As access to safe and effective COVID-19 vaccinations expands, vaccine hesitancy among people with rheumatic diseases has become increasingly important.1, 2 Before widespread vaccination programmes, potential side-effects and flares after COVID-19 vaccination were frequent reasons for vaccine hesitancy among people with rheumatic disease.3, 4 The study objective was to describe the perceptions and behaviours of people with rheumatic disease regarding COVID-19 vaccination and to identify the information sources most likely to affect their intention to be vaccinated.
The COVID-19 Global Rheumatology Alliance (C19-GRA) Vaccine Survey has been described elsewhere (appendix pp 16–93).5 Briefly, the survey was constructed collaboratively and iteratively with input from multiple patient partners and was launched globally in English on April 2, 2021. 11 translations were subsequently released (Italian, Hebrew, French, Punjabi, Russian, Spanish, Arabic, Traditional Chinese [Mandarin], Turkish, Simplified Chinese, Hindi). Rheumatologists and patient-facing organisations disseminated the survey on social media and the C19-GRA website. The survey was anonymous, the project was approved by Boston Children's Hospital Institutional Review Board and participants provided consent at the survey start.
This analysis includes 7005 vaccinated and unvaccinated respondents from 102 countries who were aged 18 years or older and provided consent, reported one or more rheumatic disease (excluding osteoarthritis and fibromyalgia), and completed the entire survey (April 2 to July 5, 2021; appendix p 2). We asked, “If one of the approved vaccines to prevent COVID-19 was available to you right now at no cost, would you agree to be vaccinated?”. Respondents answering, “Yes, I have already received at least one dose”, or “Yes, I will get it when it is available”, were classified as willing. Those answering, “No”, were classified as unwilling, and the remainder selected, “Unsure”, and were classified as unsure. Vaccination willingness was also measured on a visual analogue scale from 0 to 10, with 0 indicating not willing at all and 10 very willing (appendix p 3). Vaccination perceptions were assessed using 15 statements with five-point Likert scale response options (appendix pp 6–7). Respondents also reported factors influencing vaccination willingness and ranked information sources most likely to influence their decision, such as doctors, news media, and social media (appendix pp 4–5). Results of the survey are described by means, SDs, and proportions.
Of 7005 respondents, 5548 (79·2%) had already received a COVID-19 vaccine, 883 (12·6%) were willing to be vaccinated (when a vaccine became available to them), 275 (3·9%) were unvaccinated and unsure, and 299 (4·3%) were unvaccinated and unwilling to receive a vaccine. Of the 1457 unvaccinated respondents, 883 (60·6%) were willing to receive a vaccine and 574 (39·4%) were unsure or unwilling to receive a vaccine. The mean age for all 7005 respondents was 53·2 years (SD 14·2). Of the 7005 respondents, 5367 (76·6%) reported race or ethnicity as White, 680 (9·7%) as other, 511 (7·3%) as Hispanic or Latin American, 212 (3·0%) as Asian (south or east Asian), 124 (1·8%) as Middle Eastern or North African, 93 (1·3%) as Black, and 18 (0·3%) as American Indian, Alaska Native, Aboriginal, Indigenous, or First Nations. 6023 (86·0%) respondents were female, 954 (13·6%) were male, and 28 (0·4%) were other or preferred not to say; 3619 (51·7%) resided in the WHO Region of the Americas, and 3119 (44·5%) were taking one or more disease-modifying antirheumatic drug (DMARD). Of the total respondents, 580 (8·3%) reported previous adverse reactions to other vaccines within 2 months of the vaccination and 5295 (75·6%) respondents reported receiving regular influenza vaccinations. Demographics, clinical characteristics, comorbidities, and relevant response proportions are provided in the appendix (pp 8, 12).
