Abstract
Implantable cardioverter-defibrillators (ICDs) have revolutionized the treatment of acquired or inherited cardiac diseases associated with a high risk of sudden cardiac death due to ventricular tachyarrhythmias. Contemporary ICD devices offer reliable arrhythmia detection and discrimination algorithms and deliver highly efficient tachytherapies. Percutaneously inserted transvenous defibrillator coils with pectoral generator placement are the first-line approach in the majority of adults due to their extensively documented clinical benefit and efficiency with comparably low periprocedural implantation risks as well as the option of providing pain-free tachycardia treatment via anti-tachycardia pacing (ATP), concomitant bradycardiaprotection, and incorporation in a cardiac resynchronization therapy if indicated. Yet, expanding ICD indications particularly among younger and more complex patient groups as well as the increasingly evident long-term consequences and complications associated with intravascular lead placements promoted the development of alternative ICD configurations. Most established in daily clinical practice is the subcutaneous ICD but other innovative extravascular approaches like epicardial, pericardial, extra-pleural, and most recently substernal defibrillator coil placements have been introduced as well to overcome shortcomings associated with traditional devices and allow for individualized treatment strategies tailored to the patients characteristics and needs. The review aims to provide practical solutions for common complications encountered with transvenous ICD systems including restricted venous access, high defibrillation/fibrillation thresholds (DFTs), and recurrent device infections. We summarize the contemporary options for non-traditional extravascular ICD configurations outlining indications, advantages, and disadvantages.
Keywords: Non-traditional implantable cardioverter-defibrillator, Epicardial implantable cardioverter-defibrillator, Subcutaneous implantable cardioverter-defibrillator, Extra-pleural implantable cardioverter-defibrillator, Substernal implantable cardioverter-defibrillator, Hybrid implantable cardioverter-defibrillator configurations, High defibrillation/fibrillation threshold, Venous access crisis
Graphical Abstract
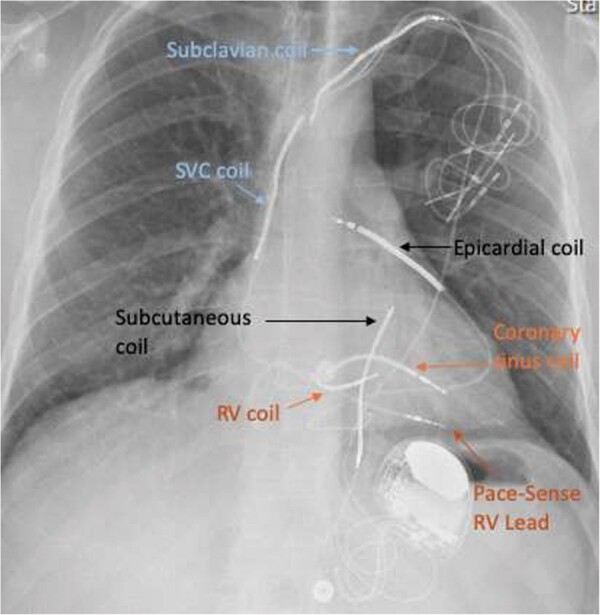
Chest X-ray with defibrillator coils in RV, SVC, subclavian vein, coronary sinus, left parasternal subcutaneous, and epicardial space. Additional pace-sense lead in RV.
Introduction
Implantable cardioverter-defibrillators (ICDs) have revolutionized the treatment of acquired or inherited cardiac diseases associated with a high risk of sudden cardiac death due to ventricular tachyarrhythmias. Contemporary ICD devices offer reliable arrhythmia detection and discrimination algorithms and deliver highly efficient tachytherapies. Percutaneously inserted transvenous defibrillator coils with pectoral generator placement are the first-line approach in the majority of adults due to their extensively documented clinical benefit and efficiency with comparably low periprocedural implantation risks as well as the option of providing pain-free tachycardia treatment via anti-tachycardia pacing (ATP), concomitant bradycardia-protection, and incorporation in a cardiac resynchronization therapy if indicated. Yet, expanding ICD indications particularly among younger and more complex patient groups as well as the increasingly evident long-term consequences and complications associated with intravascular lead placements promoted the development of alternative ICD configurations. Most established in daily clinical practice is the subcutaneous ICD but other innovative extravascular approaches like epicardial, pericardial, extra-pleural, and most recently substernal defibrillator coil placements have been introduced as well as to overcome shortcomings associated with traditional devices and allow for individualized treatment strategies tailored to the patients characteristics and needs.
Key clinical message
Transvenous implantable cardioverter-defibrillators (ICDs) inserted via the cephalic, axillary, or subclavian vein are the first-line approach for the majority of adult patients but infectious complications, intravascular lead failure, venous access restrictions, and congenital or post-surgical anatomical constraints may prevent their use.
Dedicated subcutaneous ICD systems with parasternal coil are an established safe and efficient alternative for a selected patient group and can be combined with leadless pacemakers.
Epicardial ICDs with off-label transvenous or subcutaneous defibrillation coils are a valuable option for patient unsuitable for transvenous or subcutaneous systems and it is feasible to incorporated them in a completely epicardial cardiac resynchronization system.
Extra-pleural defibrillation coils in conjunction with epicardial pace-sense electrodes are a completely extravascular alternative in the paediatric population.
Pilot studies have demonstrated feasibility of substernal defibrillator coils in adults but they are not yet commercially available.
Defibrillator coils in the coronary sinus, azygos, or hemiazygos or subclavian vein may be used to lower unacceptably high defibrillation/fibrillation thresholds with standard transvenous ICD devices.
The review aims to provide practical solutions for common complications encountered with transvenous ICD systems including restricted venous access, high defibrillation/fibrillation thresholds (DFTs) and recurrent device infections. We summarize the contemporary options for non-traditional extravascular ICD configurations outlining indications, advantages and disadvantages.
