive behavioral therapy: Systematic review. J Med Internet Res. 2017;19(11):e399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Zheng C, Chen X, Weng L, et al. Benefits of mobile apps for cancer pain management: Systematic review. JMIR Mhealth Uhealth. 2020;8(1):e17055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Cadham CJ, Jayasekera JC, Advani SM, et al. ; CISNET-SCALE Collaboration . Smoking cessation interventions for potential use in the lung cancer screening setting: A systematic review and meta-analysis. Lung Cancer. 2019;135(September):205–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Ugalde A, Haynes K, Boltong A, et al. Self-guided interventions for managing psychological distress in people with cancer—A systematic review. Patient Educ Couns. 2017;100(5):846–857. [DOI] [PubMed] [Google Scholar]
- 89. Wu C, Zheng Y, Duan Y, et al. Nonpharmacological interventions for cancer-related fatigue: A systematic review and Bayesian network meta-analysis. Worldviews Evid Based Nurs. 2019;16(2):102–110. [DOI] [PubMed] [Google Scholar]
- 90. Cillessen L, Johannsen M, Speckens AEM, Zachariae R. Mindfulness-based interventions for psychological and physical health outcomes in cancer patients and survivors: A systematic review and meta-analysis of randomized controlled trials. Psychooncology. 2019;28(12):2257–2269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Duncan M, Moschopoulou E, Herrington E, et al. ; SURECAN Investigators . Review of systematic reviews of non-pharmacological interventions to improve quality of life in cancer survivors. BMJ Open. 2017;7(11):e015860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Hewitt ME, Ganz PA, Institute of Medicine (U.S.)., American Society of Clinical Oncology (U.S.) . From Cancer Patient to Cancer Survivor: Lost in Transition: An American Society of Clinical Oncology and Institute of Medicine Symposium. Washington, DC: National Academies Press; 2006. [Google Scholar]
- 93. American Society of Clinical Oncology. Accelerating Progress against Cancer: ASCO’s Blueprint for Transforming Clinical and Translational Cancer Research. Alexandria, VA: American Society of Clinical Oncology; 2011. [Google Scholar]
- 94. Howell D, Mayer DK, Fielding R, et al. Management of cancer and health after the clinic visit: A call to action for self-management in cancer care. J Natl Cancer Inst. 2020;113(5):1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Ahern DK, Braun IM, Cooley ME, Bickmore T. Oncology informatics: behavioral and psychological sciences. In Hesse BW, Ahern DK, Beckjord E, eds. Oncology Informatics: Using Health Information Technology to Improve Processes and Outcomes in Cancer Care. Boston, MA: Elsevier; 2016:231–251. [Google Scholar]
- 96. Grassi L. Psychiatric and psychosocial implications in cancer care: The agenda of psycho-oncology. Epidemiol Psychiatr Sci. 2020;29 (January):e89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Alfano CM, Smith T, de Moor JS, et al. An action plan for translating cancer survivorship research into care. J Natl Cancer Inst. 2014;106(11):dju287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Beckjord E, Van Londen GJ, Rechis R. Survivorship. In: Hesse BW, Ahern DK, Beckjord E, eds. Oncology Informatics: Using Health Information Technology to Improve Processes and Outcomes in Cancer Care. Boston, MA: Elsevier; 2016:159–181. [Google Scholar]
- 99. Tevaarwerk AJ, Klemp JR, van Londen GJ, Hesse BW, Sesto ME. Moving beyond static survivorship care plans: A systems engineering approach to population health management for cancer survivors. Cancer. 2018;124(22):4292–4300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Perez MV, Mahaffey KW, Hedlin H, et al. ; Apple Heart Study Investigators . Large-Scale assessment of a smartwatch to identify atrial fibrillation. N Engl J Med. 2019;381(20):1909–1917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Hartshorn CM, Russell LM, Grodzinski P. National Cancer Institute alliance for nanotechnology in cancer-catalyzing research and translation toward novel cancer diagnostics and therapeutics. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2019;11(6):e1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Carayon P, Salwei ME. Moving toward a sociotechnical systems approach to continuous health information technology design: The path forward for improving electronic health record usability and reducing clinician burnout. J Am Med Inform Assoc. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Walter D, Ophir Y, Jamieson KH. Russian Twitter accounts and the Partisan polarization of vaccine discourse, 2015-2017. Am J Public Health. 2020;110(5):718–724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Ball P, Maxmen A. The epic battle against coronavirus misinformation and conspiracy theories. Nature. 2020;581(7809):371–374. [DOI] [PubMed] [Google Scholar]
- 105. Paulsen P, Fuller D. Scrolling for data or doom during COVID-19? Can J Public Health. 2020;111(4):490–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Mandl KD, Gottlieb D, Mandel JC, et al. Push button population health: the smart/hl7 fhir bulk data access application programming interface. NPJ Digit Med. 2020;3(1):151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Cutler DM. The Quality Cure: How Focusing on Health Care Quality Can Save Your Life and Lower Spending Too. Berkeley, CA: University of California Press; 2014. [Google Scholar]
- 108. Sharpless NE. COVID-19 and cancer. Science. 2020;368(6497):1290. [DOI] [PubMed] [Google Scholar]
- 109. Hesse BW, Ahern DK, Ellison M. Barn-raising on the digital frontier: The L.A.U.N.C.H. Collaborative. J Appalachian Health. 2020;2(1):6–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Rodriguez JA, Clark CR, Bates DW. Digital health equity as a necessity in the 21st Century Cures Act Era. JAMA. 2020;323(23):2381–2382. [DOI] [PubMed] [Google Scholar]