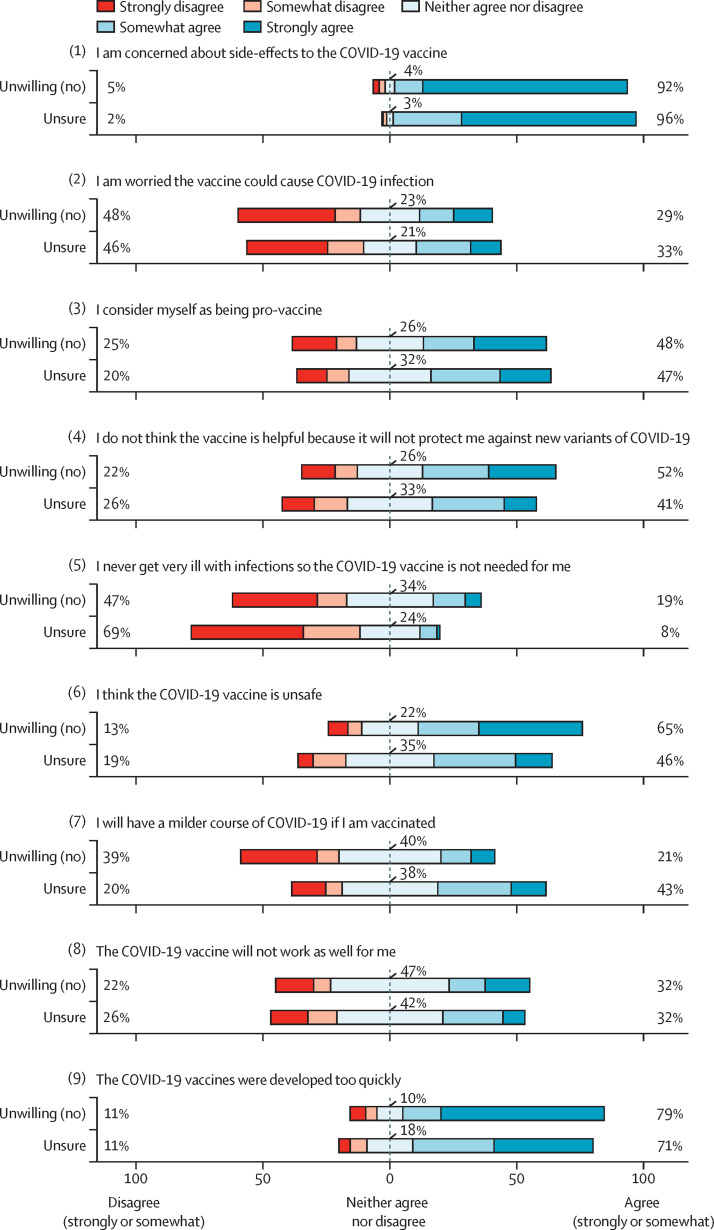
Almost all unsure or unwilling respondents expressed concerns about side-effects, safety, and the rapid development and use in clinical practice of COVID-19 vaccines. However, nearly half still considered themselves pro-vaccine, and many unwilling respondents displayed varying degrees of hesitancy (appendix p 3). Logistical challenges, cost, and efficacy concerns were less common. The majority of unsure (271 [98·5%] of 275) and unwilling (200 [66·9%] of 299) respondents reported that their willingness to be vaccinated could increase, particularly with rheumatologist endorsement and more outcomes data. 562 (97·9%) of 574 respondents who were unwilling or unsure of being vaccinated ranked the top three sources of information most likely to influence their decision for vaccination. Doctors or other health professionals were most commonly ranked in the top three among 479 (85·2%) of 562 respondents who were uncertain or unwilling respondents (appendix p 5). Patient or professional organisations were second most commonly ranked in top three sources, cited by 388 (69·0%) uncertain or unwilling respondents (appendix p 5). The news media (74 [13·2%]), political figures (17 [3·0%]), and advertisements (eight [1·4%]) were most infrequently cited in the top three sources of information most likely to influence the decision to be vaccinated (appendix p 5).
Figure 1.
Vaccination perceptions among people with rheumatic diseases who were unsure of vaccination or unwilling to become vaccinated (n=574)
Percentages were based on the total number of people for whom the question was applicable.