The need for alternatives
Due to various patient characteristics or complications (summarized in Table 1), traditional transvenous systems may be an unsuitable or contraindicated option. One of the most common causes preventing transvenous device insertions is an occluded or restricted thoracic venous access frequently observed in patients with cardiac devices in situ requiring a revision or upgrade, patients on dialysis, with prior thoracic radiotherapy or congenital heart disease with lack of venous continuity. Furthermore, endovascular leads are naturally exposed to a variety of biological and mechanical stress factors straining their electrical integrity and long-term considerations need to be taken into account particularly in the young. Even though significant technological advances in terms of intravascular hardware biocompatibility and durability have been made, HV lead survival rates remain comparatively low ranging from 91% to 99% at 2 years, 85% to 95% at 5 years, and 60% to 72% at 8 years in studies including leads subject to safety communications or recalls11. Likewise, device-related infectious events remain an important complication despite the use of preoperative antibiotics and recent demonstration of further risk reduction by the use of absorbable antibacterial envelops12. They account for 52.8% of indications for extraction13. Intracardiac shunts with risk of paradox embolism, recurrent lead displacements, high DFTs, or severe iatrogenic tricuspid valve regurgitation due to lead adherence, entanglement, leaflet perforation, or impingement with associated right heart failure represent further indications for non-traditional configurations.
Table 1.
Complications of transvenous devices
|
CIED, Cardiac implantable electronic devices; DFT, defibrillation/fibrillation threshold; ICD, implantable cardioverter-defibrillator; RCT, randomized controlled trial; SVC, Superior vena cava.
Various techniques have been developed to overcome limited vascular access and alternative intravascular defibrillator coil positions have been suggested to treat patients with high DFTs or tricuspid valve abnormalities (summarized in Table 2). If intravascular hardware should be avoided, several options for entirely extravascular ICDs are available. Experience and evidence for long-term safety and efficacy data for these novel configurations vary significantly and must be taken into consideration.
Table 2.
Overview alternative venous access options and intravascular coil positions
| Advantage | Disadvantage | ||
|---|---|---|---|
| Alternative venous access optionsa | Transhepatic |
|
|
| Transfemoral/iliacal | |||
| Inside out venous access |
|
|
|
| Transthoracic transatrial |
|
|
|
|
Coronary sinus (CS) |
|
|
| Hemi-/azygos vein |
|
||
| Left subclavian vein | |||
| Percutaneous intravascular cardioverter defibrillator 15 |
|
|
CIED, cardiac implantable electronic devices; DFT, defibrillation/fibrillation threshold; ICD, implantable cardioverter-defibrillator; RV, right ventricular; SVC, Superior vena cava; CS coronary sinus.
In occluded thoracic veins unsuitable for interventional or surgical revascularization.
In the setting of high DFTs with traditional ICDs and failure of non-invasive measures or in the presence of tricuspid valve abnormalities precluding standard RV coil placement.
Venous access options
For patients with occluded upper central venous access interventional venous revascularization with venoplasty ± stenting or vascular surgery can be an option. If a device is already in situ and requires a revision or upgrade the use of laser or mechanical recanalization tools (with or without lead extraction) can be considered. In case of an unilateral venous occlusion contralateral access and subcutaneous tunnelling of the new lead to the existing generator site may be attempted.
If the patient is deemed unsuitable or declines any of the above-mentioned solutions alternative insertion techniques have been described via a transfemoral/-iliacal16,17 or trans-hepatic access18,19 with placement of the defibrillator coils into the RV cavity and tunnelling of the lead body to an abdominal generator. If a pectoral generator placement is preferred despite the presence of complex thoracic vein occlusion the ‘inside-out’ central venous access offers an elegant percutaneous alternative20. The latter involves the use of a special needle guide inserted via the femoral vein which is used to puncture through the occluded central vein segment from within the vasculature and advancing a wire to a predefined infra- or supraclavicular exit point (see Figure 1). A further albeit more invasive option is the transatrial access with placement of defibrillator into the right ventricular cavity via a thoracotomy and atriotomy. This approach has been successfully reported even in very small patients with otherwise insufficient vessel size or lack of venous continuity and/or the concomitant need for bradytherapy rendering a subcutaneous system unsuitable.22
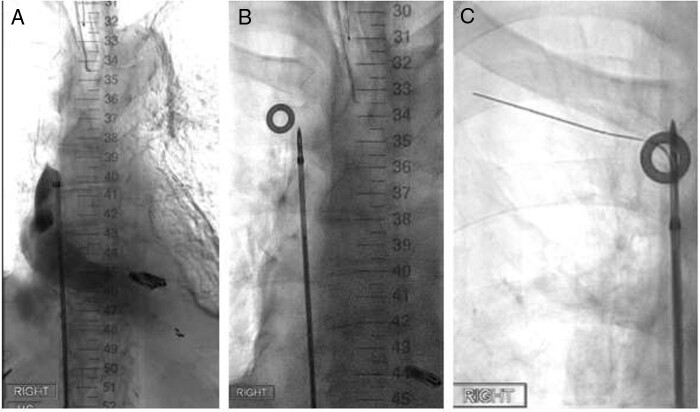
Figure 1.
Inside-out central venous access with right infraclavicular exit in superior vena cava occlusion (adapted from Ref.21). (A) Venogram via femoral working sheath demonstrates occluded SVC. (B) Inside-out venous access kit with needle guide puncturing through occluded vein segment and (C) guide wire exiting at site of radiopaque skin marker inferior to right clavicula.
Alternative intravascular defibrillator coil positions
The two main indications for non-traditional intravascular defibrillator coil placements include high defibrillation-fibrillation thresholds and tricuspid valve abnormalities.
For transvenous ICDs, the praxis of routinely adding a second coil (traditionally in the superior vena cava) has been largely abandoned as similar efficacy was demonstrated for single-coil systems with active can. The decrease in impedance and small reduction in DFT with dual-coil systems in an era of high-output ICDs with biphasic shocks was thought to be offset by an increase in long-term complications.23,24 However, for selected patients with high defibrillation fibrillation thresholds (defined as safety margin of <10 V between threshold and maximum output shock in any of the available shock vectors) with standard transvenous ICD systems (RV/SVC coils) case reports/series demonstrated that insertion of an ancillary defibrillator coil in the coronary sinus25, azygos vein26,27, hemi-azygos vein (with right-sided generator)28 or left subclavian veins29 is safe, feasible, and successful in lowering the mean DFT. The challenge is the manoeuvring across many angles and delivering large defibrillator coils to these positions (with the exception of the subclavian vein). Also, lead displacement and migration are a concern. Use of a vascular plug to anchor the coil in adequate position to prevent displacement has been described.30
Coronary sinusdefibrillator coils may also be offered to patients with tricuspid valve abnormalities (including mechanical valve prothesis precluding access to the RV cavity) provided a sufficiently large ventricular branch is present to accommodate the coil. One possible disadvantage of this position relates to providing transvenous cardiac resynchronization therapy as a small case series found delivery of left ventricular (LV) pacing via the CS unreliable and the coil itself may impede placement of or cause interference with a standard pace-sense LV lead.25 This is an important concern if both the ventricular pace/sense and HV lead need to be inserted into the coronary sinus. Alternatives for the pace/sense lead insertion in this situation would be an epicardial/pericardial or left intraventricular position.
Trans-septal access and placement of leads into the LV cavity has been described for pacing leads only31, but not for high-voltage leads due to difficulties of easing the comparatively bulky defibrillation coil through the septum into the LV cavity and the associated risks of systemic embolism.
Extravascular implantable cardioverter-defibrillator configurations
If endovascular lead placements fail or are contraindicated alternative options consist of subcutaneous, epicardial, pericardial, extra-pleural or substernal defibrillator placements, or hybrid configuration combining intra- and extravascular components. With the exception of the subcutaneous ICD no dedicated hardware is available for non-traditional coil positions. Inserted leads are usually off-label transvenous or subcutaneous coils in combination with a standard transvenous ICD generator.
Table 3 gives an overview of the available extravascular ICD configurations.
Table 3.
Summary extracardiac ICD configurations
| Configuration | Advantages | Disadvantages | Pace/sense leada | Evidenceb | |
|---|---|---|---|---|---|
| Subcutaneous | (1) Subcutaneous ICD system (Boston Scientific S-ICDTM system)—parasternal tripolar lead |
|
|
|
|
| (2) Subcutaneous single-coil or array without sensing electrodes |
|
|
Epi-or pericardial Transvenous (RV and/or LV) |
|
|
| Epicardial/pericardial |
|
|
|
|
|
| Substernal | Coil in substernal space in anterior mediastinum |
|
|
|
|
| Extra-pleural | Coil in between parietal pleura and thoracic wall |
|
|
|
|
| Hybrid | Combination of intra- and extravascular components |
|
|
|
|
ATP, anti-tachycardia pacing; CRT, cardiac resynchronization therapy; DFT, defibrillation/fibrillation threshold; ICD, implantable cardioverter-defibrillator; LV, left ventricular; MRI, magnetic resonance imaging; RV, right ventricular; VATS, Video assisted thoracoscopic surgery
Traditional transvenous (active fixation) or dedicated epicardial (active or passive fixation) pace/sense leads tunnelled and connected to ICD generator (or CRTD generator if applicable).
Main text for respective references.
Subcutaneous implantable cardioverter-defibrillator
Over two decades ago, the use of subcutaneous coils32 and patches33 in a parasternal or left dorsolateral position as an adjunct to a transvenous or epicardial system to lower high DFTs has been described and remains until today a bailout strategy for this indication including in an adult population34. The original subcutaneous array consisted of three ‘fingers’ (=coils) requiring extensive dissection with creation of three subcutaneous tunnels for placement. Later case reports demonstrated that single defibrillation coils were as efficacious35 and that in children subcutaneous array leads with an active can could safely achieve defibrillation even in the absence of a transvenous device36.
The first dedicated entirely subcutaneous ICD for adults (Cameron Health acquired by Boston Scientific in 2012) with a parasternal subcutaneous lead with an 8 cm shocking coil and a distal and proximal sensing electrode and intramuscular generator between the left latissimus dorsi and serratus anterior was introduced in Europe in 2009 and approved by the US FDA in 2012. Large registry37 and randomized trial38 data have since confirmed its safety and efficacy and established it as an alternative to the transvenous system in patients without pacing requirement and no cardiac resynchronization therapy (CRT) indication. The latter limitations are currently being challenged as case reports have shown that the combination of S-ICDTM and leadless pacemaker or WiSE-CRT (EBR Systems, Sunnyvale, CA, USA) is feasible (see Hybrid ICD systems below). Also, the initial concerns of high inappropriate shock rates in S-ICDs were recently dispersed as studies with 2nd or 3rd generation devices with improved discrimination algorithms and standardized programming algorithms report significantly lower rates (3.1% at 1 year)39 comparable to many transvenous systems. At present, DFT testing at time of insertion is still recommended but a risk stratification score to predict defibrillation success is currently being evaluated (PRAETORIAN DFT trial40) and may identify patients in whom routine DFT testing can be safely omitted.
Ongoing limitations for subcutaneous systems are the high number of unsuitable patients due to sensing issues with failed screening rates reported between 7–8% for 1 vector41 and 15% for 2 vectors42 or due to their body habitus in case of significant obesity. Also, the larger generator sizes required for the high energy shocks of up to 80 J may cause patient discomfort, bulging, and aesthetic concerns. Likewise, the extra-thoracic lead position exposes it to environmental mechanical stress with risk of compromising lead integrity43 as well as lead migration. The latter complication has been significantly reduced by the introduction of a suture sleeve at the xiphoid incision to secure the lead.44
Epicardial/pericardial implantable cardioverter-defibrillator
Due to their complete extravascular position and independency of venous patency epicardial ICD systems are usually considered in the context of lack of vascular access, recurrent endocarditis, or device associated infectious complications, tricuspid valve-related pathologies, and poor transvenous lead performance in patients not suitable for an S-ICD. Epicardial placements are more common though some authors advocate a pericardial position in order to minimize the risk of constrictive pericarditis, interference of heart movement and coronary artery damage.45
Traditionally epicardial systems have been inserted via sternotomy or left-sided thoracotomy with the benefit of unrestricted access to the hearts surface allowing for optimal electrical mapping and active lead fixation. To reduce perioperative morbidity new techniques using video-assisted thoracoscopy or sub-xiphoid access have been successfully applied and offer a minimal invasive alternative for epicardial systems. Dedicated delivery tools are lacking. For thoracoscopic epicardial lead insertion special steerable delivery tools are available only for pacing leads but not for defibrillator coils. Delivery systems for sub-xiphoidal introduction of high-voltage leads via a steerable sheath are still investigational.46
For epicardial pacing leads acceptable long-term lead performances have been described47–49; however, the opposite was found for epicardial defibrillation patches which have been largely abandoned due to high patch failure rates (up to 28% within 4 years50). Instead, the off-label deployment of contemporary transvenous and subcutaneous coils passively inserted in the pericardial space or actively sutured on the epicardial or pericardial surface has gained popularity. Theoretically, transvenous defibrillator leads afford ventricular pacing and R-wave sensing, however, in an epicardial position this has been found to be unreliable. Additional epicardial pace-sense leads are required to assure appropriate arrhythmia detection and may also deliver cardiac resynchronization therapy if indicated.