These survey results highlight the critical importance of concerns related to vaccine safety and efficacy for people with rheumatic diseases, which appear to have persisted after widespread vaccination. Vaccine-related adverse events do occur and include both mild reactions (eg, fatigue, myalgias, and headaches)2 and very rare severe adverse reactions (eg, central venous thrombosis and myocarditis).6, 7 People with rheumatic diseases might also be concerned about flares of their underlying disease, but surveys suggest that flares requiring changes to medications are uncommon.5 Many of the respondents who were unsure or unwilling also questioned the benefits of vaccination. However, formal risk–benefit assessments have consistently found that the benefits of vaccination far outweigh potential risks,8 and rheumatology professional societies have concluded that the benefits of vaccination outweigh safety concerns for people with rheumatic diseases.9
It should be emphasised that even respondents who were unwilling to be vaccinated reported varying degrees of hesitancy, and two-thirds reported that their willingness could be increased. As with earlier surveys,3 rheumatologist endorsement was commonly a crucial factor that increased willingness to be vaccinated. Patient or professional organisations were also frequently ranked highly as credible sources of information, and information about vaccination in people like them was the second most important factor in increasing willingness to be vaccinated. Taken together, these findings suggest that advice from physicians or patient and professional organisations focusing on safety and efficacy in people with similar rheumatic diseases might be most productive to encourage vaccination. Evidence-based strategies for communicating this information should be considered, such as affirming patient values, framing vaccination in terms of personal gain or altruistic behaviour, and providing a strong recommendation.10
Strengths of this study included participation across numerous countries and in multiple languages and a large sample of respondents with a wide array of rheumatic diseases. This study also has limitations, which include selection bias (ie, survey disseminated via patient-facing organisations and social media; non-response rate cannot be calculated), potentially limited generalisability ie, (White respondents from English-speaking countries with graduate or professional degrees were over-represented), and response bias (ie, vaccinated people being more willing to fill out a survey about vaccination). This was a cross-sectional descriptive study, and causal inferences are not possible.
In summary, in this large international survey of people with rheumatic diseases, most people with vaccine hesitancy would consider becoming vaccinated. Data regarding the safety and efficacy of COVID-19 vaccination among people with rheumatic diseases, which is delivered by rheumatologists or patient and professional organisations, might increase vaccine uptake. These findings highlight urgent research and educational priorities to combat vaccine hesitancy in people with rheumatic diseases.
MP, KK, and ES contributed equally and are co-first authors. JHS, JASp, and JFS contributed equally and are co-senior authors. The authors thank Berk Degirmenci, Christele Feliix, Shangyi Jin, Candace A Palmerlee, Andrea Peirce, Lisa G Rider, Esra Sari, Robert Tseng, and Leslie Wang for their invaluable contributions to the GRA Vax Survey. MP, KK, ES, SES, and JWL contributed to data collection, data quality control, and data analysis and interpretation. AAA, DA-R, SA, RPB, FB, IB, YPEC, RC, AD-G, ED, KLD, TAG, CLH, RH, BFH, EH, LK, AK, AHJK, DFLL, CL, EFM, BM, SM, MN, ADS, JASi, NS, MFU-G, JW, KJY, and EAZ-T, critically revised the manuscript and provided intellectual content. TTM, CH, MJL, ML, GF, and LT contributed to planning and data collection, reviewed the manuscript, and provided important intellectual content. SB, WC, RG, PMM, PCR, PS, ZSW, and JY contributed to the acquisition, analysis, and interpretation of the data. JASp, JFS, and JSH directed the work, designed the data collection methods, and contributed to the analysis and interpretation of the data. MP, KK, ES, SES, JWL, SB, WC, RG, PMM, PCR, PS, ZSW, JY, JASp, JFS, and JSH drafted and revised the manuscript critically for important intellectual content and gave final approval of the version to be published. SES, JWL, KK, JFS, and JASp had full access to the data and verify the credibility of the underlying data. All authors have read, revised, and approved this manuscript and take final responsibility for the decision to submit for publication. MP reports