Multiple case series and reports have documented acceptable efficiency and safety of epicardial ICDs employing standard transvenous or subcutaneous coils.51 Minimal invasive insertion techniques with lower peri-operative morbidity have further contributed to their increased use. However, they have not been investigated in randomized prospective trials and recurrent concerns regarding long-term lead performance of the defibrillator coils as well as several rare but severe complications associated with the epicardial position remain (outlined in Table 4). Dedicated follow-up in a specialized centre familiar with these systems is advised and may involve routine radiographic and echocardiographic surveillance as well as continuous monitoring via home monitoring for early identification of complications.
Table 4.
Complications of epicardial ICD devices
|
CHD, Congenital Heart disease; DFT, defibrillation/fibrillation threshold.
Generally epicardial ICDs are considered not MRI conditional with only very limited data of small case series regarding the safety of MRI scanning.57,58
Extra-pleural implantable cardioverter-defibrillator
Paediatric and adolescent population case series59–61 described successful placement of an extra-pleural defibrillator coil between the parietal pleura and thoracic wall along the 3rd intercostal space inserted via left lateral thoracotomy or sternotomy. Inserted defibrillator coils were off-label standard transvenous or subcutaneous leads and combined with epicardial pace-sense leads. Generators were placed abdominally or in a sub-cardiac pocket.
Outcome data showed reasonable efficiency and safety in follow-up of up to 5 years. The extra-pleural position prevents complication associated with intravascular leads, protects the lead body from external trauma and tension within the thoracic cage and allows for a favourable shocking vector in combination with an abdominal generator. Disadvantages include the invasive nature of the insertion including the risk of damage to the lung, the need for separate (epicardial or transvenous) pace-sense leads and relatively frequent surgical revisions including for lead failure59.
Substernal implantable cardioverter-defibrillator
To overcome the limitations of subcutaneous and epicardial ICDs in adult patients but maintaining the benefits of an extravascular position, placement of a defibrillator lead in the substernal space has been proposed. The defibrillator coil can be inserted minimal invasively via a sub-xiphoid approach with a tunnelling tool kept close to the posterior surface of the sternum and is combined with a generator in the left midaxillary line. Initial case reports described the use of transvenous SVC coils in conjunction with epicardial pacing leads62 or the use of standard subcutaneous coils with integrated sensing electrodes63. Recently a dedicated substernal defibrillator system has been developed and feasibility studies demonstrated successful defibrillation in adult patients with shock energies comparable to transvenous devices and substantially lower than subcutaneous ICDs64 allowing for smaller generator sizes. First-In-Human pilot studies yielded encouraging results65,66 and also proved feasibility of appropriate R-wave sensing and pacing capture from the substernal space allowing for complementary anti-tachycardia pacing and bradycardia protection 67.
The extravascular substernal defibrillator is an investigational device and not commercially available. A prospective open-label multicentre trial to further evaluate safety and efficacy is currently recruiting.68
Hybrid implantable cardioverter-defibrillator
Hybrid ICD systems incorporate a combination of extra- and/or intravascular components to allow for an individualized therapy tailored to the patients’ characteristics and needs. Examples are shown in Figure 2. Expertise for each of the individual components and in case of separate modular configurations considerations of the complex interaction between the systems are primordial to provide a safe and efficient therapy. Literature on multicomponent ICDs is sparse and limited to case series and reports.
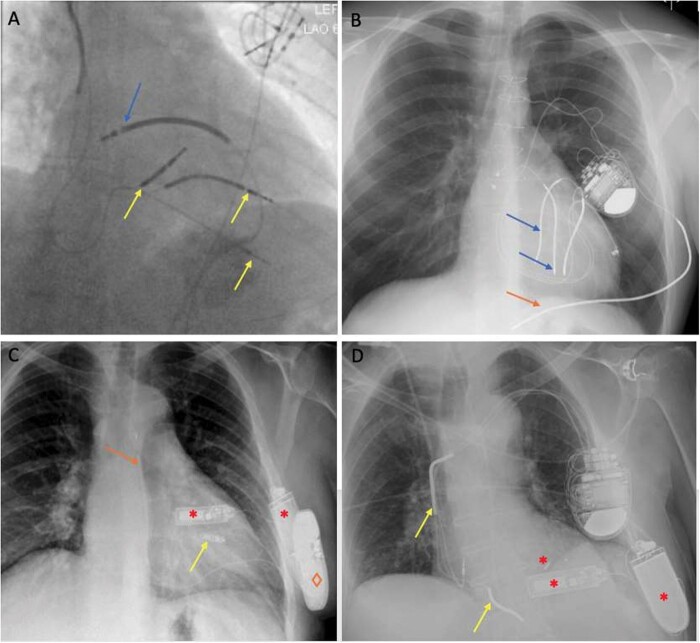
Figure 2.
Examples for hybrid ICD configurations. (A) Transvenous dual-coil ICD including coronary sinus coil and pace-sense leads (yellow arrows, two abandoned, one tunnelled to abdominal generator) with epicardial defibrillator coil (blue arrow). (B) Epicardial ICD with two dual-coil HV leads (blue arrows) and traditional subcutaneous coil (orange arrow) in posterolateral position tunnelled to left pectoral generator. (C) Subcutaneous ICDTM (orange arrow and ◊) with leadless pacemaker in RV (yellow arrow, Micra) and WiSE CRT (red *). (D) Transvenous dual-coil ICD (yellow arrows) with WiSE CRT (red *). CRT, cardiac resynchronization therapy; ICD, implantable cardioverter-defibrillator; RV, right ventricular.
The combination of subcutaneous ICDsTM with a leadless cardiac pacemaker (LCP) has recently been established as a feasible combination to offer entirely leadless bradytherapy and high-voltage tachytherapy to a larger patient population.69 Animal studies demonstrated LCPs can also afford ATP delivery commanded by an implanted subcutaneous ICD70 and clinical applications for modular cardiac rhythm management systems with integrated wireless inter-device nearfield communication are under investigation.71 These systems may be further complemented by a WiSE-CRT (‘wireless stimulation endocardially’) capable of delivering wireless LV endocardial pacing to provide completely wireless cardiac resynchronization therapy.72 Particular attention is required to confirm appropriate S-ICDTM sensing in the context of changing QRS morphologies due to breakthrough conduction, fusion, and pseudo-fusion during pacing. Re-confirmation of acceptable S-ICDTM sensing at the time of replacement of the original LCP is essential but limited by the stiff large-sized femoral delivery tools for LCP allowing only for supine testing prior to definitive deployment.
Transvenous ICD systems may be combined with the above-mentioned WiSE-CRT or complemented by a surgically inserted LV lead to provide CRT if transvenous insertion failed. Large comparative studies for surgically vs. percutaneously placed LV pacing lead insertion for CRT have shown similar outcomes and rates of reverse ventricular remodelling for the two approaches73,74.
Extra-pleural and epicardial defibrillator coils are usually inserted together with separate epicardial pace-sense leads and may also be connected to an existing transvenous system. To reduce high DFTs refractory to non-invasive interventions, subcutaneous coils, or arrays can be incorporated in the epicardial or extra-pleural high-voltage circuit.
Wearable cardioverter/defibrillators
The WCD is a non-invasive option as a bridge-to-decision or bridge-to-recovery in acute heart failure or after an acute cardiac event with estimated high risk of ventricular arrhythmias but reasonable probability of recovery over time and with optimized medical therapy. Typical indications include acute myocarditis, peripartum- or takotsubo cardiomyopathy, or acute myocardial infarction, where the decision about the necessity of a permanent ICD should ideally be deferred. Randomized controlled trial and large registries have demonstrated the clinical effectiveness of the WCD for treating ventricular arrhythmias and also highlighted the importance of patient compliance and maximizing wearing time to achieve a clinical benefit.75,76
Conclusion
Non-traditional ICD configurations offer important alternatives for patients at risk of sudden cardiac death due to ventricular tachyarrhythmias even in the most complex of cardiac patients and the growing range of options allow for more individualized treatment strategies. These possibilities need to be evaluated in the light of limited clinical experience and sparse long-term safety- and device performance data for the majority of these systems. Considerate patient selection and informed decision-making together with the patient is essential. Close follow-up in a centre with expertise for non-traditional systems is recommended to assure adequate device function and early identification of complications.
Safety, efficacy, and lead performance of non-traditional ICDs could be further improved with development of dedicated delivery tools for minimal invasive insertion techniques and special defibrillation leads designed to meet the demands of specific coil positions. Also, the combination of high-voltage systems with leadless right- or LV pacing devices requires further clinical investigation. Dedicated modular cardiac device systems with integrated wireless inter-device communication are a promising innovative solution currently under development.
Conflict of interest: The authors have no conflict of interest to declare in relation to this manuscript.
Data availability
Data is available in a public repository that issues datasets with DOIs.
Contributor Information
Johanna B Tonko, Department of Cardiology, St. Thomas’ Hospital, Westminster Bridge Rd, London SE1 7EH, UK; Department of Cardiovascular Imaging, Arrhythmia Research Group, King’s College London, School of Biomedical Engineering & Imaging Sciences, London, UK.
Christopher A Rinaldi, Department of Cardiology, St. Thomas’ Hospital, Westminster Bridge Rd, London SE1 7EH, UK; Department of Cardiovascular Imaging, Arrhythmia Research Group, King’s College London, School of Biomedical Engineering & Imaging Sciences, London, UK.
References
- 1. Lundqvist CB, Traykov V, Erba PA, Burri H, Nielsen JC, Bongiorni MG et al. EHRA International consensus document on how to prevent, diagnose and treat cardiac implantable electronic device infections. Europace 2020;22:515–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Boczar K, Ząbek A, Haberka K, Hardzina M, Dębski M, Rydlewska A et al. Venous stenosis and occlusion in the presence of endocardial leads. Adv Clin Exp Med 2016;25:83–91. [DOI] [PubMed] [Google Scholar]
- 3. Hauser RG, Hayes DL. Increasing hazard of Sprint Fidelis implantable cardioverter-defibrillator lead failure. Heart Rhythm 2009;6:605–10. [DOI] [PubMed] [Google Scholar]
- 4. Ezzat VA, Lee V, Ahsan S, Chow AW, Segal O, Rowland E et al. A systematic review of ICD complications in randomised controlled trials versus registries: is our ‘real-world’ data an underestimation? Open Heart 2015;2:e000198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hsu JC, Varosy PD, Bao H, Dewland TA, Curtis JP, Marcus GM. Cardiac perforation from implantable cardioverter defibrillator lead placement. Insights from the National. Circ Cardiovasc Qual Outcomes 2013;6:582–90. [DOI] [PubMed] [Google Scholar]
- 6. Migliore F, Zorzi A, Bertaglia E, Leoni L, Siciliano M, De Lazzari M et al. Incidence, management and prevention of right ventricular perforation by pacemaker and implantable cardioverter defibrillator leads. Pacing Clin Electrophysiol 2014;37:1602–9. [DOI] [PubMed] [Google Scholar]
- 7. Lin G, Nishimura RA, Connolly HM, Dearani JA, Sundt TM 3rd, Hayes DL. Severe symptomatic tricuspid valve regurgitation due to permanent pacemaker or implantable cardioverter‐defibrillator leads. J Am Coll Cardiol 2005;45:1672–5. [DOI] [PubMed] [Google Scholar]
- 8. Delling FN, Hassan ZK, Piatkowski G, Tsao CW, Rajabali A, Markson LJ et al. Tricuspid regurgitation and mortality in patients with transvenous permanent pacemaker leads. Am J Card 2016;117:988–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lin EF, Dalal D, Cheng A, Marine JE, Nazarian S, Sinha S et al. Predictors of high defibrillation threshold in the modern era. Pacing Clin Electrophysiol 2013;36:231–7. [DOI] [PubMed] [Google Scholar]