clinical trials participation with AbbVie and grants from Rheumatology Research Foundation, outside the submitted work. ES is a board member of the Canadian Arthritis Patient Alliance, a patient run, volunteer-based organisation whose activities are primarily supported by independent grants from pharmaceutical companies. JWL has received research grant funding from Pfizer unrelated to this work. SES reports research funding related to clinical trials from AstraZeneca (MANDARA), outside of the submitted work and is supported by the Vasculitis Clinical Research Consortium and Vasculitis Foundation outside of the submitted work. DA-R is a scientific advisor for GlaxoSmithKilne unrelated to this work. RC reports speaker fees from Janssen, Roche, Sanofi, and AbbVie, outside of the submitted work. AD-G reports grants from the Center for Disease Control and Prevention, Rheumatology Research Foundation, and Mayo Clinic, outside the submitted work. KLD is an unpaid volunteer president of the Autoinflammatory Alliance and reports grants from Novartis, Sobi, National Institutes of Health (NIH), and Horizon Bio, all received by the non-profit organisation outside of the submitted work. CLH received funding under a sponsored research agreement unrelated to the data in the paper from Vifor Pharmaceuticals. RH reports grants from AbbVie, Amgen, Boehringer Ingleheim, Johnson and Johnson, Lilly, Novartis, Pfizer, and Union Chimique Belge, all paid to Spondylitis Association of America, consultant fees from GlaxoSmithKline and Novartis, outside the submitted work. RH also owns stocks (<20 shares and representing <4% of personal investments) in AbbVie, Amgen, Bristol Myers Squibb, GlaxoSmithKline, Johnson & Johnson, Eli Lilly, Merck, Novartis, Pfizer, Teva, and Union Chimique Belge. AHJK reports personal fees from Exagen Diagnostics, Alexion Pharmaceuticals, and Aurinia Pharmaceuticals, grants from National Institutes of Health, Rheumatology Research Foundation, and Helmsley Charitable Trust, grants and personal fees from GlaxoSmithKline, outside the submitted work. EFM reports personal fees from Boehringer Ingelheim, and that Liga Portuguesa Contra as Doenças Reumaticas has received grants from AbbVie, Novartis, Lilly Portugal, Amgen Biofarmacêutica, Grünenthal, Merck Sharp & Dohme, Medac and from A Menarini Portugal–Farmacêutica; grants and non-financial support from Pfizer and Grünenthal, outside the submitted work. JASi has received consultant fees from Crealta/Horizon, Medisys, Fidia, PK Med, Two labs, Adept Field Solutions, Clinical Care options, Clearview healthcare partners, Putnam associates, Focus forward, Navigant consulting, Spherix, MedIQ, Jupiter Life Science, United BioMed, Trio Health, Medscape, WebMD, and Practice Point communications; and the National Institutes of Health, and the American College of Rheumatology. JASi owns stock options in TPT Global Tech, Vaxart pharmaceuticals, and Charlotte's Web Holdings and previously owned stock options in Amarin, Viking and Moderna pharmaceuticals. JASi is on the speaker's bureau of Simply Speaking and is a member of the executive of Outcomes Measures in Rheumatology, an organisation that develops outcome measures in rheumatology and receives funding from eight companies. JASi also serves on the FDA Arthritis Advisory Committee and is the chair of the Veterans Affairs Rheumatology Field Advisory Committee. JASi is also the editor and the Director of the University of Alabama at Birmingham Cochrane Musculoskeletal Group Satellite Center on Network Meta-analysis. MFU-G has received research support from Pfizer and Janssen, unrelated to this work. SB reports non-branded consulting fees from Novartis, AbbVie, Pfizer, and Horizon Pharma, outside the submitted work, and is a Pfizer employee as of September, 2021. RG reports personal fees from AbbVie New Zealand, Cornerstones, Janssen New Zealand, and Novartis, and personal fees and non-financial support Pfizer Australia (all <AU$10,000) outside the submitted work. PMM reports personal fees from AbbVie, Eli Lilly, Janssen, Novartis, Pfizer, and Union Chimique Belge; and grants and personal fees from Orphazyme, outside the submitted work. PCR reports personal fees from AbbVie, Gilead, Lilly, and Roche; grants and personal fees from Novartis, Union Chimique Belge, Janssen, and Pfizer; and non-financial support from Bristol Myers Squibb, outside the submitted work. PS reports honoraria from bring the social media editor for the American College of Rheumatology journals, outside the submitted work. ZSW reports grants from NIH, Bristol Myers Squibb, and Principia/Sanofi; and personal fees from Viela Bio and MedPace, outside the submitted work. JY reports