- 10. Desimone CV, Friedman PA, Noheria A, Patel NA, DeSimone DC, Bdeir S et al. Stroke or transient ischemic attack in patients with transvenous pacemaker or defibrillator and echocardiographically detected patent foramen ovale. Circulation 2013;128:1433–41. [DOI] [PubMed] [Google Scholar]
- 11. Kusumoto FM, Schoenfeld MH, Wilkoff BL, Berul CL, Birgersdotter-Green UM, Carrillo R et al. 2017 HRS expert consensus statement on cardiovascular implantable electronic device lead management and extraction. Heart Rhythm 2017;14:e504–48. [DOI] [PubMed] [Google Scholar]
- 12. Tarakji KG, Mittal S, Kennergren C, Corey R, Poole JE, Schloss E et al. Antibacterial envelope to prevent cardiac implantable device infection. N Engl J Med 2019;380:1895–905. [DOI] [PubMed] [Google Scholar]
- 13. Bongiorni MG, Kennergren C, Butter C, Deharo JC, Kutarski A, Rinaldi CA et al. ; ELECTRa Investigators. The European Lead Extraction ConTRolled (ELECTRa) study: a European Heart Rhythm Association (EHRA) registry of transvenous lead extraction outcomes. Eur Heart J 2017;38:2995–3005. [DOI] [PubMed] [Google Scholar]
- 14. Mathur G, Stables RH, Heaven D, Ingram A, Sutton R. Permanent pacemaker implantation via the femoral vein: an alternative in cases with contraindications to the pectoral approach. Europace 2001;3:56–9. [DOI] [PubMed] [Google Scholar]
- 15. Neuzil P, Reddy V, Merkely B, Geller L, Molnar L, Bednarek J et al. Implantable intravascular defibrillator: defibrillation thresholds of an intravascular cardioverter-defibrillator compared with those of a conventional ICD in humans. Heart Rhythm 2014;11:210–5. [DOI] [PubMed] [Google Scholar]
- 16. Higgins SL. Biventricular ICD placement percutaneously via the Iliac Vein: case reports and review. J Innov Card Rhythm Manag 2017;8:2784–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ellestad MH, French J. Iliac vein approach to permanent pacemaker implantation. Pacing Clin Electrophysiol 1989;12:1030–3. [DOI] [PubMed] [Google Scholar]
- 18. Myung-Jin C, Jae-Sun U, Tae Hoon K, Eue-Keun C, Boyoung J, Hui-Nam P et al. Two cases of transhepatic implantation of cardia implantable electronic device: all roads lead to Rome. Int J Arrhythm 2017;18:209–14. [Google Scholar]
- 19. Cui K, Feng Y, Li X, Fang Y. Percutaneous transhepatic venous access for ICD implantation in a patient with Ebstein's anomaly with ventricular tachycardia post-Glenn operation. J Cardiovasc Electrophysiol 2013;24:832–3. [DOI] [PubMed] [Google Scholar]
- 20. Elayi CS, Allen CL, Leung S, Lusher S, Morales GX, Wiisanen M et al. Inside-out access: a new method of lead placement for patients with central venous occlusions. Heart Rhythm 2011;8:851–7. [DOI] [PubMed] [Google Scholar]
- 21. Tonko JB, Black S, Rinaldi CA. “Inside-out” central venous access approach with infraclavicular exit for right sided CRT-D Implantation in bilateral brachiocephalic and superior vena cava occlusion. Clin Case Reports 2021;9:e03980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Molina JE. Surgical options for endocardial lead placement when upper veins are obstructed or non-usable. J Interv Card Electrophysiol 2004;11:149–54. [DOI] [PubMed] [Google Scholar]
- 23. Kumar P, Baker M, Gehi AK. Comparison of single-coil and dual-coil implantable defibrillators: a meta-analysis. JACC Clin Electrophysiol 2017;3:12–9. [DOI] [PubMed] [Google Scholar]
- 24. Aoukar PS, Poole JE, Johnson GW, Anderson J, Hellkamp AS, Mark DB et al. No benefit of a dual coil over a single coil ICD lead: evidence from the sudden cardiac death in heart failure trial. Heart Rhythm 2013;10:970–6. [DOI] [PubMed] [Google Scholar]
- 25. Rodríguez‐Mañero M, Kreidieh B, Ibarra‐Cortez SH, Álvarez P, Schurmann P, Dave AS et al. Coronary vein defibrillator coil placement in patients with high defibrillation thresholds. J Arrhythmia 2019;35:79–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Cooper JA, Latacha MP, Soto GE, Garmany RG, Gleva MJ, Chen J et al. The azygos defibrillator lead for elevated defibrillation thresholds: implant technique, lead stability and patient series. Pacing Clin Electrophysiol 2008;31:1405–10. [DOI] [PubMed] [Google Scholar]
- 27. Cesario D, Bhargava M, Valderrabano M, Fonarow GC, Wilkoff B, Shivkumar K. Azygos vein lead implantation: a novel adjunctive technique for implantable cardioverter defibrillator placement. J Cardiovasc Electrophysiol 2004;15:780–3. [DOI] [PubMed] [Google Scholar]
- 28. Nilsson KR, Jackson KP. Hemiazygous coil placement for high-defibrillation thresholds in a patient with a right-sided implantable cardioverter defibrillator. Pacing Clin Electrophysiol 2012;35:e10–12. [DOI] [PubMed] [Google Scholar]
- 29. Markewitz A, Kaulbach H, Mattke S, Müller D, Bernutz C, Hoffmann E et al. Influence of anodal electrode position on transvenous defibrillation efficacy in humans: a prospective randomized comparison. Pacing Clin Electrophysiol 1997;20:2193–9. [DOI] [PubMed] [Google Scholar]
- 30. Bar-Cohen Y, Takao CM, Wells WJ, Saxon LA, Cesario DA, Silka MJ. Novel use of a vascular plug to anchor an azygous vein ICD lead. J Cardiovasc Electrophysiol 2010;21:99–102. [DOI] [PubMed] [Google Scholar]
- 31. Van Gelder BM, Scheffer MG, Meijer A, Bracke FA. Transseptal endocardial left ventricular pacing: an alternative technique for coronary sinus lead placement in cardiac resynchronization therapy. Heart Rhythm 2007;4:454–60. [DOI] [PubMed] [Google Scholar]
- 32. Gradaus R, Block M, Seidl K, Brunn J, Isgro F, Hammel D et al. Defibrillation efficacy comparing a subcutaneous array electrode versus an “active can” implantable cardioverter defibrillator and a subcutaneous array electrode in addition to an “active can” implantable cardioverter defibrillator: results from active can versus array trials I and II. J Cardiovasc Electrophysiol 2001;12:921–7. [DOI] [PubMed] [Google Scholar]
- 33. Munsif AN, Saksena S, DeGroot P, Krol RB, Mathew P, Giorgberidze I et al. Low‐energy endocardial defibrillation using dual, triple, and quadruple electrode systems. Am J Cardiol 1997;79:1632–9. [DOI] [PubMed] [Google Scholar]
- 34. Kempa M, Budrejko S, Drelich Ł, Królak T, Raczak G, Kozłowski D. Implantation of additional subcutaneous array electrode reduces defibrillation threshold in ICD patients—preliminary results. AOMS 2013;3:440–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Madan N, Gaynor JW, Tanel R, Cohen M, Nicholson S, Vetter V et al. Single finger subcutaneous defibrillation lead and “active can”: a novel minimally invasive defibrillation configuration for implantable cardioverter defibrillator implantation in a young child. J Thoracic Cardiovasc Surg 2003;126:1657–9. [DOI] [PubMed] [Google Scholar]
- 36. Gradaus R, Hammel D, Kotthoff S, Böcker D. Non thoracotomy implantable cardioverter defibrillator placement in children: use of subcutaneous array leads and abdominally placed ICDs in children. J Cardiovasc Electrophysiol 2001;12:356–60. [DOI] [PubMed] [Google Scholar]
- 37. Boersma L, Barr C, Knops R, Theuns D, Eckardt L, Neuzil P et al. ; EFFORTLESS Investigator Group. Implant and midterm outcomes of the subcutaneous implantable cardioverter-defibrillator registry: the EFFORTLESS study. J Am Coll Cardiol 2017;70:830–41. [DOI] [PubMed] [Google Scholar]
- 38. Knops R, Olde Nordkamp L, Delnoy P, Boersma LV, Kuschyk J, El-Chami MF et al. PRAETORIAN Investigators. Subcutaneous or transvenous defibrillator therapy. N Engl J Med 2020;383:526–36. [DOI] [PubMed] [Google Scholar]
- 39. Gold MR, Lambiase PD, El-Chami MF, Knops RE, Aasbo JD, Bongiorni MG et al. Primary results from the understanding outcomes with the S ICD in primary prevention patients with low ejection fraction (UNTOUCHED) trial. Circulation 2021;143:7–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Quast AF, Baalman SW, Betts TR, Boersma LV, Bonnemeier H, Boveda S et al. Rationale and design of the PRAETORIAN-DFT trial: a prospective randomized CompArative trial of SubcutanEous ImplanTable CardiOVerter-DefibrillatoR ImplANtation with and without DeFibrillation testing. Am Heart J 2019;214:167–74. [DOI] [PubMed] [Google Scholar]
- 41. Nordkamp LRA, Warnaars JLF, Kooiman KM, de Groot JR, Rosenmöller BRAM, Wilde AAM et al. Which patients are not suitable for a subcutaneous ICD: incidence and predictors of failed QRS-T-wave morphology screening. J Cardiovasc Electrophysiol 2014;25:494–9. [DOI] [PubMed] [Google Scholar]
- 42. Randles DA, Hawkins NM, Shaw M, Patwala AY, Pettit SJ, Wright DJ. How many patients fulfil the surface electrocardiogram criteria for subcutaneous implantable cardioverter-defibrillator implantation? Europace 2014;16:1015–21. [DOI] [PubMed] [Google Scholar]
- 43. Kettering K, Mewis C, Dörnberger V, Vonthein R, Bosch RF, Seipel L et al. Experience with subcutaneous ICD leads: a comparison among three different types of subcutaneous leads. Pacing Clin Electrophysiol 2004;27:1355–61. [DOI] [PubMed] [Google Scholar]
- 44. Lewis GF, Gold MR. Safety and efficacy of the subcutaneous implantable defibrillator. J Am Coll Cardiol 2016;67:445–54. [DOI] [PubMed] [Google Scholar]
- 45. Suzuki S, Watanabe H, Yoshii S, Kaga S, Honda Y, Ishikawa N et al. Alternative technique for implanting an implantable cardioverter defibrillator in infants. Ann Thorac Surg 2008;86:1701–3. [DOI] [PubMed] [Google Scholar]
- 46. Killu AM, Naksuk N, Starek Z, DeSimone CV, Syed F, Gaba P et al. A novel defibrillation tool: percutaneously delivered, partially insulated epicardial defibrillation. JACC Clin Electrophysiol 2017;3:747–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Lau KC, Gaynor JW, Fuller SM, Smoots KA, Shah MJ. Long term atrial and ventricular epicardial pacemaker lead survival after cardiac operations in pediatric patients with congenital heart disease. Heart Rhythm 2015;12:566–73. [DOI] [PubMed] [Google Scholar]
- 48. Paech C, Kostelka M, Dähnert I, Flosdorff P, Riede FT, Gebauer RA. Performance of steroid eluting bipolar epicardial leads in paediatric and congenital heart disease patients: 15 years of single centre experience. J Cardiothorac Surg 2014;9:84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Caliskan E, Fischer F, Schoenrath F, Emmert MY, Maisano F, Falk V et al. Epicardial left ventricular leads via minimally invasive technique: a role of steroid eluting leads. J Cardiothor Surg 2017;12:95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Brady PA, Friedman PA, Trusty JM, Grice S, Hammill SC, Stanton MS. High failure rate for an epicardial ICD lead: implications for long term follow up of patients with an ICD. J Am Coll Cardiol 1998;31:616–22. [DOI] [PubMed] [Google Scholar]
- 51.Tonko JB, Blauth C, Rosenthal E, Rinaldi CA. Completely epicardial ICD and CRT-D Systems: a case series and systematic literature review. Pacing Clin Electrophysiolog 2021. Article in Press. DOI: 10.1111/pace.14318 [DOI] [PubMed] [Google Scholar]
- 52. Mah DY, Prakash A, Porras D, Fynn-Thompson F, DeWitt ES, Banka P. Coronary artery compression from epicardial leads: more common than we think. Heart Rhythm 2018;15:1439–47. [DOI] [PubMed] [Google Scholar]
- 53. Stefanelli CB, Bradley DJ, Leroy S, Dick M 2nd, Serwer GA, Fischbach PS. Implantable cardioverter defibrillator therapy for life-threatening arrhythmias in young patients. J Interv Cardiol 2002;6:235–44. [DOI] [PubMed] [Google Scholar]
- 54. Nolan RL, McAdams HP. Bronchopericardial fistula after placement of an automatic implantable cardioverter defibrillator: radiographic and CT findings. AJR Am J Roentgenol 1999;172:365–8. [DOI] [PubMed] [Google Scholar]