personal fees from Pfizer and Eli Lilly, and grants and personal fees from AstraZeneca, outside the submitted work. CH reports personal fees from AstraZeneca and Aurinia Pharmaceuticals, outside the submitted work. MJL reports grants from American College of Rheumatology, during the conduct of the study and consulting fees from AbbVie, Amgen, Actelion, Boehringer Ingelheim, Bristol Myers Squibb, Celgene, Gilead, Johnson and Johnson, Mallinckrodt, Novartis, Pfizer, Roche, Sandoz, Sanofi, Sobi, and Union Chimique Belge, outside the submitted work. JSH reports grants from Childhood Arthritis and Rheumatology Research Alliance and Rheumatology Research Alliance, and personal fees from Novartis, Pfizer, and Biogen, outside the submitted work. JASp reports grants from National Institute of Arthritis and Musculoskeletal and Skin Diseases, Rheumatology Research Foundation, and R Bruce and Joan M Mickey Research Scholar Fund; and consulting fees for AbbVie, Boehringer Ingelheim, Bristol Myers Squibb, Gilead, Inova Diagnostics, Optum, and Pfizer, unrelated to this work. JFS received research grant funding from the National Institutes of Health unrelated to this work (NIAMS R01 AR077103, and NIAID R01 AI154533). All other authors report no competing interests. This study was funded by the American College of Rheumatology (ACR). The ACR was not involved in any aspect of study design, collection, analysis, or interpretation of data, writing of the report, or the decision to submit the paper for publication. The views expressed here are those of the authors and participating members of the COVID-19 Global Rheumatology Alliance and do not necessarily represent the views of the ACR, the European Alliance of Associations for Rheumatology, the UK National Health Service, the National Institute for Health Research, or the UK Department of Health, or any other organisation. Researchers interested in performing additional analyses from survey data are invited to submit proposals through the COVID-19 Global Rheumatology Alliance at rheumcovid.org. For approved projects, we will provide summary tables and data analyses as requested. We do not currently have institutional review board approval to make the raw data available to other researchers.
Supplementary Material
References
- 1.Machingaidze S, Wiysonge CS. Understanding COVID-19 vaccine hesitancy. Nat Med. 2021;27:1338–1339. doi: 10.1038/s41591-021-01459-7. [DOI] [PubMed] [Google Scholar]
- 2.Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA COVID-19 vaccine. New Engl J Med. 2020;383:2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Felten R, Dubois M, Ugarte-Gil MF, et al. Vaccination against COVID-19: expectations and concerns of patients with autoimmune and rheumatic diseases. Lancet Rheumatol. 2021;3:e243–e245. doi: 10.1016/S2665-9913(21)00039-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vieira Rezende RP, Braz AS, Guimarães MFB, et al. Characteristics associated with COVID-19 vaccine hesitancy: a nationwide survey of 1000 patients with immune-mediated inflammatory diseases. Vaccine. 2021;39:6454–6459. doi: 10.1016/j.vaccine.2021.09.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sattui SE, Liew JW, Kennedy K, et al. Early experience of COVID-19 vaccination in adults with systemic rheumatic diseases: results from the COVID-19 Global Rheumatology Alliance Vaccine Survey. RMD Open. 2021;7 doi: 10.1136/rmdopen-2021-001814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Østergaard SD, Schmidt M, Horváth-Puhó E, Thomsen RW, Sørensen HT. Thromboembolism and the Oxford–AstraZeneca COVID-19 vaccine: side-effect or coincidence? Lancet. 2021;397:1441–1443. doi: 10.1016/S0140-6736(21)00762-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim HW, Jenista ER, Wendell DC, et al. Patients with acute myocarditis following mRNA COVID-19 vaccination. JAMA Cardiol. 2021;6:1196–1201. doi: 10.1001/jamacardio.2021.2828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rosenblum H. COVID-19 vaccines in adults: benefit-risk discussion. ACIP Meeting. July 22, 2021. https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2021-07/05-COVID-Rosenblum-508.pdf
- 9.Curtis JR, Johnson SR, Anthony DD, et al. American College of Rheumatology guidance for COVID-19 vaccination in patients with rheumatic and musculoskeletal diseases: version 1. Arthritis Rheumatol. 2021;73:1093–1107. doi: 10.1002/art.41734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Finney Rutten LJ, Zhu X, Leppin AL, et al. Evidence-based strategies for clinical organizations to address COVID-19 vaccine hesitancy. Mayo Clin Proc. 2021;96:699–707. doi: 10.1016/j.mayocp.2020.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.