- 55. Carreras EM, Duncan WJ, Djurdjev O, Campbell AI. Cardia strangulation following epicardial pacemaker implantation: a rare pediatric complication. J Thorac Cardiovasc Surg 2015;149:522–7. [DOI] [PubMed] [Google Scholar]
- 56. Roque C, Trevisi N, Silberbauer J, Oloriz T, Mizuno H, Baratto F et al. Electrical storm induced by cardiac resynchronization therapy is determined by pacing on epicardial scar and can be successfully managed by catheter ablation. Circ Arrhythm Electrophysiol 2014;7:1064–9. [DOI] [PubMed] [Google Scholar]
- 57. Vuorinen AM, Pakarinen S, Jaakkola I, Holmström M, Kivistö S, Kaasalainen T. Clinical experience of magnetic resonance imaging in patients with cardiac pacing devices: unrestricted patient population. Acta Radiol 2019;60:1414–21. [DOI] [PubMed] [Google Scholar]
- 58. Shah AD, Morris MA, Hirsh DS, Warnock M, Huang Y, Mollerus M et al. MRI safety in nonconditional pacemaker and defibrillator recipients: a meta analysis and systematic review. Heart Rhythm 2018;15:1001–8. [DOI] [PubMed] [Google Scholar]
- 59. Winkler F, Dave H, Weber R, Gass M, Balmer C. Long-term outcome of epicardial implantable cardioverter-defibrillator systems in children: results justify its preference in paediatric patients. Europace 2018;20:1484–90. [DOI] [PubMed] [Google Scholar]
- 60. Tomaske M, Prêtre R, Rahn M, Bauersfeld U. Epicardial and pleural ICD leads in children and adolescents maintain functionality over 5 years. Europace 2008;10:1152–6. [DOI] [PubMed] [Google Scholar]
- 61. Müller MJ, Dieks JK, Backhoff D, Schneider HE, Ruschewski W, Tirilomis T et al. Efficacy and safety of non-transvenous cardioverter defibrillators in infants and young children. J Interv Card Electrophysiol 2019;54:151–9. [DOI] [PubMed] [Google Scholar]
- 62. Bhagwandien RE, Kik C, Yap SC, Szili-Torok T. Substernal ICD lead implantation in a patient not suitable for subcutaneous ICD Implantation without venous access due to superior vena cava syndrome. Heart Rhythm Case Rep 2017;3:97–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Do K, Lee CC, Kiankhooy A, Chang PM, Doshi RN. Substernal subcutaneous implantable cardioverter-defibrillator lead placement for the management of inappropriate shocks. Heart Rhythm Case Rep 2019;5:407–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Chan JY, Lelakowski J, Murgatroyd FD, Boersma LV, Cao J, Nikolski V et al. Novel extravascular defibrillation configuration with a coil in the substernal space: the ASD clinical study. JACC Clin Electrophysiol 2017;3:905–10. [DOI] [PubMed] [Google Scholar]
- 65. Crozier I, Haqqani H, Kotschet E, Shaw D, Prabhu A, Roubos N et al. First-in-human chronic implant experience of the substernal extravascular implantable cardioverter-defibrillator. JACC Clin Electrophysiol 2020;6:1525–36. [DOI] [PubMed] [Google Scholar]
- 66. Boersma LV, Merkely B, Neuzil P, Crozier IG, Akula DN, Timmers L et al. Therapy from a novel substernal Lead: the ASD2 Study. JACC Clin Electrophysiol 2019;5:186–96. [DOI] [PubMed] [Google Scholar]
- 67. Sholevar DP, Tung S, Kuriachan V, Leong-Sit P, Roukoz H, Engel G et al. ; SPACE Study Investigators. Feasibility of extravascular pacing with a novel substernal electrode configuration: the substernal pacing acute clinical evaluation (SPACE) study. Heart Rhythm 2018;15:536–42. [DOI] [PubMed] [Google Scholar]
- 68. https://clinicaltrials.gov/ct2/show/NCT04060680 (14 April 2021, date last accessed).
- 69. Tjong FV, Brouwer TF, Smeding L, Kooiman KM, de Groot JR, Ligon D et al. Combined leadless pacemaker and subcutaneous implantable defibrillator therapy: feasibility, safety and performance. Europace 2016;18:1740–7. [DOI] [PubMed] [Google Scholar]
- 70. Tjong FV, Brouwer TF, Koop BE, Soltis B, Shuros A, Schmidt B et al. Acute and 3-month performance of a communicating leadless antitachycardia pacemaker and subcutaneous implantable defibrillator. JACC Clin Electrophysiol 2017;3:1487–98. [DOI] [PubMed] [Google Scholar]
- 71. Tjong FV, Koop BE. The modular cardiac rhythm management system: the EMPOWER leadless pacemaker and the EMBLEM subcutaneous ICD. Herzschrittmacherther Elektrophysiol 2018;29:355–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Sidhu BS, Gould J, Porter B, Elliott M, Mehta V, Niederer S et al. Completely leadless cardiac resynchronization defibrillator system. JACC Clin Electrophysiol 2020;6:588–9. [DOI] [PubMed] [Google Scholar]
- 73. Rickard J, Johnston DR, Price J, Tedford R, Baranowski B, Bassiouny M et al. Reverse ventricular remodelling and long term survival in patients undergoing cardiac resynchronization with surgically versus percutaneously placed left ventricular pacing leads. Heart Rhythm 2015;12:517–23. [DOI] [PubMed] [Google Scholar]
- 74. Ailawadi G, LaPar DJ, Swenson BR, Maxwell CD, Girotti ME, Bergin JD et al. Surgically placed left ventricular leads provide similar outcomes to percutaneous leads in patients with failed coronary sinus lead placement. Heart Rhythm 2010;7:619–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Olgin JE, Lee BK, Vittinghoff E, Morin DP, Zweibel S, Rashba E et al. Impact of wearable cardioverter-defibrillator compliance on outcomes in the VEST trial: as-treated and per protocol analyses. J Cardiovasc Electrophysiol 2020;31:1009–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Nguyen E, Weeda ER, Kohn CG, D’Souza BA, Russo AM, Noreika S et al. Wearable cardioverter-defibrillators for the prevention of sudden cardiac death: a meta-analysis. J Innov Card Rhythm Manag 2018;9:A7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data is available in a public repository that issues datasets with DOIs.